ABSTRACT
Considerable attention has been given to the use of chitosan (CS)‐based materials reinforced with inorganic bioactive signals such as hydroxyapatite (HA) to treat bone defects and tissue loss. It is well known that CS/HA based materials possess minimal foreign body reactions, good biocompatibility, controlled biodegradability and antibacterial property. Herein, the bioactivity of these composite systems was analyzed on in vitro bone cell models for their applications in the field of bone tissue engineering (BTE). The combination of sol–gel approach and freeze‐drying technology was used to obtain CS/HA scaffolds with three‐dimensional (3D) porous structure suitable for cell in‐growth. Specifically, our aim was to investigate the influence of bioactive composite scaffolds on cellular behavior in terms of osteoinductivity and anti‐inflammatory effects for treating bone defects. The results obtained have demonstrated that by increasing inorganic component concentration, CS/HA (60 and 70% v/v) scaffolds induced a good biological response in terms of osteogenic differentiation of human mesenchymal stem cells (hMSC) towards osteoblast phenotype. Furthermore, the scaffolds with higher concentration of inorganic fillers are able to modulate the production of pro‐inflammatory (TGF‐β) and anti‐inflammatory (IL‐4, IL‐10) cytokines. Our results highlight the possibility of achieving smart CS/HA based composites able to promote a great osteogenic differentiation of hMSC by increasing the amount of HA nanoparticles used as bioactive inorganic signal. Contemporarily, these materials allow avoiding the induction of a pro‐inflammatory response in bone implant site.
Keywords: 3D scaffolds, bone regeneration, chitosan, hydroxyapatite, in vitro inflammatory response
1. INTRODUCTION
Bone defects are characterized by bone destruction due to inflammatory processes and infections caused by microorganisms. 1 Recently, great attention has been paid to the design of structures/devices for bone regeneration, which possess the ability to stimulate the newly forming bone tissue and repair bone defects. Hydroxyapatite (HA), [Ca10(PO4)6(OH)2] is the most studied biomaterial for medical applications due to its high biocompatibility and for being the main constituent of bone tissue. 2 Its structure looks like crystalline bio‐apatite mimicking mineral component of human bone. In the field of biomedical applications, hydroxyapatite precursors are often combined with various natural and synthetic polymers to improve their mechanical and biological properties. 3 It has been reported that Chitosan (CS) represents an ideal candidate for bone tissue engineering due to its biocompatibility, bio‐resorbability, anti‐bacterial activity. 4 and the capability to chemically interact with HA nanoparticles (nHA). Indeed, Xianmiao et al, 5 proposed two mechanisms for the interaction between CS and HA due to (a) hydrogen bonding between amino and hydroxyl groups of CS with hydroxyl ions on nHA surface and (b) coordination bonding between amino group of CS and calcium of nHA. To date, current therapeutic approaches for bone substitution including bone graft transplants (autologous, homologous, or heterologous grafts and biomaterials implants) have not proved to be fully satisfactory. 6 However, many encouraging studies based on bioactive cellularized scaffolds by osteogenic cells are ongoing and may represent a tool for the design of innovative therapeutic strategies, which could adequately satisfy the clinical demands. 7 Furthermore, in order to design high‐throughput tissue‐engineered constructs useful in orthopedic field, a valid approach based on the bioactivation of scaffold is needed. This means using the combination of osteoinductive and angiogenic signals, thus influencing cell behavior in terms of both endothelial and bone regeneration. 8 , 9 In particular, the design of composite materials for hard tissue engineering is a promising strategy to reach structural and biological similarities to natural bone tissue. The combination of two or more components with different physicochemical properties can increase their practical applications, which cannot be satisfied by the single component. 7 Specifically, the mixing of bioactive ceramics and degradable polymers composite materials, which are suitable for the realization of scaffolds with high porosity, ideal for hard tissue regeneration. Three‐dimensional (3D) interconnected porosity coupled to an appropriate pore size and wide surface area promotes cell attachment, migration, cell proliferation 10 and differentiation. Here, we have evaluated in vitro biological properties of an inorganic–organic composite scaffold made of hydroxyapatite and chitosan. The composite materials were prepared using sol–gel technology, which assures a good dispersion of HA nanoparticles in the organic phase, with an increase of in vitro bioactive performance. 9 , 10 Moreover, the technology promotes synthesis of hydroxyapatite with low crystallinity and higher bioactivity using a particle dimension of about 80 nm. 11 In addition, the best composition able to drive hMSC differentiation to osteoblast phenotype and promote anti‐inflammatory response was investigated.
