Abstract
Aim
Biophenol‐rich nutraceuticals may be an adjuvant treatment for Crohn's disease (CD), ulcerative colitis (UC), symptomatic uncomplicated diverticular disease (SUDD), and irritable bowel syndrome (IBS). This systematic review and meta‐analysis aimed to determine the efficacy and safety of biophenol‐rich nutraceutical supplementation on CD, UC, SUDD, and IBS on gastrointestinal symptoms (GIS), quality of life (QoL), inflammatory and oxidative stress biomarkers, and adverse events compared to usual care or placebo.
Methods
PubMed, Embase, CINAHL, and CENTRAL were searched for randomised controlled trials until 27 April 2020. Outcomes were GIS, inflammatory and oxidative stress markers, QoL, and adverse events. The Cochrane Risk of Bias tool and GRADE were used to appraise studies. Data were pooled using Revman.
Results
Twenty‐three trials in CD, UC, and IBS patients were included. Compared with placebo, biophenol‐rich nutraceuticals improved GIS (SMD: 0.43 [95%CI: 0.22, 0.63]; GRADE: very low) in UC, CD, and IBS participants. In UC and CD participants, biophenol‐rich nutraceuticals improved CRP by 1.6 mg/L [95%CI:0.08, 3.11; GRADE: low], malondialdehyde by 1 mmol/L [95%CI:0.55, 1.38; GRADE: low]; but only resveratrol improved QoL (SMD: −0.84 [95%CI: −1.24, −0.44; GRADE: high). Resveratrol (for UC and CD participants) and peppermint oil (for IBS participants) had greater certainty in the evidence for improving GIS and QoL (GRADE: moderate to high). There was no effect on adverse events (P > .05).
Conclusions
Biophenol‐rich nutraceuticals may be an effective and safe adjuvant treatment for the management of CD, UC, and IBS; with higher certainty of evidence for resveratrol for UC and CD and peppermint oil for IBS.
Keywords: Crohn disease, diverticular diseases, inflammatory bowel diseases, irritable bowel syndrome, polyphenols, ulcerative colitis
1. INTRODUCTION
Inflammatory‐related gastrointestinal conditions, such as Crohn's disease (CD), ulcerative colitis (UC), symptomatic uncomplicated diverticular disease (SUDD), and irritable bowel syndrome (IBS), are an emerging global health concern, with incidence and prevalence predicted to increase worldwide. 1 , 2 , 3 Although CD, UC, SUDD, and IBS all have unique pathophysiology, they share gastrointestinal symptoms such as abdominal pain, cramping, bloating, diarrhoea, and/or constipation. 4 , 5 , 6 Inflammation and/or oxidative stress play a role in the pathophysiology of CD, UC, SUDD 7 , 8 ; however, not consistently in IBS. 9 From the patient perspective, burden is often introduced not only by the percieved symptoms, but lifelong financial and personal costs and treatment side effects, leading to reduced quality of life and suffering. 10 , 11 , 12 The rising prevalence of these conditions and subsequent hospitalisations also present a substantial burden to health‐care systems. 13
Current therapies for the treatment and management of inflammation‐related gastrointestinal conditions include a variety of medical, diet, and lifestyle recommendations 14 , 15 , 16 , 17 ; however, biophenols have recently gained interest as a possible adjuvant therapy for a range of conditions. 18 , 19 , 20 Biophenols, sometimes referred to as polyphenols, are phytochemicals found in foods such as extra virgin olive oil, peanuts, turmeric, ginger, tea, and peppermint. Although polyphenols are the more common term for such phytochmicals, they represent only phenolic compounds with two or more aromatic benzene rings. 21 Recently, there has been a move towards utilising the more scientifically accurate term of biophenols, which represent all plant‐derived phenols. 21 The benefits of biophenols have been suggested for the treatment of chronic conditions in humans, such as decreasing toxicity in hemodialysis, improving mental health and cognitive performance, managing nausea and vomiting in chemotherapy, or reducing cardiovascular disease risk. 19 , 22 , 23 , 24 , 25 , 26 , 27
The beneficial effects of biophenols are due to a variety of mechanisms, including their antioxidant, antiglycation, and anti‐inflammatory activities on glucose and lipid metabolism as well as cell proliferation and interactions with the gut microbiota. 28 , 29 , 30 More recently, there is a growing body of interventional research exploring the potential of biophenol‐rich nutraceuticals on patient‐centered outcomes in gastrointestinal conditions. 31 , 32 However, the efficacy and safety of biophenol‐rich nutraceuticals for such conditions has not been systematically reviewed and certainty in the body of evidence for their adjuvant treatment potential is unknown.
This systematic review and meta‐analysis aimed to determine the efficacy and safety of biophenol‐rich nutraceutical supplementation on CD, UC, SUDD, and IBS on gastrointestinal symptoms (GIS), quality of life (QoL), inflammatory and oxidative stress biomarkers, and adverse events compared to usual care or placebo.
2. METHODS
This systematic review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines 33 and was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO: 159820).
Relevant studies were identified through a systematic search of the Cochrane Central Library, Medline (via PubMed), Embase (via Ovid), and CINAHL databases for articles published since database inception to 27 April 2020. Controlled vocabulary search terms were used where appropriate to describe major biophenol classes, disease states, and study designs in combination with keywords (Table S1). A snowball search was also conducted, whereby reference lists of included studies and similar reviews were considered to identify additional studies not found in the systematic search strategy, up until 9 December 2019.
Inclusion criteria for participants were human adults aged ≥18 years with CD, UC, SUDD, or IBS. Where age range was not reported, samples were included if the mean age was ≥18 years. Inclusion criteria for types of studies were parallel or crossover randomised controlled trials (RCTs). Studies were included if the intervention group were treated with an orally consumed biophenol‐rich nutraceutical with no coadministration of any test product or therapy beyond standard care for more than 1‐week. Biophenol‐rich nutraceuticals were included if the test product was listed on the Phenol‐Explorer 3.6 database, which is an independent database compiling over 500 biophenols. 34 Studies were included if comparator groups were treated with standard care alone (if this standard care was also provided to the intervention group) or placebo. Studies were included if they were published in languages spoken by the authorship team (English and Chinese). Studies in other languages were included only if they could be translated to English using Google Translate Software. 35 Exclusion criteria for study designs were review or observational studies, studies that did not report associated outcomes, or studies which did not undergo peer review (ie, grey literature, conference papers, abstracts).
Following study deduplication using DeDuplicate, 36 EndNote, 37 and Covidence 2019, 38 the initial title and abstracts screening, then full‐text screening of articles, was conducted on Covidence by two investigators independently. Disagreements between reviewers were resolved by consensus or third investigator.
The primary outcome of interest was GIS; and secondary outcomes were inflammatory markers (e.g., C‐reactive protein (CRP), calprotectin, interleukin‐6 (IL‐6), interleukin‐8 (IL‐8)), oxidative stress markers, QoL, and adverse events. Adverse events were considered any patient‐reported side effect related or potentially related to the intervention or control conditions.
Data were extracted from relevant studies using the following parameters: author/date, study design, sample size and attrition, study duration, sample demographics (eg, age, sex, type of gastrointestinal condition), intervention characteristics (type of biophenol, dosing regimen, duration), and outcomes as described above. Extracted continuous outcome data were baseline, follow‐up, and change measure of central tendency (mean or median), variation or precision (SD, SE, or 95% confidence intervals (CI)) for both groups as well as P‐value for change over time and between groups. If mean change was not reported, it was calculated and the SD of the change was estimated using the Review Manager calculator (Versions 5.3 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). 39 Extracted categorical outcome data were number of events. Data were extracted by a single investigator and checked for accuracy by a second investigator, as were calculated data.
Internal study quality was assessed using the Cochrane Risk of Bias tool, which included assessing random sequence generation, allocation sequence concealment, blinding, and incomplete outcome data. 40 Risk of bias was assessed independently by two researchers.
Certainty in the body of evidence informing the estimated effect was assessed via the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) assessment tool. 41 Although GRADE may be applied to meta‐analysed or narratively synthesised data; due to the large number of potential outcomes included in this study only meta‐analysed outcomes were assessed via GRADE. Certainty in the body of evidence was judged by considering risk of bias, inconsistency, indirectness, imprecision, publication bias, effect size, dose‐response, and plausible confounding. Considering these factors, each pooled outcome was rated as having high, moderate, low, or very low certainty. The GRADE certainty in the estimated effect sizes was assessed by two investigators independently and judgements were confirmed by a third investigator. Publication bias was assessed via funnel plot.
Outcome data were pooled where adequately reported change data (i.e., difference from baseline to follow‐up) was available or could be calculated for two or more interventions using Review Manager (Versions 5.3 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). 39 Continuous outcomes were pooled using the inverse variance test and reported as mean difference or standardised mean difference (SMD) if different scales/measurement tools were used. SMD effect sizes of <0.4 were considered small, 0.4 to 0.7 moderate, and >0.7 large. 42 Pooled categorical outcomes were assessed using Mantel‐Haenszel test and reported as odds ratios (OR). Heterogeneity was evaluated with the I 2 statistic, where a value >50% was considered to represent substantial statistical heterogeneity. 43 P < .05 was considered as statistically significant. Pre‐registered sensitivity analyses were undertaken in models with substantial statistical heterogeneity using participant demographics, study quality, differences in measurement tools, intervention factors, or confounding variables. Type of inflammatory‐related gastrointestinal condition and type of nutraceutical administered were explored for significant subgroup effects.
3. RESULTS
The systematic search identified 3008 records and a further four were identified through the snowball search. After deduplication and title/abstract screening, the full text of 93 articles were evaluated for eligibility and 23 were included (Figure S1). Most RCTs had a low to unclear risk of bias and two studies had a high risk of bias (Figure 1; justifications Table S2). There were 10 RCTs included where funding was received from a source with a potential interest in a positive result (Tables 1 and 2).
