Abstract
Background and Aims
Environmentally triggered chronic liver inflammation can cause collagen deposits, whereas early stages of fibrosis without any specific symptoms could hardly be detectable. We hypothesized that some of the human donor grafts in clinical liver transplantation (LT) might possess unrecognizable fibrosis, affecting their susceptibility to LT‐induced stress and hepatocellular damage. This retrospective study aimed to assess the impact of occult hepatic fibrosis on clinical LT outcomes.
Approach and Results
Human (194) donor liver biopsies were stained for collagen with Sirius red, and positive areas (Sirius red–positive area; SRA) were measured. The body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes score was calculated using 962 cases of the donor data at the procurement. LT outcomes, including ischemia‐reperfusion injury (IRI), early allograft dysfunction (EAD), and survival rates, were analyzed according to SRA and BARD scores. With the median SRA in 194 grafts of 9.4%, grafts were classified into low‐SRA (<15%; n = 140) and high‐SRA (≥15%; n = 54) groups. Grafts with high SRA suffered from higher rates of IRI and EAD (P < 0.05) as compared to those with low SRA. Interestingly, high SRA was identified as an independent risk factor for EAD and positively correlated with the donor BARD score. When comparing low‐BARD (n = 692) with high‐BARD (n = 270) grafts in the same period, those with high BARD showed significantly higher post‐LT transaminase levels and higher rates of IRI and EAD.
Conclusions
These findings from the largest clinical study cohort to date document the essential role of occult collagen deposition in donor livers on LT outcomes. High‐SRA and donor BARD scores correlated with an increased incidence of hepatic IRI and EAD in LT recipients. This study provides the rationale for in‐depth and prospective assessment of occult fibrosis for refined personalized LT management.
Abbreviations
- Abs
antibodies
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- Atg5
autophagy‐related 5
- Atg7
autophagy‐related 7
- BARD
BMI, AST/ALT ratio, diabetes
- BMI
body mass index
- DCD
donation after circulatory death
- EAD
early allograft dysfunction
- FIB‐4
Fibrosis‐4
- H&E
hematoxylin and eosin
- HMGB1
high mobility group protein B1
- IR
ischemia‐reperfusion
- IRI
ischemia‐reperfusion injury
- LT
liver transplantation
- MCP1
monocyte chemoattractant protein‐1
- MELD
Model for End‐Stage Liver Disease
- NAFLD‐FS
NAFLD Fibrosis Score
- POD
postoperative day
- SRA
Sirius red–positive area
- UCLA
University of California Los Angeles
Although liver transplantation (LT) has become the standard care for patients with end‐stage liver diseases and those with hepatic malignancies, graft dysfunction is not uncommon and remains a serious complication in LT recipients. Indeed, the incidence of early allograft dysfunction (EAD) occurs in as much as 25% of all LT’s, varying from 10.8% to 36.3% between centers.( 1 ) Despite continuously improving graft survival attributable to surgical techniques and postoperative management, nearly 30% of LT recipients lose their graft within 5 years.( 2 , 3 ) With clinical studies indicating the association between EAD and ultimate graft loss,( 4 , 5 ) preventive strategies against EAD are warranted. Besides, severe donor liver shortage, evidenced by >10,000 patients registered on transplant waiting lists for a life‐saving organ, remains the major challenge.( 2 ) Moreover, the age of recipients has increased steadily in the past decade, and 25% of recipients are >65 years in the USA.( 6 ) The imbalance between ever‐growing LT candidates with advanced age and organ shortage increases patients’ acuity on transplant waiting lists.( 7 ) Although donor shortage encourages the use of extended criteria organs to increase LT access, the use of marginal livers has to be critically examined, especially for high‐acuity patients.
Chronic liver inflammation causes fibrosis, resulting from an imbalance between collagen deposition and reabsorption, ultimately leading to cirrhosis, the 12th‐leading cause of death in the USA.( 8 ) The early stage of hepatic fibrosis develops without any symptoms, recognizable clinical variables, or macroscopic abnormalities on the liver surface. A fibrotic/cirrhotic graft has generally been considered unsuitable for LT.( 9 ) However, despite numerous reports on the development of hepatic fibrosis after LT,( 10 , 11 , 12 , 13 ) the impact of collagen deposition in donor livers on recipient outcomes remains to be thoroughly investigated. Others have reported that LT recipients implanted with early‐stage fibrotic livers, diagnosed as F1 or F2 (the Knodell‐modified histological activity index; Ishak score; F0‐F4),( 14 ) showed survival comparable with nonfibrotic (F0) livers.( 15 ) This implies the acceptability of donor liver grafts with early‐stage fibrosis pathology. However, the degree of collagen deposition may be different in each donor liver, even if diagnosed at the same scores (F1 or F2) or as “nonfibrotic” (F0) by common assessment.( 14 , 16 ) Considering ever‐increasing exposure to arrays of metabolic and environmental stresses, we reasoned that some donor livers develop occult fibrosis, which, in turn, may influence hepatic susceptibility to LT stress and hepatocellular damage. Indeed, precise quantification of collagen deposition is needed to evaluate the impact of early fibrosis on LT injuries and clinical outcomes, including ischemia‐reperfusion injury (IRI) and EAD.
In this study, we aimed to determine whether occult fibrosis, assessed by Sirius red staining, correlates with LT clinical outcomes. Adult LTs (n = 962) performed at University of California Los Angeles (UCLA) were retrospectively analyzed for collagen deposition with Sirius red staining (n = 194). Unexpectedly, we found that inapparent fibrosis was significantly associated with EAD and IRI. By providing the evidence that the hidden collagen deposit at the time of procurement affects LT outcomes, this study offers insight into the management of clinical LT patients, especially in the high‐acuity patient population.
Patients and Methods
Patient Selection and Data Collection
This study was approved by the UCLA Institutional Review Board (IRB#13‐00143, 18‐000125, and 19‐000845). We performed a retrospective analysis of adult LT cases (recipient age ≥18 years; January 2012 to December 2018). Of 1,011 LTs in the study period, 962 LT cases were included in this study after excluding 49 LTs lacking detailed data (Supporting Fig. S1). All patients received standard immunosuppressive therapy, per our institutional protocol, during the perioperative period. All liver grafts were procured from donation after brain death or circulatory death (DCD) donors using standard procurement techniques and were cold‐stored in University of Wisconsin solution. We collected data including histological findings, recipient demographics, donor characteristics, graft ischemia time, and patient/graft outcomes, including postoperative laboratory data in the first 7 post‐LT days. EAD was defined by the presence of one or more of the following: bilirubin level of ≥10 mg/dL on postoperative day (POD) 7; prothrombin time/international normalized ratio ≥1.6 on POD7; or aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels of >2,000 IU/L within the first 7 days. LT rejection was diagnosed by follow‐up biopsy done per clinical standard of care. Graft survival time was calculated from the date of LT to the date of graft failure (patient death/retransplantation) or the date of the last follow‐up if graft failure did not occur.