2. MATERIALS AND METHODS
2.1. Synthesis of chitosan‐hydroxyapatite composite materials
CS/HA composite scaffolds were fabricated by in situ sol–gel synthesis of HA in the CS matrix as reported in the flow chart (Figure 1). For this study, four different compositions of samples by considering the 0, 50, 60 and 70% v/v of HA amount respect to composite were produced. Briefly, viscous CS solution [Middle molecular weight; the degree of deacetylation (DD) = 75–85%] was prepared by dissolving chitosan in 0.1 M acetic acid (AA, 99%) solution (1.5% wt) at 40°C. Simultaneously, calcium nitrate Ca(NO3)2 · 4H2O and ammonium hydrogen phosphate (NH4)2HPO4 were dissolved in distilled water (dH2O) and stirred for 2 hr. The Ca2+ solution was added to CS solution and mixed at 250 rpm for 2 hr at 40°C; then, phosphate solution was introduced to Ca2+/CS system. The alkaline environment was reached by using NH4OH solution and measured by pH‐meter. The samples were treated at 40°C for 24 hr (stirring at 85 rpm) and sonicated for 3 hr in order to obtain a homogeneous system. The CS/HA biocomposites were prepared as 3D scaffolds by combining in situ sol–gel approach with freeze‐drying technology. The sol–gel method was used to synthesize and maintain the nanoscale HA precipitates along the chitosan backbone, whilst a freeze‐drying method was applied in order to lock the structure of the composite by quenching the CS/HA gels, into Teflon molds (5 × 10 mm), at −80°C and freeze‐dried for 48 hr.
FIGURE 1.
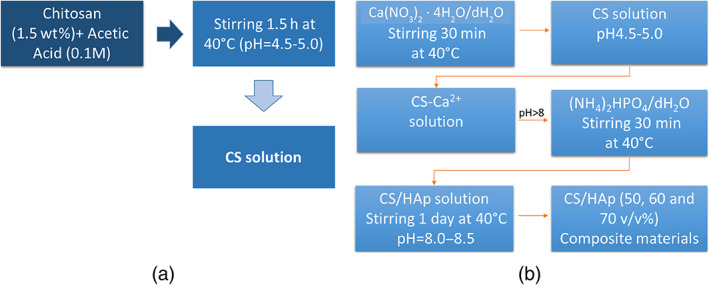
Flow chart representing the preparation of (a) chitosan and (b) CS/HA biocomposite solutions with different content of nHA (50, 60, and 70%v/v)
2.2. Morphological investigations of CS/HA composite scaffolds: SEM Analysis
Morphological properties of CS/HA composite scaffolds at different compositions were studied by scanning electron microscopy SEM (Quanta FEG200‐FEI, The Netherlands). To this end, the scaffolds were deposited on aluminum stubs gold‐coated in an automatic sputter‐coater (EMSCsOPE SC500, 20 kV) to a thickness of about 30 nm.