FIGURE 1.
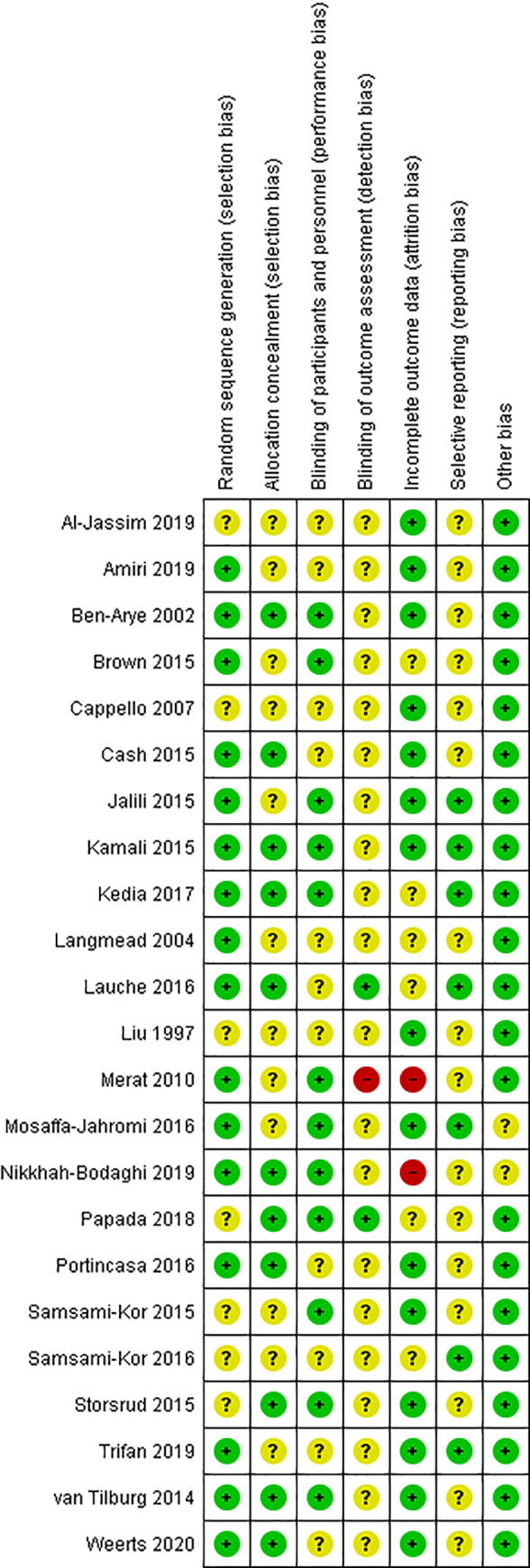
Risk of bias summary of randomised controlled trials examining the effect of biophenol‐rich nutraceuticals on gastrointestinal symptoms and related outcomes in adults with inflammatory related gastrointestinal conditions
TABLE 1.
Characteristics and findings of n = 9 included randomised controlled trials which examined orally consumed biophenol‐rich nutraceuticals in participants with inflammatory bowel disease
| Characteristics | Intervention | Comparator | Outcomes a |
|---|---|---|---|
| Yarrow (Achillea wilhelmsii C. Koch) 44 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N 49 (IG: n = 23, CG: n = 26), n = 9 (18%) attrition. Mild‐to‐moderate active UC: 53% F, μ34.5 y (range 19‐55 y). Funding and COI not described. |
n = 20 at FU. Type: Capsule with 0.124 mg/g powdered caffeic acid extract from fresh aerial parts of A. Wilhelmsii C. Koch. Dose: 2 capsules/day (1 capsule BDS). Duration: 1 month. |
n = 20 at FU. Type: Placebo capsule with hydroxypropyl methylcellulose powder. Dose: 2 capsules/day (1 capsule BDS). Duration: 1 month. |
Adverse events: IG: n = 1/23. CG: 0/26. GIS—Partial Mayo Score (score b ): IG: baseline 5.35 ± 1.69, FU 2.45 ± 2.16, change −2.9 ± 0.46, P < .001. CG: baseline 5 ± 1.94, FU 2.65 ± 1.89, change −2.35 ± 0.46, P < .001. Inflammatory marker—CRP (mg/L): IG: baseline 5.4 ± 0.3, FU 2.6 ± 1.4, change −2.8 ± 5.88, P = .04. CG: baseline 0.5 ± 0.1, FU 0.65 ± 0.35, change 0.15 ± 5.88, P = .91. P = .12 between groups. |
| Aloe vera 45 | |||
|
DBPCRCT, 2‐arms, parallel: United Kingdom, N = 44 (IG: n = 30, CG: n = 14), n:9 (20%) attrition. Mild‐to‐moderately active UC: 50%F, IG μ40 y (22‐76 y), CG μ36 y (20‐55 y). Independently funded. No COI declared. |
n = 30 at FU. Type: Aloe Vera gel (>95% of active ingredient—active ingredient not specified). Dose: 100 mL BDS, Starting with 25 to 50 mL BDS. Duration: 4 weeks |
n = 14 at FU. Type: Liquid placebo containing flavorings, no known active agents, matched for taste and appearance. Dose: Starting with 25 to 50 mL BDS. Duration: 4 weeks. |
Adverse effects: IG: n = 6/30, CG: CG: 4/14. GIS—SCCAI (score b ): IG: baseline median 6.5 (IQR: 5.2‐8.2), FU median 6.0 (IQR: 2.0‐9.0), change −0.5 ± 5.2, P = .01. CG: baseline 6.1 (4.7‐7.6), FU median 4.9 (IQR: 3.3‐7.5), change −1.2 ± 3.1 P = .33. Inflammatory marker—CRP (mg/L): IG: baseline median 5 (IQR: 4‐11), FU median 4 (IQR 4‐9), change −1 ± 4.4, P = .33. CG: baseline median 5 (IQR: 4‐8), FU median 4 (IQR: 3‐9), change −1 ± 3.7, P = .22. P > .05 between groups. Quality of life—IBDQ (score c ): IG: baseline median 4.4 (IQR: 3.2‐5.0), FU median 4.8 (3.8‐5.7), change 0.4 ± 1.4, P > .05. CG: baseline median 4.6 (IQR: 3.6‐5.1), FU median 5.8 (IQR: 4.8‐5.9), change 1.2 ± 0.8, P < .05). |
| Curcumin 46 | |||
|
DBPCRCT, 2‐arms, parallel: India, N = 62 (IG: n = 29, CG: n = 33), n = 21 (34%) attrition. Mild‐to‐moderate UC: 34%F, IG μ36 ± 12 y, CG: μ34 ± 7 y. Independently funded, no COI declared. |
n = 16 at FU. Type: Curcumin and mesalamine. Dose: 2.4 g/d (1 capsule TDS; each capsule 150 mg curcumin). Duration: 8 weeks |
n = 25 at FU. Type: Placebo and mesalamine. Dose: 2.4 g/d (1 capsule TDS). Duration: 8 weeks |
Adverse events: IG: 0/16, CG: 1/25. GIS—DAI (score b ): IG: baseline 5.2 ± 2.0, FU 3.4 ± 3.1, change −1.8 ± 0.76, P = .711. CG: baseline 5.5 ± 1.9, FU 3.8 ± 2.8, change −1.7 ± 0.76, P = .711. |
| Mastic tree (Pistacia lentiscus) 47 , 48 | |||
|
DBPCRCT, 2‐arms, parallel: Greece, N = 60 (IG = 33, CG = 27), n = 14 (23.3%) attrition. CD and UC: %F NR, 18‐67 y. Funding associated with test product; no COI declared. |
n = 26 at FU (ITT used) Type: Natural mastiha tablets. Dose: 2.8 g/d (700 mg tablet QDS). Duration: 12 weeks. |
n = 20 at FU (ITT used). Type: Placebo capsule. Dose: NR QDS. Duration: 12 weeks. |
GIS—HBI (score b ): IG: baseline 7.8 ± 2.3, FU: 4.7 ± 3.8, change −3.1±4.1, P < .001. CG: baseline 6.1 ± 1.8, FU: 4.7 ± 2.6, change −1.4 ± 2.6, P = .055. P = .0635 between groups. GIS—Partial Mayo Score b : IG: baseline 2.8 ± 1.8, FU: 2.0 ± 1.3, change −0.9 ± 2.0, P = .481. CG: baseline 3.2 ± 2.0, FU: 2.2 ± 1.6, change −1.0 ± 2.1, P = .055. P = .324 between groups. Inflammatory marker—calprotectin (ug/g): IG: baseline 1688.6 ± 1712.4, FU: 2744 ± 4910.6, change 1055.4 ± 5043.1, P = .289. CG: 2170.6 ± 444.4, FU: 3598.5 ± 3620.4, change 1427.9 ± 5606.1, P = .029. P = .348 between groups. Inflammatory marker—IL6 (pg/mL): IG: baseline 11.5 ± 12.3, FU: 15.7 ± 13.3, change 4.2 ± 9.7, P = .021. CG: baseline 14.4 ± 16.8, FU: 24.3 ± 43.8, change 9.9 ± 33.6, P = .030. P = .955 between groups. Inflammatory marker—IL10 (pg/mL): IG: baseline 3.1 ± 2.7, FU: 3.1 ± 2.7, change 0.0 ± 3.6, P = .951. CG: baseline 8.8 ± 18.9, FU: 9.5 ± 20.1, change 0.6 ± 3.4, P = .454. P = .607 between groups. Inflammatory marker—CRP (mg/L): IG: baseline 6.8 ± 8.2, FU: 5.7 ± 5.9, P = .94, change −1.2 ± 8.3, P = .41. CF: baseline 6.4 ± 7.6, FU: 5.3 ± 4.8, P = .76, change −1.1 ± 8, P = .48. P = .79 between groups. Oxidative stress marker—oxLDL (U/l): IG: baseline 160.42 ± 34.26, FU: 140.12 ± 41.91, change −20.3 ± 51.7, P = .031. CG: baseline 135.3 ± 48.38. FU: 135.45 ± 38.92, change 0.15 ± 52.76, P > .05. P > .05 between groups. Quality of life—IBDQ (score c ): IG: baseline 145.2 ± 27.3, FU: 163.4 ± 30.6, change −18.3 ± 26.4, P = .0004. CG: baseline 144.9 ± 27.3, FU: 155.1 ± 33.3, change −10.2 ± 43.6, P = .23. P > .0.5 between groups. |
| Pomegranate peel 49 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N = 78 (IG: n = 39, CG: n = 39), n = 16 (21%) attrition. UC: 45%F, IG: μ41.7 y, CG: μ37.8 y. Independently funded. No COI declared. |
n = 29 at FU. Type: Aqueous extract of the P. granatum peel. Dose: 8 mL syrup (6 g dry peel)/days (4 mL BDS). Duration: 4 weeks. |
