Summary
Background
Psoriasis is a chronic inflammatory skin disease requiring prolonged treatment. New biologic therapies require long‐term evaluation to assess the durability of their efficacy and safety profiles over time.
Objectives
To evaluate the long‐term efficacy and safety of risankizumab (RZB) for the treatment of psoriasis.
Methods
LIMMitless is an ongoing, phase III, open‐label extension study evaluating the long‐term efficacy and safety of RZB in adults with moderate‐to‐severe plaque psoriasis following multiple phase II/III studies. This analysis assessed efficacy through 172 weeks of continuous RZB treatment by examining the proportion of patients achieving ≥ 90% or 100% improvement in Psoriasis Area and Severity Index (PASI 90 and PASI 100), static Physician’s Global Assessment of clear or almost clear (sPGA 0/1) and Dermatology Life Quality Index of no effect on quality of life (DLQI 0/1). Safety was assessed by recording adverse events (AEs) through the data cutoff date. The study is registered at ClinicalTrials.gov (identifier: NCT03047395).
Results
Of 955 patients randomized to RZB 150 mg in the base studies, 897 patients continued into LIMMitless; 799 patients were still receiving treatment in LIMMitless at the time of data cutoff for this analysis. After 172 weeks of continuous RZB treatment, 85·5% of patients achieved PASI 90, 54·4% achieved PASI 100, 85·2% achieved sPGA 0/1, and 78·4% achieved DLQI 0/1 using modified nonresponder imputation. Rates of AEs leading to discontinuation and AEs of safety interest were low with long‐term treatment and comparable with those identified in the base studies.
Conclusions
Overall, long‐term continuous RZB was well tolerated and showed high and durable efficacy over 172 weeks.
Short abstract
What is already known about this topic?
Risankizumab is a humanized immunoglobulin G1 monoclonal antibody that specifically inhibits interleukin‐23 by binding to its p19 subunit.
In multiple phase II/III clinical trials in adults with moderate‐to‐severe psoriasis, risankizumab has displayed superior efficacy vs. placebo or other psoriasis treatments for up to 52 weeks.
What does this study add?
LIMMitless is an ongoing phase III open‐label extension study designed to evaluate the long‐term safety and efficacy of risankizumab treatment for up to 5 years of continuous use.
The results from this interim analysis demonstrate that risankizumab offers sustained efficacy after more than 3 years of continuous use, with no new safety signals identified.
Linked Comment: S. Gerdes and J. Albrecht. Br J Dermatol 2021; 185: 1086–1087.
Psoriasis is a chronic, immune‐mediated inflammatory skin disease that can negatively impact a patient’s quality of life and requires long‐term control. 1 , 2 Although multiple treatment options are currently available to manage psoriasis, clinicians need further guidance on the safety concerns that may arise when using long‐term therapies. Additionally, the efficacy of psoriasis treatments may also decline after prolonged periods of use. While a number of inflammatory cytokines have been implicated in the development and maintenance of psoriatic lesions, interleukin (IL)‐23 has emerged as a key disease regulator. 2 , 3 However, data demonstrating the effect of long‐term IL‐23 inhibition on the durability of response and the safety profile in patients with psoriasis are limited.
Risankizumab (RZB) is a humanized immunoglobulin G1 monoclonal anti–IL‐23 antibody developed to treat inflammatory diseases 4 and is an approved therapy for moderate‐to‐severe plaque psoriasis in adults. 5 RZB specifically inhibits IL‐23 by binding to its p19 subunit. 4 , 6 In multiple phase II/III clinical trials, RZB demonstrated superior efficacy compared with placebo or other psoriasis treatments: vs. placebo at week 16 (UltIMMa‐1, UltIMMa‐2, IMMhance and SustaIMM), 6 , 7 , 8 vs. adalimumab at weeks 16 and 44 (IMMvent study), 9 vs. fumaric acid esters at week 24 (NCT03255382), 10 vs. ustekinumab at weeks 16 and 52 (UltIMMa‐1 and UltIMMa‐2) 6 and vs. secukinumab at week 52 (IMMerge). 11
LIMMitless (open‐Label extension study to assess the safety and efficacy of rIsankizuMab for Maintenance in moderate‐to‐severe plaque type psoriasis) is an ongoing, phase III, single‐arm, multicentre, open‐label extension (OLE) study that builds on the findings derived from the shorter‐term base studies described in the methods section and evaluates long‐term RZB treatment. The LIMMitless study will eventually provide treatment data on up to 5 years of continuous RZB therapy. Our current analysis evaluates the long‐term efficacy of RZB therapy through 172 weeks of continuous treatment and safety through the cutoff date for this interim analysis, using integrated data from the LIMMitless study and the preceding base studies.
Patients and methods
Study design and treatment
LIMMitless is an ongoing, phase III, single‐arm, global, multicentre, OLE trial to evaluate the long‐term efficacy and safety of RZB in adults with moderate‐to‐severe psoriasis who have participated in multiple phase II/III studies. The LIMMitless study is designed for all patients to receive RZB 150 mg subcutaneously every 12 weeks for 252 weeks. To date, efficacy and safety assessments have been performed at study visits occurring every 12 weeks until week 156 and then every 24 weeks thereafter. At weeks 168, 192, 216 and 240, patients self‐administer RZB at home after proper training. The study is registered at ClinicalTrials.gov (identifier: NCT03047395).