3. BIOLOGICAL INVESTIGATIONS: CELL–MATERIAL INTERACTIONS
3.1. Cell proliferation
Biological investigations were performed at different time points of cell–material interaction. The scaffolds were prepared for cell seeding by soaking first in 70% ethanol (1 hr) and then in 1% antibiotic/antimycotic in PBS (2 hr) and pre‐wetting in medium (2 hr). To evaluate cell proliferation, cells (20,000 cells/scaffold) were seeded in triplicate onto different CS/HA scaffolds (from 50 to 70%) using basal culture medium. Later, the culture medium in cell‐loaded materials was removed after 1, 3, 7, 14, and 21 days of cell culture and in vitro cell viability was checked by Alamar blue assay (AbD Serotec, Milano, Italy), which is useful to quantify the metabolic activity of live cells. 12 In brief, an aliquot of 500 μl of Alamar Blue diluted 1:10 in phenol red‐free medium was added to each well and incubated for 4 hr at 37°C, 5% CO2. Later, 100 μl of this solution was transferred into a 96 well plate for colorimetric analysis. Wells without any cells were used to correct any background interference from the redox indicator. The optical density was immediately measured with a spectrophotometer (Victor X3, Perkin Elmer) at wavelengths of 540 and 600 nm. Cell viability, expressed as percent of Alamar Blue reduction, was evaluated in according to the manufacturer's protocol. The culture medium during experimental time was changed every 2 days.
3.2. Alkaline phosphatase and osteocalcin expression as markers of osteogenic differentiation
The effect of CS/HA scaffolds on osteogenic differentiation of hMSCs was evaluated by measuring early and later markers of differentiation such as alkaline phosphatase (ALP) and osteocalcin (OCN), respectively. ALP levels were tested on cell lysates (50 μl) by measuring the activity of ALP enzyme, which catalyzes the cleavage of a phosphate group and releases p‐nitrophenol from p‐nitrophenyl phosphate using the SensoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, DBA, Italy), according to the manufacturer's instructions. The absorbance was measured in a 96‐well plate at 405 nm on a plate reader to determine enzyme concentrations per scaffold at 3, 7, and 14 days of cell–material interaction. The ALP values were corrected for the number of live cells attached on each scaffold. Thus, double stranded DNA (dsDNA) was used as a marker for cell number and measured by PicoGreen_dsDNA quantification kit (Invitrogen, The United States). The ALP results were reported as nanograms of ALP normalized to the micrograms of total DNA content (ng ALP/μg DNA). Furthermore, OCN levels were quantified at day 14 using a commercially available kit (Quantikine Human Osteocalcin Immunoassay R&D system, Italy), following the manufacturer's instructions. hMSC cells were cultured on tissue culture plate (control), neat scaffolds, and CS/HA at different concentrations in basal medium for 14 days of culture time. The levels of OCN secreted into the culture medium were determined using an enzyme‐linked immunoassay kit.
3.3. Effect of CS/HA scaffolds on basal inflammatory response
The effect of CS/HA scaffolds on inflammatory response in basal conditions was also evaluated by quantifying the secretion of pro‐inflammatory and anti‐inflammatory cytokines using commercial ELISA kits (Affimetrix Italia, SRL) according to the manufacturer's instructions. In this way, cells at density of 1.5 × 104 cells/scaffold were seeded on the scaffolds and after 3 days of incubation, cell medium was collected and used to evaluate TGF‐β, interleukin (IL)‐10, and interleukin (IL)‐4 levels.
3.4. Statistical analysis
Statistical analyses were performed using GraphPad Prism, version 5.00 (GraphPad Software, La Jolla, California). Data were compared using a Student's t‐test and a two‐way ANOVA, with a Bonferroni post‐test. The results are expressed as mean ± SD. Values of p < .05 were considered significant.
4. RESULTS AND DISCUSSION
4.1. Morphological investigations of 3D scaffolds through SEM analysis
In the field of regenerative medicine, the fabrication of tissue substitutes possessing the same geometrical parameters, composition, and functionality of the original tissue constitutes one of the most difficult goal to achieve. 13 , 14 , 15 Here, we proposed composite scaffolds prepared at room temperature (RT) where the HA was in situ precipitated in CS matrix in order to improve the bioactive properties of polymer. To this end, CS/HA composites at different concentration of HA starting from 50 to 70% (v/v) were studied in order to choose the best formulation in terms of biological properties. SEM investigations demonstrated that HA crystals are well embedded in CS matrix and this feature is more expressed in biocomposites with higher HA amount (Figure 2). However, by increasing the inorganic amount (60 and 70% v/v), some HA clusters were observed and HA crystals look like needles stuck on a support. This HA morphology was related to needle formation as a result of HA crystallization process and it is an index of future bone mineralization events as reported in a previous study. 16
FIGURE 2.