n = 33 at FU. Type: Placebo syrup: USP simple syrup formula + approved additives (Amaranth). Matched for appearance and smell. Dose: 8 mL of the placebo syrup (4 mL BDS). Duration: 4 weeks. |
Adverse events: Urticaria: IG: n = 2/29, CG: n = 2/33. Nausea: IG: n = 2/29, CG: n = 1/33. Increased appetite: IG: n = 2/29, CG: n = 3/33. P > .05 between groups. GIS—LCAI (score b ): IG: baseline 6.34 ± 1.98, FU: 4.68 ± 3.48, change −1.68 ± 3.85, P = .019. CG: baseline 5.57 ± 1.75, FU 4.18 ± 2.62, change −1.39 ± 2.41, P = .002. |
| Resveratrol 50 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N = 56 (IG: n = 28, CG: n = 28), n = 3 (5%) attrition. Mild‐to‐moderate active UC: %F NR, IG: μ37.43 ± 16.54 y, CG: μ38.78 ± 11.65y. Independently funded. No COI declared. |
n = 27 at FU (ITT). Type: Resveratrol supplements. Dose: 500 mg trans‐resveratrol, 1 capsule daily. Duration: 6 weeks. |
n = 26 at FU (ITT). Type: Placebo capsule (medium chain triglycerides), 1 daily. Duration: 6 weeks. |
Adverse events: IG: n = 0/28, CG: n = 0/28. GIS—SCCAI (score b ): IG: baseline 11.67 ± 2.72, FU: 8.14 ± 2.1, change −3.53 ± 1.81, P < .001. CG: baseline 10.88 ± 2.69, FU: 9.34 ± 2.65, −1.54 ± 1.81, P < .001. Oxidative stress marker—MDA (mmol): IG: baseline 5.62 ± 1.18, FU: 3.42 ± 1.01, change −2.2 ± 4.16, P = .0094. CG: baseline 5.26 ± 1.15, FU: 6.93 ± 1.12; change 1.67 ± 4.16, P = .0430. Oxidative stress marker—SOD (U/mL): IG: baseline 122.28 ± 11.55, FU 125.77 ± 10.97, change 3.49, P < .01. CG: baseline 120.94 ± 16.13, FU 113.47 ± 114.85, change −7.47, P < .01. P = .01 between groups. Oxidative stress marker—TAC (U/mL): IG: baseline 9.87 ± 1.51, FU: 11.97 ± 1.61, change −2.1 ± 2.9, P = .0007. CG: baseline 10.02 ± 2.02, FU 9.41 ± 1.82,change 0.61 ± 2.9, P = .2755. P < .001 between groups. Quality of life—IBDQ‐9 (score c ): IG: baseline 34.85 ± 7.67, FU: 47.64 ± 8.59, change 12.79 ± 8.74, P < .01. CG: baseline 35.67 ± 9.89, FU: 41.08 ± 8.59, change 5.41 ± 8.74, P < .01. |
| Resveratrol 51 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N = 50 (IG: n = 25, CG: n = 25), n = 1 (2%) attrition. Mild to moderate active UC: %F NR, IG μ38 y, CG μ39 y. Funding and COI not described. |
n = 25 at FU. Type: Resveratrol capsule. Dose: 500 mg/d/capsule, 1 daily. Duration: 6 weeks. |
n = 24 at FU. Type: Placebo capsule (medium‐chain triglyceride) Dose: NR, 1 daily. Duration: 6 weeks. |
GIS—SCCAI (score b ): IG: baseline 12.34 ± 2.51, FU: 8.41 ± 2.1, change −3.93 ± 2.23, P < .001. CG: baseline 10.76 ± 2.55, FU: 9.34 ± 2.65, change −1.42 ± 2.23, P < .001. P < .001 between groups. Inflammatory marker—CRP (ng/ml): IG: baseline 4764.25 ± 2260.48, FU: 2584.50 ± 1792.80, change −2179.75. CG: baseline 3158.67 ± 2419.55, CU 3538.42 ± 2348.93, change 389.75. P < .001 between groups. Inflammatory marker—TNF‐α (pg/mL): IG: baseline 19.70 ± 12.80, FU: 17.20 ± 10.09, change −2.5, P < .01. CG: 20.53 ± 13.34, FU: 23.59 ± 14.82, change 3.06, P < .01. P≤ .001 between groups. Quality of life—IBDQ‐9 (score c ): IG: baseline 32.72 ± 7.52, FU: 47.64 ± 8.59, change 14.92 ± 10.9, P < .01. CG: baseline 35.54 ± 9.50, FU: 41.08 ± 8.59, change 5.54 ± 10.91, P < .01. P < .001 between groups. |
| Wheat grass 52 | |||
|
DBPCRCT, 2‐arms, parallel: Israel, N = 24 (IG: n = 12, CG: n = 12)m n = 3 (12%) attrition. Active UC, involving left colon: 33%F, μ35 y. Funding and COI not described. |
n = 10 at FU. Type: 100 mL unstandardised fresh wheat grass juice. Dose: Increased from 20 mL/d, optimal dose 100 mL/d by d 5. Duration: 4 weeks. |
n = 11 at FU. Type: Placebo juice. Dose: Increased from 20 mL/d, optimal dose 100 mL/d by d 5. Duration: 4 weeks. |
Adverse events: IG: n = 7/10, CG: n = 0/11. |
| Ginger (Zingiber officinale) 53 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N = 64 (IG: n = 32, CG: n = 32), n = 18 (28.1%) attrition. CD and UC: 35%F, IG: μ41.4 y, CG: μ39.2 y. Independently funded. No COI reported. |
n = 24 at FU. Type: Fried ginger powder capsule. Dose: 2000 mg/d (500 mg/capsule QID). Duration: 12 weeks. |
n = 24 at FU. Type: Placebo (maltodextrin powder) capsule. Dose: 2000 mg/d (500 mg/capsule QID). Duration: 12 weeks. |
GIS—SCCAI (score b ): IG: baseline 7.6 ±4.03, FU 4.05 ± 1.23, change −3.55 ± 3.56, P = .438. CG: baseline 6.2 ± 3.22, FU 5.55 ± 2.39, change −0.65 ± 3.56, P = .194. P = .017 between groups. Oxidative stress marker—MDA (mmol): IG: baseline 8.33 ± 1.82, FU: 3.87 ± 1.95, change −4.46 ± 2.84, P = .016. CG: baseline 7.88 ± 2.24, FU 6.38 ±2.42, change −1.5 ± 2.84, P = .000. P < .001 between groups. Oxidative stress marker –TAC (U/mL): IG: baseline 1.9 ± 1.2, FU: 2.16 ± 1.16, −0.26 ± 0.58, P = .04. CG: baseline 1.99 ± 1.33, FU: 2.17 ± 1.17, change −0.18 ± 0.58, P = .14. P = 0.64 between groups. Quality of life: IBDQ (score c ): IG: baseline 44.22 ± 9.79, FU 47.23 ± 9.24, change 3.01 ± 9.6, P = .039. CG: baseline 43.12 ± 6, FU 41.87, change −1.25 ± 9.6, P = .636. P = .140 between groups. |
Abbreviations: BDS, twice daily; CD, Crohn's disease; CG, control group; COI, conflict of interest; CRP, c‐reactive protein; DAI, disease activity index; d, day; DBPCRCT, double blind placebo controlled randomised controlled trial; F, female; FU, follow‐up; GIS, gastrointestinal symptoms; HBI, Harvey‐Bradshaw Index; IBDQ, inflammatory bowel disease questionnaire; IG, intervention group; IQR, interquartile range; ITT, intention to treat; LCAI, Lichtiger colitis activity index; MDA, malondialdehyde; NR, not reported; QDS, four times daily; SCCAI, Simple Clinical Colitis Activity Index; SOD, superoxide dismutase; TAC, total antioxidant capacity; TDS, thrice daily; TNF‐α, tumour necrosis factor alpha; UC, ulcerative colitis; y, years.
Continuous outcome data reported as mean (μ) ± SD and categorical outcome data reported as number of events/number of participants, unless otherwise specified.
Higher score indicates worse symptoms/activity.
Higher score indicates better symptoms.
TABLE 2.
