Abstract
Extranodal natural killer/T‐cell lymphoma, nasal‐type (ENKTL) is a distinct subtype of non‐Hodgkin lymphoma and most of the patients presented localized disease. Combined modality therapy (CMT), namely chemotherapy combined with radiotherapy, has been recommended for patients with early‐stage ENKTL. However, the optimal CMT has not been fully clarified. This study reports the efficacy and toxicity of sequential P‐GEMOX (pegaspargase, gemcitabine and oxaliplatin) and radiotherapy in a large Chinese cohort comprising of 202 patients diagnosed with early‐stage ENKTL from six medical centers. The observed best overall response rate was 96.0% and 168 (83.2%) patients achieved complete remission. With a median follow‐up of 44.1 months, the 3‐year progression‐free survival (PFS) and overall survival (OS) were 74.6% and 85.2%, respectively. Multivariate analysis suggested that extensive primary tumor (PFS, hazard ratio [HR] 3.660, 95% CI 1.820–7.359, p < 0.001; OS, HR 3.825, 95% CI 1.442–10.148, p = 0.007) and Eastern Cooperative Oncology Group performance status ≥ 2 (PFS, 3.042, 95% CI 1.468–6.306, p = 0.003; OS, HR 3.983, 95% CI 1.678–9.457, p = 0.02) were independent prognostic factors for survival outcomes. Among the established prognostic models for ENKTL, the nomogram‐revised risk index model had optimal prognostic risk stratification ability (PFS, p < 0.001; OS, p < 0.001) and relatively balanced population distribution. The adverse events of this CMT were well‐tolerated and manageable. In conclusion, sequential P‐GEMOX and radiotherapy showed favorable efficacy with acceptable toxicity, and could be an effective treatment option for early‐stage ENKTL patients.
1. INTRODUCTION
Extranodal natural killer/T‐cell lymphoma, nasal‐type (ENKTL) is an aggressive extranodal non‐Hodgkin lymphoma and is most commonly diagnosed in East Asia and South America. 1 , 2 Among the patients with newly‐diagnosed ENKTL, over 70% present with early‐stage disease involving the upper aerodigestive tract (UADT) such as nasopharynx, nasal cavity, and the Waldeyer's ring. 3
Radiotherapy is an important modality for successful treatment of localized ENKTL, however, a large number of patients still experience recurrence after radiotherapy alone, suggesting that the current treatment modality is insufficient and more efficacious treatment strategies should be explored. 4 , 5 , 6 , 7 Thus, combined modality therapy (CMT), namely chemotherapy combined with radiotherapy, has been recommended for patients with localized ENTKL and fit for chemotherapy. 8 So, CMT protocols include concurrent chemoradiotherapy (CCRT), sequential chemoradiotherapy and sandwich chemoradiotherapy. 9 , 10 Currently, no prospective, randomized controlled studies directly comparing various CMT protocols have been reported. A recent international multicenter study revealed comparable clinical outcomes between sequential chemoradiotherapy and CCRT in the era of non‐anthracycline chemotherapy. 11 Moreover, several studies have confirmed the efficacy of sequential asparaginase‐based or pegaspargase‐based chemotherapy and radiotherapy for early‐stage patients. 12 , 13 , 14 As chemotherapy is more easily accessible than immediate radiotherapy in the routine clinical practice, the “chemotherapy‐first” CMT deserves further exploration.
Previous studies showed that high expression of P‐glycoprotein in ENKTL cells could lead to chemo‐resistance, while asparaginase could produce anti‐tumor effect via unique mechanisms that overcome the resistance. 15 , 16 , 17 , 18 L‐asparaginase or pegaspargase‐based chemotherapy regimens have shown promising efficacy in ENKTL. 19 , 20 , 21 , 22 , 23 , 24 The P‐GEMOX (pegaspargase, gemcitabine and oxaliplatin) demonstrated comparable efficacy with moderate toxicities. 22 , 23 , 24 In a real‐world retrospective study from China, the P‐GEMOX regimen yielded an objective response rate (ORR) of 89.4% and complete remission (CR) rate of 62.4% in newly‐diagnosed ENKTL patients. 23 In a randomized, phase II clinical trial comparing P‐GEMOX plus thalidomide regimen and AspaMetDex (pegaspargase, methotrexate, and dexamethasone) regimen for ENKTL, the two regimens had similar response rate and long‐term survival outcomes while P‐GEMOX had better tolerability. 24 Therefore, the P‐GEMOX regimen was shown to be a safe and effective regimen for ENKTL.
Considering that the optimal CMT for early‐stage ENKTL and the effectiveness of the P‐GEMOX regimen remain obscured, efforts should be made to provide additional evidence on this issue. In this study, we performed a retrospective analysis using a large‐scale, multicenter cohort comprising of newly‐diagnosed early‐stage ENKTL patients to evaluate the efficacy and toxicity of sequential P‐GEMOX and radiotherapy in this population.