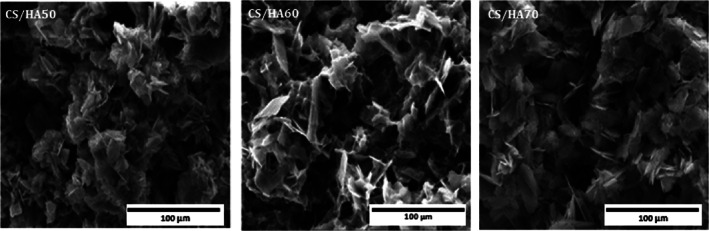
SEM investigations on CS/HA at different compositions (CS/HA 50–60 and 70% v/v). The images are representative of three experiments
4.2. Biological investigations
4.2.1. Effect of 3D scaffolds on cell proliferation and osteogenesis processes
Bone substitutes should possess the ability to promote the growth of newly forming‐bone tissue stimulating niche hMSC to produce more mature bone cell‐phenotypes by preventing foreign body reactions. 17 Our aim was to evaluate the effect of CS/HA based scaffolds on cell behavior in terms of viability and hMSC differentiation towards osteoblast phenotype. Cell viability, in terms of percent of Alamar Blue reduction, for neat CS and CS/HA composite scaffolds, at different concentrations (50–60–70% v/v), was evaluated. In particular, it was observed that CS/HA composite scaffolds induced a reduction in hMSC viability compared to cells grown on neat CS (Figure 3). This latter effect was likely due to differentiation of hMSC to osteoblast phenotype over culture time induced by the presence of HA used as bioactive signal. 18 However, all scaffolds showed no cytotoxic effect at each time point analyzed. The ability of osteoinductive biomaterials to block cell proliferating behavior by favoring differentiation processes was widely discussed over past years. Indeed, previous studies described how several 3D scaffolds functionalized by bioactive signals were able to drive undifferentiated cell lines towards more mature bone and endothelial phenotypes by changing cell proliferation response. 19
FIGURE 3.
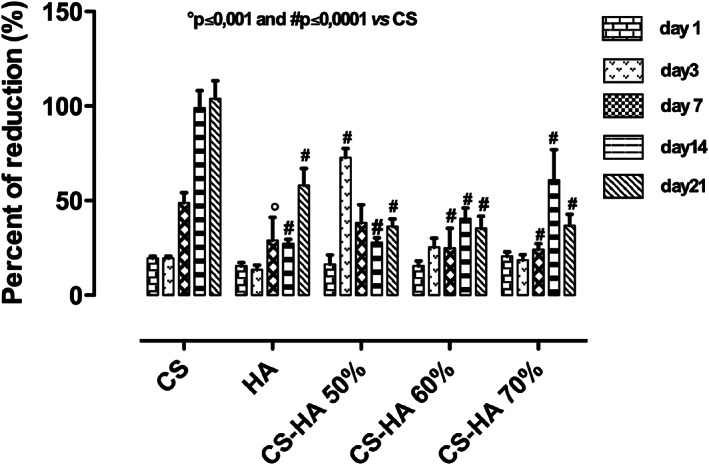
hMSCs viability seeded onto CS and CS/HA based scaffolds materials at 1, 3, 14, and 21 days of cell culture compared to plate control (hMSCs). Results are mean ± SD and are representative of four experiments
The expression of early marker of osteogenic differentiation as ALP, at days 3, 7, and 14 of cell culture was investigated. Particularly, neat CS did not increase ALP values compared to HA alone where, a time dependent increasing in ALP expression was observed (Figure 4). Regarding CS/HA based scaffolds, the composition CS/HA 50% v/v was able to maintain high ALP levels over culture time. By contrast CS/HA 60 and CS/HA 70% v/v compositions decreased ALP values at 3 and 7 days of cell culture, respectively. In particular, CS/HA 50 and 60% show higher ALP expression than CS and HA alone in early days of cell–material interaction. This result demonstrated that the combination of biodegradable and biocompatible polymer, such as chitosan, with bioactive and osteoinductive HA nanoparticles promoted a good effect on cell behavior. However, the best effect in terms of ALP expression was observed in presence of CS/HA 50% v/v composition and this was due to its higher homogeneous distribution between CS and HA thus allowing better structure stability. Indeed, higher scaffold stability influences cell response by promoting a good cell attachment, migration and differentiation. 