Histological Assessment of Liver Fibrosis/Collagen Detection by Sirius Red Staining
One hundred ninety‐four formalin‐fixed, paraffin‐embedded unstained LT biopsy specimens were obtainable from consented patients for Sirius red staining and further assessment. Biopsies during LT were performed routinely on all cases (not individual clinical indication) during the earlier years of the study period. This practice was subsequently adjusted, and biopsies were only done for ongoing research or data‐bank collection on consented patients. Tru‐cut needle biopsies were obtained from the left lobe during LT, and formalin‐fixed, paraffin‐embedded LT biopsy specimens were stained with the Picro Sirius Red Stain Kit (ab150681; Abcam, Cambridge, MA) in one run for this study. Sirius red–positive area (SRA; %) was calculated as follows. The images of the whole liver biopsy area in each specimen were captured with 200× magnification (the average number of pictures in each sample was 50.0) by the All‐in‐One Fluorescence Microscope BZ‐X800 (Keyence, Itasca, IL). Then, the positive area and the entire biopsy area were calculated using ImageJ software (NIH, Bethesda, MD). The detailed analysis method is available in the Supporting Information (Supporting Fig. S2). Additionally, liver fibrosis stage was assessed by hematoxylin and eosin (H&E) and trichrome staining, according to the Metavir system.( 16 )
Noninvasive Evaluation of Liver Fibrosis
The following scores in donor livers were evaluated: AST/ALT ratio,( 17 ) (APRI),( 18 ) Fibrosis‐4 (FIB‐4) score,( 19 ) NAFLD Fibrosis Score (NAFLD‐FS),( 20 ) and body mass index (BMI), AST/ALT ratio, diabetes (BARD) scores.( 21 ) Correlations between each score and LT outcomes were analyzed.
Evaluation of Histological Hepatic IRI on Postreperfusion LT Biopsy
Four hundred sixty‐six liver biopsies were obtained after portal reperfusion (before surgical closure of the abdomen), and then the degree of hepatic IRI was evaluated by H&E staining (Supporting Table S1). These specimens were reviewed and assessed by a blinded transplant hepatopathologist and graded semiquantitatively for IRI severity as previously described by our group.( 22 , 23 )
Western Blotting
Forty‐nine protein samples were available for extraction from liver tissues, and their concentration was measured using the BCA Protein Assay Kit (ThermoFisherScientific, Waltham, MA). Equal amounts of protein were electrophoresed, blotted, incubated with primary antibodies (Abs), secondary horseradish peroxidase–conjugated Abs, and developed. Primary Abs detecting high mobility group protein B1 (HMGB1; #6893, clone D3E5), autophagy‐related 7 (Atg7; #8558, clone D12B11), autophagy‐related 5 (Atg5; #12994, clone D5F5U), and β‐actin (#12620, clone D6A8; Cell Signaling Technology, Danvers, MA) were used. The densitometry quantification was performed to compare target protein expression levels in multiple human LT samples, as reported.( 24 , 25 , 26 ) Briefly, in a preliminary study, one of the biopsy samples expressing all target proteins was chosen and assigned as a control sample. Equal amounts of protein lysate from each sample were then applied to each well or gel, and target band intensity is expressed as relative band intensity to that of the positive control in the same gel. Target relative protein value was further normalized according to β‐actin intensity.
Immunofluorescence
Paraffin‐embedded postreperfusion liver biopsy sections were stained with rabbit anti‐HMGB1 Ab (ab18256; Abcam) and mouse anti‐COL1A (collagen type I alpha) Ab (sc‐59772; Santa Cruz, Dallas, TX). Signals were visualized with secondary Alexa Fluor Abs.( 24 , 26 )
Quantitative RT‐PCR Analysis
RNA extracted with the RNeasy Mini Kit (Qiagen) was reverse‐transcribed into complementary DNA. Quantitative PCR was performed using QuantStudio 3 (Applied Biosystems). Target gene expression was normalized to the housekeeping gene, glyceraldehyde 3‐phosphate dehydrogenase.( 24 , 26 )
Statistical Analysis
Group comparisons were performed using the Mann‐Whitney U test for continuous values and the chi‐square or Fisher’s exact test for categorical variables, as appropriate. Spearman’s correlation coefficient (r) and or the Cochran‐Armitage test for trend were used to evaluate the strength of the linear relationship between variables. Survival curves were generated by the Kaplan‐Meier method, and differences in survival rates were analyzed using the log‐rank test. To identify predictors for EAD, a logistic regression model was used for multivariate analysis. A P value of <0.05 was considered statistically significant, and the CI was determined at 95%. All analyses were performed using IBM SPSS Statistics (version 26; IBM Corporation, Armonk, NY) and R software (version 3.5.0; R Development Core Team).
Results
Quantification of Collagen Deposition in Human LT
One hundred ninety‐four hepatic allograft biopsy specimens obtained during LT surgery were stained with Sirius red to evaluate the extent of collagen deposition in human donor livers. SRA percentage was calculated by dividing the positive area by the entire biopsy area. Representative Sirius red staining images are shown in Fig. 1A‐D (A, SRA <5%; B, SRA = 9.5%; C, SRA = 14.1%; D, SRA = 20.4%). Figure 1E shows the distribution of SRA. A median of SRA was 9.4% (range, 1.1‐35.9) for 194 cases under investigation.
FIG. 1.
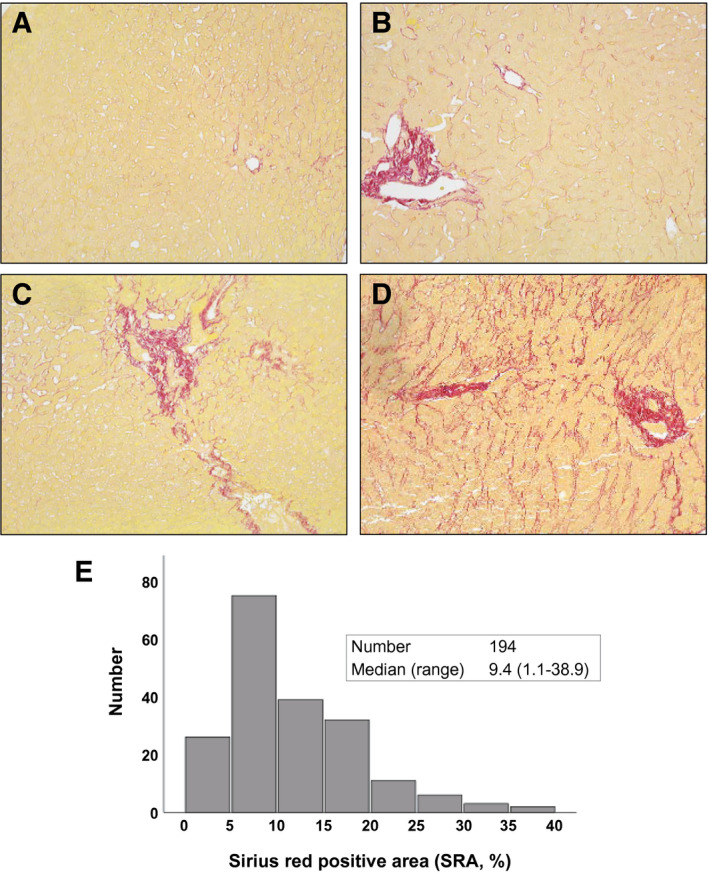
Collagen deposit evaluation by Sirius red staining in human LT. Liver biopsy samples were collected from human donor livers (n = 194) during LT surgery. Biopsy specimens were stained with Sirius red, and the percentage of SRA (%) was calculated in the entire biopsy. Representative cases are shown: (A) SRA <5%; (B) SRA = 9.5%; (C) SRA = 14.1%; (D) SRA = 20.4%; and (E) SRA distribution. Median SRA was 9.4% (range, 1.1‐35.9).