Characteristics and findings of n = 14 included randomised controlled trials which examined orally consumed biophenol‐rich nutraceuticals in participants with irritable bowel syndrome
| Characteristics | Intervention | Comparator | Outcomes a |
|---|---|---|---|
| Aloe vera 54 | |||
|
DBPCRCT, 2‐arms, parallel: Sweden, N = 68 (IG = 33, CG = 35), 7.4% attrition. IBS: 75%F, IG: μ43.9 y, μ44.2 y. No funding or COI reported. |
n = 32 at FU. Type: 250 mg Aloe barbadensis Mill. Extract (AVH200), 60 mg ascorbic acid dissolvable tablet. Dose: 500 mg/d (250 mg BD). Duration: 4‐weeks. |
n = 31 at FU. Type: 60 mg ascorbic acid and excipients dissolvable table. Dose: 120 mg/d (60 mg BD). Duration: 4‐weeks. |
GIS—IBS‐SSS (score b ): IG: baseline 315 ± 83, FU: 257 ± 107, change −58 ± 1.1.9, P = .003. CG: baseline 276 ± 88, FU: 253 ± 100, change −23 ± 69.0, P > .05. P = .03 between groups. |
| Anise oil 55 | |||
|
DBPCRCT, 3‐arms [2 eligible], parallel: Iran, N = 80 (IG = 40, CG = 40), 6.25% attrition. IBS: 48.8%, IG μ34.15 y, CG μ32.35 y. No funding or COI declared. |
n = 38 at FU. Type: Enteric coated anise oil capsule. Dose: 600 mg/d (200 mg TDS). Duration: 4 weeks. |
n = 37 at FU. Type: Enteric coated placebo capsule. Dose: NR. Duration: 4 weeks. |
Adverse event: IG: n = 0/38. CG n = 0/37. GIS—Abdominal discomfort VAS (score b ) IG: baseline 5.97 ± 2.35, FU 1.82 ± 1.43, change −4.15 ± 4.8. CG: baseline 5.27 ± 2.12, FU 3.33±1.74, change −1.94 ± 2.7. P < .0001 between groups. Quality of life—IBS‐QOL (score c ): IG: baseline 86.20 ± 27.13, FU 64.38±23.10, change −21.8. CG baseline 83.30 ± 21.64, FU 72.82±18.20, change −10.5. P < .0001 between groups. |
| Mixed biophenols 56 | |||
|
DBPCRCT, 2‐arms, cross‐over, no washout: Romania, N = 60 (IG: n = 30, CG: n = 30), 0% attrition. IBS‐D: 73%F, μ35 y. No funding or COI declared. |
n = 30 at FU. Type: Gelsectan capsule (containing xyloglucan, pea protein and tannins from grape seed extract, and xylo‐oligosaccharides) Dose: not clear, 1 capsule BD. Duration: 28 days. |
n = 30 at FU. Type: Placebo capsule (not clear). Dose: not clear, 1 capsule BD. Duration: 28 days. |
Adverse events: IG: n = 0/30, CG: n = 0/30. GIS—incidence normal stools: IG baseline 1/60, FU 54/60, P = .0019. CG baseline 26/60, FU 7/60, P = .0001. On day 28, the proportion of patients with normal stools, GIS—IBS‐D incidence abdominal pain: IG baseline 38/60, FU: 0/60, P = .002. CG 25/60, FU 27/60, P = .027. GIS—IBS incidence bloating: IG baseline 38/60, FU 1/60, P = .028. CG baseline 25/60, FU 27/60, P = .041. Quality of life—IBS‐QOL and EQ‐5D‐3L: data represented graphically only. |
| Mixed biophenols 57 | |||
|
DBPCRCT, 2‐arms, cross‐over, 1‐week washout: Germany, N = 30 (IG: n = 15, CG: n = 15), n = 11 (34%) attrition. IBS‐D: 59%F, μ50.3 ± 11.9 y. No funding or COI declared. |
n = 20 in the second phase; n = 5 in the third phase. Type: Ayurvedic herbal compound preparation made from curry (Murraya Koenigii), pomegranate (Punica granatum) and turmeric (Curcuma longa) in a 6:3:1 ratio. Dose: 10 g (5 g of powder dissolved in 100 mL of warm water BD). Duration: 4 weeks. |
n = 6 in the second phase. n = 17 in the third phase. Type: Placebo (hay Graminis flores dep. and Maidis stigmata). Dose: 140 g. Duration: 4 weeks. |
Adverse events: IG: n = 9/15. CG: n = 4/15. GIS—IBS‐SSS (score b ): IG: baseline 217.4 ± 91.4, FU: 220.3 ± 90.0, change 2.9 ± 33.64, P = .74. CG: baseline 226.0 ± 81.8, FU 215.0 ± 89.9, change −11 ± 33.64, P = .23. No significant differences between the IG and CG phases. Quality of life—IBS‐QOL (score c ): IG: baseline 69.9 ± 21.6, FU: 72.1 ± 19.0, 2.2 ± 65.83, P = .89. CG: baseline 68.2 ± 19.1 CG: FU 67.4 ± 22.3, change −0.8 ± 65.83, P = .96. P > .05 between groups. |
| Mixed biophenols 58 | |||
|
DBPCRCT, 2‐arms, parallel: United States, N: 16 (IG: n = 8, CG: n = 8), n = 3 (19%) attrition. IBS‐C: 81%F, μ38 y (23‐57 y). Funding and authors affiliated with test product. |
n = 7 at FU. Type: blended extracts in capsule (Quebracho, Conker tree, M. balsamea Willd, peppermint oil). Dose: Quebracho 150 mg (80‐82% polyphenol content), Conker tree—470 mg (20% saponin content), M. balsamea Willd oil—0.2 mL; pure peppermint oil (content quantity not specified). Duration: 2 weeks. |
n = 6 at FU. Type: Placebo capsule. Dose: NR. Duration: 2 weeks. |
Adverse events: IG: n = 0/8, CG: n = 0/8. GIS—Constipation and bloating (score c ): IG: change 2.62±0.886, P < .001. CG: change 0.28 ± 0.39, P = .141. |
| Mixed biophenols 59 | |||
|
DBPCRCT, 2‐arms, parallel: Italy, N = 21 (IG n = 60, CG n = 61), 4.1% attrition. IBS: 63.6%F, IG μ41.4 y, CG μ39.4 y. Funding associated with test product. No COI declared. |
n = 58 at FU. Type: blended essential oils (curcumin and fennel) capsule. Dose: 84 mg/d curcumin and 50 mg fennel essential oil (42 mg curcumin and 25 mg fennel essential oil/capsule BD). Duration: 4 weeks. |
n = 58 at FU. Type: Placebo capsule. Dose: not clear BD. Duration: 4 weeks. |
Adverse events: IG: 1.7%. CG 3.4%. GIS—IBS‐SSS (score b ): IG: baseline 255.7 ± 39.9, FU: 127.8 ± 77.4, change −127.9 ± 119.89. CG: baseline 263.2 ± 34.3, FU: 195.5 ± 88.0, change −67.7 ± 119.89. P < .001. P < .05 between groups. Quality of life—IBS‐QOL (score c ): IG: change 17.4 ± 19.2. CG: change 7.7 ± 18.0, P = .003. |
| Peppermint oil 60 | |||
|
DBPCRCT, 2‐arms, parallel: Italy, N = 57 (IG: n = 28, CG: n = 29), n = 7 (12%) attrition. IBS: 76%F, μ41 y (20–60). No funding or COI declared. |
n = 24 at FU. Type: Enteric‐coated, gastro‐protected peppermint oil capsules. Dose: 550 mg (225 mg peppermint oil +45 mg of Natrasorb BD). Duration: 4 weeks. |
n = 26 at FU. Type: Placebo capsules. Dose: 55 mg maltodextrin (225 mg of maltodextrin with mint flavour BD). Duration: 4 weeks. |
Adverse events: IG: n = 1/24. CG: n = 0/26. GIS—incidence ≥50% reduction of total IBS symptoms score: IG: n = 18/24, P < .01. CG: n = 10/26, P < .05. P < .05 between groups. |
| Peppermint oil 61 | |||
|
DBPCRCT, 2‐arms, parallel: United States, N = 72 (IG: n = 35, CG: n = 37), n = 2 (2.7%) attrition. IBS‐M and IBS‐D: 75%F, IG μ40.2 y, CG μ41 y. Independently funded. COI declared. |
n = 34 at FU. Type: Peppermint oil capsules. Dose: 540 mg (180 mg TDS). Duration: 4 weeks. |
n = 36 at FU. Type: Placebo capsules. Dose: NR TDS. Duration: 4 weeks. |
Adverse events: IG: n = 2/34, CG: n = 4/36. GIS—TISS (score b ): IG: change −1.16 ± 0.897, P = .0246. CG: change −0.70 ± 0.737. GIS—incidence of severe symptoms: IG change −66.8%. CG: change −24.9%. P = .0282 between groups. |
| Peppermint oil 62 | |||
|
DBPCRCT, 2‐arms, parallel: China, N = 110 (IG: n = 55, CG: n = 55), n = 9 (8.1%) attrition. IBS: 40%F, 18‐70 y. Funding and COI were not described. |
n = 52 at FU. Type: Enteric coated peppermint oil capsule Dose: 561‐748 mg/d (187 mg/capsule x 3‐4/d) Duration: 4‐weeks. |
n = 49 at FU. Type: Placebo capsule Dose: 3‐4/d, dose not reported. Duration: 4‐weeks. |
GIS—Incidence abdominal pain: IG: n = 41/51(79%) participants improved. CG: n = 21/49(43%) participants improved. Significant difference between groups (P < .05). |
| Peppermint oil 63 | |||
|
DBPCRCT, 3‐arms, parallel: Netherlands, N = 189 (unclear number of patients in each group), 5.8% attrition. IBS: 78%F, μ34.4 y. No funding or COI declared. |
IG 1: n = 62 at FU. Type: Peppermint oil capsule. Dose:182 mg/d (60.3 mg TDS). Duration: 8‐weeks. IG 2: n = 62 at FU. Type: Peppermint oil capsule. Dose:182 mg/d (60.3 mg TDS). Duration: 8 weeks. |
n = 64 at FU. Type: Placebo capsule. Dose: Not reported. Duration: 8 weeks. |