20 , 21 Conversely, after 14 days of cell culture, the highest OCN levels, as later marker of osteogenic differentiation, 22 , 23 were observed in presence of composite CS scaffolds at 60% and 70% of HA. Indeed, OCN values increased in a HA concentration dependent manner. Therefore, this study underlined that by increasing the amount of bioactive solid signal, osteogenic differentiation of hMSC at longer time of cell–materials interaction was achieved (Figure 5). Furthermore, the choice of an effective osteoinductive signal for 3D scaffolds functionalization promotes attainment of later phases of osteogenesis through the release of specific osteogenic marker such as OCN. Indeed, OCN expression is index of mineralization events that are crucial for obtaining an effective bone tissue regeneration. 24 , 25 , 26
FIGURE 4.
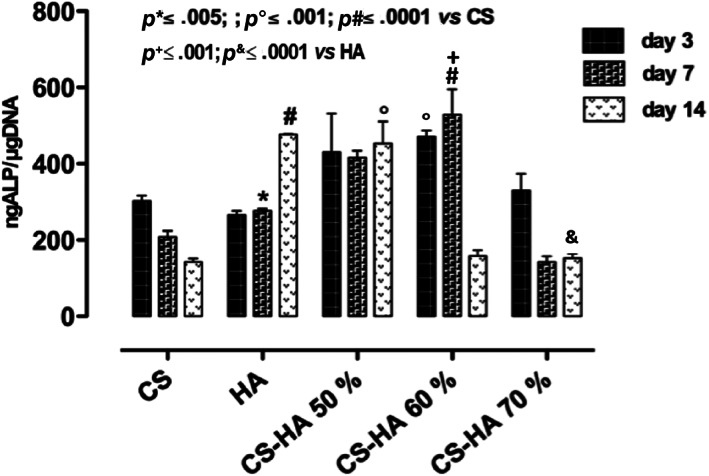
ALP expression, at different time points, for hMSCs seeded on neat CS, HA, and CS/HA scaffolds at different compositions (50–60–70% v/v) at 3, 7, and 14 days of cell culture. Results are mean ± SD and are representative of four experiments
FIGURE 5.
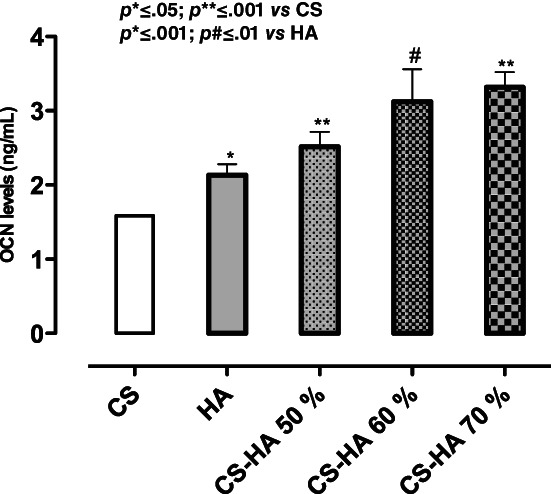
Osteocalcin expression from hMSCs seeded on CS, HAp, and CS/HA based scaffolds (50–70%) after day 14 of in vitro cell culture. Results are mean ± SD and are representative of three experiments
4.2.2. Effect of 3D scaffolds on basal inflammatory response
Herein, biological studies showed CS‐based scaffolds effect on inflammatory response in physiological conditions by assessing pro‐inflammatory and anti‐inflammatory cytokines (TGF‐β, IL‐10, and IL‐4) secretion. It is well known that bone implant could often induce a foreign body reaction associated to an inflammation process. 27 , 28 For this purpose, in bone tissue engineering context, we have assessed scaffold related effects on cytokine production induced during an inflammatory reaction. In particular, the production of pro‐inflammatory (TGF‐β) and anti‐inflammatory (IL‐10 and IL‐4) cytokines was studied. Transforming growth factor‐beta (TGF‐β) plays a pivotal role in bone remodeling. Indeed, TGF‐β promotes matrix protein synthesis and critically influences the balance between bone cells responsible for bone formation and resorption. 