Patients who were initially randomized to receive RZB (week 0, week 4, and every 12 weeks thereafter) in one of the five phase II/III studies that included a continuous RZB treatment arm (UltIMMa‐1, UltIMMa‐2, SustaIMM, NCT03255382 or IMMvent) were included in this analysis. 6 , 8 , 9 , 10 Three of the base studies were 52 weeks long (UltIMMa‐1, UltIMMa‐2 and IMMvent), while SustaIMM was 44 weeks long and NCT03255382 was 24 weeks long. Our interim analysis assessed the efficacy of continuous RZB use through 172 weeks (week 120 of the LIMMitless study), while safety was assessed through the cutoff date (26 March 2020; up to 208 weeks of treatment).
The study is being conducted in accordance with the Good Clinical Practice Guideline as defined by the International Conference on Harmonisation, the Declaration of Helsinki and/or all applicable federal and local regulations, and all patients provided written informed consent. All protocols were approved by an institutional review board (Quorom Review IRB, Seattle, WA, USA).
Patients
Eligible patients were adults with a history of moderate‐to‐severe chronic plaque psoriasis. To enrol in the study, patients must have completed one of the preceding studies and met all inclusion criteria for long‐term, open‐label RZB treatment. Patients were ineligible to enrol if they had developed guttate, erythrodermic, pustular or drug‐induced psoriasis during the base study; used prohibited medication; had a known diagnosis or evidence of tuberculosis; developed a malignancy during the base study; were diagnosed with HIV or hepatitis B or C infection; had > 8 weeks’ time elapse since completion of the base study; or had any evidence of other medical conditions that might compromise the quality of the study data (e.g. pregnancy, chronic alcohol or drug abuse, organ transplant, abnormal clinical laboratory values, or history of hypersensitivity to a systemically administered biologic therapy).
Assessments
Efficacy was evaluated using the Psoriasis Area and Severity Index (PASI) and the Static Physician’s Global Assessment (sPGA), both of which are established clinical measures for psoriasis severity. For all efficacy analyses, baseline refers to the last nonmissing value before the first dose of study drug in the preceding base studies. Efficacy assessments included the proportion of patients achieving ≥ 90% improvement in PASI (PASI 90) or 100% improvement in PASI (PASI 100), and the proportion of patients achieving sPGA of clear or almost clear (sPGA 0/1). Other efficacy assessments included the percentage improvement in PASI from baseline of the base study and quality of life as determined by the proportion of patients who achieved a score of 0 or 1 on the Dermatology Life Quality Index (DLQI; measured every 24 weeks). Safety assessments were performed continuously throughout the trial by monitoring of adverse events (AEs) and tabulated using MedDRA system organ class and preferred terms (version 22·1). AEs were calculated as the number of events per 100 patient‐years (PY) to account for differences in base study lengths.
Statistical analysis
The expected sample size was 2200, which included patients from all treatment groups in the base studies, based on estimated rates of base study completion and consent for the extension study and an anticipated dropout rate of 10–15%. Safety and efficacy were assessed in all patients who received at least one dose of study drug during the LIMMitless study. Data were described using summary statistics and were analysed using SAS® software (SAS Institute Inc., Cary, NC, USA).
Efficacy was calculated using three different statistical methods to impute missing data. In the modified nonresponder imputation (mNRI) method, which was the primary method of analysis, a mixed‐effect model was used to impute the missing values with efficacy outcome as the response variable and visit and baseline measurement as the fixed effects; patients were then characterized as responders or nonresponders based on the imputed values. Regardless of imputed values, patients who discontinued because of worsening of psoriasis were counted as nonresponders thereafter. Sensitivity analyses were performed using two additional imputation methods. Using the last observation carried forward (LOCF) method, missing data were imputed from the completed evaluation from the most recent visit. Using the observed cases (OC) method, patients with missing data at a visit were excluded from the analysis for that visit and there was no data imputation.
Results
Patients
In total, 955 patients with moderate‐to‐severe plaque psoriasis were randomized to receive RZB 150 mg and completed one of the preceding base studies; of these, 897 patients were enrolled in the LIMMitless study (Figure 1). The demographics and baseline disease characteristics at the time of base study enrolment were representative of the psoriasis population (Table 1). In total, 70·6% of the patients were male and the average age was 46·9 years. Before enrolling in the base study, 30·9% of patients had no prior systemic therapy use, while 37·8% had received a biologic treatment (19·5% had received a tumour necrosis factor antagonist). At baseline, the mean PASI was 20·5 and the proportions of patients with sPGA of moderate or severe were 80·6% and 18·6%, respectively. The mean baseline DLQI total score was 13·9, indicating that psoriasis had a very large effect on the patients’ quality of life. 12
Figure 1.