Adverse events incidence: IG1 4.26 ±0.37. IG2 4.54± 0.45. CG 2.78 ±0.34. GIS—IBS‐SSS (score b ): IG1: baseline 284.52±95.28, FU 192.99±121.65, change −91.53±66.28, P = .00. IG2 baseline 285.42±92.47, FU 201.05±120.17, change −84.37±63.34 P = .00. CG baseline 276.53±93.44, FU 226.80±119.76, change −49.73 ± 66.28, P = .00. P = .020 between IG1 and CG. P = .22 between IG2 and CG. Quality of life—IBS QOL (score c ): IG1: baseline 68.8 ± 2.6, FU: 75.9 ± 2.6, change 7.1, P = .066. IG2: baseline 69.8 ± 2.5, FU 75.5 ± 2.5, change 5.7, P = .374. CG baseline 70.5 ± 2.5, FU 75.0 ± 2.5. |
| Peppermint oil 64 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N = 0 (IG = 45, CG = 45), 33.3% attrition. IBS: 75%F, μ36 y. Independently funded. COI not described. |
n = 33 at FU. Type: Peppermint oil capsule Dose: 546 mg/d (182 mg TDS). Duration: 8 weeks. |
n = 27 at FU. Type: Placebo capsule. Dose: NR TDS. Duration: 8 weeks. |
Adverse events: IG: n = 19/33. CG n = 14/27. GIS—incidence abdominal pain free: IG: baseline n = 0/33, FU n = 14/33. CG: baseline n = 0/27, FU n = 6/2. P < .001 between groups. Quality of life—SF‐36 (score c ): IG baseline 57.8 ± 29.3, FU: 63.0 ± 28.2. CG baseline 48.9 ± 29.8, FU: 53.7 ± 26.4. P = .194 between groups. |
| Soy isoflavones 65 | |||
|
DBPCRCT, 2‐arms, parallel: Iran, N = 67 (IG: n = 35, CG: n = 32), n = 22 (33%) attrition. IBS: 72%F, IG: μ45.5 y, CG: 40.0 y. Independently funded. No COI declared. |
n = 22 at FU. Type: Soy isoflavones capsule Dose: 40 mg of isoflavones (10 mg of diadzein, 8.5 mg genistein, 1.5 mg glycetin BD). Duration: 6 weeks. |
n = 23 at FU. Type: Placebo capsule (corn starch). Dose: 1 capsule BD. Duration: 6 weeks. |
Adverse events: IG: n = 0/22. CG: n = 0/23. GIS—IBS‐SSS (score b ): IG: baseline 23.64 ± 8.17, FU: 12.77 ± 8.16, change −10.87 ± 7.85, P = .00. CG: baseline 24.78 ± 11.82, FU: 19.74 ± 12.08, change −5.04 ± 7.85, P = .00. No difference between groups P = .068. Quality of life—IBS‐QOL (score c ): IG: baseline 64.41 ± 27.78, FU 41.68 ± 28.47, change −22.73 ± 228.31, P = .65. CG: baseline 46.70 ± 31.37, FU: 44.17 ± 33.47, change −2.53 ± 228.31, P = .96. P = .009 between groups. |
| Ginger (Zingiber officinale) 66 | |||
|
DBPCRCT, 3‐arms, parallel: Iran, N = 50 (IG = 25, CG = 25), 2% attrition. IBS: age and sex NR. Funding and COI NR. |
IG1: n = 15 at FU. Type: Ginger capsule. Dose: 1 g/day. Duration: 28 days. IG 2: n = 15 at FU. Type: Ginger capsule. Dose: 2 g/day. Duration: 28 days. |
n = 14 at FU. Type: Placebo capsule. Dose: NR. Duration: 28 days. |
Adverse events: IG: 16.7%. CG: 35.7%. GIS—IBS‐SSS (score b ): IG1: baseline 260.0 ± 65.5, FU: 191.3 ± 95.8, change −68.7 ± 84.3, P = .007. IG2: baseline 222.7±53.3, FU: 198.9 ± 88.9, change −23.8 ± 73.9, P = .233. CG: baseline 253.2 ± 65.9, FU: 165.0±49.3, change—88.2 ± 78.2, P = .001. P > .05 between groups. |
| Ginger (Zingiber officinale) 67 | |||
|
DBPCRCT, 3‐arms (2 eligible), parallel: Iran, N = 45 (IG1 n = 15, CG n = 15), 0% attrition. IBS: age and sex NR. No funding or COI declared. |
IG2: n = 15 FU. Type: Ginger root powder capsule. Dose: 1 g/day. Duration: 20 days. |
n = 15 at FU. Type: Placebo (brown sugar) capsule. Dose: NR. Duration: 20 days. |
GIS—Abdominal pain severity (score b ): IG: baseline 39.6 ± 3.29, FU: 31.73 ± 3.21, change −7.9 ± 13.5, P < .05. CG: baseline 42.46 ± 2.94, change −4.8 ± 8.7, FU: 37.66 ± 2.89, P > .05. GIS—Abdominal distention severity (score b ): IG: baseline 68.73 ± 3.68, FU: 50.00 ± 2.88. CG: baseline 66.33 ± 2.72, FU: 59.33 ± 2.43. GIS—Constipation severity (score b ): IG: baseline 70.66 ± 3.09, FU: 49.13 ± 2.7. |
Abbreviations: BDS, twice daily; CG, control group; COI, conflict of interest; CRP, c‐reactive protein; d, day; DBPCRCT, double blind placebo controlled randomied controlled trial; F, female; FU, follow‐up; GIS, gastrointestinal symptoms; IBS, irritable bowel syndrome; IBS‐c, irritable bowel syndrome constipation dominant; IBS‐d, irritable bowel syndrome diarrhoea dominant; IBS‐m, irritable bowel syndrome mixed constipation and diarrhoea; IBS‐SSS, irritable bowel syndrome symptom scoring scale; IG, intervention group; IQR, interquartile range; 4ITT, intention to treat; NR, not reported; QDS, four times daily; QOL, quality of life; SF‐36, short form 36; TDS, thrice daily; TISS, total IBS symptom score; VAS, visual analogue scale; y, years.
Continuous outcome data reported as mean (μ) ± standard deviation and categorical outcome data reported as number of events/number of participants, unless otherwise specified.
Higher scores indicate worse symptoms.
Higher scores indicate better symptoms.
All 23 included RCTs were placebo‐controlled; 21 were parallel and two were cross‐over (Tables 1 and 2). Two parallel RCTs in participants with IBS had two intervention groups; therefore, a total of 25 interventions arms were included. Most of the studies were from Iran (n = 10 RCTs), followed by the United States (n = 2 RCTs), and Italy (n = 2 RCTs). All other RCTs were from different countries across Europe, the Middle East, and Asia.
Total study sample sizes ranged from n = 16 58 to n = 189 63 (N = 1566 total participants; Tables 1 and 2). Of the 25 interventions, 16 (64%) recruited participants with active IBS (Table 2), seven (28%) with mild to moderate UC, and two (8%) with inflammatory bowel disease (IBD; i.e., UC or CD) (Table 1). No included RCT recruited participants with SUDD.
Peppermint oil was the most studied intervention (n = 5 RCTs in IBS participants), followed by ginger (Zingiber officinale) (n = 2 RCTs in IBS participants, n = 1 RCT in IBD participants), resveratrol (n = 2 RCTs in IBD participants), and aloe vera (n = 1 RCT in IBS participants, n = 1 RCT in IBD participants). Four RCTs, all in IBS participants, used an intervention product composed of mixed biophenols (a blend of two to four biophenols in powder or capsule form). Other interventions were reported by a single study (curcumin, wheat grass, anise oil, soy isoflavones, mastic tree (Pistacia lentiscus), yarrow (Achillea wilhelmsii C. Koch), pomegranate peel; Tables 1 and 2). Study durations ranged from 2 to 12 weeks. The most common mode of nutraceutical delivery was capsule form (n = 17 RCTs, 74%). Other modes of delivery included tablet (n = 2 studies), drink (n = 2 studies), syrup (n = 1 study), and gel form (n = 1 study). Of the four studies that reported compliance, adherence to the prescribed supplements ranged from 67% 64 to 100% 50 , 51 , 56 with no difference between intervention and control groups (P > .05).
Gastrointestinal symptom scores were measured using condition‐specific tools including visual analogue scales (VAS), Irritable Bowel Syndrome Symptom Scoring Scale, the Simple Clinical Colitis Activity Index, the Disease Activity Index, Lichtiger Colitis Activity Index, Partial Mayo Score, and Harvey‐Bradshaw Index (Table 2). Twenty‐three intervention groups assessing GIS that were able to be pooled with meta‐analysis used yarrow, aloe vera, curcumin, mastic tree, pomegranate peel, resveratrol, ginger, anise oil, peppermint oil, soy isoflavones, or mixed biophenols.
Meta‐analysis found that compared with placebo, biophenol‐rich nutraceuticals significantly improved GIS with a moderate effect size (SMD: 0.43 [95%CI: 0.22, 0.63], P < .0001, I 2: 64%, GRADE: very low; Table S3, n = 18 studies, n = 21 intervention groups, n = 1187 participants; Figure 2). Sensitivity analysis did not substantially improve statistical heterogeneity. GIS were not different according to subgroup by type of condition (IBD vs IBS; P = .52). Four studies that recruited participants with IBS measured GIS incidence; all of which reported significant improvements in the intervention (n = 3 studies used peppermint oil, n = 1 study used a mixed biophenol formulation (gelsectan) (Table 2). There were sufficient studies pooled for GIS to generate a funnel plot, which suggests some positive effects of the intervention have not been published (Figure S2).
FIGURE 2.