29 In this study, we observed the TGF‐β peak in the presence of CS alone after 3 days of cell culture. Meanwhile, all the composite scaffolds did not increase TGF‐β values compared to control suggesting the ability of biocomposites to prevent inflammatory events in basal conditions (Figure 6). Unlike TGF‐β, IL‐10 cytokine family exerts host defense effects including the inhibition of injuries caused by infection or inflammation by promoting tissue‐healing. 30 IL‐4 is a multifunctional pleiotropic cytokine, which functionally regulates cell proliferation, apoptosis and gene expression in several cell lines such as lymphocytes, macrophages, and fibroblasts, as well as epithelial and endothelial cells. 31 Here, we reported the effect of CS/HA scaffolds on basal IL‐10 and IL‐4 secretion. Results suggested that IL‐10 and IL‐4 levels modulation was obtained in presence of composite CS/HA based scaffolds at higher concentration (CS/HA 60–70%) after 3 days of cell seeding (Figure 7a,b). This effect was related to HA concentration and probably due to the different amount of Ca2+ able to interact with calcium depended receptors. 32 Meanwhile, CS/HA 50% showed effect only on IL‐10 at the same time point (Figure 7a).
FIGURE 6.
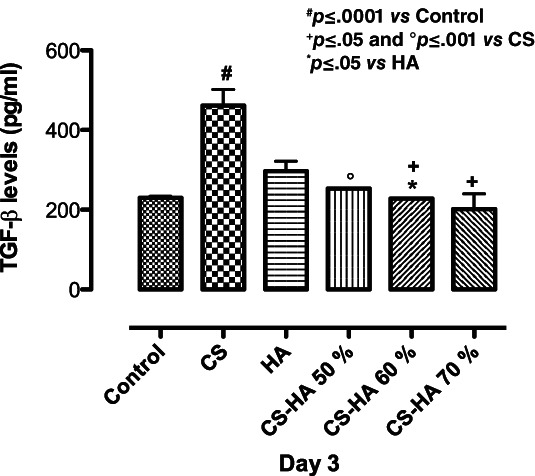
Effect of CS, HA, and CS/HA based scaffolds on basal TGF‐β levels production after 3 days of cell culture. Results are mean ± SD and are representative of three experiments
FIGURE 7.
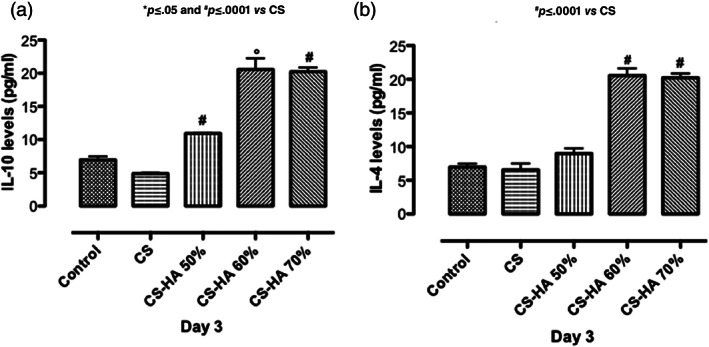
Effect of HA, CS, and CS/HA based scaffolds at different composition on IL‐10 (a) and IL‐4 (b) basal secretion after 3 days of cell culture. Results are mean ± SD and are representative of three experiments
5. CONCLUSIONS
The present study has demonstrated the possibility to develop biocomposite scaffolds with high amount of hydroxyapatite nanoparticles by combining sol–gel technology and freeze‐drying process. In particular, the results indicated that CS/HA scaffolds are able to support cell proliferation and differentiation thanks to important chemical features of CS and bioactive/osteoinductive properties of HA. Moreover, biocomposites at higher concentration of inorganic fillers (60 and 70%) showed an impressive effect on osteogenic differentiation of hMSC towards mature osteoblast phenotype and were able to prevent, on in vitro cell culture model, pro‐inflammatory events. Indeed, CS/HA biocomposites showed a good effect on the expression of anti‐inflammatory cytokines (IL‐10 and IL‐4), meanwhile it was able to decrease pro‐inflammatory cytokine (TGF‐β) levels. This study emphasized the concept of CS/HA composites in a way such that by increasing the amount of HA nanoparticles (as bioactive inorganic solid signals in the CS matrix), high levels of hMSC osteogenic differentiation could be attained by avoiding the establishment of pro‐inflammatory conditions.