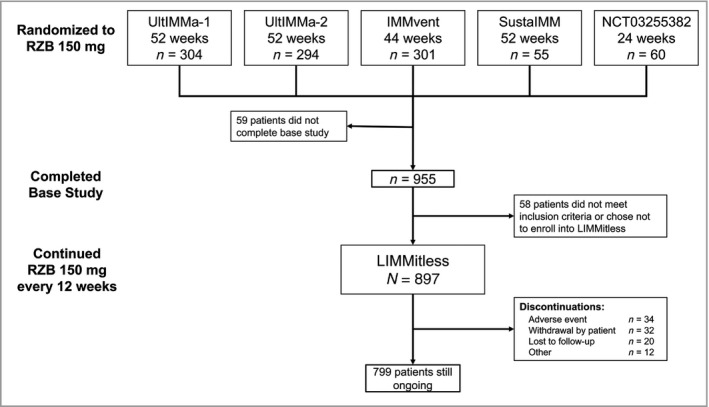
Patient disposition. RZB, risankizumab.
Table 1.
Demographics and baseline disease characteristics (n = 897)a
| Characteristic | Continuous risankizumab 150 mg |
|---|---|
| Male, n (%) | 633 (70·6) |
| Age (years) | |
| Mean (SD) | 46·9 (13·5) |
| Median (IQR) | 47·0 (37·0–57·0) |
| Weight (kg) | |
| Mean (SD) | 88·5 (22·4) |
| Median (IQR) | 85·2 (72·7–102·0) |
| Psoriatic arthritis,b,c n (%) | 195 (23·3) |
| Prior treatment, n (%) | |
| Naive to systemic therapyc | 259 (30·9) |
| Prior biologic therapyc | 317 (37·8) |
| Prior TNF antagonist therapy | 163 (19·5) |
| BSA involvement (%) | |
| Mean (SD) | 26·7 (16·5) |
| Median (IQR) | 21·0 (15·0–33·0) |
| PASI | |
| Mean (SD) | 20·5 (8·0) |
| Median (IQR) | 18·0 (14·7–23·7) |
| sPGA, n (%) | |
| Moderate (3) | 723 (80·6) |
| Severe (4) | 167 (18·6) |
| DLQI, mean (SD) | 13·9 (7·2) |
BSA, body surface area; DLQI, Dermatology Life Quality Index; IQR, interquartile range; sPGA, static Physician’s Global Assessment; TNF, tumour necrosis factor. aBaseline at the start of the base study. bDiagnosed or suspected. cBased on n = 838 as this information was not collected in NCT03255382; 59 patients from NCT03255382 continued into LIMMitless.
Efficacy
Analyses of efficacy demonstrated substantial improvements in psoriasis severity during the base studies that were maintained during the LIMMitless study. All three statistical methods used for efficacy analyses (mNRI, LOCF and OC) demonstrated consistent results. After 16 weeks of RZB treatment, > 75% of patients achieved PASI 90 and > 40% achieved PASI 100; after 52 weeks of RZB treatment, > 86% of patients achieved PASI 90 and > 58% of patients achieved PASI 100, and these levels remained largely constant through 172 weeks of treatment (Figure 2). sPGA of 0 or 1 was achieved by > 87% of patients after 16 weeks of treatment and was generally maintained throughout the OLE study (Figure 3).
Figure 2.
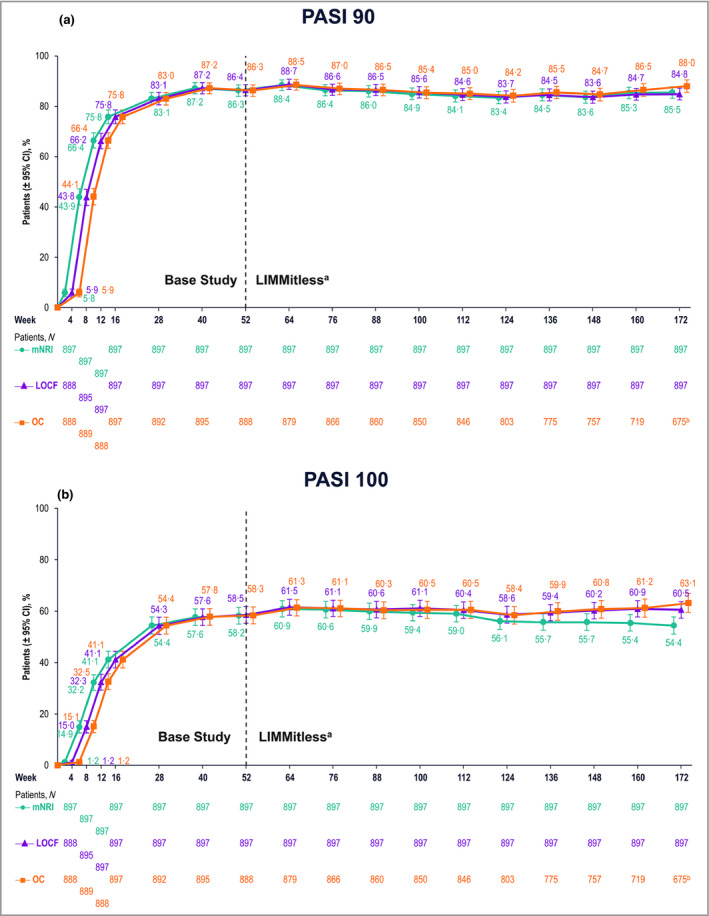
Patients achieving improvement in Psoriasis Area and Severity Index (PASI). Proportions of patients achieving (a) ≥ 90% improvement in PASI and (b) 100% improvement in PASI. aBecause of differences in base study lengths, some patients enrolled in LIMMitless earlier than 52 weeks. b675 of the 722 ongoing patients completed the assessment visit at week 172; 47 ongoing patients have reached the assessment window but have not yet completed the assessment visit at week 172. CI, confidence interval; LOCF, last observation carried forward; mNRI, modified nonresponder imputation; OC, observed cases.