Collagen Deposition in Donor Liver Correlates With EAD After LT
To determine whether inapparent collagen deposition in donor livers influences LT outcomes, 194 grafts were initially classified into five groups according to SRA of: (1) 0 < SRA < 5%; (2) 5 ≤ SRA < 10%; (3) 10 ≤ SRA < 15%; (4) 15 < SRA ≤ 20%; and (5) 20% ≤ SRA. SRA showed a positive correlation with the incidence of EAD (P = 0.025; Fig. 2A). We next aimed to search for an optimal SRA cut‐off value for EAD. Using the receiver operating characteristic (ROC) curve, the sensitivity and specificity for each cut‐off value of SRA for EAD were obtained (Fig. 2B,C). The cut‐off value of 15% of SRA showed the maximized sum of sensitivity and specificity.
FIG. 2.
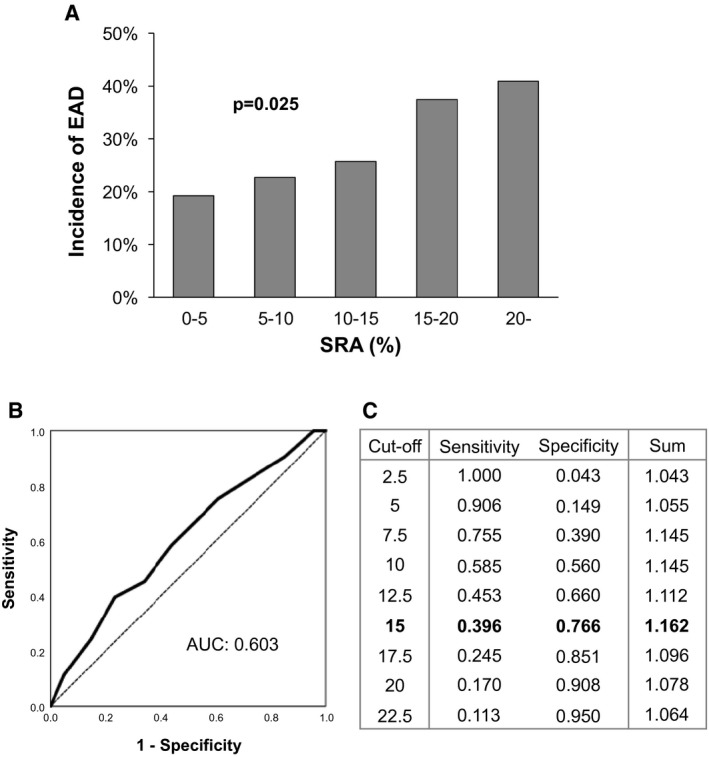
Correlation between SRA and EAD and the determination of the optimal cut‐off value for EAD. The incidence of EAD was positively correlated with SRA (A). Based on the ROC curve (B), the optimal cut‐off value for EAD (SRA = 15%) was obtained according to the maximized sum of sensitivity and specificity (C).
Collagen Deposition in the Donor Liver Correlates With LT Early Injury
Based on a cut‐off value of 15%, 194 grafts with evaluable SRA were classified into low‐SRA (<15%; n = 140) versus high‐SRA (≥15%; n = 54) groups. Demographics of recipient/donor/graft are shown in Table 1. The high‐SRA group showed a significantly higher rate of diabetes as donor comorbidity (14.8% vs. 5.7%; P = 0.039). There were no differences in graft ischemia time, rate of HCV antibody‐positive donor, rate of large droplet macrovesicular (≥30%) steatosis, and liver fibrosis according to the Metavir system between the two groups. There were no differences in recipient factors, including age, sex, BMI, comorbidity underlying liver disease, preoperative length of hospital stay, laboratory data, and Model for End‐Stage Liver Disease (MELD) score.
TABLE 1.
Demographics in 194 Human LTs With SRA <15% versus SRA ≥15%
| SRA <15% | SRA ≥15% | P Value | |
|---|---|---|---|
| n = 140 | n = 54 | ||
| Recipient factors | |||
| Age (years) | 58 (18‐78) | 60 (24‐74) | 0.086 |
| Sex (female/male) | 47/93 | 20/34 | 0.649 |
| BMI (kg/m2) | 26.9 (16.1‐47.5) | 26.6 (17.5‐41.1) | 0.818 |
| Comorbidity | |||
| DM | 49 (35.0) | 16 (29.6) | 0.478 |
| HTN | 60 (42.9) | 24 (44.4) | 0.841 |
| CAD | 25 (17.9) | 10 (18.5) | 0.914 |
| Underlying liver disease | 0.591 | ||
| HBV | 7 (5.0) | 2 (3.7) | |
| HCV | 55 (39.3) | 22 (40.7) | |
| EtOH | 24 (17.1) | 10 (18.5) | |
| NASH | 16 (11.4) | 8 (14.8) | |
| ALF | 9 (6.4) | 3 (5.6) | |
| PBC | 2 (1.4) | 3 (5.6) | |
| Others | 27 (19.3) | 6 (11.1) | |
| Preoperative hospital stay (days) | 5 (0‐168) | 3 (0‐56) | 0.978 |
| Preoperative ICU admission | 75 (53.6) | 26 (48.1) | 0.498 |
| Laboratory data at LT | |||
| AST (IU/L) | 67 (18‐6,705) | 57 (22‐1,918) | 0.180 |
| ALT (IU/L) | 35 (9‐6,850) | 37 (8‐3,705) | 0.993 |
| Laboratory MELD | 30.9 (6.4‐46.2) | 31.4 (6.4‐45.9) | 0.922 |
| Donor factors | |||
| Age (years) | 35 (13‐74) | 43 (16‐72) | 0.074 |
| Sex (female/male) | 62/78 | 18/36 | 0.165 |
| BMI (kg/m2) | 26.3 (17.3‐53.0) | 25.8 (19.6‐38.7) | 0.907 |
| Comorbidity | |||
| DM | 8 (5.7) | 8 (14 .8) | 0.039 |
| HTN | 36 (25.7) | 14 (25.9) | 0.976 |
| CAD | 8 (5.8) | 2 (3.7) | 0.729 |
| Cause of death | 0.814 | ||
| Trauma | 49 (35.0) | 22 (40.7) | |
| CVS | 48 (34.3) | 18 (33.3) | |
| Anoxia | 41 (29.3) | 13 (24.1) | |
| Others | 2 (1.4) | 1 (1.9) | |
| Laboratory data at procurement | |||
| AST (IU/L) | 38 (9‐483) | 49 (12‐260) | 0.113 |
| ALT (IU/L) | 32 (8‐730) | 32 (11‐74) | 0.759 |
| DCD | 7 (5.0) | 3 (5.6) | >0.999 |
| HCV Ab positive | 3 (2.1%) | 2 (3.7%) | 0.619 |
| Cold ischemia time (min) | 440 (138‐878) | 419 (234‐757) | 0.590 |
| Warm ischemia time (min) | 48 (24‐79) | 49 (23‐77) | 0.834 |
| Large droplet steatosis ≥30% | 4 (2.9) | 4 (7.4) | 0.222 |
| Fibrosis on H&E and trichrome (≥F1) | 1 (0.7) | 0 | >0.999 |
Bold represent statistical significance.
Abbreviations: ALF, acute liver failure; CAD, coronary artery disease; CVS, cerebrovascular stroke; DM, diabetes mellitus; EtOH, alcohol‐associated (ethanol) liver disease; HTN, hypertension; ICU, intensive care unit; PBC, primary biliary cholangitis.
