Abstract
Species extinction risk is accelerating due to anthropogenic climate change, making it urgent to protect vulnerable species through legal frameworks in order to facilitate conservation actions that help mitigate risk. Here, we discuss fundamental concepts for assessing climate change risks to species using the example of the emperor penguin (Aptenodytes forsteri), currently being considered for protection under the US Endangered Species Act (ESA). This species forms colonies on Antarctic sea ice, which is projected to significantly decline due to ongoing greenhouse gas (GHG) emissions. We project the dynamics of all known emperor penguin colonies under different GHG emission scenarios using a climate‐dependent meta‐population model including the effects of extreme climate events based on the observational satellite record of colonies. Assessments for listing species under the ESA require information about how species resiliency, redundancy and representation (3Rs) will be affected by threats within the foreseeable future. Our results show that if sea ice declines at the rate projected by climate models under current energy system trends and policies, the 3Rs would be dramatically reduced and almost all colonies would become quasi‐extinct by 2100. We conclude that the species should be listed as threatened under the ESA.
Keywords: climate risk assessments; Endangered Species Act; foreseeable future; population projections; resiliency, redundancy and representation (3Rs); sea ice projections; species distribution; treatment of scientific uncertainty
We recommend that emperor penguins should now be listed as threatened under the Endangered Species Act. Indeed, by 2100, under emissions scenarios resulting from current energy‐system trends and policies, emperor penguins will be in danger of extinction throughout its entire range (color of colonies on the map). Extreme environmental perturbations occurring at colonies, magnify global population declines and accelerate the time to extinction (colored lines on right panels). If the world would take aggressive actions to reduce greenhouse gases emission now and the Paris Agreement objectives are met, declines will be much less severe and the species will persist longer.

1. INTRODUCTION
Climate change is increasing the stress on species and ecosystems, and climate‐related local extinctions are already widespread (Wiens, 2016). Species extinction risk will accelerate with continued global warming, threatening 16%–30% of species under current climate policies (Román‐Palacios & Wiens, 2020; Urban, 2015). Rapid cuts in greenhouse gas (GHG) emissions to limit warming to 1.5°C under the international Paris Agreement, adopted by 195 parties in 2015, are by far the most important action for preventing catastrophic species losses (IPCC, 2018; Warren et al., 2018). In tandem, conservation actions can increase species’ resilience to climate stress including protecting important habitat, increasing habitat connectivity, and reducing non‐climate stressors (Heller & Zavaleta, 2009; Mawdsley et al., 2009).
The US Endangered Species Act (ESA) is the world's strongest environmental law focused on preventing extinction and facilitating recovery of imperiled species (Rohlf, 1989). The ESA has increasingly been applied to provide protection for species threatened primarily, or in part, by climate change, with the polar bear (Ursus maritimus), in 2008, being the first species listed principally due to global warming (Table 1). For climate‐threatened species, listing under the ESA mandates use of science‐based, enforceable tools to reduce climate threats and increase resilience, including habitat protection and recovery planning by Federal agencies to avoid jeopardizing listed species or adversely modifying their critical habitat, and a prohibition on killing or harming listed species (Moritz et al., 2008; Povilitis & Suckling, 2010). For climate‐threatened species occurring outside of US jurisdiction, some of these protections do not currently apply, but ESA listing still confers benefits such as promoting research and conservation actions (Foley et al., 2017). Further, ESA listing would require all US Federal agencies to evaluate and ensure that their activities do not jeopardize the species or their habitat, which could include limiting GHG emissions for species endangered by climate change. The Services are currently not conducting these analyses but efforts are underway to change this (Harvard Law School, 2021).
TABLE 1.
Species considered under the US Endangered Species Act in relation to climate change, showing the year and outcome of the listing decision, the primary threat to the species related to climate change, the time period used when considering the foreseeable future, and the agency undertaking the evaluation
| Species | Year | Decision | Climate threat | Foreseeable future | Agency | Ref |
|---|---|---|---|---|---|---|
| Polar bear (Ursus maritimus) | 2008 | Threatened | Sea ice loss | 2050 | FWS | 1 |
| Emperor penguin (Aptenodytes forsteri) | 2008 | Not listed | Sea ice loss | 2080–2100 | FWS | 2 |
| Ribbon seal (Histriophoca fasciata) | 2008 | Not listed | Sea ice loss | 2050 | NMFS | 3 |
| American pika (Ochotona princeps) | 2010 | Not listed | Temperature rise | 2025–2050 | FWS | 4 |
| Pacific walrus (Odobenus rosmarus divergens) | 2011 | Warranted but precluded | Sea ice loss | 2100 | FWS | 5 |
| Bearded seal (Erignathus barbatus nauticus) (Beringia and Okhotsk DPS) | 2012 | Threatened | Sea ice loss | 2100 | NMFS | 6 |
| Ringed seal (Phoca hispida) (Arctic, Okhotsk, Baltic, Ladoga subspecies) | 2012 | Threatened (Arctic, Okhotsk, Baltic); endangered (Ladoga) | Sea ice loss | 2100 | NMFS | 7 |
| North American wolverine (Gulo gulo luscus) (contiguous US DPS) | 2013 | Proposed listing as threatened (later withdrawn) | Snowpack loss | 2099 | FWS | 8 |
| 20 coral species | 2014 | Threatened | Ocean warming and acidification | 2100 | NMFS | 9 |
| Florida Keys mole skink (Plestiodon egregius egregius) | 2017 | Not listed | Sea level rise | 2060 | FWS | 10 |
| ʻIʻiwi (Drepanis coccinea) | 2017 | Threatened | Temperature rise | 2100 | FWS | 11 |
| Pacific walrus (Odobenus rosmarus divergens) | 2017 | Not listed | Sea ice loss | 2060 | FWS | 12 |
| Cedar Key mole skink (Plestiodon egregius insularis) | 2018 | Not listed | Sea level rise | 2050 | FWS | 13 |
| Meltwater lednian stonefly (Lednia tumana) | 2019 | Threatened | Glacier loss | 2050 | FWS | 14 |
| Western glacier stonefly (Zapada glacier) | 2019 | Threatened | Glacier loss | 2050 | FWS | 15 |
| North American wolverine (Gulo gulo luscus) (contiguous US DPS) | 2020 | Listing proposal withdrawn | Snowpack loss | 38–50 years | FWS | 16 |
1: USFWS (2008c); 2: USFWS (2008a); 3: NMFS (2008); 4: USFWS (2010); 5: USFWS (2011); 6: NMFS (2012b); 7: NMFS (2012a); 8: USFWS (2013); 9: NMFS (2014); 10: USFWS (2017c); 11: USFWS (2017d); 12: USFWS (2017c); 13: USFWS (2018b); 14: USFWS (2019); 15: USFWS (2019); 16: USFWS (2020b).
Abbreviations: FWS, US Fish and Wildlife Service; NMFS, National Marine Fisheries Service, DPS, distinct population segment.
The emperor penguin (Aptenodytes forsteri) is an iconic species threatened by climate change (Trathan et al., 2020). Climate models project significant declines in Antarctic sea ice to which the emperor penguin life cycle is closely tied (Barbraud & Weimerskirch, 2001; Jenouvrier et al., 2012, 2020). Indeed, emperor penguins breed on land‐fast sea ice (stable sea ice locked to the coast, ice shelves, or islands) during the austral winter around the Antarctic continent (Figure 1). During the nonbreeding season, remnant fast ice or large floes in the pack ice (sea ice floes that can move with ocean currents or the wind, but which may merge and combine), serve as a platform where adult emperor penguins rest, seek refuge from predators, and molt. Emperor penguins also spend much of their time foraging within the pack ice, both during the breeding season (Kirkwood & Robertson, 1997; Wienecke & Robertson, 1997) and post breeding (Goetz et al., 2018; Kooyman et al., 2004; Labrousse et al., 2019; Rodary et al., 2000). Sea ice concentration also influences the presence and abundance of some emperor penguin prey species (Bluhm et al., 2017; La Mesa et al., 2010; Meyer et al., 2017; Vacchi et al., 2012). Therefore, variations in sea ice concentration affect the survival and reproduction of emperor penguins both directly (e.g., early fast sea ice breakup can jeopardize chick survival) and indirectly through the food web.
FIGURE 1.
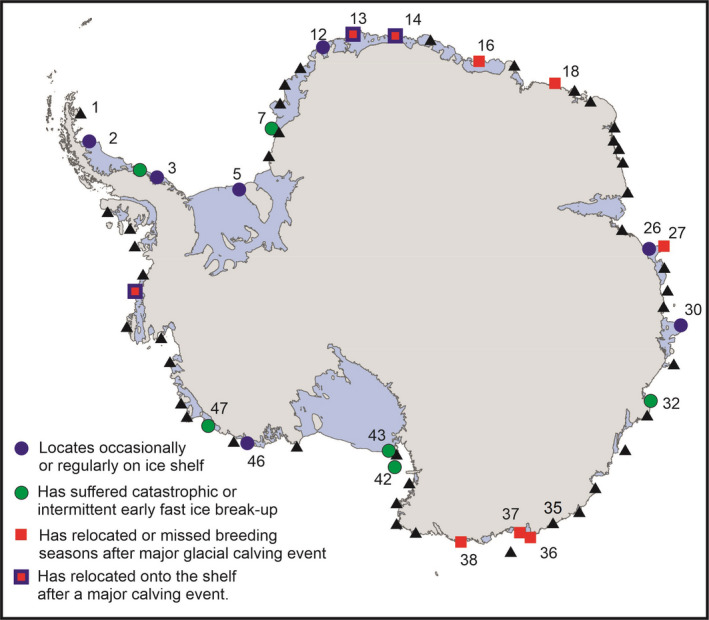
Colony locations where environmental perturbations to emperor penguin breeding sites have been recorded in the satellite record. These include early sea ice loss, before mid‐December, which will result in chick loss and sometimes, total breeding failure. In one case, Ledda Bay, #47, ice loss is a regular occurrence and breeding is intermittent at this site. A further type of perturbation is glacial calving, which will lead to colony relocation and, sometimes, total breeding failure (Mertz Glacier, #36). Finally, relocation of the colony onto ice shelves will often happen when sea ice does not form at the beginning of the breeding season, or breaks up during the season. Breeding on ice shelves is presumed to take more energy, be more exposed to wind and have a cost to reproductive success. Colony locations are taken from Fretwell and Trathan (2021)
The need for legal recognition and enhanced precautionary management for emperor penguins is now urgent, particularly given continued increases in GHG emissions (Friedlingstein et al., 2020; Jones et al., 2021; Nisbet et al., 2019). Starting in 2012, the International Union for Conservation of Nature listed the emperor penguin as Near Threatened due to climate change threats (BirdLife International, 2021). In 2008, the US Fish and Wildlife Service (FWS) determined that the emperor penguin did not warrant listing under the ESA, in part because of uncertainty in future predictions of sea ice conditions and a lack of significant population decline at the time (US Fish and Wildlife Service, 2008a) (Table 1). Based on new scientific research, the Center for Biological Diversity petitioned for protection again in 2011 (Center for Biological Diversity, 2011). The FWS is now under a court deadline to conduct a full scientific review of emperor penguin status and decide whether the listing is warranted by July 2021. Previous modelling efforts to project the effects of climate change on the status of emperor penguin populations (Jenouvrier et al., 2009, 2012, 2014, 2020) were not designed to provide assessments relevant to any legal framework. The analysis described below is specifically tailored for decision‐making under the ESA, and expands upon previous research by assessing the effects of annual extreme climate‐related perturbations through exploration of various climate scenarios.
Extreme events influence species population dynamics and geographic representation (de Pol et al., 2017; Jenouvrier et al., 2015) which are important criteria in the evaluation of species’ extinction risk. The most obvious perturbations directly affecting emperor penguins’ population viability are late formation and early loss of the fast ice on which a given colony is located. The former may delay the onset of breeding, the latter may reduce breeding success if the fast ice breaks out before the chicks are ready to fledge. For example, at Ledda Bay in the Amundsen Sea (#47 on Figure 1), early break out of the fast ice has occurred in multiple years resulting in intermittent breeding as observed from satellite imagery (LaRue et al., 2015; Trathan et al., 2020). Further, late formation of sea ice in the autumn, can also lead to delayed breeding and/or reduced breeding success, or the relocation of colonies onto icebergs or ice shelves (Fretwell et al., 2014; Figure 1). On the other hand, extensive fast ice cover during the rearing period can reduce breeding success, as breeding adults are forced to cover long distances on fast ice to reach foraging grounds, which decreases chick feeding frequencies and growth, and increases chick mortality (Barbraud et al., 2015; Labrousse et al., 2021) with important consequences for emperor penguin population recovery (Jenouvrier et al., 2009).
At some sites, perturbations in local atmospheric or oceanographic conditions have rendered sites uninhabitable, sometimes for several consecutive seasons (Figure 1). For example, at the second largest colony, Halley Bay (#7, Figure 1) a recent shift in the local environment caused the ice to break up too early for chicks to fledge successfully for four consecutive seasons (Fretwell & Trathan, 2019). Other major perturbations, including the calving of glaciers, ice tongues, and ice shelves, can compromise fast ice stability, forcing colonies to relocate (LaRue et al., 2015; e.g., Mertz Glacier #36, Figure 1). There are 18 colonies associated with ice tongues that presently break off every 10–50 years (Figure S1). In addition, when ice shelves calve, the formation of very large, tabular icebergs may block penguin access to foraging grounds, potentially disrupting breeding success due to the increased distances to feeding areas and destroying fast ice breeding platforms. This was observed in the Ross Sea in 2001, when the largest ever recorded iceberg‐destroyed habitat at Cape Crozier (#44 Figure 1), leading to mortality of both chicks and adults, and low breeding performance for several consecutive years (the colony failed totally in 2001, and in the years to 2004, reduced chick production ranged from 0% to 40% of the chicks previously produced in 2000; Kooyman et al., 2007).
These observations exemplify extreme changes that we might expect as ocean and air temperatures warm. Plausibly such perturbations may be most evident where the environment is changing rapidly; however, the fact that large and southern colonies have already experienced such drastic change (Schmidt & Ballard, 2020) suggests the frequency of similar events may increase in the future. As we currently lack information to accurately estimate the frequency and amplitude of these perturbations and how they will change in the future, we developed four new conservative demographic extreme event scenarios. Importantly, as emperor penguin movements between colonies in response to these stochastic and extreme events influence population dynamics (Jenouvrier et al., 2017), we also consider nine dispersal scenarios combining different dispersal rates, behaviors, and distances.