Figure 3.
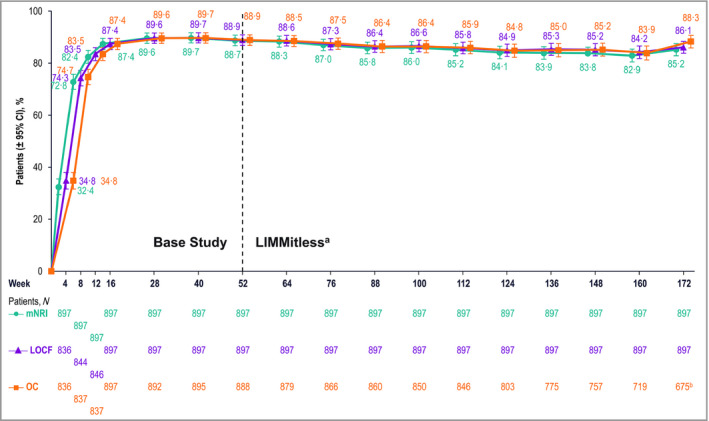
Patients achieving static Physician’s Global assessment of clear or almost clear (0 or 1). aBecause of differences in base study lengths, some patients enrolled in the LIMMitless study earlier than 52 weeks. b675 of the 722 ongoing patients completed the assessment visit at week 172; 47 ongoing patients have reached the assessment window but have not yet completed the assessment visit at week 172. CI, confidence interval; LOCF, last observation carried forward; mNRI, modified nonresponder imputation; OC, observed cases.
Following 16 weeks of RZB treatment, the mean percentage change from baseline in PASI was > 92%, increasing to > 95% throughout the OLE study (Figure S1; see Supporting Information). The proportions of patients with PASI 90 and PASI 100 responses after 172 weeks of continuous treatment were similar, regardless of patient baseline characteristics (Tables S1 and S2; see Supporting Information). Among patients who entered the LIMMitless OLE study with PASI 90 and PASI 100 responses, > 90% and > 67%, respectively, maintained that response through 120 weeks of treatment in the LIMMitless study (i.e. 172 weeks of continuous RZB treatment; Figure 4).
Figure 4.
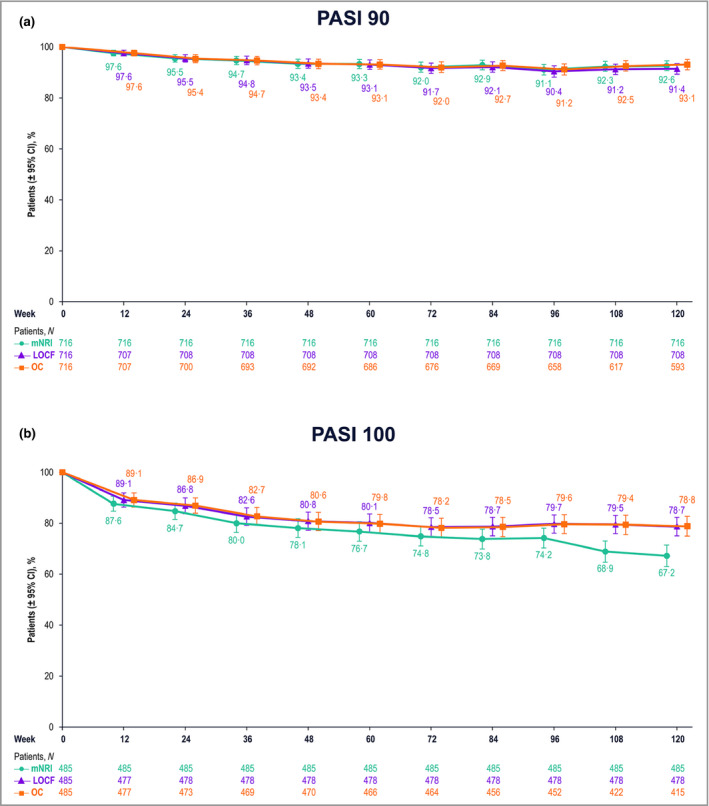
Patients maintaining Psoriasis Area and Severity Index (PASI) response from 52 to 172 weeks of continuous risankizumab treatment. Proportions of patients maintaining (a) ≥ 90% improvement in PASI and (b) 100% improvement in PASI. CI, confidence interval; LOCF, last observation carried forward; mNRI, modified nonresponder imputation; OC, observed cases.
Quality of life
Quality of life was evaluated using DLQI, results from which showed substantial improvements during the base studies that were maintained during the LIMMitless study. After 16 weeks of RZB treatment, > 66% of patients achieved a DLQI score of 0 or 1 (DLQI 0/1); this percentage increased to > 77% after 52 weeks of treatment and > 78% after 172 weeks of treatment (Figure 5). More than half of patients (56·9%) achieved both PASI 90 and DLQI 0/1 after 16 weeks of treatment; this percentage increased to ≥ 70% in the LIMMitless study and remained stable through 172 weeks of treatment (Figure 6).