The high‐SRA group showed higher trends in post‐LT maximal transaminase levels (Fig. 3A,B). In parallel, the high‐SRA group had a significantly higher incidence of mild‐to‐severe IRI (66.7% vs. 50.0%; P = 0.037) and EAD (38.9% vs. 22.9%; P = 0.025). There were no relationships between SRA and acute rejection or biliary complications, including leakage and stricture after LT. Subanalysis by recipient and donor age revealed that recipients aged >60 years implanted with high‐SRA grafts experienced a higher incidence of EAD than those with low‐SRA grafts (42.9% vs. 17.5%; P = 0.012; Fig. 3C). In comparison, SRA did not affect the incidence of EAD in recipients aged <60 years (34.6% vs. 26.5%; P = 0.424). From the point of view of the donor’s age, high‐SRA grafts from donors >40 years rendered a significantly higher incidence of EAD as compared to low‐SRA grafts (44.8% vs. 22.4%; P = 0.031), whereas, regardless of SRA, livers from donors <40 years had no impact on the rates of EAD (32.0% vs. 23.3%; P = 0.374). Upon log‐rank testing, there were no significant differences in graft survival after LT between the two groups. However, in terms of short‐term outcomes, the high‐SRA group exhibited a significantly inferior graft survival compared to the low‐SRA group (88.9% vs. 95.7%; P = 0.042; Supporting Fig. S3A) at 3 months after LT (P value obtained by comparing graft failure incidence within 3 months as binary outcomes). Similarly, recipients >60 years implanted with high‐SRA livers showed inferior 3‐month graft survival compared to those with low‐SRA (89.3% vs. 94.7%; P = 0.087; Supporting Fig. S3B). Also, recipients implanted with high‐SRA livers from donors >40 years exhibited significantly worse short‐term LT survival as compared to low‐SRA donor livers (86.2% vs. 98.3%; P = 0.011, Supporting Fig. S3C). There were no differences in short‐ and long‐term graft survival rates in subgroup analysis by the donor (<40 years) and recipient (<60 years) age (Supporting Fig. S3D,E).
FIG. 3.
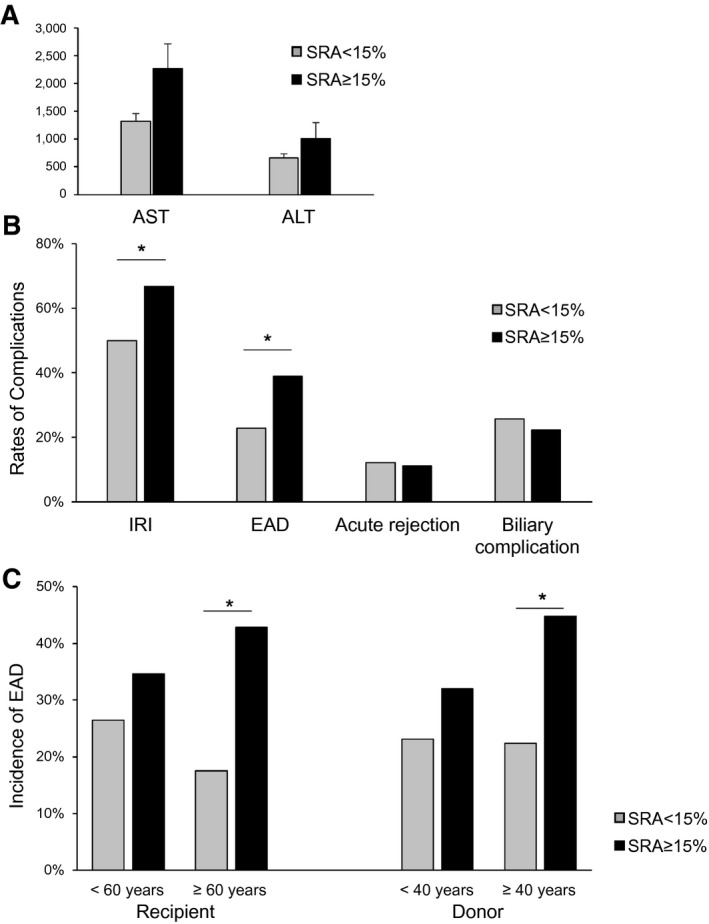
SRA in LT correlates with postoperative liver damage and subanalysis of EAD by recipient and donor age. One‐hundred ninety‐four grafts with evaluable SRA were classified into SRA <15% and SRA ≥15%. (A) Grafts with SRA ≥15% had higher trends in post‐LT maximal transaminase level. (B) Grafts with SRA ≥15% had significantly higher rates of mild‐to‐severe hepatic IRI and EAD (P < 0.05). Meanwhile, there were no differences in rates of acute rejection and biliary complications, including leakage and stricture. (C) Subanalysis by recipient and donor age showed significantly higher rates of EAD in grafts with SRA ≥15% compared to grafts with SRA <15% in recipients aged ≥60 years and donors ≥40 years. However, no statistical differences in EAD rates were shown in subanalysis for young‐age recipients (<60 years) and donors (<40 years). *P < 0.05.
SRA is an Independent Predictive Factor for EAD
To determine whether high SRA is a predictor of EAD, we conducted a multivariate analysis. Supporting Table S2 shows the univariate analysis for EAD risk factors (comparing grafts with EAD vs. non‐EAD). Grafts with EAD had significantly higher donor BMI (29.2 vs. 25.7 kg/m2; P = 0.013), longer cold ischemia time (500 vs. 419 minutes; P = 0.010), and higher rates of donor hypertension (35.8% vs. 20.0%; P = 0.049), large droplet macrovesicular steatosis (≥30%; 11.3% vs. 1.4%; P = 0.006), and high SRA (39.6% vs. 23.4%; P = 0.025). On multivariate analysis, high SRA was identified as an independent predictive factor of EAD (odds ratio = 2.258; 95% CI, 1.090‐4.675; P = 0.028), along with longer cold ischemia time and large droplet macrovesicular (≥30%) steatosis (Table 2).
TABLE 2.
Multivariate Analysis for Risk Factors for EAD in Human LT
| Factors | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Donor BMI (per kg/m2) | 1.030 (0.974‐1.090) | 0.298 |
| Donor HTN | 1.731 (0.801‐3.744) | 0.163 |
| Cold ischemia time (per min) | 1.004 (1.001‐1.007) | 0.005 |
| Large droplet macrovesicular steatosis (≥30%) | 8.472 (1.450‐49.492) | 0.018 |
| High SRA (≥15%) | 2.258 (1.090‐4.675) | 0.028 |
Bold represent statistical significance.
Abbreviation: HTN, hypertension.
SRA Negatively Correlates With Autophagy‐Related Protein and HMGB1 Expressions in LT
In the current study, donors with high‐SRA livers were older than their low‐SRA counterparts (Table 1). Given that autophagy is known to be involved in aging and liver pathology,( 27 ) we reasoned that high‐SRA livers might be more susceptible to peritransplant ischemia‐reperfusion (IR) stress attributable to the impairment of autophagy induction resulting from aging. We screened for western‐assisted Atg5, Atg7, and HMGB1 protein expression patterns at 2 hours postreperfusion in 49 LT cases. High‐SRA livers showed decreased expression of Atg7 (P = 0.0058) and Atg5 (P = 0.0206; Fig. 4A,B). Besides, SRA was negatively correlated with HMGB1 expression (P = 0.041; Fig. 4C). Representative western‐assisted HMGB1 expression in low‐SRA and high‐SRA grafts is shown in Fig. 4D. To confirm the localization of HMGB1 and type 1 collagen, we evaluated liver specimens by immunohistochemistry. Consistent with western blotting data, the lower HMGB1 expression with higher type 1 collagen deposits were preferential for high‐SRA livers (Fig. 4E). In postreperfusion LT biopsy samples, SRA correlated positively, albeit insignificantly, with gene expression coding for activated monocyte/macrophage markers monocyte chemoattractant protein‐1 (MCP1) and CD68 (Supporting Fig. S4). Figure 4F depicts a simplified mechanistic scheme of the peritransplant hepatocellular damage in a high‐SRA liver graft.