2. METHODS
2.1. Patients
The data of adult patients diagnosed with early‐stage ENKTL between January 2010 and December 2019 were retrieved. Eligibility criteria included: (1) patients pathologically diagnosed with ENKTL based on the World Health Organization classification of lymphoma; (2) aged ≥ 18 years; (3) classified as stage I–II according to the Ann Arbor staging system, and; (4) received P‐GEMOX chemotherapy regimen followed by radiotherapy. The participating medical centers included (1) the Sun Yat‐sen University Cancer Center; (2) Fujian Provincial Cancer Hospital & Institute; (3) Peking University Third Hospital; (4) Jiangxi Cancer Hospital; (5) First Affiliated Hospital of Guangxi Medical University, and; (6) Second Affiliated Hospital of Suzhou University.
This study was conducted in compliance with the Declaration of Helsinki and was approved by the institutional review board. Written informed consent was given by all the included participants.
2.2. Treatment
The P‐GEMOX regimen was administrated as follows: pegaspargase 2000 IU/m2 intramuscular on day 1, gemcitabine 1000 mg/m2 intravenous on day 1 and 8, oxaliplatin 130 mg/m2 intravenous on day 1 and was repeated every 21 days. Patients who achieved at least stable disease (SD) subsequently underwent radiotherapy. Patients with progressive disease to P‐GEMOX chemotherapy were given salvage treatments. The number of P‐GEMOX chemotherapy cycles offered to each patient was according to their baseline characteristics, responses to P‐GEMOX and tolerability, and were at the discretion of the treating physicians. Involved site radiotherapy was performed following the guidelines of the International Lymphoma Radiation Oncology Group. 25 Patients in this study received intensity‐modulated radiation therapy (IMRT) or volumetric‐modulated arc radiotherapy (VMAT). The gross target volume covered the primary tumor and involved lymph nodes defined by imaging examinations before chemotherapy. The clinical target volume covered the involved primary tumor sites and adjacent structures.
2.3. Definitions and outcomes
All baseline characteristics were evaluated before initial treatment. Extensive primary tumor was defined as a primary lesion beyond the nasal cavity which extended to adjacent structures; a modification based on the study of Li et al. 7 to assess the condition of the local tumor. Regional lymph node involvement was defined as N1–N3 according to the TNM staging system. Circulating Epstein–Barr virus (EBV)‐DNA in the peripheral plasma was detected by quantitative polymerase chain reaction and any detectable EBV‐DNA load was defined as positive. B symptoms were defined as unexplained weight loss of > 10% within the 6 months before diagnosis, unexplained fever with temperature above 38°C, and night sweats. UADT involvement was defined as the tumor involvement of nasal cavity, nasopharynx, paranasal sinuses, oral cavity, oropharynx, and the Waldeyer's ring. Elevated lactate dehydrogenase (LDH) was defined as serum LDH > 245 U/L. Several widely‐used prognostic systems, including the international prognostic index (IPI), the Korean prognostic index (KPI), the prognostic index of natural killer lymphoma (PINK), the PINK model with circulating EBV‐DNA (PINK‐E) and the nomogram‐revised risk index (NRI) were implemented to evaluate the prognosis of the early‐stage ENKTL patients who received sequential P‐GEMOX and radiotherapy in this study and to compare their clinical utility. 26 , 27 , 28 , 29
Treatment response was evaluated after every two cycles of P‐GEMOX and after radiotherapy. The proportion of patients who achieved an objective response, CR, partial remission (PR), SD or PD was assessed using positron emission tomography/computed tomography, contrast‐enhanced computed tomography or contrast‐enhanced magnetic resonance imaging, according to the 2014 Lugano criteria. 30 Progression‐free survival (PFS) was calculated from the time of initial diagnosis of ENKTL to disease progression, relapse, death or until the last follow‐up. Overall survival (OS) was calculated from the time of initial diagnosis of ENKTL to death from any cause or until the last follow‐up. Event‐free survival (EFS) was calculated from the time of initial diagnosis of ENKTL to disease progression, relapse, discontinuation of therapy due to toxicity, death or until the last follow‐up. Time to first response was calculated from the time of treatment initiation of P‐GEMOX to first objective response. Duration of response (DOR) was calculated from the time of first objective response to disease progression, relapse, death or until the last follow‐up. Time to next therapy was calculated from the time of treatment initiation of P‐GEMOX to the start of next line therapy, death or until the last follow‐up. Adverse events were assessed based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
2.4. Statistical analysis
Both PFS and OS were analyzed using the Kaplan–Meier method and compared using the log‐rank test. The following clinical parameters were estimated for prognostic performance by univariate analysis: age, gender, Ann Arbor stage, Eastern Cooperative Oncology Group performance status (ECOG‐PS), B symptoms, extensive primary tumor, UADT involvement, circulating EBV‐DNA and LDH. All parameters with a p < 0.10 in univariate analysis were included in multivariate analysis using stepwise forward Cox regression (for survival outcome events) or Logistic regression (for response events) model. Categorical data were compared using the chi‐square test or Fisher exact test. For all statistical tests, two‐tailed p value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 25.0 software.