Figure 5.
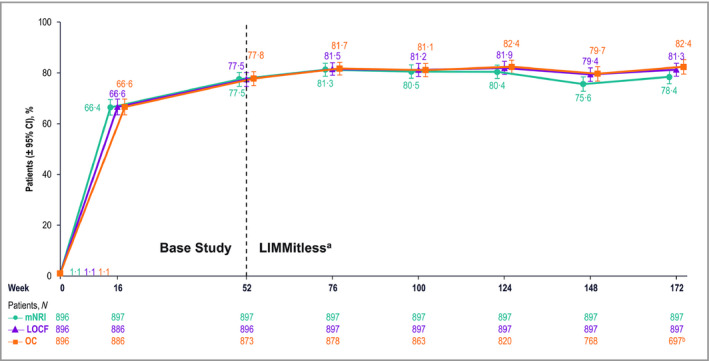
Patients achieving a Dermatology Life Quality Index score of 0 or 1 (no effect on quality of life). aBecause of differences in base study lengths, some patients enrolled in the LIMMitless study earlier than 52 weeks. b697 of the 722 ongoing patients completed the assessment visit at week 172; 25 ongoing patients have reached the assessment window but have not yet completed the assessment visit at week 172. CI, confidence interval; LOCF, last observation carried forward; mNRI, modified nonresponder imputation; OC, observed cases.
Figure 6.
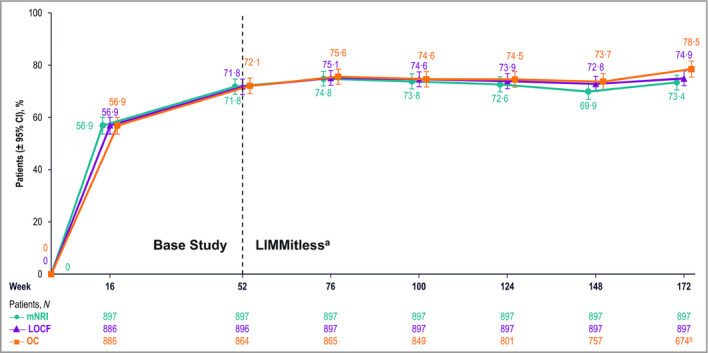
Patients achieving both ≥ 90% improvement in Psoriasis Area and Severity Index and Dermatology Life Quality Index score of 0 or 1 (no effect on quality of life). aBecause of differences in base study lengths, some patients enrolled in the LIMMitless study earlier than 52 weeks. b674 of the 722 ongoing patients completed the assessment visit at week 172; 48 ongoing patients have reached the assessment window but have not yet completed the assessment visit at week 172. CI, confidence interval; LOCF, last observation carried forward; mNRI, modified nonresponder imputation; OC, observed case.
Safety
Compared with data from the initial 16 weeks of trials in the psoriasis clinical development programme (402·2 PY), rates of treatment‐emergent AEs remained stable with long‐term treatment through the OLE study (up to 208 weeks of exposure by the cutoff date, 3106·2 PY; Table 2). Incidence rates of AEs leading to discontinuation of RZB were low (1·4 events per 100 PY), as were the rates of serious AEs (6·7 events per 100 PY). There were five deaths during the OLE study (one from natural causes, one car accident, one cardiopulmonary arrest, one sudden cardiac death and one unknown cause); none of these deaths were considered by the investigator to be related to the study drug. The standardized mortality ratio for treatment‐emergent deaths at the time of data cutoff was 0·32 (95% confidence interval 0·18–0·53). The most frequently reported treatment‐emergent AEs through the cutoff date were nasopharyngitis (31·0%; 17.3 events per 100 PY), upper respiratory tract infections (20·0%; 10.7 events per 100 PY) and arthralgia (10·7%; 3.8 events per 100 PY; Table 3).
Table 2.