FIG. 4.
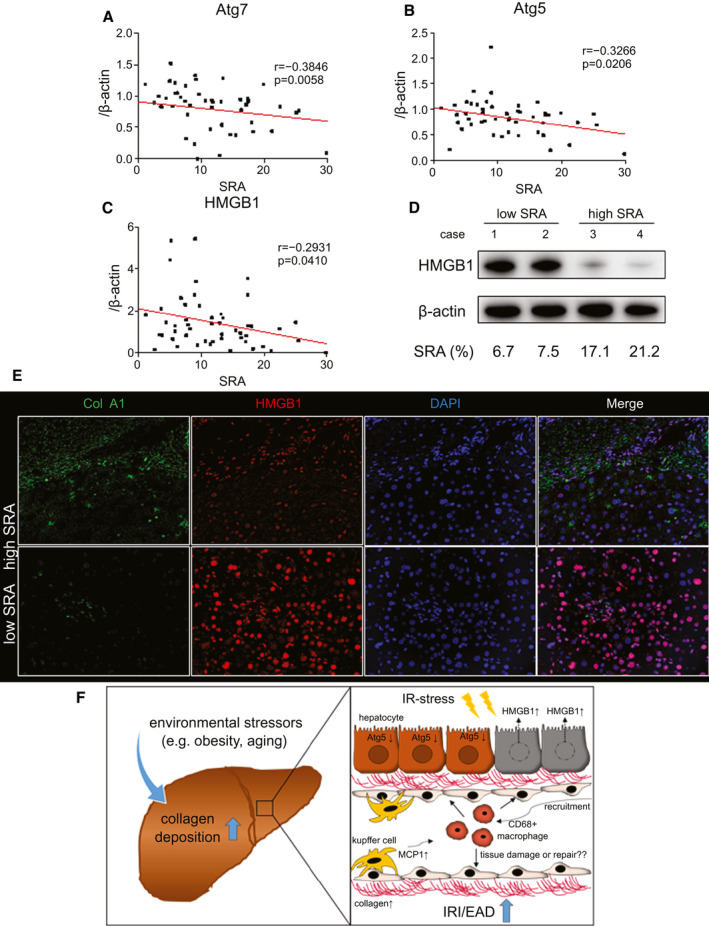
Hepatic SRA negatively correlates with Atg7, Atg5, and HMGB1 levels in human LT. Forty‐nine human LT biopsy samples were collected and analyzed at 2 hours postreperfusion. (A,B) Pretransplant hepatic SRA was correlated negatively with posttransplant western‐assisted expression of autophagy‐related proteins (Atg7, P = 0.005; Atg5, P = 0.020). (C) SRA was negatively correlated with HMGB‐1 expression (P = 0.041). (D) Representative HMGB1 expression profiles in high‐ versus low‐SRA cases (case 1/2, low SRA; case 3/4, high SRA). (E) Representative immunofluorescence staining for type 1 collagen and HMGB1 (n = 2 per group; original magnification, ×200). (F) Proposed mechanistic scheme of IR‐related hepatocellular injury in a high‐SRA donor liver. Environmental factors, such as obesity or aging, increase the intrahepatic collagen deposition. High‐SRA livers fail to induce autophagy against IR stress leading to the hepatocellular damage with enhanced HMGB1 release. IR stress in high‐SRA livers releases a higher amount of MCP1 and recruits CD68‐positive macrophages.
BARD Score Correlates With LT Damage and SRA
We next asked whether noninvasive fibrosis scores, such as BARD, FIB‐4, APRI (AST to platelet ratio index), and NAFLD‐FS, indonors correlated with LT outcomes as well as extent of graft fibrosis, evaluated by Sirius red staining. Five liver fibrotic scores at the time of procurement of 194 cases were analyzed for correlation with graft damage (IRI and EAD) and SRA. AST/ALT ratio, NAFLD‐FS, and FIB‐4 were all significantly higher in SRA ≥15% grafts when compared with the SRA <15% group (Supporting Fig. S5). However, those scores failed to show a significant correlation with the incidence of EAD and IRI, except for NAFLD‐FS in IRI (P = 0.035). The BARD score was positively correlated with EAD (P = 0.018), IRI (P = 0.010), and SRA (P = 0.008; Fig. 5A‐C). Thus, we used the BARD score as a noninvasive liver fibrotic measure to predict LT clinical outcomes. Percentages of donor BARD score in each group were as follows: low‐SRA: 0, 17.1% (n = 24); 1, 12.9% (n = 18); 2, 45.0% (n = 63); 3, 23.6% (n = 33); and 4, 1.4% (n = 2); high‐SRA: 0, 7.4% (n = 4); 1, 11.1% (n = 6); 2, 40.7% (n = 22); 3, 31.5% (n = 17); and 4, 9.3% (n = 5).
FIG. 5.
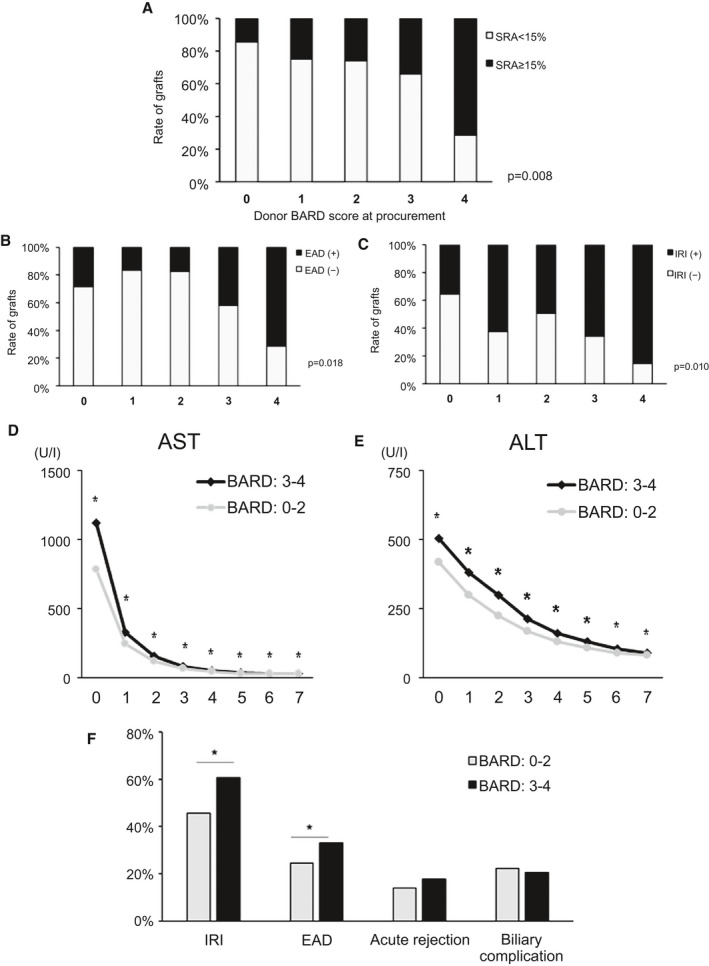
Donor BARD score correlates with LT damage. (A‐C) Under the analysis of 194 cases, donor BARD score positively correlated with SRA (P = 0.008), EAD (P = 0.018), and IRI (P = 0.010). (D,E) Nine hundred sixty‐two human donor livers were classified into BARD: 0‐2 versus BARD: 3‐4. LT recipients with donor BARD score 3‐4 had significantly higher AST/ALT levels at POD1‐7 (P < 0.05). (F) LT recipients with donor BARD score 3‐4 had significantly higher rates of mild‐severe IRI (466 cases) and EAD (P < 0.05). There were no differences in rates of acute rejection or biliary complications. *P < 0.05.