ACKNOWLEDGMENTS
This study has received funding from bilateral cooperation CNR‐CINVESTAV (Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional) – Mexico 2017–2018 with CHITOCARB project. The authors also thank Mrs. Mariarosaria Bonetti for lab technical support and data elaboration and Roberta Marzella for support to project management of IPCB‐CNR.
Soriente, A. , Fasolino, I. , Gomez‐Sánchez, A. , Prokhorov, E. , Buonocore, G. G. , Luna‐Barcenas, G. , Ambrosio, L. , & Raucci, M. G. (2022). Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. Journal of Biomedical Materials Research Part A, 110(2), 266–272. 10.1002/jbm.a.37283
Funding information Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional; Consiglio Nazionale delle Ricerche
Contributor Information
Ines Fasolino, Email: ines.fasolino@cnr.it.
Maria Grazia Raucci, Email: mariagrazia.raucci@cnr.it.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Shakir M, Jolly R, Khan MS, Iram NE, Khan HM. Nano‐hydroxyapatite/chitosan–starch nanocomposite as a novel bone construct: synthesis and in vitro studies. Int J Biol Macromol. 2015;80:282‐292. [DOI] [PubMed] [Google Scholar]
- 2. Wan Jeffrey B, Bahman N‐T, Saeid B. Overview of hydroxyapatite–graphene nanoplatelets composite as bone graft substitute: mechanical behavior and in‐vitro biofunctionality. Crit Rev Solid State. 2018;43:177‐212. [Google Scholar]
- 3. Szcześ A, Hołysz L, Chibowski E. Synthesis of hydroxyapatite for biomedical applications. Adv Colloid Interface Sci. 2017;249:321‐330. [DOI] [PubMed] [Google Scholar]
- 4. Sultankulov B, Berillo D, Sultankulova K, Tokay T, Saparov A. Progress in the development of chitosan‐based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9(9):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xianmiao C, Yubao L, Yi Z, Li Z, Jidong L, Huanan W. Properties and in vitro biological evaluation of nanohydroxyapatite/chitosan membranes for bone guided regeneration. Mater Sci Eng C. 2009;29:29‐35. [Google Scholar]
- 6. Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240‐4250. [DOI] [PubMed] [Google Scholar]
- 7. Ramakrishna S, Mayer J, Wintermantell E. Biomedical application of polymer‐composite materials: a review. Compos Sci Technol. 2001;61:1189‐1224. [Google Scholar]
- 8. Liu X, Chen W, Zhang C, et al. Co‐seeding human endothelial cells with human‐induced pluripotent stem cell‐derived mesenchymal stem cells on calcium phosphate scaffold enhances osteogenesis and vascularization in rats. Tissue Eng Part A. 2017;23(11–12):546‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soriente A, Amodio SP, Fasolino I, et al. Chitosan/PEGDA based scaffolds as bioinspired materials to control in vitro angiogenesis. Mater Sci Eng C. 2020;118:111420. [DOI] [PubMed] [Google Scholar]
- 10. Raucci MG, D'Antò V, Guarino V. Biomineralized porous composite scaffolds prepared by chemical synthesis for bone tissue regeneration. Acta Biomater. 2010;6:4090‐4099. [DOI] [PubMed] [Google Scholar]
- 11. Raucci MG, Guarino V, Ambrosio L. Hybrid composite scaffolds prepared by sol–gel method for bone regeneration. Compos Sci Technol. 2010;70:1861‐1868. [Google Scholar]
- 12. Raucci MG, Giugliano D, Alvarez‐Perez MA, Ambrosio L. Effects on growth and osteogenic differentiation of mesenchymal stem cells by the strontium‐added sol‐gel hydroxyapatite gel materials. J Mater Sci Mater. 2015;26(2):90. [DOI] [PubMed] [Google Scholar]