Summary of treatment‐emergent adverse events
| Primary psoriasis safety pool (16 weeksa) | LIMMitless study (up to 208 weeks at time of data cutoff) | ||
|---|---|---|---|
| RZB 150 mg | Placebo | Continuous RZB 150 mg | |
| Number of patients | 1306 | 300 | 897 |
| Number of PY | 402·2 | 92·0 | 3106·2 |
| Events (events per 100 PY) | |||
| Any TEAE | 1279 (318·0) | 261 (283·7) | 5295 (170·5) |
| Serious AE | 40 (9·9) | 16 (17·4) | 209 (6·7) |
| AE leading to discontinuation of study medication | 11 (2·7) | 9 (9·8) | 44 (1·4) |
| Deaths | 2 (0·5) | 0 | 5 (0·2)b |
| TEAEs of safety interest | |||
| Adjudicated MACE | 1 (0·2) | 1 (1·1) | 5 (0·2) |
| Serious infections | 7 (1·7) | 1 (1·1) | 37 (1·2) |
| Malignant tumours (including NMSC) | 6 (1·5) | 1 (1·1) | 25 (0·8)c |
| NMSC | 3 (0·7) | 1 (1·1) | 15 (0·5) |
| Excluding NMSC | 3 (0·7) | 0 | 10 (0·3) |
| Serious hypersensitivity reactions | 0 | 0 | 3 (<0·1)d |
| Candida infection | 2 (0·5) | 0 | 20 (0·6)e |
| Systemic candidiasis | 0 | 0 | 0 |
AE, adverse event; MACE, major adverse cardiac event; NMSC, nonmelanoma skin cancer; PY, patient‐years; RZB, risankizumab; TEAE, treatment‐emergent adverse event. aPrimary psoriasis safety pool includes NCT02054481, 20 UltIMMa‐1, 6 UltMMa‐2, 6 IMMhance (NCT02672852) 7 and IMMvent. 9 bDue to natural causes (n = 1), accident (n = 1), cardiac arrest (n = 1), sudden cardiac death (n = 1) and cause unknown (n = 1); none related to risankizumab. cMalignancies were colorectal adenocarcinoma (n = 2), metastatic colon cancer (n = 1), basal cell carcinoma (n = 9), Bowen disease (n = 1), breast cancer (n = 3), endometrial cancer (n = 1), oral metastatic squamous cell carcinoma with lymph node metastasis (n = 1), metastatic squamous cell carcinoma (n = 1), prostate cancer (n = 2) and squamous cell carcinoma of the skin (n = 4). dSerious hypersensitivity reactions (all of which were unrelated to study drug) were para‐phenylenediamine allergy (n = 1; mild, attributed to hair dye application), generalized microbial eczema (n = 1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone) and Stevens–Johnson syndrome (n = 1; severe, attributed to addition of chlorpromazine). eTwenty Candida‐related events were reported in 16 patients. More than half occurred in patients with known risk factors such as diabetes, steroid use and prior Candida episodes. None were serious or led to study drug discontinuation.
Table 3.
Most frequently reported treatment‐emergent adverse events with incidence > 1 event per 100 patient‐years
| Preferred term | Continuous risankizumab 150 mg |
|---|---|
| n = 897; PYs = 3106·2 | |
| Nasopharyngitis | 537 (17·3) |
| Upper respiratory tract infection | 332 (10·7) |
| Arthralgia | 119 (3·8) |
| Headache | 96 (3·1) |
| Hypertension | 87 (2·8) |
| Back pain | 86 (2·8) |
| Influenza | 78 (2·5) |
| Sinusitis | 68 (2·2) |
| Bronchitis | 62 (2·0) |
| Gastroenteritis | 60 (1·9) |
| Urinary tract infection | 51 (1·6) |
| Diarrhoea | 49 (1·6) |
| Cough | 44 (1·4) |
| Contact dermatitis | 42 (1·4) |
| Oropharyngeal pain | 37 (1·2) |
| Injection‐site erythema | 36 (1·2) |
| Fatigue | 35 (1·1) |
| Osteoarthritis | 33 (1·1) |
| Pruritus | 33 (1·1) |
| Tinea pedis | 33 (1·1) |
The data are presented as the number of events per 100 PYs. PY, patient‐years.
There were 37 cases of serious infections (Table S3; see Supporting Information) and 25 cases of malignant tumours (10 excluding nonmelanoma skin cancer). The incidence of malignancies did not increase with longer durations of RZB treatment, and the most frequently reported malignancies were similar to those observed in the general population (breast cancer and colorectal cancer); there were no haematological malignancies, including leukaemias or lymphomas. The ratio of squamous cell carcinoma to basal cell carcinoma was 1 : 2. There were no reported cases of systemic Candida infection, inflammatory bowel disease or active tuberculosis. There were 20 cases of Candida infection reported (0·6 events per 100 PY): seven cases of oral candidiasis, seven cases of genital candidiasis, four cases of oesophageal candidiasis, one case of intestinal candidiasis and one case of skin candidiasis. In summary, no new safety concerns were identified.
Discussion
In this study, we demonstrate that long‐term treatment with RZB provides durable efficacy beyond 3 years of follow‐up in patients with moderate‐to‐severe plaque psoriasis. Most patients had clinically relevant improvements in disease severity, and nearly 90% of patients achieved PASI 90 after 52 weeks of continuous RZB treatment. This degree of response remained stable through 120 further weeks of continuous RZB treatment. The improvements observed in patient quality of life during the base studies were maintained at consistently high levels throughout the OLE study. At each timepoint through 172 weeks of treatment, > 75% of patients reported no effect on their quality of life (per responses on the DLQI).
These results demonstrate sustained improvements in psoriasis severity with long‐term RZB therapy. Other studies have reported prolonged efficacy with different biologic psoriasis treatments; however, comparisons between studies must be made with caution because of differences in study designs and patient populations. Results from a 5‐year evaluation from the BioCAPTURE registry evaluating the efficacy of adalimumab (a tumour necrosis factor inhibitor), etanercept (a tumour necrosis factor inhibitor) and ustekinumab (an IL‐12/23 inhibitor) showed that treatment with any of these biologic agents resulted in sustained reductions in mean PASI. 13
The level of sustained response observed in this study is greater than that observed for adalimumab over 3 to > 4 years of treatment in clinical studies. 14 , 15 Similar trends were observed for the IL‐23 inhibitor guselkumab, which was analysed in the phase III VOYAGE 1 and 2 studies, where 82·1% and 77·2% of patients, respectively, achieved PASI 90 response at 3 years. 16 Among IL‐17 inhibitors, the proportions of patients who achieved long‐term PASI 90 response in clinical trials were numerically lower. In the phase III SCULPTURE study, in which secukinumab was analysed, 66·4% of patients achieved PASI 90 response at 5 years. 17 In the phase III UNCOVER‐3 study, in which ixekizumab (every 4 weeks) was analysed, 66·4% of patients achieved PASI 90 response at 4 years. 18 In a phase II study in which brodalumab was analysed, around 65% of patients achieved PASI 90 at 5 years. 19
RZB was generally well tolerated throughout the LIMMitless study; safety findings were similar to those observed during the base studies, with no new safety signals identified and low discontinuation rates after more than 3 years of continuous RZB treatment.