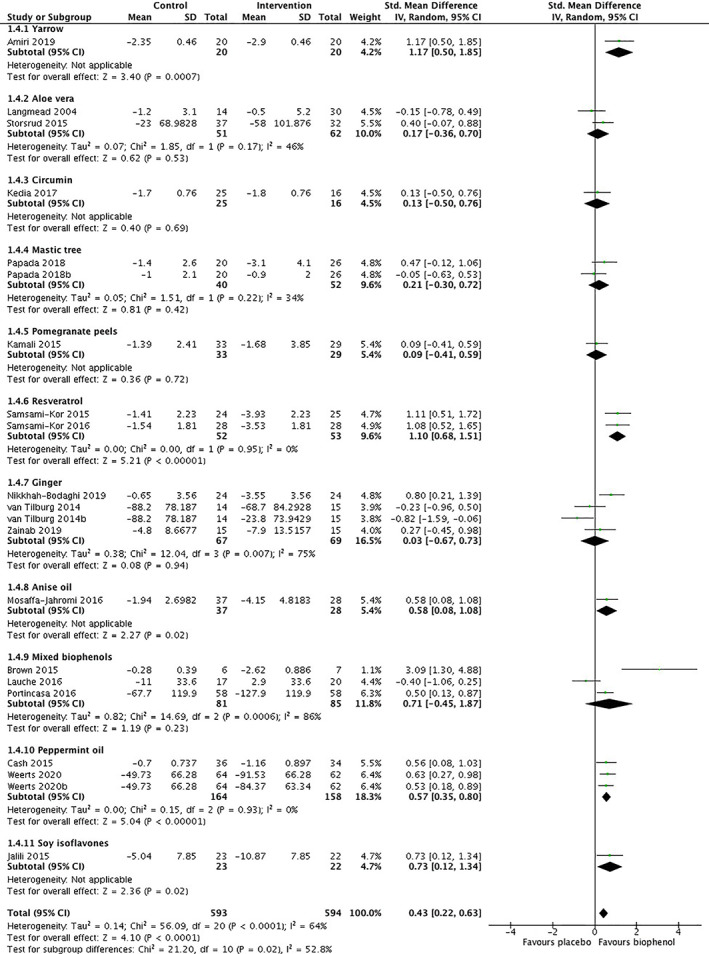
Gastrointestinal symptoms of adults with inflammatory bowel disease or irritable bowel syndrome after treatment with biophenol‐rich nutraceuticals or placebo per nutraceutical type
Subgroup analysis of nutraceutical type found significant differences in GIS between groups (P = .02, I 2: 53%). Compared with placebo, peppermint oil significantly improved GIS in participants with IBS with a moderate effect size (SMD: 0.57 [95%CI: 0.35, 0.80], P < .0001, I 2: 0%, n = 2 studies, n = 3 intervention groups, n = 322 participants, GRADE: moderate, Figure 2) and resveratrol improved GIS in participants with IBD with a large effect size (SMD: 1.10 [95%CI: 0.68, 1.51], P < .00001, I 2: 0%, n = 1 study, n = 2 intervention groups, n = 105 participants, GRADE: moderate, Figure 2). Other nutraceutical types with two or more interventions which showed no significant effect on GIS were aloe vera (SMD: 0.17 [95%CI: −0.36, 0.70], P = .53, I 2: 46%, n = 2 studies, n = 2 intervention groups, n = 113 participants, GRADE: very low, Figure 2), mastic tree (SMD: 0.21 [95%CI: −0.30, 0.72], P = .42, I2: 34%, n = 1 study, n = 2 intervention groups, n = 92 participants, GRADE: very low, Figure 2), ginger (SMD: 0.03 [95%CI: −0.67, 0.73], P = .94, I 2: 75%, n = 3 studies, n = 4 intervention groups, n = 136 participants, GRADE: very low, Figure 2), and mixed biophenols (SMD: 0.71 [95%CI: −0.45, 1.87], P = .23, I 2: 86%, n = 3 studies, n = 3 intervention groups, n = 166 participants, GRADE: very low, Figure 2).
Quality of life was mostly measured with condition specific tools such as the Inflammatory Bowel Disease Questionnaire and the Irritable Bowel Syndrome Quality of Life (Table 2). Pooling 12 intervention groups which measured and reported QoL (Tables 1 and 2) found that biophenol‐rich nutraceuticals did not improve QoL compared with placebo (SMD: −0.18 [95%CI: −0.41, 0.06], P = .14, I 2: 62%; GRADE: very low; Table S3). Condition subgroups were not significantly different (IBD vs IBS; P = 0.32); however, a significant subgroup difference was found with nutraceutical type (P = .002, Figure S3). Compared with placebo, resveratrol significantly improved QoL in participants with IBD (SMD: −0.84 [95%CI: −1.24, −0.44], P < .0001, I 2: 0%, n = 2 studies, n = 2 intervention groups, n = 105 participants, GRADE: high, Figure S3). Other nutraceutical types with two or more interventions, which showed no significant effect on QoL were peppermint oil and mixed biophenols.
No studies which recruited participants with IBS measured inflammatory or oxidative stress markers. The pro‐inflammatory marker CRP was reported by four studies (participants with UC: n = 3 studies, UC and CD: n = 1 study). Interventions assessing CRP used yarrow, mastic tree, resveratrol, or aloe vera. Compared with placebo, biophenol‐rich nutraceuticals significantly improved CRP by 1.6 mg/L (95%CI: 0.08, 3.11; P = .04; I 2: 18%, GRADE: low;Table S3, n = 4 studies, n = 4 interventions, n = 193 participants). Compared with placebo, biophenol‐rich nutraceuticals (resveratrol (n = 1 study) and ginger (n = 1 study)) significantly improved oxidative stress marker malondialdehyde by 1 mmol/L ([95%CI: 0.55, 1.38]; P < .00001; I 2: 0%, n = 2 studies, n = 2 interventions, n = 102 participants; GRADE: low) but had no effect on total antioxidant capacity (SMD: 1.36 [95%CI: −0.24, 1.30], P = .17 I2: 73%, 2 studies, 2 interventions, 102 participants; GRADE: very low). Too few studies were included in these models to conduct a sensitivity analysis. One study reported significant positive effects of resveratrol on inflammatory marker, tumour necrosis factor alpha, 51 and oxidative stress marker, superoxide dismutase. 50 Other oxidative stress markers measured were oxidised LDL, which was significantly improved with mastic tree. Other inflammatory markers measured were IL‐6, IL‐10, and calprotectin, which showed no significant effect (Table 1). 47 , 48
Adverse events were reported in 19 studies. A total of 46 and 80 adverse events were reported in control and intervention groups, respectively; however, when pooled, there was no significant difference between groups (OR: 0.68 [95%CI: 0.40, 1.15], P = .15, I 2: 21%; GRADE: low; Table S3). Adverse events were not different according to subgroup by type of condition (IBD vs IBS; P = .27) or nutraceutical type (P = .16). Adverse events were mild and mostly included gastrointestinal distress. No serious adverse events were reported.
4. DISCUSSION
This review found that adjuvant biophenol‐rich nutraceutical supplementation may improve GIS, inflammation, oxidative stress, and QoL in adults diagnosed with IBD, and GIS in participants diagnosed with IBS. Using the GRADE framework, there was low certainty that the pooled effect sizes represent the true effect due to statistical heterogeneity, publication bias, and/or imprecision. Factors leading to the substantial clinical heterogeneity included varying medical status, pathophysiology of the gastrointestinal condition, and lifestyle of participants as well as the use of different outcome measurement tools and biophenol sources. Subgroup analyses resolved some sources of heterogeneity, and found high certainty that resveratrol improved QoL in IBD with a large effect size, and moderate certainty that peppermint oil and resveratrol improved GIS with moderate and large effect sizes for IBS and IBD, respectively.
The pooled effect size of all biophenols on GIS was moderate (SMD 0.43), suggesting substantial clinical benefit of intervention, particularly peppermint oil and resveratrol. Despite the use of validated tools in measuring GIS, it is important to recognise that there is no universally accepted endpoint to assess GIS severity. This is due to several factors including the subjectivity of GIS experienced by patients, variations in the types of rating scales such as VAS vs Likert scales, the treatment of scales as continuous or ordinal, and the ceiling and floor effects of different tools. 68 Although the inconsistency found in the pooling of GIS scores across the 21 intervention groups may be contributed to by the pooling of patients with IBS and IBD, two distinct conditions, subgroup analysis did not identify any statistically significant difference in their perceptions of GIS. Publication bias contributed to the decreased certainty in the effect on GIS; however, the direction of bias suggests that the true effect size may be larger than the estimate.
The evidence supporting the use of biophenol‐rich nutraceuticals for subjective GIS improvement in IBD is strengthened by the finding of decreased objective inflammatory and oxidative stress biomarkers. Previous studies have shown the effect of biophenol‐rich nutraceuticals on improving oxidative stress and inflammation in chronic disease states including liver disease, cardiovascular disease, and kidney disease. 19 , 27 , 69 Although only measured by few studies with a limited cumulative sample size and wide confidence intervals, decreasing certainty in the estimated effects, this finding may explain the mechanism by which GIS were improved. 70 Although inflammatory and oxidative stress markers are not consistent biomarkers for IBS, future studies should measure these outcomes to better explore how inflammation and oxidative stress may play a role within IBS subtypes and if these may be modified by biophenols.