This analysis is relevant to other species endangered by climate change that may be considered for protection under legal frameworks globally. We discuss fundamental concepts to do with climate risk assessments for species, including the selection of climate models and GHG emission scenarios to evaluate climate threats, the treatment of scientific uncertainty, the meaning of what constitutes a foreseeable future, and the conservation biology principles of resiliency, redundancy, and representation for evaluating extinction risk in response to climate threats. We apply these concepts to the emperor penguin as a case study, providing new projections of future population dynamics and status under a range of emissions scenarios, factoring in extreme climate events.
2. METHODS
Our new analysis builds upon past work (Jenouvrier et al., 2010, 2012, 2014, 2017, 2020), but integrates recent published knowledge about colony dynamics including models that reflect extreme perturbations, something hitherto not included. Specifically, our model includes the effect of sea ice concentrations on vital rates (survival and reproduction) and accounts for differences in the impact of sea ice concentrations on adult survival for males and females to project the intrinsic population growth rate at each colony (Figure S2). In addition, a meta‐population model is used to describe the demography and dispersal behaviors of emperor penguins across their Antarctic range (Jenouvrier et al., 2017, 2020). Our analysis of the impact of sea ice concentration on emperor population dynamics at each colony uses as much information as is available. Despite advancement in satellite imagery, assessment of some relevant sea ice features at each colony are not available (e.g., fast ice, polynya, and presence of icebergs), which influence breeding, feeding, and foraging of emperor penguins. Current Atmosphere‐Ocean General Circulation Models (AOGCMs), on which our sea ice environment projections are based, do not represent these sea ice features and project sea ice concentrations over relatively coarse spatial grids (100‐ to 200‐km resolution). Hence, to link our meta‐population model to the output of AOGCMs, we use sea ice concentration anomalies over similarly large spatial scales around each colony (see details in Jenouvrier et al., 2012; Section 2.3).
2.1. Climate projections
2.1.1. Emissions scenarios
In its 2016 guidance on ESA decisions involving species affected by climate change, the National Marine Fisheries Service (NMFS) determined that for significant uncertainty in future emission trajectories, the agency would assume conditions similar to the status quo until new information suggests a change is appropriate, and therefore would use Representative Concentration Pathway (RCP) 8.5 as representing the trajectory under current policies (National Marine Fisheries Service, 2016) (Figure 2). This is consistent with Schwalm, Huntzinger, et al. (2020) who recommended that RCP8.5 be used for assessing the climate and future risks, at least through to mid‐century, given what is presently known about biotic feedbacks, the current GHG emission path, and the success of past forecasts to anticipate human behavior. The total cumulative CO2 emissions since 2005 projected under RCP8.5 by 2020 are in close agreement with historical observed total cumulative CO2 emissions (Schwalm et al., 2020a). In addition, the total cumulative CO2 emissions since 2005 projected under RCP8.5 by 2050 agree well with energy forecasts under current and stated policies by 2050, with still highly plausible levels of CO2 emissions by 2100 (Schwalm et al., 2020a). In contrast, RCP4.5 provides an underestimate of physical climate risk (Schwalm et al., 2020a). Here we used five emission scenarios to project the population dynamics of emperor penguins to place bounds on socio‐economic uncertainties, including two scenarios to examine the temporal climate dynamics that would result from meeting the Paris Agreement objectives (Sanderson et al., 2017). We refer to these various scenario by the projected global warming increase (°C) above pre‐industrial levels: Scenario 4.3°C [RCP8.5], Scenario 2.6°C [new scenario, see Section 2.1.3], Scenario 2.4°C [RCP4.5], Scenario 2.0°C [Paris <2.0°C] and Scenario 1.5°C [Paris 1.5°C].
FIGURE 2.
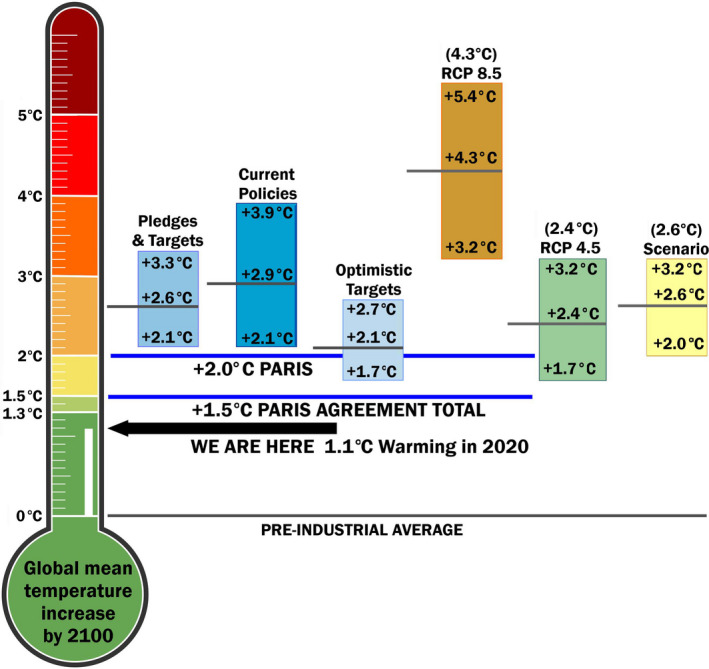
Comparison of climate scenarios with the global mean temperature targets under current policies. The thermometer comes from the Climate Action Tracker (Stockwell et al., 2021), which is an independent scientific analysis to quantify and evaluate climate change mitigation commitments, and assess whether countries are on track for meeting them. For the climate scenarios (orange, green, and yellow boxes), the likely range of global mean temperature values are the global warming increase (°C) projections from the Fifth Assessment Report Table SPM‐2 in: Summary for Policymakers corrected to be relative to pre‐industrial level (Stocker et al., 2013). For RCP scenarios, it is the mean and likely range for 2081–2100, and for the new climate scenario, it is the mean and likely range for 2046–2065. In the text we refer to each scenario by the mean temperature, for example, for RCP4.5 we refer to it as the 2.4°C Scenario. In addition, we used two climate change scenarios meeting the Paris Agreement objectives that produce stable equilibrium global mean temperature at 1.5 and 2.0°C above pre‐industrial levels (horizontal lines, Sanderson et al., 2017). The blue boxes represent the likely range of global median temperature values provided by the Climate Action Tracker analysis in May 2021 to provide the likelihood of temperature goals being met under various policy scenarios. RCP, Representative Concentration Pathway
2.1.2. AOGCMs climate outputs
The climate outputs from multiple AOGCMs are publicly available in a standardized format on the Coupled Model Intercomparison Project (CMIP) website (https://www.wcrp‐climate.org/). CMIP5 provides a framework for coordinated climate change experiments for assessment in the IPCC Fifth Assessment Report in 2014 using four RCPs describing future GHG concentration trajectories based on socio‐economic assumptions. Other recent GHG emissions forcing scenarios have been developed and used for climate projections in CMIP6 for the Sixth Assessment Report due to be released in 2022 (Hausfather & Peters, 2020a). These “Shared Socioeconomic Pathways” (O’Neill et al., 2016) differ in the future time series of specific prescribed climate forcers, such as GHG and aerosol emissions, but bracket the same radiative forcing range (i.e., global heat gained by the Earth System) as the RCP scenarios. Large ensemble simulations using Shared Socioeconomic Pathways scenarios were not available at the time of our penguin assessment; thus, we use climate projections driven with RCP emissions forcing and special Paris Target emissions instead.
To diagnose uncertainties related to natural climate variability—a noise from unforced variability generated internally within the climate system (e.g., weather) or associated with external forces to the climate system (e.g., volcanoes)—require multiple climate ensemble members from a single AOGCM. We also wanted to use emission scenarios specifically designed to assess the Paris Climate Agreement targets. Large ensembles and simulations with specialized Paris Target scenario emissions scenarios are not typically available from CMIP AOGCMs but have been performed for the Community Earth System Model, version 1 (CESM1). Therefore, we use results exclusively from the CESM1 as discussed further below.
The population growth rates are largely influenced by the Antarctic sea ice concentrations during the laying season (April–May), which are very well simulated in the CESM model. While there are some biases in the sea ice average projected by CESM for the colonies located in the eastern Weddell Sea and in the west Pacific Ocean during non‐breeding (January–March), the population growth rate is not influenced by these local and seasonal sea ice concentrations (Jenouvrier et al., 2020). In comparison, the growth rate is highly influenced by sea ice concentrations during the chick‐rearing season (August–December), but the magnitude of the population growth rate response to sea ice varies considerably amongst colonies and years. There is little impact of sea ice concentrations during the rearing season for colonies located in the west Pacific Ocean, where the largest differences occur between the averaged sea ice concentrations simulated by CESM and observations (Jenouvrier et al., 2020).
2.1.3. Sea ice projections
The sea ice concentrations used as inputs to the meta‐population models are obtained from several sets of ensemble simulations from the CESM1 with the Community Atmosphere Model version 5 (Hurrell et al., 2013) (CESM1–CAM5). These include the CESM1 Large Ensemble (Kay et al., 2015) with 40 ensemble members from 1920 to 2100 which are run with the RCP8.5 emissions scenario from 2005 to 2100 [4.3°C Scenario]; the CESM1 Medium Ensemble (Sanderson et al., 2018) which includes 15 ensemble members from 2005 to 2080 forced with the RCP4.5 emissions scenario [2.4°C Scenario]; and Paris target ensembles run from 2005 to 2100 which are designed to reach 1.5 and 2°C global warming by 2100 (Sanderson et al., 2017). These specialized sets of large ensemble and Paris target experiments are not available from other AOGCMs and thus we only use CESM1–CAM5 simulated ice concentrations for our analysis. Therefore, we are not able to consider the influence of climate model structural uncertainty associated with the variations in simulated sea ice concentration across different AOGCMs. Nevertheless, when compared to observational products, CESM1 produces a good simulation of the annual cycle of total Antarctic sea ice extent (Eayrs et al., 2020), associated melt and growth rates (Eayrs et al., 2020), and processes, such as wind variations, that drive sea ice variability (e.g., Landrum et al., 2017), making it a useful tool for our analysis.
To account for resilience in sea ice processes (Ridley et al., 2012), we developed a new climate scenario [Scenario 2.6°C] which conservatively assumes that sea ice concentrations will remain at a steady level for 50 years from 2050 to 2100 (Figure 3). Indeed, Ridley et al. (2012) suggested that sea ice will continue to decline for ~20 years and that sea ice loss pause for an additional ~30 years after the maximum global temperature is reached. Estimating such loss requires specific climate experiments that are beyond the scope of our study. Hence, to construct such sea ice forecasts from 2050 to 2100, sea ice concentrations are sampled randomly from 2045 to 2055 concentrations simulated by the CESM1 large ensemble under RCP8.5 emissions, as the total cumulative CO2 emissions consistent with RCP8.5 are in close agreement with historical total cumulative CO2 emissions and the best match out to 2050 under current and stated policies (Schwalm et al., 2020a).
FIGURE 3.
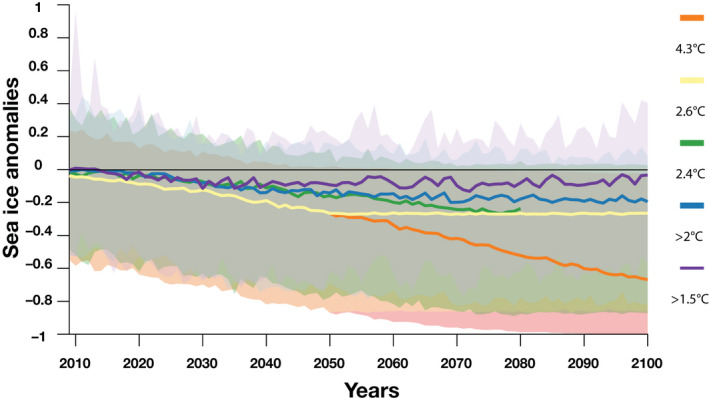
Sea ice concentration anomalies relative to historical level for each climate scenario (colored lines; orange—Scenario 4.3°C [RCP8.5], yellow—Scenario 2.6°C [new scenario], green—Scenario 2.4°C [RCP4.5], blue—Scenario 2.0°C [Paris >2.0°C] and purple—Scenario 1.5°C [Paris 1.5°C]) projected from the Community Earth System Model from 2009 to 2100 (except for Scenario 2.4°C [RCP4.5] as projections are available only to 2080). The median and 90% confidence envelope are calculated across all seasons of the penguin life cycle and across all emperor penguin colonies. The zero black horizontal line is provided to assess the decreasing trend over time. RCP, Representative Concentration Pathway
2.2. Population projections of emperor penguin
2.2.1. Sea ice‐dependent meta‐population models
The sea ice‐dependent meta‐population model projects the population size at each colony from 2009 to 2100 (except for Scenario 2.4°C [RCP4.5] for which CESM climate outputs are available only until 2080), hence, allowing assessment of the conservation status at each colony, and the regional and global population sizes. Here, we present for the first time the regional population projections and Table S1 shows the details of the colony name included in each of the five regions. This model was built over a decade of research (Jenouvrier et al., 2010, 2012, 2014, 2017; see Supplementary Methods) based on long‐term dataset on breeding emperor penguins at Pointe Géologie (#35, Figure 1). This colony has been monitored every year from 1962 onwards allowing the estimation of breeding success and breeding pair number (Barbraud & Weimerskirch, 2001). Survival estimates are based on individual longitudinal data from capture–recapture dataset from 1968 to 2000 (see details in Jenouvrier et al., 2012). In a nutshell, this sea ice‐dependent meta‐population model includes nine dispersal scenarios combining different dispersal rates, behaviors, and distances. Specifically, it assumes that individuals only emigrate from poor‐quality breeding sites when environmental conditions lead to negative intrinsic population growth rate (Jenouvrier et al., 2017; Figure S2). With an informed search, using information gained while searching, individuals select breeding habitats that maximize fitness within a specific dispersal range; this behavior occurs among some colonial seabirds that prospect for breeding sites using the presence and reproductive success of residents (Doligez et al., 2002). In contrast, random search behavior results in undirected movements with respect to habitat quality. The short‐distance dispersal scenario allows for regional movements among colonies, while long‐distance dispersal creates a more connected meta‐population across the entire range (see Jenouvrier et al., 2017 for more details).