The main strengths of this study include long‐term follow‐up for over 172 weeks of continuous RZB therapy, from a consistent population of patients who require long‐term treatment and were treated with RZB since the base studies. This study also includes a large patient population with a low discontinuation rate, which provides reliable and consistent data. Additionally, while the methods used to analyse missing data in OLE studies are not standardized, our study used three different statistical methods to handle missing data, all of which yielded consistent results. Limitations of this study include the open‐label and single‐arm study design.
Overall, the results from the ongoing LIMMitless trial demonstrate substantial and durable maintenance of response with RZB over time in patients with moderate‐to‐severe plaque psoriasis, with a safety profile similar to that observed in the pivotal studies. The response will be further investigated in the remaining time of this OLE study.
Author Contribution
Kim Papp: Conceptualization (lead); Methodology (lead). Mark Lebwohl: Methodology (lead). Lluís Puig: Methodology (lead). Mamitaro Ohtsuki: Methodology (lead). Stefan Beissert: Conceptualization (supporting); Methodology (supporting). Jiewei Zeng: Data curation (lead); Formal analysis (lead); Validation (lead). Simone Rubant: Methodology (lead). Ranjeeta Sinvhal: Methodology (lead). Yiwei Zhao: Data curation (lead); Methodology (lead). Ahmed M Soliman: Methodology (lead). Gabriela Alperovich: Data curation (lead); Methodology (lead). Craig Leonardi: Methodology (lead).
Supporting information
Figure S1 Mean percentage change from baseline in Psoriasis Area and Severity Index.
Table S1 Patients achieving ≥ 90% improvement in Psoriasis Area and Severity Index by patient subgroup.
Table S2 Patients achieving 100% improvement in Psoriasis Area and Severity Index by patient subgroup.
Table S3 Treatment‐emergent serious infections.
Acknowledgments
AbbVie and the authors thank all of the study investigators for their contributions, and the patients who participated in this study. AbbVie funded the research for this study and provided writing support for this manuscript. Medical writing assistance, funded by AbbVie, was provided by Callie A.S. Corsa, PhD, and Jennifer C. Jaworski, MS, of JB Ashtin.
Appendix 1. Conflicts of interest
K.A.P. has received research funds from AbbVie, Amgen, Arcutis, Astellas, Bausch Health, Baxalta, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus, Dermavant, Dermira, EMD Serono, Forward Pharma, Galderma, Genentech, Gilead, Incyte, Janssen, LEO Pharma, Lilly, Merck, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma and UCB; is a consultant for AbbVie, Amgen, Arcutis, Astellas, Bausch Health, Baxalta, Baxter, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus, Dermavant, Dermira, EMD Serono, Forward Pharma, Galderma, Genentech, Gilead, Incyte, Janssen, LEO Pharma, Lilly, Meiji Seika Pharma, Merck, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda and UCB; is a speaker for AbbVie, Amgen, Arcutis, Astellas, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Incyte, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi Genzyme and UCB; and is a committee member for the PSOLAR and PURE registries. M.G.L. is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Evommune, Incyte, Janssen, LEO Pharma, Lilly, Ortho Dermatologics, Pfizer and UCB; and is a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Avotres, BirchBioMed, BMD Skincare, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant, Dr. Reddy’s Laboratory (Promius), EMD Serono, Evelo Biosciences, Evommune, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, Kyowa Kirin, LEO Pharma, Meiji Seika Pharma, Menlo Therapeutics, Mitsubishi Tanabe Pharma, NeuroDerm, Pfizer, Theravance and Verrica. L.P. has received consultancy or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, EMD Serono, Gebro, Janssen, LEO Pharma, Lilly, Merck, Mylan, Novartis, Pfizer, Regeneron, Roche, Samsung Bioepis, Sandoz, Sanofi and UCB. M.O. has received honoraria or fees for serving on advisory boards or speakers’ bureaus, or for consulting, and grants for investigator activities from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Maruho, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sanofi, Sun Pharma, Torii Pharmaceutical and UCB. S.B. has received honoraria as an advisory board member for AbbVie, Actelion, Almirall, Amgen, Celgene, Galderma, Janssen, LEO Pharma, Lilly, Menlo Therapeutics, Merck, Novartis, Pfizer, Sanofi and UCB; and as a speaker for AbbVie, Actelion, Almirall, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Roche and Sandoz. J.Z., S.R., R.S., Y.Z. and A.M.S. are full‐time employees of AbbVie, and may hold AbbVie stock and/or stock options. G.A. is a former employee of AbbVie, and may hold AbbVie stock and/or stock options. C.L. has received honoraria as a consultant or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, LEO Pharma, Lilly, Ortho Dermatologics, Pfizer, Sandoz, UCB and Vitae Pharmaceuticals; and as a speaker for AbbVie, Amgen, Celgene, Lilly, Novartis, Sun Pharma and UCB; and has received fees for services as a principal investigator for Actavis, Amgen, Boehringer Ingelheim, Celgene, Cellceutix, Coherus, Corrona, Dermira, Galderma, Glenmark, Janssen, LEO Pharma, Lilly, Merck, Novartis, Novella Clinical, Pfizer, Sandoz, Sienna Biopharmaceuticals, Stiefel, UCB and Warner Chillcott.