Emerging evidence suggests that biophenols may beneficially modulate inflammation, oxidation, and the gut‐brain axis via stimulation of the abundance of health promoting bacterial species and inhibition of pathogenic species in the gut. 71 Gut bacteria metabolites are linked to having important roles in reducing inflammation and oxidation as well as maintaining the health of gastrointestinal cells. Furthermore, gastrointestinal conditions such as IBD and IBS have been associated with alterations in the gut microbiota, which is thought to be related to activation of gut pain sensory pathways. 72 , 73 , 74 Gut microbiota are also thought to influence bioavailability and metabolism of biophenols, suggesting that an individual's microbiota profile may influence biophenol treatment efficacy. 71 The gut microbiota play an important role in the gut‐brain axis, which involves a bidirectional communication network between the central nervous system and the enteric nervous system. This allows the brain to influence intestinal activities, and the gut to influence mood, cognition, and mental health, which may explain the positive effects of resveratrol biophenols on QoL. 75
In this review, 19 articles reported adverse events that were mild and GIS‐related such as nausea, difficulty passing stools, loose stools, bloating, and heart burn. Meta‐analysis found no difference between intervention groups and placebo and these symptoms are also consistent with those that people with IBS and IBD commonly experience due to their underlying disease. Furthermore, no severe adverse events were associated with the intervention, suggesting that biophenol‐rich nutraceuticals are safe to be used in patients with IBD and IBS and potential benefits may outweigh possible discomfort. However, due to included studies in this review being of short duration (4‐12 weeks), caution should be taken if taken for long‐term use.
The evidence identified is limited by small sample sizes of individual studies, and broad clinical heterogeneity of both samples, gastrointestinal pathophysiology, and intervention types. Although conclusions may be drawn about the general effect of biophenol‐rich nutraceuticals, the evidence exploring the comparative effects of different types of biophenols is more limited, except for peppermint oil and resveratrol. Likewise, the current evidence is insufficient to determine optimal dosing regimens due to clinical and statistical heterogeneity. Although publication bias was detected for GIS only, it is likely to also exist for other outcomes in which there were insufficient studies to generate funnel plots. Publication bias may have been impacted by the exclusion of otherwise eligible studies on the basis of publication language. Confounding effects of dietary intakes were not reported or accounted for in multivariable models in the identified RCTs. As some studies did not report mean changes or SDs within groups, these values had to be calculated for use in the meta‐analysis, and as such, SDs are often overestimated. Finally, this study did not develop GRADE clinical recommendations.
RCTs of longer duration of 6 to 12 months, which explore optimal dosing regimens, and confirm safety are a priority, as are RCTs which deliver interventions to patients with SUDD, and explore the effect of sub‐types of IBS. Studies that explore the mechanisms of how biophenols influence biomarkers and clinical presentation of inflammatory‐related gastrointestinal conditions are needed, including exploration of the impact on the microbiome. RCTs should be sufficiently powered or allow for multivariable models to account for confounding factors, including dietary intake and lifestyle.
Biophenol‐rich nutraceutical supplementation used as an adjuvant therapy appears safe and may improve GIS, inflammation, oxidative stress, and QoL in patients with IBD and IBS, with higher certainty of evidence for peppermint oil for IBS and resveratrol for IBD. Further research is required to strengthen confidence in the body of evidence, identify ideal dosing regimens, and confirm long‐term safety.
CONFLICT OF INTEREST
The authors declare no actual or potential conflicts of interest.
AUTHOR CONTRIBUTIONS
The study concept was developed by Skye Marshall and contributed to by Joanna Giang and Xiao Lan. Joanna Giang and Xiao Lan drafted the manuscript and led the screening, data extraction, quality assessments, meta‐analysis, and GRADE assessments. Skye Marshall contributed to screening, meta‐analysis, and GRADE assessment. Megan Crichton and Wolfgang Marx contributed to quality assessments and data extraction. All authors contributed to manuscript revision. Skye Marshall provided supervision and oversight to all study components and led the response to peer review.
Supporting information
Table S1: Full search strategies with controlled vocabulary search terms and keywords.
Table S2: Risk of bias assessment justifications of randomized controlled trials of biophenol‐rich nutraceuticals for adults with inflammatory related gastrointestinal conditions
Figure S1: PRISMA flowchart of the systematic search strategy to identify randomized controlled trials of biophenol‐rich nutraceuticals for adults with inflammatory related gastrointestinal condition.
Figure S2: Funnel plot of randomized controlled trials which compare biophenol‐rich nutraceuticals vs placebo on gastrointestinal symptoms in patients with irritable bowel syndrome or inflammatory bowel disease.
Figure S3: Pooled quality of life scores of adults with inflammatory bowel disease or irritable bowel syndrome after treatment with biophenol‐rich nutraceuticals or placebo per nutraceutical type.
Table S3: GRADE assessment of the certainty in the body of evidence for biophenol‐rich nutraceutical interventions for inflammatory bowel disease and irritable bowel syndrome.
Giang J, Lan X, Crichton M, Marx W, Marshall S. Efficacy and safety of biophenol‐rich nutraceuticals in adults with inflammatory gastrointestinal diseases or irritable bowel syndrome: A systematic literature review and meta‐analysis. Nutrition & Dietetics. 2022;79(1):76-93. doi: 10.1111/1747-0080.12672
Joanna Giang and Xiao Lan contributed to this work equally.
REFERENCES
- 1. Corsetti M, Whorwell P. The global impact of IBS: time to think about IBS‐specific models of care? Therap Adv Gastroenterol. 2017;10(9):727‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. M'Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamoto‐Furusho JK, Sarmiento‐Aguilar A, Toledo‐Mauriño JJ, et al. Incidence and prevalence of inflammatory bowel disease in Mexico from a nationwide cohort study in a period of 15 years (2000‐2017). Medicine (Baltimore). 2019;98(27):e16291‐e16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence‐based medicine. World J Gastroenterol. 2014;20(22):6759‐6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fakhoury M, Negrulj R, Mooranian A, Al‐Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim DH, Cheon JH. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017;17(1):25‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fengming Y, Jianbing W. Biomarkers of inflammatory bowel disease. Dis Markers. 2014;2014:710915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gallo A, Ianiro G, Montalto M, Cammarota G. The role of biomarkers in diverticular disease. J Clin Gastroenterol. 2016;50:S26‐S28. [DOI] [PubMed] [Google Scholar]
- 9. Kim JH, Lin E, Pimentel M. Biomarkers of irritable bowel syndrome. J Neurogastroenterol Motil. 2017;23(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eberhardt F, Jenkins‐Chapman J, Nucera R, Dalwood P, Canavan R, Marshall S. A lack of knowledge and a fear of food triggers suffering in patients with a history of acute diverticulitis: an interpretative phenomenological analysis. Clin Nutr. 2019;38(S1):S180. [Google Scholar]
- 11. Buono JL, Carson RT, Flores NM. Health‐related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2017;15(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gracie DJ, Williams CJ, Sood R, et al. Negative effects on psychological health and quality of life of genuine irritable bowel syndrome–type symptoms in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017;15(3):376‐384. e375. [DOI] [PubMed] [Google Scholar]
- 13. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eberhardt F, Crichton M, Dahl C, et al. Role of dietary fibre in older adults with asymptomatic (AS) or symptomatic uncomplicated diverticular disease (SUDD): systematic review and meta‐analysis. Maturitas. 2019.57‐67 [DOI] [PubMed] [Google Scholar]
- 15. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence‐based clinical practice guidelines for inflammatory bowel disease. Journal of gastroenterology. 2018;53:305‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Staudacher HM, Lomer MC, Farquharson FM, et al. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153(4):936‐947. [DOI] [PubMed] [Google Scholar]
- 17. Nunan D, Ordóñez‐Mena JM, Roberts N, Thomas E, Mahtani K. Physical activity for treatment of irritable bowel syndrome: cochrane systematic review. Br J Gen Pract. 2019;69(suppl 1):bjgp19X703205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marx W, McCarthy AL, Marshall S, et al. Supplemental prophylactic intervention for chemotherapy‐induced nausea and emesis (SPICE) trial: protocol for a multi‐centre double‐blind placebo‐controlled randomized trial. Nutr Diet. 2018;77(1):144‐150. [DOI] [PubMed] [Google Scholar]
- 19. Marx W, Kelly J, Marshall S, Nakos S, Campbell K, Itsiopoulos C. The effect of polyphenol‐rich interventions on cardiovascular risk factors in haemodialysis: a systematic review and meta‐analysis. Nutrients. 2017;9(12):1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marx W, George ES, Mayr HL, et al. Effect of high polyphenol extra virgin olive oil on markers of cardiovascular disease risk in healthy Australian adults (OLIVAUS): a protocol for a double‐blind randomised, controlled, cross‐over study. Nutr Diet. 2020;77(5):523‐528. [DOI] [PubMed] [Google Scholar]
- 21. Obied HK. Biography of biophenols: past, present and future. Funct Foods Health Dis. 2013;3(6):230‐241. [Google Scholar]
- 22. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chong MF, Macdonald R, Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr. 2010;104(Suppl 3):S28‐S39. [DOI] [PubMed] [Google Scholar]
- 24. Crichton M, Marshall S, Marx W, McCarthy AL, Isenring E. Efficacy of ginger (Zingiber officinale) in ameliorating chemotherapy‐induced nausea and vomiting and chemotherapy‐related outcomes: a systematic review update and meta‐analysis. J Acad Nutr Diet. 2019;119(12):2055‐2068. [DOI] [PubMed] [Google Scholar]
- 25. Marx W, Kelly JT, Marshall S, et al. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta‐analysis of randomized controlled trials. Nutr Rev. 2018;76(6):432‐443. [DOI] [PubMed] [Google Scholar]
- 26. Travica N, D'Cunha NM, Naumovski N, et al. The effect of blueberry interventions on cognitive performance and mood: a systematic review of randomized controlled trials. Brain Behav Immun. 2020;85:96‐105. [DOI] [PubMed] [Google Scholar]
- 27. George ES, Marshall S, Mayr HL, et al. The effect of high‐polyphenol extra virgin olive oil on cardiovascular risk factors: a systematic review and meta‐analysis. Crit Rev Food Sci Nutr. 2019;59(17):2772‐2795. [DOI] [PubMed] [Google Scholar]
- 28. Karković Marković A, Torić J, Barbarić M, Jakobušić BC. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules. 2019;24(10):2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeh W‐J, Hsia S‐M, Lee W‐H, Wu C‐H. Polyphenols with antiglycation activity and mechanisms of action: a review of recent findings. J Food Drug Anal. 2017;25(1):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cardona F, Andrés‐Lacueva C, Tulipani S, Tinahones FJ, Queipo‐Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24(8):1415‐1422. [DOI] [PubMed] [Google Scholar]
- 31. Larussa T, Imeneo M, Luzza F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J Gastroenterol. 2017;23(14):2483‐2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao X, Liu J, Li L, Liu W, Sun M. A brief review of nutraceutical ingredients in gastrointestinal disorders: evidence and suggestions. Int J Mol Sci. 2020;21(5):1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. [DOI] [PubMed] [Google Scholar]
- 34. Neveu V, Perez‐Jiménez J, Vos F, et al. Phenol‐explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Google . Google translate. https://translate.google.com/ 2017.