In addition, here, we develop four scenarios of extreme environmental pertations:
Extreme events with the historical observed frequency that will produce a complete breeding failure at a colony in a given year;
Extreme events with the historical observed frequency that will reduce adult survival by 10% and produce a complete breeding failure at a colony in a given year;
Extreme events that will increase in frequency in the future proportionally to the loss of sea ice and produce a complete breeding failure at a colony in a given year;
Extreme events that will increase in frequency in the future proportionally to the loss of sea ice and will reduce adult survival by 10% and produce a complete breeding failure at a colony in a given year.
We consider five different plausible future emission scenarios with associated projected changes in sea ice habitat, and account for the uncertainties in both climate and demographic processes resulting in 180 scenarios (5 climate scenarios × 4 extreme event scenarios × 9 dispersal scenarios) and 360,000 simulated population trajectories.
2.2.2. Frequency of extreme events
To estimate the frequency of extreme events, we calculated true absence for the number of colonies during the last 10 years using very high‐resolution satellite imagery. It is important to note that this is a conservative estimate, as complete breeding failure may occur at colonies even while breeding pairs may be still present at the colony (e.g., in 2013 at Pointe Géologie #35; Barbraud et al., 2015), hence, such extreme events are not detected from satellite imagery. As a consequence, a true absence of a colony underestimates the frequency of complete breeding failure. We estimated a frequency of f = 3.6% as the number of colony absences across all colonies across all years divided by the total number of colonies × years (M. LaRue, S. Labrousse, P. Fretwell, P. Trathan, D. Ortega, R. Foster‐Dyer, M. Nixon, E. Devane, B. Horstman, L. Viollat, & S. Jenouvrier, unpublished data).
For scenario 1 where historical extreme events caused complete breeding failures, we assume that f is constant throughout the century, and we sample extreme events within a binomial distribution with probability f. To account for the fact that this frequency of extreme events may change with future global warming, we assume that the future frequency increase is likely proportional to sea ice concentration decline. First, we calculated the sea ice concentration threshold T si that corresponds to the observed frequency of extreme events f during the 10‐year historical period for laying or rearing seasons. Second, we classified each year as extreme or not, by comparing the sea ice concentration in that year to T si. If sea ice concentrations are lower than T si, we sample into a binomial distribution with a probability of 50%, an event about as likely as not, to characterize if such an event is extreme or not. This allows us to account for uncertainties in our assumption that extreme events frequency is related to sea ice, but as a consequence reduces the frequency of extreme events by half during the historical period.
2.2.3. Impact of extreme events on reproduction and survival
In our population model, both reproduction and survival depend upon sea ice concentrations (see Jenouvrier et al., 2012 for details). These demographic rates are included in a nonlinear, stochastic, sea ice‐dependent, two‐sex, stage‐classified matrix A to project the intrinsic growth rate of the population (Figure S2; Supplementary Methods). Here, we associate each environmental state (extreme or not) with a set of population matrices based on the occurrence of extreme events: A EX [A EX1, … A EX k ], with k the number of extreme climate years, and A ORD includes all other non‐extreme years. In the set of A EX the reproduction is reduced to zero and adult survival may be reduced by 10% in the case of extreme events that affect both survival and reproduction. We do not account for the fact that for many perturbations major consequences may last for several years (e.g., giant iceberg; Kooyman et al., 2007). Hence, our scenarios underestimate the consequences of lower reproduction and the cost of relocation on both reproduction and likely survival in the consecutive years following a major perturbation.
2.2.4. The 3Rs: Resiliency, redundancy and representation
Under the Species Status Assessment framework developed by the USFWS, the status of a species is evaluated with respect to resiliency, redundancy, and representation, also known as the 3Rs (Redford et al., 2011; Shaffer & Stein, 2000; Wolf et al., 2015). Resiliency is associated with population size, growth rate, and habitat quality. The percent median global population decline relative to its initial size (Table S2; Figure 4), which is a function of the habitat quality (measured as the intrinsic growth rate [Figure S2] in our model) in our model was used to describe resiliency in the Species Status Assessment. Representation is related to distribution within the species’ ecological settings and we used the number of ecological settings, that is, regions, with suitable habitat (population growth rate of regional population size >0) (Table S2; Figure 5). To characterize redundancy, which is related to the number, distribution, and resilience of populations, we used the proportion of quasi‐extinct colonies across the entire continent (Table S2; Figure 6). We scale up these values between 0% and 100%.
FIGURE 4.
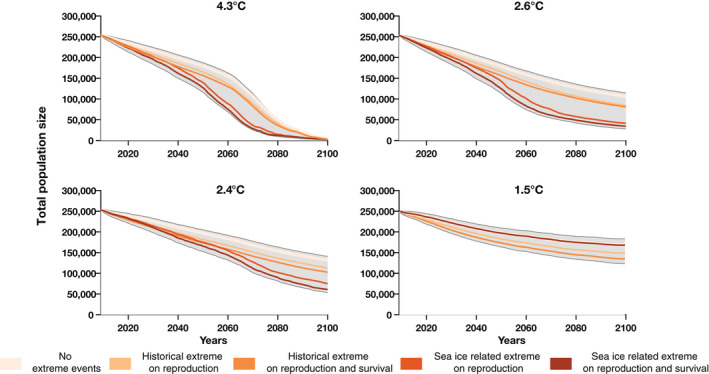
Total number of breeding pairs of emperor penguins from 2009 to 2100 projected for various climate scenarios (panels—Scenario 4.3°C [RCP8.5], Scenario 2.6°C C [new scenario], Scenario 2.4°C [RCP4.5], Scenario 1.5°C [Paris 1.5°C]) for various demographic scenarios of extreme events (colored lines). RCP, Representative Concentration Pathway
FIGURE 5.
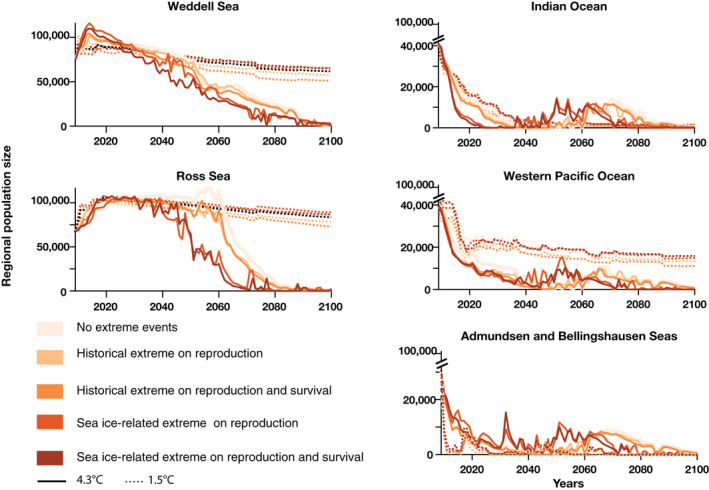
Regional number of breeding pairs of emperor penguins from 2009 to 2100 projected for two climate scenarios (plain lines 4.3°C Scenario [RCP8.5]; dotted line 1.5°C [Paris 1.5°C]) for various demographic scenarios of extreme events (colored lines) and regions (panels). RCP, Representative Concentration Pathway
FIGURE 6.
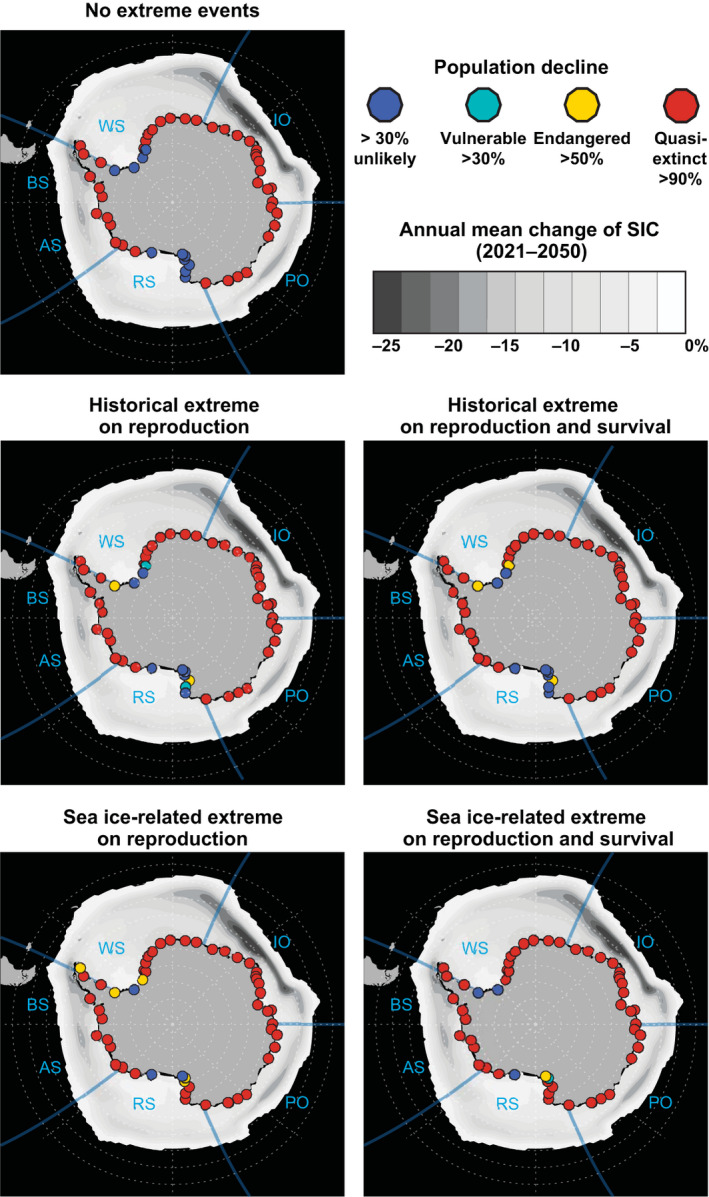
Conservation status of emperor penguin colonies by 2080 and annual mean change of sea ice concentration (SIC) between the 20th and mid‐21st centuries for the 2.6°C Scenario. Panels show each extreme events scenario with all dispersive scenarios combined. SIC projections were obtained from the Community Earth System Model. Dots show the location of colonies (Figure 1; Table S2). Dot colors show the projected International Union for Conservation of Nature Red List conservation status. Following Jenouvrier et al. (2014, 2020), “vulnerable” (green) is a likely population decline by more than 30%; “endangered” (yellow) is a likely population decline by more than 50%; “quasi‐extinct” (red) is a likely population decline by more than 90%. Blue color refers to populations that are not likely to decline by more than 30%. A likely outcome is defined by IPCC as a probability >66%. AS, Amundsen Sea; BS, Bellingshausen Sea; IO, Indian Ocean; RS, Ross Sea; WPO, Western Pacific Ocean; WS, Weddell Sea
2.3. Uncertainties in projections
Our population viability analyses are projections; they are expressed as conditional statements based on the structure of the model producing the results. Here we link climate models to a species life cycle model, with long‐term and statistically rigorous estimates of the functional relationship between sea ice concentrations and vital rates (reproduction, survival, etc.) at Pointe Géologie (#35 Table S1), extended to all other colonies, with sea ice concentrations measured over large spatial scales (Jenouvrier et al., 2014). The species has existed over geological time, surviving previous glacial and interglacial periods probably by migrating to suitable habitat as conditions change (Younger et al., 2015, 2017). In addition, within the 13 years (2009−2021) of the satellite record, at least 9 of ~61 colonies are known to “blink” (disappear in some years, reappear in others, such as Ledda Bay, #47 Figure 1). Therefore, we include individual dispersive behaviors and extreme perturbations that were documented using satellite imagery (Trathan et al., 2020) in our metapopulation model to capture these potential dynamics observed over geological and historical scales (Cole et al., 2019). Importantly, our model includes multiple sources of stochasticity and uncertainties related to climate and demography (Jenouvrier et al., 2012, 2020), including the chaotic temporal evolution of the coupled ocean–atmosphere system (“natural variability”), and demographic parameter “unexplained” temporal variance in demographic rates that are not accounted for by sea ice (Jenouvrier et al., 2020).
Our model makes assumptions about the ecology of emperor penguins based on over 60 years of research (see discussion in Jenouvrier et al., 2014). It assumes ecological carrying capacity remains constant over time (Jenouvrier et al., 2017). However, a probable impact of sea ice loss will be on Antarctic trophic food web structure, including on emperor penguin prey. Decreased foraging habitat and availability (or abundance) of prey will reduce carrying capacity. On the other hand, as in the Arctic (Kaartvedt & Titelman, 2018), new species may colonize high‐latitude waters, constituting new resources for opportunistic foraging (Trathan et al., 2020) by emperor penguins. Emperor penguins breed on unstable habitat. When sea ice is sub‐optimal, breeding on land or on ice shelves is sometime possible, but most likely will result in higher energy expenditure (longer foraging trips, greater exposure to cold and wind, etc.). Thus, although the species may adapt in part, it is uncertain whether this is a long‐term solution as birds would still be subject to the consequences of an altered food web. With time, many uncertainties will decrease as the response of emperor penguins to climate change becomes progressively apparent.
Accurate measurement and modelling of environmental features that directly affect emperor penguin life cycle, such as fast ice extent remains challenging, especially at the circumpolar scale (Fraser et al., 2021), and these features are not projected by AOGCMs. Trends in overall sea ice extent (largely contributed by the trend in pack ice extent) are potentially independent of what might be happening with coastal fast ice: for example, altered winds may lead to more extensive large‐scale sea ice, but possibly reduced fast ice (Ainley et al., 2010). Understanding and projecting fast ice is still limited by a paucity of studies investigating the role of environmental factors driving fast ice changes, most of which consider only one‐dimensional (i.e., thermodynamic) drivers of fast ice thickness (Brett et al., 2020; Heil, 2006; Hoppmann et al., 2015; Lei et al., 2010), and do not consider fast ice extent/distribution. Moreover, other complexities affect both fast ice trends and processes driving them, such as the profound and unpredictable effects that large tabular icebergs can have on regional fast ice extent (Fogwill et al., 2016). Nevertheless, an analysis of circumpolar fast ice extent shows similarities with overall sea ice extent (Fraser et al., 2021), suggesting that in the long term, sea ice extent and sea ice concentrations at the large scale probably determines the ultimate condition of fast ice as a breeding platform for penguins. Future work should entail a better understanding of the projected changes in Antarctic fast ice dynamics, and other sea ice features such as icebergs and ice tongues, that ultimately affect the emperor penguin habitat.