3. RESULTS
3.1. Baseline characteristics
Between January 2010 and December 2019, a total of 202 patients with newly diagnosed early‐stage ENKTL at six medical centers in China were included in this study. Their median age was 44.0 years (range, 18–73 years). The patient distributions of the IPI, PINK, and PINK‐E model were unbalanced with most patients classified into a low‐risk group, while the distributions of the KPI and NRI model were relatively more symmetrical. The baseline characteristics of the patients at initial diagnosis are summarized in Table 1.
TABLE 1.
Baseline characteristics of early‐stage ENKTL patients in this study (N = 202)
| Characteristic | Number (%) |
|---|---|
| Age | |
| Median (range) | 44.0 (18–73) |
| ≤60 years | 176 (87.1%) |
| >60 years | 26 (12.9%) |
| Sex | |
| Male | 140 (69.3%) |
| Female | 62 (30.7%) |
| Ann Arbor stage | |
| I | 112(55.4%) |
| II | 90 (44.6%) |
| ECOG‐PS | |
| 0–1 | 188 (93.1%) |
| ≥2 | 14 (6.9%) |
| B symptoms | |
| No | 121 (59.9%) |
| Yes | 81 (40.1%) |
| Extensive primary tumor | |
| No | 89 (44.1%) |
| Yes | 113 (55.9%) |
| Regional lymph node involvement | |
| No | 113 (55.9%) |
| Yes | 89 (44.1%) |
| UADT involvement | |
| No | 7 (3.5%) |
| Yes | 195 (96.5%) |
| Circulating EBV‐DNA | |
| Negative | 67 (33.2%) |
| Positive | 117 (57.9%) |
| Unknown or missing | 18 (8.9%) |
| Elevated LDH | |
| No | 147 (72.8%) |
| Yes | 55 (27.2%) |
| IPI | |
| Low‐risk | 186 (92.1%) |
| Intermediate‐low‐risk | 16 (7.9%) |
| Intermediate‐high‐risk | 0 (0.0%) |
| High‐risk | 0 (0.0%) |
| KPI | |
| Group 1 | 56 (27.7%) |
| Group 2 | 80 (39.6%) |
| Group 3 | 53 (26.2%) |
| Group 4 | 13 (6.4%) |
| PINK | |
| Low‐risk | 170 (84.2%) |
| Intermediate‐risk | 31 (15.3%) |
| High‐risk | 1 (0.5%) |
| PINK‐E | |
| Low‐risk | 162 (80.2%) |
| Intermediate‐risk | 21 (10.4%) |
| High‐risk | 1 (0.5%) |
| Unknown | 18 (8.9%) |
| NRI | |
| Low‐risk | 41 (20.3%) |
| Intermediate‐low‐risk | 75 (37.1%) |
| Intermediate‐high‐risk | 41 (20.3%) |
| High risk | 45 (22.3%) |
Note: Data are shown as number (%) or median (range). The sum of some percentages may not equal 100% because of rounding. EBV, Epstein–Barr virus; ECOG‐PS, Eastern Cooperative Oncology Group performance status; ENKTL, extranodal natural killer/T‐cell lymphoma, nasal‐type; IPI, international prognostic index; KPI, Korean prognostic index; LDH, lactate dehydrogenase; NRI, nomogram‐revised risk index; PINK, prognostic index of natural killer lymphoma; PINK‐E, PINK model with circulating EBV‐DNA; UADT, upper aerodigestive tract.
3.2. Treatment and response
In the study population, the median number of P‐GEMOX cycles was four (range, 2–6). Except for seven patients who had progressive disease (PD) after chemotherapy, a total of 195 patients received radiation therapy. Among them, the percentages of patients who underwent IMRT and VMAT were 91.8% and 8.2%, respectively. The median radiation dose was 54.6 Gy (interquartile range [IQR], 50.4–54.6 Gy). None of our patients underwent autologous stem cell transplantation after sequential P‐GEMOX and radiotherapy.
After P‐GEMOX induction chemotherapy, the ORR for all patients was 94.1%, with 63.9% of patients achieving CR and 30.2% partial remission (PR). Seven patients had PD and were subjected to second‐line therapy. For the 61 patients who had PR after chemotherapy, 65.6% (40/61) achieved CR after radiotherapy. Among the five patients who had SD after chemotherapy, four of them received four cycles of P‐GEMOX and one patient received six cycles, subsequently three patients attained PR after radiotherapy. Overall, the best ORR after P‐GEMOX chemotherapy followed by radiotherapy was 96.0%, with a CR rate of 83.2%. The response rates are summarized in Table S1.