- 36. Rathbone J, Carter M, Hoffmann T, Glasziou P. Better duplicate detection for systematic reviewers: evaluation of systematic review assistant‐deduplication module. Syst Rev. 2015;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. EndNote X9 [computer program]. Windows, macOS; 2019.
- 38. Covidence Systematic Review Software [computer program]. Melbourne, Australia: Veritas Health Innovation; 2019. [Google Scholar]
- 39. Review Manager Web (RevMan Web) [computer program]. The Cochrane Collaboration; 2019.
- 40. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith J, Dransfield A. Patient and carer involvement in healthcare education, service delivery and research: avoiding tokenism. Evid Based Nurs. 2019;22(3):65‐66. [DOI] [PubMed] [Google Scholar]
- 42. Higgins JPT. Green S. 17.8.2 study summaries using more than one patient‐reported outcome. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK: The Cochrane Collaboration and John Wiley & Sons Ltd; 2011. [Google Scholar]
- 43. Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews cross‐sectional study. BMC Med Res Methodol. 2011;11(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amiri M, Navabi J, Shokoohinia Y, et al. Efficacy and safety of a standardized extract from Achillea wilhelmsii C. Koch in patients with ulcerative colitis: a randomized double blind placebo‐controlled clinical trial. Complement Ther Med. 2019;45:262‐268. [DOI] [PubMed] [Google Scholar]
- 45. Langmead L, Feakins RM, Goldthorpe S, et al. Randomized, double‐blind, placebo‐controlled trial of oral Aloe vera gel for active ulcerative colitis. Aliment Pharmacol Ther. 2004;19(7):739‐747. [DOI] [PubMed] [Google Scholar]
- 46. Kedia S, Bhatia V, Thareja S, et al. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: results from a randomized double blind placebo controlled trial. World J Gastrointest Pharmacol Ther. 2017;8(2):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Papada E, Forbes A, Amerikanou C, et al. Antioxidative efficacy of a Pistacia lentiscus supplement and its effect on the plasma amino acid profile in inflammatory bowel disease: a randomised, double‐blind, placebo‐controlled trial. Nutrients. 2018;10(11):1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Papada E, Gioxari A, Amerikanou C, et al. Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: a double‐blind and placebo‐controlled randomised trial. Phytother Res. 2019;33(2):360‐369. [DOI] [PubMed] [Google Scholar]
- 49. Kamali M, Tavakoli H, Khodadoost M, et al. Efficacy of the Punica granatum peels aqueous extract for symptom management in ulcerative colitis patients. A randomized, placebo‐controlled, clinical trial. Complement Ther Clin Pract. 2015;21(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 50. Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A. Resveratrol supplementation and oxidative/anti‐oxidative status in patients with ulcerative colitis: a randomized, double‐blind placebo‐controlled pilot study. Arch Med Res. 2016;47(4):304‐309. [DOI] [PubMed] [Google Scholar]
- 51. Samsami‐Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti‐inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double‐blind, placebo‐controlled pilot study. Arch Med Res. 2015;46(4):280‐285. [DOI] [PubMed] [Google Scholar]
- 52. Ben‐Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double‐blind placebo‐controlled trial. Scand J Gastroenterol. 2002;37(4):444‐449. [DOI] [PubMed] [Google Scholar]
- 53. Nikkhah‐Bodaghi M, Maleki I, Agah S, Hekmatdoost A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: a randomized, placebo‐controlled, clinical trial. Complement Ther Med. 2019;43:1‐6. [DOI] [PubMed] [Google Scholar]
- 54. Storsrud S, Pontén I, Simren M. A pilot study of the effect of Aloe barbadensis mill. Extract (AVH200®) in patients with irritable bowel syndrome: a randomized, double‐blind, placebo‐controlled study. J Gastrointest Liver Dis. 2015;24:275‐280. [DOI] [PubMed] [Google Scholar]
- 55. Mosaffa‐Jahromi M, Lankarani KB, Pasalar M, Afsharypuor S, Tamaddon A‐M. Efficacy and safety of enteric coated capsules of anise oil to treat irritable bowel syndrome. J Ethnopharmacol. 2016;194:937‐946. [DOI] [PubMed] [Google Scholar]
- 56. Trifan A, Burta O, Tiuca N, Petrisor DC, Lenghel A, Santos J. Efficacy and safety of gelsectan for diarrhoea‐predominant irritable bowel syndrome: a randomised, crossover clinical trial. United Eur Gastroenterol J. 2019;7(8):1093‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lauche R, Kumar S, Hallmann J, et al. Efficacy and safety of ayurvedic herbs in diarrhoea‐predominant irritable bowel syndrome: a randomised controlled crossover trial. Complement Ther Med. 2016;26:171‐177. [DOI] [PubMed] [Google Scholar]
- 58. Brown K, Scott‐Hoy B, Jennings L. Efficacy of a Quebracho, Conker tree, and M. balsamea Willd blended extract in a randomized study in patients with irritable bowel syndrome with constipation. J Gastroenterol Hepatol Res. 2015;4:1762‐1767. [Google Scholar]
- 59. Portincasa P, Bonfrate L, Scribano ML, et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J Gastrointestin Liver Dis. 2016;25(2):151‐157. [DOI] [PubMed] [Google Scholar]
- 60. Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo‐controlled randomized trial. Dig Liver Dis. 2007;39(6):530‐536. [DOI] [PubMed] [Google Scholar]
- 61. Cash B, Epstein M, Shah S, Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61(2):560‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric‐coated peppermint‐oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32(6):765‐768. [DOI] [PubMed] [Google Scholar]
- 63. Weerts Z, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double‐blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158(1):123‐136. [DOI] [PubMed] [Google Scholar]
- 64. Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric‐coated, delayed‐release peppermint oil on irritable bowel syndrome. Dig Dis Sci. 2010;55(5):1385‐1390. [DOI] [PubMed] [Google Scholar]
- 65. Jalili M, Vahedi H, Janani L, Poustchi H, Malekzadeh R, Hekmatdoost A. Soy isoflavones supplementation for patients with irritable bowel syndrome: a randomized double blind clinical trial. Middle East J Dig Dis. 2015;7:170‐176. [PMC free article] [PubMed] [Google Scholar]
- 66. van Tilburg MA, Palsson OS, Ringel Y, Whitehead WE. Is ginger effective for the treatment of irritable bowel syndrome? A double blind randomized controlled pilot trial. Complement Ther Med. 2014;22(1):17‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Al‐Jassim ZG. Using brewer's yeast and ginger in the management of constipation‐predominant irritable bowel syndrome: a randomized double‐blind placebo‐controlled trial. Asian J Pharm Clin Res. 2019;12(3):372‐376. [Google Scholar]
- 68. Miller LE. Study design considerations for irritable bowel syndrome clinical trials. Ann Gastroenterol. 2014;27(4):338‐345. [PMC free article] [PubMed] [Google Scholar]
- 69. Faghihzadeh F, Hekmatdoost A, Adibi P. Resveratrol and liver: a systematic review. J Res Med Sci. 2015;20(8):797‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schoepfer AM, Vavricka S, Zahnd‐Straumann N, Straumann A, Beglinger C. Monitoring inflammatory bowel disease activity: clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J Crohn's Colitis. 2012;6(4):412‐418. [DOI] [PubMed] [Google Scholar]
- 71. Ma G, Chen Y. Polyphenol supplementation benefits human health via gut microbiota: a systematic review via meta‐analysis. J Funct Foods. 2020;66:103829. [Google Scholar]
- 72. Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39(10):1033‐1042. [DOI] [PubMed] [Google Scholar]
- 73. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome‐gut‐brain axis disorder? World J Gastroenterol. 2014;20(39):14105‐14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mayer EA, Savidge T, Shulman RJ. Brain‐gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146(6):1500‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cryan JF, O'Riordan KJ, Cowan CSM, et al. The microbiota‐gut‐brain axis. Physiol Rev. 2019;99(4):1877‐2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Full search strategies with controlled vocabulary search terms and keywords.
Table S2: Risk of bias assessment justifications of randomized controlled trials of biophenol‐rich nutraceuticals for adults with inflammatory related gastrointestinal conditions
Figure S1: PRISMA flowchart of the systematic search strategy to identify randomized controlled trials of biophenol‐rich nutraceuticals for adults with inflammatory related gastrointestinal condition.
Figure S2: Funnel plot of randomized controlled trials which compare biophenol‐rich nutraceuticals vs placebo on gastrointestinal symptoms in patients with irritable bowel syndrome or inflammatory bowel disease.
Figure S3: Pooled quality of life scores of adults with inflammatory bowel disease or irritable bowel syndrome after treatment with biophenol‐rich nutraceuticals or placebo per nutraceutical type.
Table S3: GRADE assessment of the certainty in the body of evidence for biophenol‐rich nutraceutical interventions for inflammatory bowel disease and irritable bowel syndrome.