Appendix 2. Data availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial‐level data (analysis datasets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial dataset can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.AbbVie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.
Funding sources AbbVie Inc. funded this study, and participated in the study design, research, analysis, data collection, interpretation of data and review and approval of the publication. All authors had access to the data and participated in the development, review, critique and approval of the manuscript throughout the editorial process and approved the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. Medical writing support was paid for by AbbVie.
Conflicts of interest statements can be found in Appendix 1.
A statement of data availability can be found in Appendix 2.
References
- 1. Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 2015; 64:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol 2017; 13:525–34. [DOI] [PubMed] [Google Scholar]
- 3. Gooderham MJ, Papp KA, Lynde CW. Shifting the focus – the primary role of IL‐23 in psoriasis and other inflammatory disorders. J Eur Acad Dermatol Venereol 2018; 32:1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh S, Kroe‐Barrett RR, Canada KA et al. Selective targeting of the IL23 pathway: generation and characterization of a novel high‐affinity humanized anti‐IL23A antibody. MAbs 2015; 7:778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skyrizi (risankizumab‐rzaa) [package insert]. Chicago, IL: AbbVie Inc., 2020. [Google Scholar]
- 6. Gordon KB, Strober B, Lebwohl M et al. Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet 2018; 392:650–61. [DOI] [PubMed] [Google Scholar]
- 7. Blauvelt A, Leonardi CL, Gooderham M et al. Efficacy and safety of continuous risankizumab therapy versus treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohtsuki M, Fujita H, Watanabe M et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol 2019; 46:686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reich K, Gooderham M, Thaçi D et al. Risankizumab compared with adalimumab in patients with moderate‐to‐severe plaque psoriasis (IMMvent): a randomised, double‐blind, active‐comparator‐controlled phase 3 trial. Lancet 2019; 394:576–86. [DOI] [PubMed] [Google Scholar]
- 10. Thaçi D, Eyerich K, Pinter A et al. Direct comparison of risankizumab and fumaric acid esters in patients with moderate‐to‐severe plaque psoriasis who were naive to systemic therapy. Presented at the 28th European Academy of Dermatology and Venereology Congress, Madrid, Spain, 9–13 October 2019.
- 11. Warren RB, Blauvelt A, Poulin Y et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate‐to‐severe plaque psoriasis (IMMerge): results from a phase III, randomized, open‐label, efficacy‐assessor‐blinded clinical trial. Br J Dermatol 2021; 184:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hongbo Y, Thomas CL, Harrison MA et al. Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol 2005; 125:659–64. [DOI] [PubMed] [Google Scholar]
- 13. Zweegers J, Groenewoud JMM, van den Reek J et al. Comparison of the 1‐ and 5‐year effectiveness of adalimumab, etanercept and ustekinumab in patients with psoriasis in daily clinical practice: results from the prospective BioCAPTURE registry. Br J Dermatol 2017; 176:1001–9. [DOI] [PubMed] [Google Scholar]
- 14. Asahina A, Ohtsuki M, Etoh T et al. Adalimumab treatment optimization for psoriasis: results of a long‐term phase 2/3 Japanese study. J Dermatol 2015; 42:1042–52. [DOI] [PubMed] [Google Scholar]
- 15. Gordon K, Papp K, Poulin Y et al. Long‐term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open‐label extension study for patients from REVEAL. J Am Acad Dermatol 2012; 66:241–51. [DOI] [PubMed] [Google Scholar]
- 16. Reich K, Griffiths CEM, Gordon KB et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol 2020; 82:936–45. [DOI] [PubMed] [Google Scholar]
- 17. Bissonnette R, Luger T, Thaçi D et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate‐to‐severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol 2018; 32:1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebwohl MG, Gordon KB, Gallo G et al. Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER‐3 study. J Eur Acad Dermatol Venereol 2020; 34:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lebwohl MG, Blauvelt A, Menter A et al. Efficacy, safety, and patient‐reported outcomes in patients with moderate‐to‐severe plaque psoriasis treated with brodalumab for 5 years in a long‐term, open‐label, phase II study. Am J Clin Dermatol 2019; 20:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papp KA, Blauvelt A, Bukhalo M et al. Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med 2017; 376:1551–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Mean percentage change from baseline in Psoriasis Area and Severity Index.
Table S1 Patients achieving ≥ 90% improvement in Psoriasis Area and Severity Index by patient subgroup.
Table S2 Patients achieving 100% improvement in Psoriasis Area and Severity Index by patient subgroup.
Table S3 Treatment‐emergent serious infections.