To determine the impact of the donor BARD score on clinical outcomes, we assessed 962 human LT cases and calculated the BARD score from donor parameters at the time of liver procurement. These were classified into low‐BARD (0‐2) versus high‐BARD (3‐4) groups (Table 3). The high‐BARD group had significantly older donor age (44 vs. 32 years; P < 0.001), higher BMI (30.7 vs. 24.6 kg/m2; P < 0.001), and higher rates of the following comorbidities: diabetes (24.1% vs. 3.3%; P < 0.001); hypertension (46.3% vs. 19.1%; P < 0.001); and coronary artery disease (7.8% vs. 2.6%; P < 0.001). Additionally, the high‐BARD group had significantly higher/lower rates of cerebrovascular stroke (41.9% vs. 27.5%)/anoxia (21.5% vs. 29.3%) as the cause of death (P < 0.001) and higher/lower AST (42 vs. 36 IU/L; P = 0.001)/ALT (27 vs. 33 IU/L; P < 0.001), respectively. Donor grafts characterized by a BARD score of 3‐4 had significantly higher AST/ALT levels at POD1‐7 (P < 0.05; Fig. 5D,E). Also, grafts with a high BARD score (3‐4) had significantly higher rates of mild‐to‐severe IRI (60.7% vs. 45.6%; P = 0.003, in 466 grafts with evaluable IRI) and EAD (33.0% vs. 24.8%; P = 0.008). There were no differences in acute rejection and biliary complication rates (Fig. 5F).
TABLE 3.
Demographics in 962 Human LTs With Donor BARD Score: 0‐2 versus 3‐4
| Factors | BARD: 0‐2 | BARD: 3‐4 | P Value |
|---|---|---|---|
| n = 692 | n = 270 | ||
| Recipient factors | |||
| Age (years) | 57 (18‐78) | 58 (22‐80) | 0.526 |
| Sex (female/male) | 294 (42.5)/398 (57.5) | 87 (32.2)/183 (67.8) | 0.003 |
| BMI (kg/m2) | 27.0 (13.6‐49.9) | 27.3 (16.1‐57.1) | 0.051 |
| Comorbidity | |||
| DM | 233 (33.7) | 91 (33.7) | 0.992 |
| HTN | 299 (43.2) | 113 (41.9) | 0.702 |
| CAD | 119 (17.2) | 42 (15.6) | 0.54 |
| Underlying liver disease | 0.312 | ||
| HBV | 31 (4.5) | 15 (5.6) | |
| HCV | 196 (28.3) | 87 (32.2) | |
| EtOH | 149 (21.5) | 54 (20.0) | |
| NASH | 90 (13.0) | 41 (15.2) | |
| ALF | 40 (5.8) | 14 (5.2) | |
| PBC | 19 (2.7) | 11 (4.1) | |
| Others | 167 (24.1) | 48 (17.8) | |
| Preoperative hospital stay (days) | 27 (0‐394) | 25 (1‐717) | 0.476 |
| Preoperative ICU admission | 402 (58.1) | 138 (51.1) | 0.04991 |
| Laboratory data at LT | |||
| AST (IU/L) | 65 (14‐12,606) | 64 (12‐6,917) | 0.524 |
| ALT (IU/L) | 34 (6‐6,850) | 32 (4‐6,619) | 0.756 |
| Laboratory MELD | 34.3 (6.4‐51.0) | 33.3 (6.4‐48.9) | 0.046 |
| Donor factors | |||
| Age (years) | 32 (7‐76) | 44 (11‐74) | <0.001 |
| Sex (female/male) | 263 (38.0)/429 (62.0) | 104 (38.5)/166 (61.5) | 0.883 |
| BMI (kg/m2) | 24.6 (13.4‐49.6) | 30.7 (18.9‐53.0) | <0.001 |
| Comorbidity | |||
| DM | 23 (3.3) | 65 (24.1) | <0.001 |
| HTN | 132 (19.1) | 125 (46.3) | <0.001 |
| CAD | 18 (2.6) | 21 (7.8) | <0.001 |
| Cause of death | <0.001 | ||
| Trauma | 285 (41.2) | 95 (35.2) | |
| CVS | 190 (27.5) | 113 (41.9) | |
| Anoxia | 203 (29.3) | 58 (21.5) | |
| Others | 14 (2.0) | 4 (1.5) | |
| Laboratory data at procurement | |||
| AST (IU/L) | 36 (5‐859) | 42 (7‐776) | 0.001 |
| ALT (IU/L) | 33 (5‐750) | 27 (5‐689) | <0.001 |
| DCD | 28 (4.1) | 12 (4.5) | 0.776 |
| HCV Ab positive | 12 (1.7) | 4 (1.5) | >0.999 |
| Cold ischemia time (min) | 450 (138‐909) | 454 (162‐1,252) | 0.47 |
| Warm ischemia time (min) | 47 (16‐153) | 48 (18‐138) | 0.299 |
| Large droplet steatosis ≥30% (n = 466) | 9/331 (2.7) | 4/135 (3.0) | 0.885 |
Bold represent statistical significance.
Abbreviations: ALF, acute liver failure; CAD: coronary artery disease; CVS, cerebrovascular stroke; DM, diabetes mellitus; EtOH, alcohol‐associated (ethanol) liver disease; HTN, hypertension; ICU, intensive care unit; PBC, primary biliary cholangitis.
Discussion
Our UCLA‐based retrospective study in 194 LT patients provides evidence that inapparent collagen levels in donor tissue at the time of liver procurement may influence not only allograft quality, but also clinical outcomes. These results are evident by (1) high‐SRA (≥15%) grafts experiencing significantly higher rates of IRI and EAD; (2) high SRA being identified as an independent predictive factor of EAD; (3) SRA negatively correlating with the hepatic expression of HMGB1, Atg5, and Atg7 in LT; and (4) SRA showing a positive correlation with the gene expressions of proinflammatory macrophage markers (MCP1 and CD68). These results indicate that human donor livers with early‐stage hepatic collagen deposition may be particularly susceptible to LT‐related stress and dysfunction. Consistent with the Wadhera et al. study,( 15 ) SRA was not a prognostic factor for long‐term LT survival in our series. However, it is difficult to conclude about the safety of the early‐stage fibrotic grafts because of the differences between the studies: (1) donor background: All of the donor livers except one case were diagnosed as F0 in our study, whereas their study included livers with F0‐F2, and compared F0 versus F1, F2; (2) fibrosis evaluation: They used H&E staining or trichrome staining instead of Sirius red staining as in our study; and (3) recipient background: In our single‐center study, the median MELD score was 31, that is, considerably higher than Wadhera et al.’s (25‐27) or nation‐wide MELD scores (18‐25) among 11 United Network for Organ Sharing regions (January 2015 to December 2016),( 28 ) demonstrating the higher acuity of our LT patient cohort. Indeed, we found that high‐SRA livers at the time of procurement showed increased incidence of IRI and EAD and experienced inferior short‐term survival. Also, recipients implanted with high‐SRA livers from donors aged ≥40 years experienced a higher incidence of EAD than low‐SRA grafts from donors aged ≥40 years and showed inferior short‐term LT survival. Meanwhile, the recipient group aged >60 years with high‐SRA grafts showed a higher frequency of EAD and a worse short‐term graft survival than those with low‐SRA grafts. In agreement with a previous report,( 29 ) these results suggest that younger donor livers may be safely used regardless of SRA. The high‐SRA livers may not be suitable, especially for older recipients. Thus, inapparent graft fibrosis may be safe for recipients with low acuity (e.g., younger age) while influencing clinical outcomes meaningfully when transplanted into severely ill recipients (e.g., older patients). If this is the case, histological hepatic collagen detection should be considered essential for personalized LT management in the high‐acuity patient cohort, even if the grafts are assessed as nonfibrotic by histology.