3. RESULTS AND DISCUSSION
Scientific advice to decision‐makers should be tailored to the applicable policies and laws governing decision‐making. Here we present important concepts related to the protection of species endangered by climate change, using as an example the emperor penguin, a flagship species threatened by sea ice decline. We develop a framework specifically tailored to the needs of the ESA and consider fundamental concepts for assessing species’ climate change‐driven extinction risk (e.g., foreseeable future, 3Rs, Figure 7). This study provides the “best available science” for projecting emperor penguin populations in the context of future climate change. We include new information about the impact of environmental perturbation on colony dynamics and a new climate scenario accounting for the resiliency of sea ice, while limiting uncertainties in socio‐economic pathways by using 2050 sea ice levels (Figures 2 and 3). The study was designed to assess the 3Rs (Figures 8, 9, 10) and inform policymakers about whether the emperor penguin warrants listing under the ESA and we discuss recommendations and ESA protections for emperor penguin.
FIGURE 7.
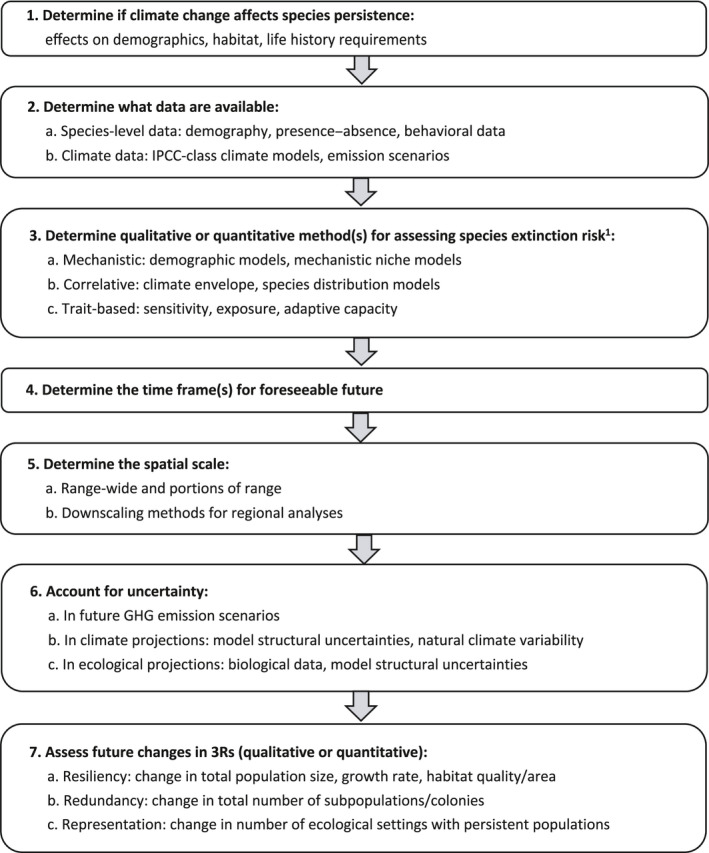
Steps for assessing species’ climate change‐driven extinction risk under the ESA. Note 1 refers to Foden et al. (2019)
FIGURE 8.
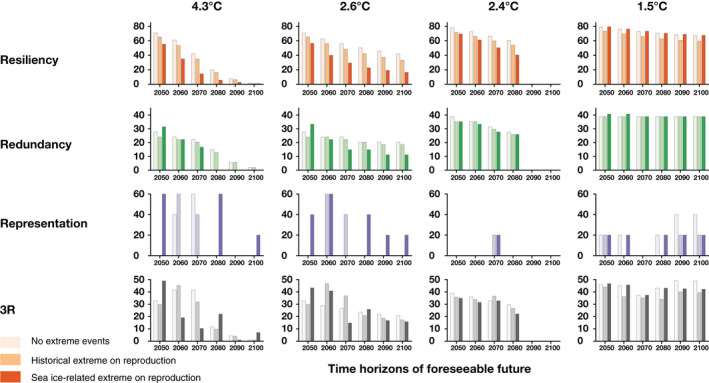
Projected resiliency, redundancy and representation which describe the demography, spatial distribution, and the diversity of ecological settings for emperor penguins, shown at decadal scales between 2050 and 2100 under different greenhouse gas emission scenarios (Scenario 4.3°C [RCP8.5], Scenario 2.6°C [new scenario], Scenario 2.4°C [RCP4.5], Scenario 1.5°C [Paris 1.5°C]). For each time period and each emission scenario, different extreme event scenarios are shown (pale bars—no extreme events, mid‐color bars—extreme events affecting reproduction, and dark bars—sea ice extreme events affecting reproduction). Each R is expressed in percentage and the 3Rs is the arithmetic average of those three percentages. RCP, Representative Concentration Pathway
FIGURE 9.
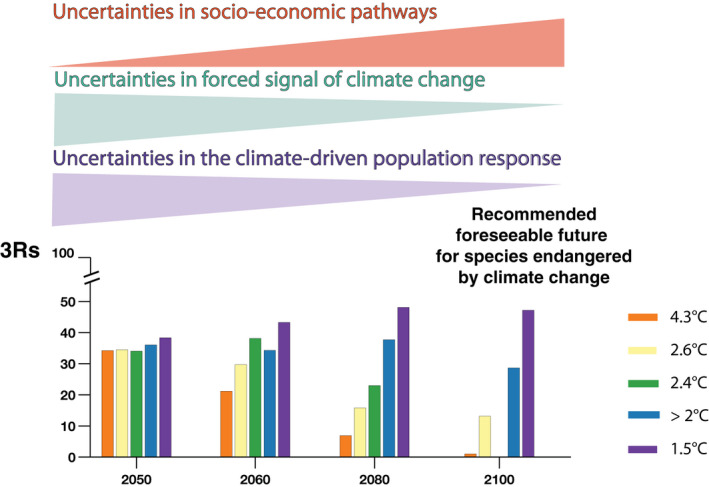
Projected 3Rs (Resiliency, Redundancy, and Representation in %) for emperor penguins at decadal scales between 2050 and 2100 under different greenhouse gas emission scenarios (Scenario 4.3°C [RCP8.5], Scenario 2.6°C [new scenario], Scenario 2.4°C [RCP4.5], Scenario 2.0°C [Paris >2.0°C] and Scenario 1.5°C [Paris 1.5°C]). A graphic depiction is shown (triangles) for the increasing uncertainty over future socio‐economic pathways, and decreasing uncertainties for the directionality of climate change (forcing signals in climate change) and climate‐driven signals in population declines. RCP, Representative Concentration Pathway
FIGURE 10.
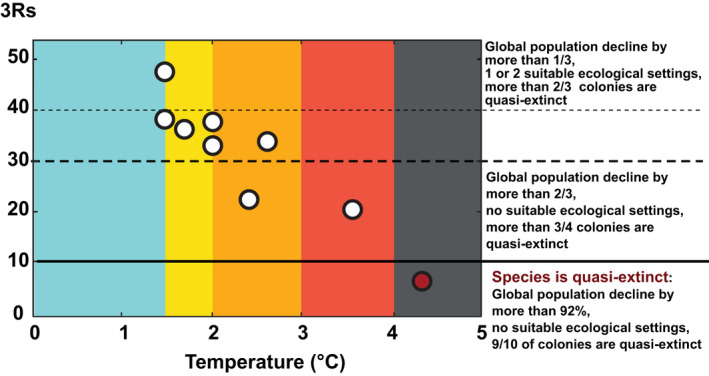
Projected 3Rs at different average global temperature increases. Those average global temperature increases correspond to the global mean temperature values from the Fifth Assessment Report global warming increase (°C) projections corrected to be relative to pre‐industrial level for 2081–2100, and for 2046–2065 (Figure 2). The colors refer to the Climate Action Tracker thermometer from Figure 2. The percentage thresholds (horizontal plain and dotted lines) are arbitrary but illustrate some decline in global population size, numbers of quasi‐extinct colonies and ecological settings (see text)
3.1. Climate risk assessment
Assessing risk to species due to climate change requires projections of future environmental conditions, which are subject to plausible emissions scenarios (Figure 7). AOGCMs are the best tools to provide these projections (Jenouvrier, 2013; Stock et al., 2011) and have been shown to skillfully predict observed changes in the climate system over the last several decades (Buis, 2020). However, while the models are proficient, they have biases and uncertainties that need to be considered (Knutti, 2008) (Section 2).
In addition to evaluating AOGCM model performance, future policy decisions must be based on a range of possible emission scenarios resulting from current and projected energy system trends and policies, as well as the species responses in relation to such scenarios. A range of emissions scenarios (including Representative Concentration Pathways, Shared Socioeconomic Pathways, and special scenarios) that reach different levels of radiative forcing over the 21st century are available. Figure 2 shows the results of the Climate Action Tracker (Stockwell et al., 2021) analysis, which tracks progress toward reaching global climate targets under the Paris Agreement. The emissions pledges and targets pathway that includes governments’ Nationally Determined Contributions and some long‐term targets has an 78% probability of exceeding 2°C, while the current policy pathways have a >97% probability of exceeding 2°C. The effect of net zero emissions targets adopted or under discussion in 131 countries could result in a warming as low as 2.0°C by 2100 (Figure 2). Recent climate actions, including the announcements at US President Biden's Leaders Summit on Climate, have improved the Climate Action Tracker's warming estimate by 0.2°C in May 2021 and targets are now estimated to be 2.4°C.
Our new scenario (2.6°C; Figures 2 and 3) is intended to demonstrate probable effects on sea ice and therefore emperor penguins by 2100 if governments act now to control GHG emissions by 2050. This optimistic scenario reflects the current window of opportunity, demonstrating that even with immediate positive action, inherent lags in the climate system will continue to have impacts into the future once carbon neutrality is reached. The 2.6°C scenario developed here assumes that CO2 emissions are in accord with RCP 8.5 scenarios until 2050 for which emissions are in closest agreement with historical total cumulative CO2 emissions and is the best match until the mid‐century under current and stated policies (Schwalm et al., 2020a) (Section 2). To consider a longer time horizon under this new scenario, we extended the sea ice projections from 2050 to 2100 by assuming that sea ice decline will pause for the next 50 years due to the lagged responses of sea ice to GHG emissions (Section 2). Indeed, Ridley and Hewitt (2014) showed that minimum extents of Antarctic sea ice lags behind peak concentrations of CO2 by ~20 years, followed by a 30 year pause in sea ice decline, while atmospheric concentrations of CO2 would return to preindustrial levels.
While there is a debate about which scenario is most relevant for climate risk assessment studies, emissions scenarios have not diverged that much by 2050, but do so more during the second half of the century with increasing uncertainties in socio‐economic pathways (Hausfather & Peters, 2020b; Schwalm et al., 2020a, 2020b). Given this, the choice of scenario is less of an issue when only looking at sea ice concentration projections to 2050 (Figure 3), and our new scenario is an optimistic projection as it assumes that sea ice loss pauses at 2050 values and remains at these levels out to 2100. As there is little consensus on the likelihood of GHG emissions scenarios, we account for uncertainties in socio‐economic pathways by using several emissions scenarios, recognizing that climate assessments under the ESA should use an emissions scenario that represents the trajectory under current policies (National Marine Fisheries Service, 2016).
3.2. Factors for assessing species’ climate risk under the ESA
Two US agencies (collectively, “the Services”) implement the ESA: the FWS manages land and freshwater species whereas NMFS manages most marine and anadromous species. Species may be listed under the ESA as endangered or threatened depending upon the level and timeframe of threats. “Endangered” means the species “is in danger of extinction throughout all or a significant portion of its range” (Endangered Species Act, 1973a), whereas “threatened” means the species is “likely to become an endangered species within the foreseeable future” in all or a significant portion of its range (Endangered Species Act, 1973b). The threatened category and its consideration of the foreseeable future is of particular relevance to climate‐affected species that may not currently be in danger of extinction, but are projected to become endangered in the future as climate threats increase in all or portions of their range. In making listing decisions, relevant agencies must assess the status and threats to species relying solely on the best scientific and commercial data available (Endangered Species Act, 1973c).
3.2.1. Best‐available science and scientific uncertainty
The ESA requires that listing decisions be based on the best available science (Endangered Species Act, 1973c) rather than conclusive evidence (U.S. District Court for the District of Columbia, 1997). This requirement is particularly relevant for climate threat assessments that rely on climate and ecological projections that have inherent variability and uncertainty. Despite the ESA’s intent to give “the benefit of the doubt to the species” (United States Court of Appeals et al., 1988), the Services have, in multiple cases, relied on uncertainties in climate projections, projected habitat change, and species’ adaptive capacity as a basis to deny listing (National Marine Fisheries Service, 2008; US Fish and Wildlife Service, 2008b, 2010, 2017a, 2017b, 2018a, 2020a) (Table 1).
3.2.2. Foreseeable future for evaluating climate threats
The ESA does not define “foreseeable future”, and the Services’ interpretations have changed over time (Li et al., 2020). According to the law's intent, foreseeable future concerns the “ability to forecast population trends” so that species can be protected “before the danger [of extinction] becomes imminent” (U.S. Senate, 1973). In 2009, the Department of Interior interpreted “foreseeable future” as the timeframe over which the Services can “reasonably rely on predictions about the future in making determinations about the future conservation status of the species” (Department of Interior, 2009). In 2016 FWS recommended using a time period “long enough to encompass multiple generations so the species responses can be predicted” and “appropriate for the information available on the stressors and conservation efforts that are likely to occur and predictions of the species responses to these future environmental changes” (US Fish and Wildlife Service, 2016). In 2019 the Services finalized regulations, currently being challenged in court, that defined the foreseeable future for the first time as “only so far into the future as the Services can reasonably determine that both the future threats and the species’ responses to those threats are likely,” where likely means “more likely than not” (US Fish and Wildlife Service and National Marine Fisheries Service, 2019).