- 13. Rampersad SN. Multiple applications of Alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12(9):12347‐12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang K, Wang S, Zhou C, et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20(5):575‐592. [DOI] [PubMed] [Google Scholar]
- 16. Soriente A, Fasolino I, Raucci MG, et al. Effect of inorganic and organic bioactive signals decoration on the biological performance of chitosan scaffolds for bone tissue engineering. J Mater Sci Mater. 2018;7:62. [DOI] [PubMed] [Google Scholar]
- 17. Raucci MG, Fasolino I, Pastore SG, et al. Antimicrobial imidazolium ionic liquids for the development of minimal invasive calcium phosphate‐based bio‐nanocomposites. ACS Appl Mater Interfaces. 2018;10(49):42766‐42776. [DOI] [PubMed] [Google Scholar]
- 18. Lopes DL, Martins‐Cruz C, Oliveira MB, Mano JF. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raucci MG, Demitri C, Soriente A, Fasolino I, Sannino A, Ambrosio L. Gelatin/nano‐ hydroxyapatite hydrogel scaffold prepared by sol‐gel technology as filler to repair bone defects. J Biomed Mater Res Part A. 2018;106:2007‐2019. [DOI] [PubMed] [Google Scholar]
- 20. Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: a review. Bioact Mater. 2019;4:271‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu L, Luo D, Liu Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int J Oral Sci. 2020;12(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Q, Chang B, Zheng G, Du S, Li X. Quercetin stimulates osteogenic differentiation of bone marrow stromal cells through miRNA‐206/connexin 43 pathway. Am J Transl Res. 2020;12(5):2062‐2070. [PMC free article] [PubMed] [Google Scholar]
- 23. Catanzano O, Soriente A, La Gatta A, et al. Macroporous alginate foams crosslinked with strontium for bone tissue. Carbohydr Polym. 2018;202:72‐83. [DOI] [PubMed] [Google Scholar]
- 24. Polo‐Corrales L, Latorre‐Esteves M, Ramirez‐Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5(1):1‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsao YT, Huang YJ, Wu HH, Liu YA, Liu YS, Lee OK. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int J Mol Sci. 2017;18(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fasolino I, Raucci MG, Soriente A, et al. Osteoinductive and anti‐inflammatory properties of chitosan‐based scaffolds for bone regeneration. Korean J Couns Psychother. 2019;105:110046. [DOI] [PubMed] [Google Scholar]
- 28. Trindade R, Albrektsson T, Tengvall P. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res. 2016;18(1):192‐203. [DOI] [PubMed] [Google Scholar]
- 29. van der Kraan PM. Differential role of transforming growth factor‐beta in an osteoarthritic or a healthy joint. J Bone Metab. 2018;25(2):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luzina IG, Keegan AD, Heller NM, Rook GA, Shea‐Donohue T, Atamas SP. Regulation of inflammation by interleukin‐4: a review of "alternatives". J Leukoc Biol. 2012;92(4):753‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burmeister AR, Marriott I. The Interleukin‐10 family of cytokines and their role in the CNS. Front Cell Neurosci. 2018;12:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hagenacker T, Ledwig D, Büsselberg D. Feedback mechanisms in the regulation of intracellular calcium ([Ca2+]i) in the peripheral nociceptive system: role of TRPV‐1 and pain related receptors. Cell Calcium. 2008;43(3):215‐227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