Hepatic IRI, an inevitable event during LT, is thought to cause hepatocellular damage leading to EAD in humans.( 30 , 31 ) To further understand the mechanism of how high SRA may affect graft damage, we assessed the protein expressions of HMGB1, Atg5, and Atg7 at 2 hours postreperfusion in human LT biopsy samples and found that they negatively correlated with SRA. HMGB1, a well‐characterized damage‐associated molecular pattern, has been identified as a potent proinflammatory sentinel.( 32 , 33 ) We have reported that hepatocyte Toll‐like receptor 4–dependent HMGB1 expression/release correlated with LT outcomes,( 26 ) and the disulfide‐HMGB1 redox form was instrumental in promoting sterile inflammation in IR‐stressed human LT.( 33 ) The high‐SRA group showed less intrahepatic HMGB1 expression and had higher posttransplant transaminase release, indicating that high‐SRA livers are more vulnerable to IR‐related injury and readily release HMGB1 to the extracellular space. Local innate immune cells (e.g., Kupffer cells or infiltrating macrophages) sense HMGB1 through their pattern recognition receptors and promote the inflammatory cascade. Autophagy associates not only with aging( 34 ) or liver fibrosis,( 27 ) but also with liver IRI.( 35 , 36 ) Others reported that autophagic activity declines with aging, whereas Atg5 overexpression exhibited an antiaging phenotype and extended the life span in mice.( 37 ) In 194 cases subjected to SRA analysis, donors with high‐SRA (>15%) grafts were older than those with low‐SRA, and SRA negatively correlated with Atg5/Atg7. Taken together, increased susceptibility of the high‐SRA group to IRI might be explained by the impairment of age‐related hepatic autophagy. However, the question arises of why high‐SRA grafts did not affect long‐term survival despite the higher incidence of IRI and EAD. There are some ways to reconcile this discordance. First, we and others previously reported that histological IRI severity was an indicator of short‐term hepatocellular injury after LT.( 23 ) Indeed, only severe IRI might develop EAD leading to ultimate graft failure.( 31 ) This indicates that early phases of LT‐related injury, such as IRI or EAD, may not affect long‐term outcomes if reversible or mitigated because of the advances in surgical techniques and postoperative management. Second, a recent experimental study by Konishi et al.( 38 ) demonstrated that CCL4‐induced fibrotic livers subjected to warm IRI had significantly higher serum transaminase levels as compared to normal livers, whereas fibrotic livers recovered more quickly than nonfibrotic livers after IRI. In addition, Zhao et al. documented that both in experimental and clinical settings, renal‐transplant–related IRI contributed to long‐term outcomes through the mechanisms of renal atrophy, vascular injury, and subsequent fibrosis.( 39 ) Collectively, one may envision that fibrotic human liver grafts are susceptible to IR stress, whereas some donor livers may recover more quickly without IRI sequelae, such as de novo fibrosis as compared to nonfibrotic grafts. Although some grafts may fail to recover from “irreversible” IRI, it awaits future study whether high‐SRA grafts could recover promptly from IR‐related hepatocellular damage. Konishi et al. also showed that murine fibrotic livers after IRI were accompanied by the increase of infiltrating Ly6C‐low macrophages with high phagocytosis ability. In our current study, SRA showed a positive, albeit insignificant, correlation with hepatic expression of mRNA coding for MCP1 and CD68. This indicates an increased frequency of macrophage populations in high‐SRA human livers. Future studies need to elucidate which macrophage subpopulations infiltrate high‐SRA livers, whether their phenotypes are pro‐ or anti‐inflammatory, and may undergo a functional shift to contribute to long‐term outcomes.
To the best of our knowledge, our study is the largest to demonstrate a correlation between inapparent fibrotic SRA status (n = 194) and donor BARD score (n = 962) at LT procurement. In our 962‐patient cohort, increased hepatic BARD score was accompanied by significantly higher post‐LT serum transaminase levels and higher IRI/EAD rates. The high BARD score in the donor demographics was correlated with older donor age, higher BMI, and higher rates of hypertension, diabetes, and coronary artery disease. Meanwhile, high‐SRA livers had a higher rate of diabetes as comorbidity and were from older donors, and also SRA positively correlated with the BARD score. Collectively, these findings imply that environmental factors, such as aging or diabetes, increase the inapparent collagen deposition in donor livers and the BARD score may potentially reflect donor liver quality. However, further investigations need to address whether the BARD score can substitute for SRA assessment because high SRA, rather than high BARD score, was one of the independent predictive factors of EAD. Hence, the histological SRA assessment in the donor liver seems superior to BARD score evaluation to predict LT short‐term outcomes.
In the present study, we used Sirius red with paraffin‐embedded slides as a reliable method for evaluating collagen deposition in human LT.( 40 , 41 ) This method is time‐consuming and may not be clinically suitable. However, Sirius staining was successfully used in frozen muscle tissue sections.( 42 ) Hence, we need to validate whether SRA stains in liver‐frozen and paraffin sections are equally valuable for analyses. If consistent, we believe SRA, along with macrosteatosis determination, will be one of the most promising indicators of the donor liver quality. We also anticipate clinical incorporation of SRA staining to facilitate decision making about graft usability and donor recipient matching for grafts placed on a normothermic machine, given that there would be ample time to obtain the staining results before performing the transplant. We also envision a scenario where the SRA stain findings might help guide early postoperative management and decision making in a manner similar to IRI or EAD where graft dysfunction and retransplantation may be a consideration. Although our findings need to be validated in the external cohort, it appears worthwhile to assess hidden fibrosis in donor livers as a predictive factor in developing IRI and EAD after LT. By shedding light on hidden collagen deposition in donor liver tissue, these findings provide a more refined analysis of donor organ quality and warrant in‐depth studies in animals and humans.
Author contributions
Concept/design: Hirao, Ito, Kadono, Kojima, Naini, Nakamura, Kaldas, Kupiec‐and Weglinski. Experiments/procedures: Hirao, Ito, Kadono, Kojima, Naini, Nakamura, and Kageyama. Clinical data collection/analysis: Ito, Naini, and Kaldas. Writing of article: Hirao, Ito, Kadono, Kojima, Kaldas, and Kupiec‐Weglinski. Critical review: all authors. Funding: Busuttil, Kaldas, and Kupiec‐Weglinski.
Supporting information
Supplementary Material
Acknowledgment
We thank UCLA students Stephanie Younan, Michelle Lu, and Damla Oncel for clinical data collection and assistance with histological staining.
Supported by NIH PO1 AI120944.
Potential conflict of interest: Nothing to report.
Contributor Information
Jerzy W. Kupiec‐Weglinski, Email: JKupiec@mednet.ucla.edu.