The Services defined foreseeable future for climate threats as 2050 for the polar bear, but subsequently increased the foreseeable future timeframe to 2100 for other climate‐threatened species including the Pacific walrus (Odobenus rosmarus divergens), ringed seal (Phoca hispida) and bearded seal (Erignathus barbatus) (Table 1). Continuing inconsistencies in timeframe appear to be related to decision‐making by different agencies. For example, listings for climate‐threatened species made by NMFS have been based on a 2100 timeframe (ringed seal, bearded seal, 20 coral species; Table 1), whereas FWS used a 2050 timeframe for three of the four species listed to date (polar bear, meltwater lednian stonefly (Lednia tumana), western glacier stonefly (Zapada glacier)) while using 2100 for ‘i'iwi (Drepanis coccinea). NMFS has repeatedly concluded that climate projections through 2100 represent the best scientific data to inform the assessment of climate change impacts (National Marine Fisheries Service, 2012, 2014). In explaining a foreseeable future through 2100, NMFS emphasized that, while the magnitude of warming is influenced by the assumed emissions scenario, trends in warming through the end of the century are “clear and unidirectional” under all climate projections and considered emissions scenarios (National Marine Fisheries Service, 2012, 2014).
Critically, climate change resulting from increases in CO2 emissions is largely irreversible for up to 1000 years after emissions cease (Solomon et al., 2009). For the emperor penguin, we emphasize that even if humankind stopped emitting any GHGs today, the lag effect due to atmospheric attenuation means that climate change will continue to affect sea ice well into the future (Figure 3). Even though we recommend that foreseeable future for species endangered by climate change should be considered to extend to at least 2100, consistent with other studies (Li et al., 2020), here, we also include results for shorter time periods to highlight the increasing threat over time.
3.2.3. 3Rs: Resiliency, redundancy, and representation
In 2016, the FWS formalized a Species Status Assessment process to support ESA decision‐making, including listing decisions (Smith et al., 2018; US Fish and Wildlife Service, 2016). Under this framework, the current and future statuses of the species are evaluated with respect to the 3Rs: resiliency, redundancy, and representation (Redford et al., 2011; Shaffer & Stein, 2000; Wolf et al., 2015). The FWS does not specify minimum acceptable thresholds for the 3Rs, but defines resiliency as the ability to withstand stochastic disturbance, which may be measured through population size, growth rate, and connectivity among populations. Redundancy describes the ability to withstand catastrophic events, and considers the number, distribution, resiliency, and connectivity of populations. Representation describes the ability to adapt to changing environmental conditions, and is related to capturing the geographic, genetic, and life history variation that exists across the species’ ecological settings. Together, the 3Rs encompass the aspects that contribute to species persistence (e.g., demography, spatial distribution, diversity) and are important for assessing climate threats in the foreseeable future.
3.3. Threats to emperor penguins: Loss of sea ice
Given the species’ reliance upon sea ice for breeding, molting, and feeding, the most important threat for emperor penguins is climate change, which would lead to Antarctic sea ice losses over this century (Ainley et al., 2010; Trathan et al., 2020). To forecast species responses to climate change, it is critical to evaluate model performance and account for uncertainties in climate processes (Dietze, 2017). From 1979 to 2018, climate models typically simulate loss of Antarctic sea ice while observations showed little change (Roach et al., 2020; Turner et al., 2013). AOGCMs that simulate large ice loss exhibit stronger‐than‐observed global warming, suggesting a role for global biases in the discrepancy in observed and modelled ice trends (Roach et al., 2020; Rosenblum & Eisenman, 2017). Regional trends, particularly the observed decrease in the Bellingshausen Sea and the expansion in the Ross Sea, are also not typically captured in the models (Hobbs et al., 2016). However, some models, such as the CESM1 used in this study (Section 2), compare well with observations in, for example, the annual cycle (Eayrs et al., 2020; Raphael et al., 2020), regional and seasonal ice distributions (Jenouvrier et al., 2020), and the relationship of ice to the Amundsen Sea Low (Landrum et al., 2017). Additionally, CESM simulations indicate that internal ocean variability can drive increasing sea ice despite rising GHGs (Singh et al., 2019). The range of Antarctic sea ice conditions relevant for the emperor penguin simulated by the CESM Large Ensemble (Kay et al., 2015) overlaps very well with the range of observations over the historical period, except in a few regions and seasons of the penguin annual cycle for which the population growth rate response to sea conditions is small (see figures 1 and 2 in Jenouvrier et al., 2020; Section 2).
Future projections consistently simulate Antarctic sea ice loss across seasons, suggesting a predictable GHG‐forced signal. Although there is considerable structural model uncertainty in the magnitude of projected sea ice loss, the sign of change is consistent, and ~75% of CMIP5 models reach a near ice‐free state in February under RCP8.5 forcing by 2100 (Collins et al., 2013). The influence of forcing scenario is apparent in sea ice projections, and multi‐model means clearly diverge by 2100 (Roach et al., 2020).
Figure 3 details sea ice concentrations anomalies relative to historical levels across all seasons of the penguin life cycle and all emperor penguin colonies simulated by CESM. Sea ice concentrations are clearly projected to decline, with differences amongst climate scenarios increasing over time, with most median trajectories starting to diverge around 2050. As such, by 2050, the differences amongst scenarios is relatively small with Antarctic sea ice declining by 23% (median across seasons and colonies) under Scenario 4.3°C (RCP8.5) and 13% under Scenario 2.0°C (Paris agreement) (Table S2). However, by 2100, under Scenario 4.3°C, Antarctic sea ice concentrations at colonies are projected to decline by ~63% relative to historical levels. In contrast, under Scenario 2.0°C, the percent decline in sea ice concentrations is 19% by 2100.
3.4. Extreme climate events and colony dynamics of emperor penguins
Major perturbations at emperor penguin colonies affect the size of the vital rates and breeding population, and consequently species’ 3Rs. Extreme events magnify global population declines (Figure 4), especially if they affect both reproduction and survival. The largest differences between the global population medians projected with or without extreme events occur between 2060 and 2080, under the 4.3°C Scenario and the 2.6°C Scenario, with a percent decrease of the global population size of at least 50% relative to a scenario without extreme events.
If the frequency of extreme events is set to a constant historical frequency and affects only reproduction, on average across the five climate scenarios and six decades (2050−2100), the global population medians with extreme events decrease by ~12% relative to the median without extreme events. This percentage decrease is larger when the extreme events affect both reproduction and survival: 18%. For example, under the 1.5°C Scenario, by 2100, 170,000 breeding pairs are projected without extreme events, while 150,226 and 136,507 breeding pairs are projected if extreme events affect only reproduction, or both reproduction and survival respectively.
If the frequency of extreme events depends on sea ice, and increases in the future, the difference in the percentage decrease in an environment with and without extreme events is ~24%, and 29% on average across climate scenarios and decades if the extreme event affects only reproduction or both reproduction and survival, respectively. For example, under the 4.3°C Scenario, by 2080, 50,359 breeding pairs are projected without extreme events, while 14,813 and 12,111 breeding pairs are projected if the extreme events affect only reproduction or both reproduction and survival, respectively. However, the median global population trajectories do not differ between scenarios (with or without extreme events) when dramatic loss of sea ice leads to the global population extinction (e.g., 4.3°C Scenario by 2100), or sea ice loss is minimal and the sea ice‐dependent frequency of extreme events is very small (e.g., 1.5°C Scenario).
The impact of extreme events at regional scales is complex (Figure 5), and the regional population dynamics differ among extreme scenarios when projected sea ice losses are large (4.3°C Scenario) and the frequency of extreme events is proportional to sea ice loss. For example, in the Ross Sea, emperor penguin habitat is projected to be unsuitable 10 years earlier (around ~2050) when sea ice‐dependent extreme events are included (Figure S2). As such, emperor penguins disperse to other regions, and regional increases are projected in the Indian Ocean and western Pacific Ocean, and the Amundsen Sea and Bellingshausen Sea. However, since none of these regions provide sustainable habitat, movements amongst colonies results in large global population declines (Figure S2; Figures 4 and 5).
Despite a strong impact of extreme events on the population dynamics of emperor penguins, the number of quasi‐extinct colonies differs among extreme scenarios only in a few cases (Table S3). Including historical frequency of extreme events has little effect on the number of quasi‐extinct colonies, but sea ice‐dependent frequency of extreme events could substantially change the status of several colonies, for example, eight colonies change by 2080 under the 4.3°C Scenario. These eight colonies are located in the Weddell Sea and Ross Sea, and are all projected to be quasi‐extinct with the extreme sea ice‐dependent scenarios, while they are projected to be only endangered or vulnerable without extreme events. It is important to note that whilst the proportion of quasi‐extinct colonies does not vary much at the continental scale between extreme event scenarios, extreme events do change the dynamics of the status of endangerment at each colony (Figure 6).
3.5. Emperor penguins and 3Rs
The 3Rs (resiliency, redundancy, and representation) describe the demography, spatial distribution, and the diversity of ecological settings that are important factors contributing to species persistence by allowing for various qualitative and quantitative methods to assess each of these conservation principles (Smith et al., 2018; Wolf et al., 2015) (Section 2; Figure 7). Here, the highest value (100%) represents maximum resiliency (the global population has not declined relative to its initial size), or maximum redundancy (none of the colonies are quasi‐extinct), or maximum representation (no ecological setting will eventually disappear). The USFWS does not specify minimum acceptable thresholds for resiliency, redundancy, and representation, and there is currently no consensus on how to quantify the 3Rs all together. To summarize the impact of global warming on the 3Rs, we average these three percentage measures to provide an overall quantification of the 3Rs across climate and demographic scenarios, and for different lengths of time into the foreseeable future. This simple 3Rs measure (Figures 9 and 10) should be interpreted alongside and in context with the individual measures (3Rs, Figure 8).
The 3Rs are projected to decline dramatically throughout the century across emission scenarios (Figures 8 and 9). The loss of the 3Rs will be larger for higher emission climate scenarios and becomes larger for longer time horizons of a foreseeable future. For example, by 2080, the 3Rs measure is 23% for Scenario 2.4°C (RCP4.5) but only 7% for Scenario 4.3°C (RCP8.5), and the 3Rs decline from 34% in 2050 to 1% in 2100 under Scenario 4.3°C (Figure 9). Specifically, all ecological settings will eventually disappear by 2100 under all emissions scenarios resulting from current energy system trends and policies (Figure 5; Table S2). The global population growth rate is projected to decrease regardless of the climate and demographic scenarios, with resiliency between 53% and 73% in 2050, and between 1% and 54% by 2100, depending upon the GHG emission scenario. For example, under Scenario 2.4°C, the global population growth rate is projected to decrease by 1% per year by 2080—a half‐life of 47 years (Table S2).
For short time horizons of foreseeable future, the 3Rs are still projected to be low, with at least two‐thirds of colonies being quasi‐extinct by 2050 under all emissions scenarios resulting from current energy system trends and policies (70% colonies under Scenario 4.3°C and Scenario 2.6°C, 65% colonies under Scenario 2.4°C and Scenario 2.0°C by 2050; Table S2, Figure S4). Even with Paris Agreement pledges to keep emissions “relatively low” by 2035, or achieve neutrality by 2050, the 3Rs measure remains below 38%, even by 2050, because of projected losses of representation and resiliency.
The extreme event scenarios affect the 3Rs in complex ways (Figure 8). Although extreme events affect the global population size negatively and reduce resiliency (Figure 4), redundancy, and representation may increase due to complex dispersive processes driving local and regional population dynamics (Figures 5 and 6). For example, population movement occurs from the Ross Sea to the Indian Ocean and western Pacific Ocean, and to the Amundsen Sea and Bellingshausen Sea by mid‐century. Sea ice‐dependent extreme events can increase representation and redundancy as the regional populations increase, reducing the number of colonies that go quasi‐extinct.
Overall, the 3Rs are most often reduced by extreme events, especially if extremes are not sea ice dependent. Cases including extreme events but showing almost no change in the averaged 3Rs measure only occur when the frequency of extreme events is very small (i.e., under low emission scenarios when the frequency is sea ice‐dependent).
3.6. Recommendations for the protection of species endangered by climate change
Our results show that the longer current GHG emissions levels continue, the more certain that sea ice loss and climate‐driven signals in population dynamics become (Figure 9). By 2050, the emperor penguin will be in danger of extinction throughout a significant portion of its range regardless of emission scenario. By 2100, our projections diverge, depending upon emission scenario (Figure 9); as such, under emissions scenarios resulting from current energy system trends and policies, including under our new Scenario 2.6°C, the emperor penguin will be in danger of extinction throughout its entire range. Accordingly, we recommend that the emperor penguin should now be listed as threatened under the ESA.
An ESA listing would provide emperor penguins with important benefits, in addition to ensuring that US Federal agencies’ activities (including GHG emissions) do not jeopardize the species or their habitat. Listing would highlight that without stronger reductions of GHG emissions, the emperor penguin will move toward local and possibly global extinction. Listing would spur research and promote international cooperation on conservation strategies, increase funding including personnel and training assistance for conservation programs, and provide concrete tools for threat reductions. For example, it would provide a mechanism to evaluate and reduce harm to emperor penguins by US fisheries operating in the region managed by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) that might inadvertently lead to changes in the ecosystem's trophic structure. This is significant since the United States is the world's largest consumer of krill products and toothfish products (Dissostichus spp.). The United States is a Member of CCAMLR and Party to the Antarctic Treaty System, both of which promote conservation, regulate human activities, facilitate spatial protection, and allow for the designation of specially protected species. The protection of emperor penguins under the ESA could play an influential role in these international conservation management fora, and policy decisions.
Ultimately, the most important action to ensure the continued viability of emperor penguins is to rapidly reduce GHG emissions to limit further warming (Figure 10). Near‐term climate policy decisions during this decade that successfully achieve the Paris Agreement targets would provide refugia for the emperor penguin, halting dramatic global population declines. In the Antarctic, the emperor penguin is now the proverbial “canary in the coal mine”; that is, it is a sentinel species (Boersma, 2008) sensitive to the effects of climate change, through sea ice, and is thus a leading signal of the impacts that may be expected for other species. Moreover, the emperor penguin also signals how well global society is acting to control GHG emissions. The future of emperor penguins and all biota on earth ultimately depends upon the decisions made today (Rintoul et al., 2018).
4. CONCLUSION
The world is facing a profound climate crisis and we need to act now to avoid the most catastrophic impacts; global society must therefore listen to science and meet the moment (Biden, 2021). Natural systems provide the ecosystem services that support people and sustain their livelihoods, as well as supporting the wildlife that form an intrinsic part of these systems. Sustaining these systems now requires legal frameworks that are appropriate protect them based on the best available scientific evidence. Long‐term ecological studies, such as that for the emperor penguin, are critical for providing robust science to document ecological responses to environmental change. Interdisciplinary science is also necessary to project population viability and species persistence in a future warming world. Such investments in science provide knowledge which must now inform legal frameworks, because with knowledge comes responsibility. Continuing to strengthen international climate action and biodiversity protection frameworks is key, but in the meantime, immediate efforts must also focus on the effective legal tools already in place, such as the ESA.
CONFLICT OF INTEREST
Two co‐authors, Shaye Wolf and Noah Greenwald, are employees and members of the non‐profit conservation organization that petitioned the Fish and Wildlife Service to consider the scientific case for listing the emperor penguin under the Endangered Species Act. They do not stand to gain or lose financially through this publication. The other authors declare no competing interests.
AUTHOR CONTRIBUTION
SJ and PT conceived of and designed the study. SJ performed the demographic analysis, MH computed the sea ice forecasts, ML, PF, PT estimated the observed frequency of extreme events. CJ, SW reviewed the policy literature with the help of NG. SJ, PT, CJ, SW, MH, SL, BW wrote the manuscript; SJ, PF, MH produced the figures. All authors contributed substantially to the interpretation of the results and edited the manuscript.
DATA AVAILABILITY STATEMENT
The demographic data (capture–recapture and breeding success) used to build the sea ice‐dependent meta‐population models in previous studies (Barbraud & Weimerskirch, 2001; Jenouvrier et al., 2010, 2012, 2014, 2017) are available upon reasonable request from C. Barbraud and S. Jenouvrier. Sea ice model projection data are available from the Earth System Grid through the Climate Data Gateway at the National Center for Atmospheric Research. All datasets and code for generating the figures and tables will be available following publication on the U.S. Antarctic Program Data Center (USAP‐DC) repository for the COLLABORATIVE RESEARCH: A Multi‐scale Approach to Understanding Spatial and Population Variability in Emperor Penguins.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We are very grateful to C. Schwalm, N. Huber, J. Kooyman, R. Reisinger, an anonymous colleague and anonymous reviewers for thoughtful discussions. We acknowledge support of NASA (80NSSC20K1289) to SJ, MH, and of NSF—OPP (1744794) to SJ, ML.
Jenouvrier, S. , Che‐Castaldo, J. , Wolf, S. , Holland, M. , Labrousse, S. , LaRue, M. , Wienecke, B. , Fretwell, P. , Barbraud, C. , Greenwald, N. , Stroeve, J. , & Trathan, P. N. (2021). The call of the emperor penguin: Legal responses to species threatened by climate change. Global Change Biology, 27, 5008–5029. 10.1111/gcb.15806
Judy Che‐Castaldo Judy, Shaye Wolf, Marika Holland, and Sara Labrousse contributed equally.
Michelle LaRue, Barbara Wienecke, Peter Fretwell, and Christophe Barbraud contributed equally.
REFERENCES
- Ainley, D. , Russell, J. , Jenouvrier, S. , Woehler, E. , Lyver, P. O. , Fraser, W. R. , & Kooyman, G. L. (2010). Antarctic penguin response to habitat change as Earth’s troposphere reaches 2°C above preindustrial levels. Ecological Monographs, 80(1), 49–66. 10.1890/08-2289.1 [DOI] [Google Scholar]
- Barbraud, C. , Delord, K. , & Weimerskirch, H. (2015). Extreme ecological response of a seabird community to unprecedented sea ice cover. Royal Society Open Science, 2(5), 140456. 10.1098/rsos.140456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbraud, C. , & Weimerskirch, H. (2001). Emperor penguins and climate change. Nature, 411, 183–186. 10.1038/35075554 [DOI] [PubMed] [Google Scholar]
- Biden, J. (2021). Executive order on tackling the climate crisis at home and abroad. Retrieved from www.whitehouse.gov/briefing‐room/presidential‐actions/2021/01/27/executive‐order‐on‐tackling‐the‐climate‐crisis‐at‐home‐and‐abroad/ [Google Scholar]
- BirdLife International . (2021). Species factsheet: Aptenodytes forsteri . BirdLife International. http://www.birdlife.org [Google Scholar]
- Bluhm, B. A. , Swadling, K. M. , & Gradinger, R. (2017). Sea ice as a habitat for macrograzers. Sea Ice, 3, 394–414. [Google Scholar]
- Boersma, P. D. (2008). Penguins as marine sentinels. BioScience, 58(7), 597–607. 10.1641/B580707 [DOI] [Google Scholar]
- Brett, G. M. , Irvin, A. , Rack, W. , Haas, C. , Langhorne, P. J. , & Leonard, G. H. (2020). Variability in the distribution of fast ice and the sub‐ice platelet layer near mcmurdo ice shelf. Journal of Geophysical Research: Oceans, 125(3). 10.1029/2019JC015678 [DOI] [Google Scholar]
- Buis, A. (2020). Study confirms climate models are getting future warming projections right. Retrieved from https://climate.nasa.gov/news/2943/study‐confirms‐climate‐models‐are‐getting‐future‐warming‐projections‐right/ [Google Scholar]
- Center for Biological Diversity . (2011). Petition to list the emperor penguin (Aptenodytes forsteri) as threatened or endangered under the Endangered Species Act (2011). Retrieved from https://www.biologicaldiversity.org/species/birds/penguins/pdfs/Emperor_penguin_ESA_petition_2011_V5.pdf [Google Scholar]
- Cole, T. L. , Dutoit, L. , Dussex, N. , Hart, T. , Alexander, A. , Younger, J. L. , Clucas, G. V. , Frugone, M. J. , Cherel, Y. , Cuthbert, R. , Ellenberg, U. , Fiddaman, S. R. , Hiscock, J. , Houston, D. , Jouventin, P. , Mattern, T. , Miller, G. , Miskelly, C. , Nolan, P. , … Waters, J. M. (2019). Receding ice drove parallel expansions in Southern Ocean penguins. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26690. 10.1073/pnas.1904048116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, M. , Knutti, R. , Arblaster, J. , Dufresne, J.‐L. , Fichefet, T. , Friedlingstein, P. , Gao, X. , Gutowski, W. J. , Johns, T. , & Krinner, G. (2013). Long‐term climate change: Projections, commitments and irreversibility. In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate change 2013—The physical science basis: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (pp. 1029–1136). Cambridge University Press. [Google Scholar]
- Department of Interior . (2009). The meaning of “foreseeable future” in section 3 (20) of the Endangered Species Act. [Google Scholar]
- Dietze, M. (2017). Ecological forecasting. Princeton University Press. 10.1515/9781400885459 [DOI] [Google Scholar]
- Doligez, B. , Danchin, E. , & Clobert, J. (2002). Public information and breeding habitat selection in a wild bird population. Science, 297(5584), 1168. 10.1126/science.1072838 [DOI] [PubMed] [Google Scholar]
- Eayrs, C. , Faller, D. , & Holland, D. M. (2020). Mechanisms driving the asymmetric seasonal cycle of Antarctic Sea Ice in the CESM Large Ensemble. Annals of Glaciology, 61(82), 171–180. 10.1017/aog.2020.26 [DOI] [Google Scholar]
- Endangered Species Act . (1973a). 16 U.S.C. § 1532 (6). Retrieved from https://www.law.cornell.edu/uscode/text/16/1532 [Google Scholar]
- Endangered Species Act . (1973b). 16 U.S.C. § 1532 (20). Retrieved from https://www.law.cornell.edu/uscode/text/16/1532 [Google Scholar]
- Endangered Species Act . (1973c). 16 U.S.C. § 1533 (b) (1) (A). Retrieved from https://www.law.cornell.edu/uscode/text/16/1533 [Google Scholar]
- Foden, W. B. , Young, B. E. , Akçakaya, H. R. , Garcia, R. A. , Hoffmann, A. A. , Stein, B. A. , Thomas, C. D. , Wheatley, C. J. , Bickford, D. , Carr, J. A. , Hole, D. G. , Martin, T. G. , Pacifici, M. , Pearce‐Higgins, J. W. , Platts, P. J. , Visconti, P. , Watson, J. E. M. , & Huntley, B. (2019). Climate change vulnerability assessment of species. Wiley Interdisciplinary Reviews: Climate Change, 10(1), e551. 10.1002/wcc.551 [DOI] [Google Scholar]
- Fogwill, C. J. , van Sebille, E. , Cougnon, E. A. , Turney, C. S. M. , Rintoul, S. R. , Galton‐Fenzi, B. K. , Clark, G. F. , Marzinelli, E. M. , Rainsley, E. B. , & Carter, L. (2016). Brief communication: Impacts of a developing polynya off Commonwealth Bay, East Antarctica, triggered by grounding of iceberg B09B. The Cryosphere, 10(6), 2603–2609. 10.5194/tc-10-2603-2016 [DOI] [Google Scholar]
- Foley, C. M. , Lynch, M. A. , Thorne, L. H. , & Lynch, H. J. (2017). Listing foreign species under the Endangered Species Act: A primer for conservation biologists. BioScience, 67(7), 627–637. 10.1093/biosci/bix027 [DOI] [Google Scholar]
- Fraser, A. D. , Massom, R. A. , Handcock, M. S. , Reid, P. , Ohshima, K. I. , Raphael, M. N. , Cartwright, J. , Klekociuk, A. R. , Wang, Z. , & Porter‐Smith, R. (2021). 18 year record of circum‐Antarctic landfast sea ice distribution allows detailed baseline characterisation, reveals trends and variability. The Cryosphere Discussions, 2021, 1–23. 10.5194/tc-2021-121 [DOI] [Google Scholar]
- Fretwell, P. T. , & Trathan, P. N. (2019). Emperors on thin ice: Three years of breeding failure at Halley Bay. Antarctic Science, 31(3), 133–138. 10.1017/S0954102019000099 [DOI] [Google Scholar]
- Fretwell, P. T. , & Trathan, P. N. (2021). Discovery of new colonies by Sentinel2 reveals good and bad news for emperor penguins. Remote Sensing in Ecology and Conservation, 7(2), 139–153. [Google Scholar]
- Fretwell, P. T. , Trathan, P. N. , Wienecke, B. , & Kooyman, G. L. (2014). Emperor penguins breeding on iceshelves. PLoS One, 9, e85285. 10.1371/journal.pone.0085285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlingstein, P. , O'Sullivan, M. , Jones, M. W. , Andrew, R. M. , Hauck, J. , Olsen, A. , Peters, G. P. , Peters, W. , Pongratz, J. , Sitch, S. , Le Quéré, C. , Canadell, J. G. , Ciais, P. , Jackson, R. B. , Alin, S. , Aragão, L. E. O. C. , Arneth, A. , Arora, V. , Bates, N. R. , … Zaehle, S. (2020). Global carbon budget 2020. Earth System Science Data, 12(4), 3269–3340. 10.5194/essd-12-3269-2020 [DOI] [Google Scholar]
- Goetz, K. , McDonald, B. , & Kooyman, G. (2018). Habitat preference and dive behavior of non‐breeding emperor penguins in the eastern Ross Sea, Antarctica. Marine Ecology Progress Series, 593, 155–171. 10.3354/meps12486 [DOI] [Google Scholar]
- Harvard Law School . (2021). Animal law and policy program, request to revoke memoranda and regulations regarding consideration of greenhouse emissions under the Endangered Species Act. Retrieved from https://animal.law.harvard.edu/wp‐content/uploads/Scientists‐and‐Legal‐Scholars‐Letter‐on‐the‐Endangered‐Species‐Act‐and‐Climate‐Change.pdf [Google Scholar]
- Hausfather, Z. , & Peters, G. P. (2020a). Emissions—The ‘business as usual’ story is misleading. Nature, 577(7792), 618–620. 10.1038/d41586-020-00177-3 [DOI] [PubMed] [Google Scholar]
- Hausfather, Z. , & Peters, G. P. (2020b). RCP8.5 is a problematic scenario for near‐term emissions. Proceedings of the National Academy of Sciences of the United States of America, 117(45), 27791–27792. 10.1073/pnas.2017124117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, P. (2006). Atmospheric conditions and fast ice at Davis, East Antarctica: A case study. Journal of Geophysical Research, 111(C5), C05009. 10.1029/2005JC002904 [DOI] [Google Scholar]
- Heller, N. E. , & Zavaleta, E. S. (2009). Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biological Conservation, 142(1), 14–32. 10.1016/j.biocon.2008.10.006 [DOI] [Google Scholar]
- Hobbs, W. R. , Massom, R. , Stammerjohn, S. , Reid, P. , Williams, G. , & Meier, W. (2016). A review of recent changes in Southern Ocean sea ice, their drivers and forcings. Global and Planetary Change, 143, 228–250. 10.1016/j.gloplacha.2016.06.008 [DOI] [Google Scholar]
- Hoppmann, M. , Nicolaus, M. , Hunkeler, P. A. , Heil, P. , Behrens, L.‐K. , König‐Langlo, G. , & Gerdes, R. (2015). Seasonal evolution of an ice‐shelf influenced fast‐ice regime, derived from an autonomous thermistor chain. Journal of Geophysical Research: Oceans, 120(3), 1703–1724. 10.1002/2014JC010327 [DOI] [Google Scholar]
- Hurrell, J. W. , Holland, M. M. , Gent, P. R. , Ghan, S. , Kay, J. E. , Kushner, P. J. , Lamarque, J.‐F. , Large, W. G. , Lawrence, D. , Lindsay, K. , Lipscomb, W. H. , Long, M. C. , Mahowald, N. , Marsh, D. R. , Neale, R. B. , Rasch, P. , Vavrus, S. , Vertenstein, M. , Bader, D. , … Marshall, S. (2013). The community earth system model: A framework for collaborative research. Bulletin of the American Meteorological Society, 94(9), 1339–1360. 10.1175/BAMS-D-12-00121.1 [DOI] [Google Scholar]