Fady M. Kaldas, Email: FKaldas@mednet.ucla.edu.
References
Author names in bold designate shared co‐first authorship.
- 1. Hudcova J, Scopa C, Rashid J, Waqas A, Ruthazer R, Schumann R. Effect of early allograft dysfunction on outcomes following liver transplantation. Clin Transplant 2017;31:e12887. [DOI] [PubMed] [Google Scholar]
- 2. Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. Liver. Am J Transplant 2016;16(Suppl. 2):69‐98. [DOI] [PubMed] [Google Scholar]
- 3. Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg 2013;258:409‐421. [DOI] [PubMed] [Google Scholar]
- 4. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010;16:943‐949. [DOI] [PubMed] [Google Scholar]
- 5. Wadei HM, Lee DD, Croome KP, Mai ML, Golan E, Brotman R, et al. Early allograft dysfunction after liver transplantation is associated with short‐ and long‐term kidney function impairment. Am J Transplant 2016;16:850‐859. [DOI] [PubMed] [Google Scholar]
- 6. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait‐listing in the era of direct‐acting antiviral therapy. Hepatology 2017;65:804‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su F, Yu L, Berry K, Liou IW, Landis CS, Rayhill SC, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post‐transplantation outcomes, and transplant‐related survival benefit. Gastroenterology 2016;150:441‐453.e6; quiz, e16. [DOI] [PubMed] [Google Scholar]
- 8. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest 2017;127:55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melin C, Miick R, Young NA, Ortiz J, Balasubramanian M. Approach to intraoperative consultation for donor liver biopsies. Arch Pathol Lab Med 2013;137:270‐274. [DOI] [PubMed] [Google Scholar]
- 10. Kelly D, Verkade HJ, Rajanayagam J, McKiernan P, Mazariegos G, Hübscher S. Late graft hepatitis and fibrosis in pediatric liver allograft recipients: current concepts and future developments. Liver Transpl 2016;22:1593‐1602. [DOI] [PubMed] [Google Scholar]
- 11. Pelletier SJ, Iezzoni JC, Crabtree TD, Hahn YS, Sawyer RG, Pruett TL. Prediction of liver allograft fibrosis after transplantation for hepatitis C virus: persistent elevation of serum transaminase levels versus necroinflammatory activity. Liver Transpl 2000;6:44‐53. [DOI] [PubMed] [Google Scholar]
- 12. Chopra KB, Demetris AJ, Blakolmer K, Dvorchik I, Laskus T, Wang LF, et al. Progression of liver fibrosis in patients with chronic hepatitis C after orthotopic liver transplantation. Transplantation 2003;76:1487‐1491. [DOI] [PubMed] [Google Scholar]
- 13. Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, et al. HCV‐related fibrosis progression following liver transplantation: increase in recent years. J Hepatol 2000;32:673‐684. [DOI] [PubMed] [Google Scholar]
- 14. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696‐699. [DOI] [PubMed] [Google Scholar]
- 15. Wadhera V, Harimoto N, Lubezky N, Gomatos I, Facciuto M, Gonzalez D, et al. The impact of donor liver allograft fibrosis on patients undergoing liver transplantation. Clin Transplant 2018;32:e13187. [DOI] [PubMed] [Google Scholar]
- 16. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20(1 Pt. 1):15‐20. [PubMed] [Google Scholar]
- 17. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44‐48. [DOI] [PubMed] [Google Scholar]
- 18. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 19. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 20. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 21. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander‐Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441‐1447. [DOI] [PubMed] [Google Scholar]
- 22. Sosa RA, Zarrinpar A, Rossetti M, Lassman CR, Naini BV, Datta N, et al. Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation. JCI Insight 2016;1:e89679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito T, Naini BV, Markovic D, Aziz A, Younan S, Lu M, et al. Ischemia‐reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am J Transplant 2021;21:614‐625. [DOI] [PubMed] [Google Scholar]
- 24. Nakamura K, Kageyama S, Ito T, Hirao H, Kadono K, Aziz A, et al. Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J Clin Invest 2019;129:3420‐3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa RA, et al. Macrophage heme oxygenase‐1‐SIRT1‐p53 axis regulates sterile inflammation in liver ischemia‐reperfusion injury. J Hepatol 2017;67:1232‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura K, Kageyama S, Kaldas FM, Hirao H, Ito T, Kadono K, et al. Hepatic CEACAM1 expression indicates donor liver quality and prevents early transplantation injury. J Clin Invest 2020;130:2689‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allaire M, Rautou PE, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol 2019;70:985‐998. [DOI] [PubMed] [Google Scholar]
- 28. Croome KP, Lee DD, Burns JM, Keaveny AP, Taner CB. Intraregional model for end‐stage liver disease score variation in liver transplantation: disparity in our own backyard. Liver Transpl 2018;24:488‐496. [DOI] [PubMed] [Google Scholar]
- 29. Feng S, Goodrich NP, Bragg‐Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783‐790. [DOI] [PubMed] [Google Scholar]
- 30. Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec‐Weglinski JW. Ischaemia‐reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ali JM, Davies SE, Brais RJ, Randle LV, Klinck JR, Allison MED, et al. Analysis of ischemia/reperfusion injury in time‐zero biopsies predicts liver allograft outcomes. Liver Transpl 2015;21:487‐499. [DOI] [PubMed] [Google Scholar]
- 32. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG‐1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248‐251. [DOI] [PubMed] [Google Scholar]
- 33. Sosa RA, Terry AQ, Kaldas FM, Jin YP, Rossetti M, Ito T, et al. Disulfide‐HMGB1 drives ischemia‐reperfusion injury in human liver transplantation. Hepatology 2021;73:1158‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbosa MC, Grosso RA, Fader CM. Hallmarks of aging: an autophagic perspective. Front Endocrinol (Lausanne) 2018;9:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Lin W, Zhuang L. CD5L‐induced activation of autophagy is associated with hepatoprotection in ischemic reperfusion injury via the CD36/ATG7 axis. Exp Ther Med 2020;19:2588‐2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han YF, Zhao YB, Li J, Li L, Li YG, Li SP, et al. Stat3‐Atg5 signal axis inducing autophagy to alleviate hepatic ischemia‐reperfusion injury. J Cell Biochem 2018;119:3440‐3450. [DOI] [PubMed] [Google Scholar]
- 37. Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 2013;4:2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Konishi T, Schuster RM, Goetzman HS, Caldwell CC, Lentsch AB. Fibrotic liver has prompt recovery after ischemia‐reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2020;318:G390‐G400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao H, Alam A, Soo AP, George AJT, Ma D. Ischemia‐reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine 2018;28:31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang YI, de Boer WB, Adams LA, MacQuillan G, Rossi E, Rigby P, et al. Image analysis of liver collagen using sirius red is more accurate and correlates better with serum fibrosis markers than trichrome. Liver Int 2013;33:1249‐1256. [DOI] [PubMed] [Google Scholar]
- 41. Cabibi D, Bronte F, Porcasi R, Ingrao S, Giannone AG, Maida M, et al. Comparison of histochemical stainings in evaluation of liver fibrosis and correlation with transient elastography in chronic hepatitis. Anal Cell Pathol (Amst) 2015;2015:431750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar A, Yamauchi J, Girgenrath T, Girgenrath M. Muscle‐specific expression of insulin‐like growth factor 1 improves outcome in Lama2Dy‐w mice, a model for congenital muscular dystrophy type 1A. Hum Mol Genet 2011;20:2333‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