- IPCC . (2018). Global warming of 1.5°C. In Masson‐Delmotte V., Zhai P., Pörtner H.‐O., Roberts D., Skea J., Shukla P. R., Pirani A., Moufouma‐Okia W., Péan C., Pidcock R., Connors S., Matthews J. B. R., Chen Y., Zhou X., Gomis M. I., Lonnoy E., Maycock T., Tignor M., & Waterfield T. (Eds.), An IPCC Special Report on the impacts of global warming of 1.5°C above pre‐industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Intergovernmental Panel on Climate Change. [Google Scholar]
- Jenouvrier, S. (2013). Impacts of climate change on avian populations. Global Change Biology, 19(7), 2036–2057. 10.1111/gcb.12195 [DOI] [PubMed] [Google Scholar]
- Jenouvrier, S. , Caswell, H. , Barbraud, C. , Holland, M. , Strœve, J. , & Weimerskirch, H. (2009). Demographic models and IPCC climate projections predict the decline of an emperor penguin population. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1844–1847. 10.1073/pnas.0806638106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenouvrier, S. , Caswell, H. , Barbraud, C. , & Weimerskirch, H. (2010). Mating behavior, population growth, and the operational sex ratio: A periodic two‐sex model approach. The American Naturalist, 175(6), 739–752. 10.1086/652436 [DOI] [PubMed] [Google Scholar]
- Jenouvrier, S. , Garnier, J. , Patout, F. , & Desvillettes, L. (2017). Influence of dispersal processes on the global dynamics of Emperor penguin, a species threatened by climate change. Biological Conservation, 212, 63–73. 10.1016/j.biocon.2017.05.017 [DOI] [Google Scholar]
- Jenouvrier, S. , Holland, M. , Iles, D. , Labrousse, S. , Landrum, L. , Garnier, J. , Caswell, H. , Weimerskirch, H. , LaRue, M. , Ji, R. , & Barbraud, C. (2020). The Paris Agreement objectives will likely halt future declines of emperor penguins. Global Change Biology, 26(3), 1170–1184. 10.1111/gcb.14864 [DOI] [PubMed] [Google Scholar]
- Jenouvrier, S. , Holland, M. , Stroeve, J. , Barbraud, C. , Weimerskirch, H. , Serreze, M. , & Caswell, H. (2012). Effects of climate change on an emperor penguin population: Analysis of coupled demographic and climate models. Global Change Biology, 18(9), 2756–2770. 10.1111/j.1365-2486.2012.02744.x [DOI] [PubMed] [Google Scholar]
- Jenouvrier, S. , Holland, M. , Stroeve, J. , Serreze, M. , Barbraud, C. , Weimerskirch, H. , & Caswell, H. (2014). Projected continent‐wide declines of the emperor penguin under climate change. Nature Climate Change, 4(8), 715–718. 10.1038/nclimate2280 [DOI] [Google Scholar]
- Jenouvrier, S. , Péron, C. , & Weimerskirch, H. (2015). Extreme climate events and individual heterogeneity shape life‐history traits and population dynamics. Ecological Monographs, 85(4), 605–624. 10.1890/14-1834.1 [DOI] [Google Scholar]
- Jones, M. W. , Andrew, R. M. , Peters, G. P. , Janssens‐Maenhout, G. , De‐Gol, A. J. , Ciais, P. , Patra, P. K. , Chevallier, F. , & Le Quéré, C. (2021). Gridded fossil CO2 emissions and related O2 combustion consistent with national inventories 1959–2018. Scientific Data, 8(1), 2. 10.1038/s41597-020-00779-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartvedt, S. , & Titelman, J. (2018). Planktivorous fish in a future Arctic Ocean of changing ice and unchanged photoperiod. ICES Journal of Marine Science, 75(7), 2312–2318. 10.1093/icesjms/fsx248 [DOI] [Google Scholar]
- Kay, J. E. , Deser, C. , Phillips, A. , Mai, A. , Hannay, C. , Strand, G. , Arblaster, J. M. , Bates, S. C. , Danabasoglu, G. , Edwards, J. , Holland, M. , Kushner, P. , Lamarque, J.‐F. , Lawrence, D. , Lindsay, K. , Middleton, A. , Munoz, E. , Neale, R. , Oleson, K. , … Vertenstein, M. (2015). The Community Earth System Model (CESM) large ensemble project: A community resource for studying climate change in the presence of internal climate variability. Bulletin of the American Meteorological Society, 96(8), 1333–1349. 10.1175/BAMS-D-13-00255.1 [DOI] [Google Scholar]
- Kirkwood, R. , & Robertson, G. (1997). The foraging ecology of female emperor penguins in winter. Ecological Monographs, 67(2), 155–176. [Google Scholar]
- Knutti, R. (2008). Hotter or not? Should we believe model predictions of future climate change? Significance, 5(4), 159–162. 10.1111/j.1740-9713.2008.00320.x [DOI] [PubMed] [Google Scholar]
- Kooyman, G. , Ainley, D. , Ballard, G. , & Ponganis, P. (2007). Effects of giant icebergs on two emperor penguin colonies in the Ross Sea, Antarctica. Antarctic Science, 19, 31–38. 10.1017/S0954102007000065 [DOI] [Google Scholar]
- Kooyman, G. , Siniff, D. , Stirling, I. , & Bengtson, J. (2004). Moult habitat, pre‐ and post‐moult diet and post‐moult travel of Ross Sea emperor penguins. Marine Ecology Progress Series, 267, 281–290. 10.3354/meps267281 [DOI] [Google Scholar]
- La Mesa, M. , Catalano, B. , Russo, A. , Greco, S. , Vacchi, M. , & Azzali, M. (2010). Influence of environmental conditions on spatial distribution and abundance of early life stages of Antarctic silverfish, Pleuragramma antarcticum (Nototheniidae), in the Ross Sea. Antarctic Science, 22(03), 243–254. 10.1017/S0954102009990721 [DOI] [Google Scholar]
- Labrousse, S. , Fraser, A. D. , Sumner, M. , Le Manach, F. , Sauser, C. , Horstmann, I. , Devane, E. , Delord, K. , Jenouvrier, S. , & Barbraud, C. (2021). Landfast ice: A major driver of reproductive success in a polar seabird. Biology Letters, 17(6), 20210097. 10.1098/rsbl.2021.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse, S. , Fraser, A. D. , Sumner, M. , Tamura, T. , Pinaud, D. , Wienecke, B. , Kirkwood, R. , Ropert‐Coudert, Y. , Reisinger, R. , Jonsen, I. , Porter‐Smith, R. , Barbraud, C. , Bost, C.‐A. , Ji, R. , & Jenouvrier, S. (2019). Dynamic fine‐scale sea icescape shapes adult emperor penguin foraging habitat in East Antarctica. Geophysical Research Letters, 46(20), 11206–11218. 10.1029/2019GL084347 [DOI] [Google Scholar]
- Landrum, L. L. , Holland, M. M. , Raphael, M. N. , & Polvani, L. M. (2017). Stratospheric ozone depletion: An unlikely driver of the regional trends in Antarctic sea ice in austral fall in the late twentieth century. Geophysical Research Letters, 44(21). 10.1002/2017GL075618 [DOI] [Google Scholar]
- LaRue, M. A. , Kooyman, G. , Lynch, H. J. , & Fretwell, P. (2015). Emigration in emperor penguins: Implications for interpretation of long‐term studies. Ecography, 38(2), 114–120. 10.1111/ecog.00990 [DOI] [Google Scholar]
- Lei, R. , Li, Z. , Cheng, B. , Zhang, Z. , & Heil, P. (2010). Annual cycle of landfast sea ice in Prydz Bay, east Antarctica. Journal of Geophysical Research, 115(C2), C02006. 10.1029/2008JC005223 [DOI] [Google Scholar]
- Li, Y.‐W. , Roman, J. , Wilcove, D. S. , Male, T. , & Doremus, H. (2020). Species protection will take more than rule reversal. Science, 370(6517), 665–666. 10.1126/science.abb3806 [DOI] [PubMed] [Google Scholar]
- Mawdsley, J. R. , O’Malley, R. , & Ojima, D. S. (2009). A review of climate‐change adaptation strategies for wildlife management and biodiversity conservation. Conservation Biology, 23(5), 1080–1089. 10.1111/j.1523-1739.2009.01264.x [DOI] [PubMed] [Google Scholar]
- Meyer, B. , Freier, U. , Grimm, V. , Groeneveld, J. , Hunt, B. P. V. , Kerwath, S. , King, R. , Klaas, C. , Pakhomov, E. , Meiners, K. M. , Melbourne‐Thomas, J. , Murphy, E. J. , Thorpe, S. E. , Stammerjohn, S. , Wolf‐Gladrow, D. , Auerswald, L. , Götz, A. , Halbach, L. , Jarman, S. , … Yilmaz, N. I. (2017). The winter pack‐ice zone provides a sheltered but food‐poor habitat for larval Antarctic krill. Nature Ecology & Evolution, 1(12), 1853–1861. 10.1038/s41559-017-0368-3 [DOI] [PubMed] [Google Scholar]
- Moritz, A. T. , Siegel, K. R. , Cummings, B. R. , & Rodgers, W. H. (2008). Biodiversity, Baking and Boiling, Endangered Species Act Turning Down the Heat. Tulsa Law Review, 44, 33. [Google Scholar]
- National Marine Fisheries Service . (2008). Endangered and threatened wildlife; notice of 12‐month finding on a petition to list the ribbon seal as a threatened or endangered species. Federal Register, 73, 79825–79828. [Google Scholar]
- National Marine Fisheries Service . (2012a). Endangered and threatened species; threatened status for the arctic, Okhotsk, and Baltic subspecies of the ringed seal and endangered status for the Ladoga subspecies of the ringed seal. Federal Register, 77, 76706–76738. [Google Scholar]
- National Marine Fisheries Service . (2012b). Endangered and threatened species; threatened status for the Beringia and Okhotsk distinct population segments of the Erignathus barbatus nauticus subspecies of the bearded seal. Federal Register, 77, 76740–76768. [Google Scholar]
- National Marine Fisheries Service . (2014). Endangered and threatened wildlife and plants: Final listing determinations on proposal to list 66 reef‐building coral species and to reclassify Elkhorn and staghorn corals. Federal Register Rules and Regulations, 79, 53851–54123. [Google Scholar]
- National Marine Fisheries Service . (2016). Revised guidance for treatment of climate change in NMFS Endangered Species Act decisions. Retrieved from https://media.fisheries.noaa.gov/dam‐migration/pr_climate_change_guidance_june_2016.pdf [Google Scholar]
- Nisbet, E. G. , Manning, M. R. , Dlugokencky, E. J. , Fisher, R. E. , Lowry, D. , Michel, S. E. , Myhre, C. L. , Platt, S. M. , Allen, G. , Bousquet, P. , Brownlow, R. , Cain, M. , France, J. L. , Hermansen, O. , Hossaini, R. , Jones, A. E. , Levin, I. , Manning, A. C. , Myhre, G. , … White, J. W. C. (2019). Very strong atmospheric methane growth in the 4 years 2014–2017: Implications for the Paris agreement. Global Biogeochemical Cycles, 33(3), 318–342. 10.1029/2018GB006009 [DOI] [Google Scholar]
- O’Neill, B. C. , Tebaldi, C. , van Vuuren, D. P. , Eyring, V. , Friedlingstein, P. , Hurtt, G. , Knutti, R. , Kriegler, E. , Lamarque, J.‐F. , Lowe, J. , Meehl, G. A. , Moss, R. , Riahi, K. , & Sanderson, B. M. (2016). The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geoscientific Model Development, 9(9), 3461–3482. 10.5194/gmd-9-3461-2016 [DOI] [Google Scholar]
- Povilitis, A. , & Suckling, K. (2010). Addressing climate change threats to endangered species in U.S. recovery plans. Conservation Biology, 24(2), 372–376. 10.1111/j.1523-1739.2010.01447.x [DOI] [PubMed] [Google Scholar]
- Raphael, M. N. , Handcock, M. S. , Holland, M. M. , & Landrum, L. L. (2020). An assessment of the temporal variability in the annual cycle of daily Antarctic sea ice in the NCAR Community Earth System Model, version 2: A comparison of the historical runs with observations. Journal of Geophysical Research: Oceans, 125(11). 10.1029/2020JC016459 [DOI] [Google Scholar]
- Redford, K. H. , Amato, G. , Baillie, J. , Beldomenico, P. , Bennett, E. L. , Clum, N. , Cook, R. , Fonseca, G. , Hedges, S. , Launay, F. , Lieberman, S. , Mace, G. M. , Murayama, A. , Putnam, A. , Robinson, J. G. , Rosenbaum, H. , Sanderson, E. W. , Stuart, S. N. , Thomas, P. , & Thorbjarnarson, J. (2011). What does it mean to successfully conserve a (vertebrate) species? BioScience, 61(1), 39–48. 10.1525/bio.2011.61.1.9 [DOI] [Google Scholar]
- Ridley, J. K. , & Hewitt, H. T. (2014). A mechanism for lack of sea ice reversibility in the Southern Ocean. Geophysical Research Letters, 41(23), 8404–8410. 10.1002/2014GL062167 [DOI] [Google Scholar]
- Ridley, J. K. , Lowe, J. A. , & Hewitt, H. T. (2012). How reversible is sea ice loss? The Cryosphere, 6(1), 193–198. 10.5194/tc-6-193-2012 [DOI] [Google Scholar]
- Rintoul, S. R. , Chown, S. L. , DeConto, R. M. , England, M. H. , Fricker, H. A. , Masson‐Delmotte, V. , Naish, T. R. , Siegert, M. J. , & Xavier, J. C. (2018). Choosing the future of Antarctica. Nature, 558(7709), 233–241. 10.1038/s41586-018-0173-4 [DOI] [PubMed] [Google Scholar]
- Roach, L. A. , Dörr, J. , Holmes, C. R. , Massonnet, F. , Blockley, E. W. , Notz, D. , Rackow, T. , Raphael, M. N. , O’Farrell, S. P. , Bailey, D. A. , & Bitz, C. M. (2020). Antarctic sea ice area in CMIP6. Geophysical Research Letters, 47(9). 10.1029/2019GL086729 [DOI] [Google Scholar]
- Rodary, D. , Bonneau, W. , Le Maho, Y. , & Bost, C. A. (2000). Benthic diving in male emperor penguins Aptenodytes forsteri foraging in winter. Marine Ecology Progress Series, 207, 171–181. 10.3354/meps207171 [DOI] [Google Scholar]
- Rohlf, D. J. (1989). The Endangered Species Act: A guide to its protections and implementation. Stanford Environmental Law Society. [Google Scholar]
- Román‐Palacios, C. , & Wiens, J. J. (2020). Recent responses to climate change reveal the drivers of species extinction and survival. Proceedings of the National Academy of Sciences of the United States of America, 117(8), 4211–4217. 10.1073/pnas.1913007117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum, E. , & Eisenman, I. (2017). Sea ice trends in climate models only accurate in runs with biased global warming. Journal of Climate, 30(16), 6265–6278. 10.1175/JCLI-D-16-0455.1 [DOI] [Google Scholar]
- Sanderson, B. M. , Oleson, K. W. , Strand, W. G. , Lehner, F. , & O’Neill, B. C. (2018). A new ensemble of GCM simulations to assess avoided impacts in a climate mitigation scenario. Climatic Change, 146(3–4), 303–318. 10.1007/s10584-015-1567-z [DOI] [Google Scholar]
- Sanderson, B. M. , Xu, Y. , Tebaldi, C. , Wehner, M. , O'Neill, B. , Jahn, A. , Pendergrass, A. G. , Lehner, F. , Strand, W. G. , Lin, L. , & Knutti, R. (2017). Community climate simulations to assess avoided impacts in 1.5 and 2 C futures. Earth System Dynamics, 8(3), 827–847 [Google Scholar]
- Schmidt, A. E. , & Ballard, G. (2020). Significant chick loss after early fast ice breakup at a high‐latitude emperor penguin colony. Antarctic Science, 32(3), 180–185. 10.1017/S0954102020000048 [DOI] [Google Scholar]
- Schwalm, C. R. , Glendon, S. , & Duffy, P. B. (2020a). RCP8.5 tracks cumulative CO2 emissions. Proceedings of the National Academy of Sciences of the United States of America, 117(33), 19656–19657. 10.1073/pnas.2007117117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm, C. R. , Glendon, S. , & Duffy, P. B. (2020b). Reply to Hausfather and Peters: RCP8.5 is neither problematic nor misleading. Proceedings of the National Academy of Sciences of the United States of America, 117(45), 27793–27794. 10.1073/pnas.2018008117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm, C. R. , Huntzinger, D. N. , Michalak, A. M. , Schaefer, K. , Fisher, J. B. , Fang, Y. , & Wei, Y. (2020). Modeling suggests fossil fuel emissions have been driving increased land carbon uptake since the turn of the 20th century. Scientific Reports, 10(1), 9059. 10.1038/s41598-020-66103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, M. L. , & Stein, B. A. (2000). Safeguarding our precious heritage [Chapter 11]. In Stein B. A., Kutner L. S., & Adams J. S. (Eds.), Precious heritage: The status of biodiversity in the United States (pp. 301–321). Oxford University Press. [Google Scholar]
- Singh, H. A. , Polvani, L. M. , & Rasch, P. J. (2019). Antarctic sea ice expansion, driven by internal variability, in the presence of increasing atmospheric CO2 . Geophysical Research Letters, 46(24), 14762–14771. 10.1029/2019GL083758 [DOI] [Google Scholar]
- Smith, D. , Allan, N. L. , McGowan, C. P. , Szymanski, J. A. , Oetker, S. , & Bell, H. (2018). Development of a species status assessment process for decisions under the U.S. Endangered Species Act. Journal of Fish and Wildlife Management, 9, 302–320. [Google Scholar]
- Solomon, S. , Plattner, G.‐K. , Knutti, R. , & Friedlingstein, P. (2009). Irreversible climate change due to carbon dioxide emissions. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1704–1709. 10.1073/pnas.0812721106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, C. A. , Alexander, M. A. , Bond, N. A. , Brander, K. M. , Cheung, W. W. L. , Curchitser, E. N. , Delworth, T. L. , Dunne, J. P. , Griffies, S. M. , Haltuch, M. A. , Hare, J. A. , Hollowed, A. B. , Lehodey, P. , Levin, S. A. , Link, J. S. , Rose, K. A. , Rykaczewski, R. R. , Sarmiento, J. L. , Stouffer, R. J. , … Werner, F. E. (2011). On the use of IPCC‐class models to assess the impact of climate on Living Marine Resources. Progress in Oceanography, 88(1–4), 1–27. 10.1016/j.pocean.2010.09.001 [DOI] [Google Scholar]
- Stocker, T. , Qin, D. , Plattner, G. , Tignor, M. , Allen, S. K. , Boschung, J. , Nauels, A. , Xia, Y. , Bex, V. , & Midgley, P. M. (2013). IPCC, 2013: Summary for policymakers in climate change 2013: The physical science basis, contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press. [Google Scholar]
- Stockwell, C. , Geiges, A. , Ramalope, D. , Gidden, M. , Hare, B. , Fekete, H. , Gonzales‐Zuñiga, S. , Hans, F. , Höhne, N. , Baxter, C. , Arslan, D. , & Beer, M. (2021). Climate action tracker | Climate summit momentum: Paris commitments improved warming estimate to 2.4°C. The Climate Action Tracker. https://climateactiontracker.org/documents/853/CAT_2021‐05‐04_Briefing_Global‐Update_Climate‐Summit‐Momentum.pdf [Google Scholar]
- Trathan, P. N. , Wienecke, B. , Barbraud, C. , Jenouvrier, S. , Kooyman, G. , Le Bohec, C. , Ainley, D. G. , Ancel, A. , Zitterbart, D. P. , Chown, S. L. , LaRue, M. , Cristofari, R. , Younger, J. , Clucas, G. , Bost, C.‐A. , Brown, J. A. , Gillett, H. J. , & Fretwell, P. T. (2020). The emperor penguin—Vulnerable to projected rates of warming and sea ice loss. Biological Conservation, 241, 108216. 10.1016/j.biocon.2019.108216 [DOI] [Google Scholar]
- Turner, J. , Bracegirdle, T. J. , Phillips, T. , Marshall, G. J. , & Hosking, J. S. (2013). An initial assessment of Antarctic sea ice extent in the CMIP5 models. Journal of Climate, 26, 1473–1484. 10.1175/JCLI-D-12-00068.1 [DOI] [Google Scholar]
- U.S. District Court for the District of Columbia . (1997). Defenders of Wildlife v. Babbitt, 958 F. Supp. 670. 670, 679–680. [Google Scholar]
- U.S. Senate . (1973). Report No. 93‐307, Endangered Species Act of 1973 at 3. Retrieved from https://nctc.fws.gov/courses/csp/csp3116/resources/ESA_Section_7_Legislative_History/Part_1_pages_298‐341.pdf [Google Scholar]
- United States Court of Appeals , Circuit, N. , & U.S. District Court for the District of Columbia . (1988). Conner v. Burford, 848 F.2d 1441. 1454. Retrieved from https://law.resource.org/pub/us/case/reporter/F2/848/848.F2d.1441.85‐3937.85‐3929.html [Google Scholar]
- Urban, M. C. (2015). Accelerating extinction risk from climate change. Science, 348(6234), 571–573. 10.1126/science.aaa4984 [DOI] [PubMed] [Google Scholar]
- US Fish and Wildlife Service . (2008a). Endangered and threatened wildlife and plants; 12‐month finding on a petition to list four penguin species as threatened or endangered under the Endangered Species Act and proposed rule to list the Southern Rockhopper Penguin in the Campbell Plateau portion of its range. Federal Register, 73, 77264–77301. [Google Scholar]
- US Fish and Wildlife Service . (2008b). Endangered and threatened wildlife and plants; 12‐month finding on a petition to list four penguin species as threatened or endangered under the Endangered Species Act and proposed rule to list the Southern Rockhopper Penguin in the Campbell Plateau portion of its range. Federal Register, 73, 772296–776299. [Google Scholar]
- US Fish and Wildlife Service . (2008c). Endangered and threatened wildlife and plants; determination of threatened status for the polar bear (Ursus maritimus) throughout its range. Federal Register, 73, 28211–28303. [Google Scholar]
- US Fish and Wildlife Service . (2010). Endangered and threatened wildlife and plants; 12‐month finding on a petition to list the American Pika as threatened or endangered. Federal Register, 75, 6453. [Google Scholar]
- US Fish and Wildlife Service . (2010). Endangered and threatened wildlife and plants; 12‐month finding on a petition to list the American Pika as threatened or endangered. Federal Register, 75, 6438‐6471. [Google Scholar]
- US Fish and Wildlife Service . (2011). Endangered and threatened wildlife and plants; 12‐month finding on a petition to list the pacific walrus as endangered or threatened. Federal Register, 76, 7633–7679. [Google Scholar]
- US Fish and Wildlife Service . (2013). Endangered and threatened wildlife and plants; threatened status for the distinct population segment of the North American wolverine occurring in the contiguous United States. Federal Register, 78, 7863–7890. [Google Scholar]
- US Fish and Wildlife Service . (2016). USFWS Species Status Assessment Framework: An integrated analytical framework for conservation. Retrieved from https://www.fws.gov/endangered/improving_esa/pdf/SSA%20Framework%20v3.4‐8_10_2016.pdf [Google Scholar]
- US Fish and Wildlife Service . (2017a). Endangered and threatened wildlife and plants; 12‐month findings on petitions to list 25 species as endangered or threatened species. Federal Register, 82, 46639. [Google Scholar]
- US Fish and Wildlife Service . (2017b). Endangered and threatened wildlife and plants; 12‐month findings on petitions to list 25 species as endangered or threatened species. Federal Register, 82, 46644. [Google Scholar]
- US Fish and Wildlife Service . (2017c). Endangered and threatened wildlife and plants; 12‐month findings on petitions to list 25 species as endangered or threatened species. Federal Register, 82, 46618–46645. [Google Scholar]
- US Fish and Wildlife Service . (2017d). Endangered and threatened wildlife and plants; threatened species status for the ʻIʻiwi (Drepanis coccinea). Federal Register, 82, 43873–43885. [Google Scholar]
- US Fish and Wildlife Service . (2018a). Endangered and threatened wildlife and plants; 12‐month findings on petitions to list 13 species as endangered or threatened species. Federal Register, 83, 65129. [Google Scholar]
- US Fish and Wildlife Service . (2018b). Endangered and threatened wildlife and plants; 12‐month findings on petitions to list 13 species as endangered or threatened species. Federal Register, 83, 65127‐65134. [Google Scholar]
- US Fish and Wildlife Service . (2019). Endangered and threatened wildlife and plants; threatened species status for meltwater Lednian Stonefly and Western Glacier Stonefly with a section 4 (d) rule. Federal Register, 84, 64210–64227. [Google Scholar]
- US Fish and Wildlife Service . (2020a). Endangered and threatened wildlife and plants; withdrawal of the proposed rule for the North American Wolverine. Federal Register, 85, 64618. [Google Scholar]
- US Fish and Wildlife Service . (2020b). Endangered and threatened wildlife and plants; withdrawal of the proposed rule for the North American Wolverine. Federal Register, 85, 64618–64648. [Google Scholar]
- US Fish and Wildlife Service and National Marine Fisheries Service . (2019). Endangered and threatened wildlife and plants; regulations for listing species and designating critical habitat. Federal Register, 84, 45020–45053. [Google Scholar]
- Vacchi, M. , DeVries, A. L. , Evans, C. W. , Bottaro, M. , Ghigliotti, L. , Cutroneo, L. , & Pisano, E. (2012). A nursery area for the Antarctic silverfish Pleuragramma antarcticum at Terra Nova Bay (Ross Sea): First estimate of distribution and abundance of eggs and larvae under the seasonal sea‐ice. Polar Biology, 35(10), 1573–1585. 10.1007/s00300-012-1199-y [DOI] [Google Scholar]
- van de Pol, M. , Jenouvrier, S. , Cornelissen, J. H. C. , & Visser, M. E. (2017). Behavioural, ecological and evolutionary responses to extreme climatic events: Challenges and directions. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 372(1723). 10.1098/rstb.2016.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, R. , Price, J. , VanDerWal, J. , Cornelius, S. , & Sohl, H. (2018). The implications of the United Nations Paris Agreement on climate change for globally significant biodiversity areas. Climatic Change. 10.1007/s10584-018-2158-6 [DOI] [Google Scholar]
- Wienecke, B. , & Robertson, G. (1997). Foraging space of emperor penguins Aptenodytes forsteri in Antarctic shelf waters in winter. Marine Ecology Progress Series, 159, 249–263. 10.3354/meps159249 [DOI] [Google Scholar]
- Wiens, J. J. (2016). Climate‐related local extinctions are already widespread among plant and animal species. PLOS Biology, 14(12), e2001104. 10.1371/journal.pbio.2001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, S. , Hartl, B. , Carroll, C. , Neel, M. C. , & Greenwald, D. N. (2015). Beyond PVA: Why recovery under the endangered species act is more than population viability. BioScience, 65(2), 200–207. 10.1093/biosci/biu218 [DOI] [Google Scholar]
- Younger, J. L. , Clucas, G. V. , Kao, D. , Rogers, A. D. , Gharbi, K. , Hart, T. , & Miller, K. J. (2017). The challenges of detecting subtle population structure and its importance for the conservation of emperor penguins. Molecular Ecology, 26(15), 3883–3897. 10.1111/mec.14172 [DOI] [PubMed] [Google Scholar]
- Younger, J. L. , Clucas, G. V. , Kooyman, G. , Wienecke, B. , Rogers, A. D. , Trathan, P. N. , Hart, T. , & Miller, K. J. (2015). Too much of a good thing: Sea ice extent may have forced emperor penguins into refugia during the last glacial maximum. Global Change Biology, 21(6), 2215–2226. 10.1111/gcb.12882 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The demographic data (capture–recapture and breeding success) used to build the sea ice‐dependent meta‐population models in previous studies (Barbraud & Weimerskirch, 2001; Jenouvrier et al., 2010, 2012, 2014, 2017) are available upon reasonable request from C. Barbraud and S. Jenouvrier. Sea ice model projection data are available from the Earth System Grid through the Climate Data Gateway at the National Center for Atmospheric Research. All datasets and code for generating the figures and tables will be available following publication on the U.S. Antarctic Program Data Center (USAP‐DC) repository for the COLLABORATIVE RESEARCH: A Multi‐scale Approach to Understanding Spatial and Population Variability in Emperor Penguins.