In subgroup analysis, a statistical significantly lower CR rate to P‐GEMOX chemotherapy was observed in patients with extensive primary tumor (p = .035) (Table S2). We then included extensive primary tumor (p = 0.035), ECOG‐PS (p = 0.090) and circulating EBV‐DNA (p = 0.054) in the Logistic regression model to identify independent prognostic factors that can accurately predict response. However, none of the factors were independently associated with a higher CR rate to P‐GEMOX chemotherapy (Table S2).
3.3. Survival outcomes and prognostic factors
With a median follow‐up of 44.1 months (95% confidence interval [CI], 37.8–50.4 months), the median PFS and OS were not reached. The 3‐year PFS and OS rates for the entire cohort were 74.6% and 85.2%, and the estimated 5‐year PFS and OS rates were 65.9% and 80.5%, respectively (Figure 1A,B). The median EFS was not reached, with 3‐year and estimated 5‐year EFS rates of 74.7% and 66.5% (Figure S1A). The median time to 1st response was 1.47 months (95% CI, 1.41–1.53 months) (Figure S1B). The median DOR was not reached, and the 3‐year DOR was 77.0% (Figure S1C).
FIGURE 1.
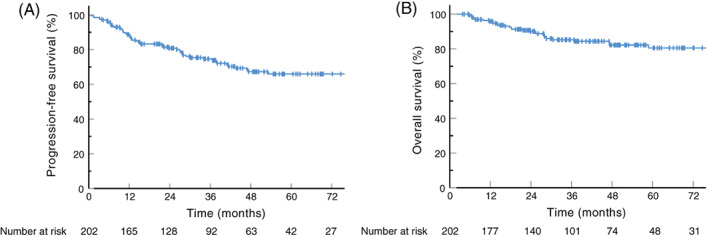
Survival curves of the investigated 202 patients with early‐stage ENKTL. (A) Progression‐free survival of the entire cohort. (B) Overall survival of the entire cohort. ENKTL, extranodal natural killer/T‐cell lymphoma, nasal‐type [Color figure can be viewed at wileyonlinelibrary.com]
In the univariate analysis for all patients, PFS was found significantly associated with ECOG‐PS (p < 0.001), extensive primary tumor (p < 0.001) and circulating EBV‐DNA (p = .025) (Table S3). We further used the information of 184 patients with available EBV‐DNA data in multivariate analysis. In multivariate Cox regression model, ECOG‐PS (HR 3.042, 95%CI 1.468–6.306, p = 0.003) and extensive primary tumor (HR 3.660, 95%CI 1.820–7.359, p < 0.001) were the independent prognostic factors for PFS (Table S4). The OS was significantly associated with age (p = 0.008), ECOG‐PS (p < 0.001), extensive primary tumor (p = 0.001) and circulating EBV‐DNA (p = 0.022) in univariate analysis (Table S3). These four clinical parameters were included in multivariate analysis of the 184 patients who had available circulating EBV‐DNA records. Finally, extensive primary tumor (HR 3.825, 95%CI 1.442–10.148, p = 0.007) and ECOG‐PS (HR 3.983, 95%CI 1.678–9.457, p = 0.002) were found independently associated with OS (Table S4).
The median time to next therapy was not reached, and the 3‐year rate was 74.5% (Figure S2A). Among the 53 patients experienced PD, the information on the salvage regimens of 43 patients were collected. The salvage regimens included asparaginase‐containing chemotherapy (N = 13), non‐asparaginase based chemotherapy (N = 5), programmed death protein‐1 (PD‐1)/chidamide‐containing regimens (N = 21), and other regimens (N = 4) (included programmed death 1 ligand 1 inhibitor, mitoxantrone, and selinexor). No significant differences were observed in OS among the four groups (p = 0.415) (Figure S2B).
3.4. Prognostic value of established model
We compared the prognostic significance of IPI, KPI, PINK, PINK‐E, and NRI to select the optimal prognostic prediction model for early‐stage ENKTL patients receiving sequential P‐GEMOX and radiotherapy.
The IPI model demonstrated satisfactory PFS (p = 0.005) and OS (p < 0.001) differences between the low‐risk and intermediate‐low‐risk group, however, 92.1% of all patients were classified into the low‐risk group (Figure 2A,B). For the KPI model, distinction in survival outcomes was observed between groups, but the severity of the prognostic risk was not consistent with the grading of the index stratification: group two had an obvious trend for longer PFS than group one (p = 0.129), and no statistical survival differences were detected between group one and group four (PFS, p = 0.389; OS, p = 0.271) (Figure 2C,D). The difference of OS (p = 0.012) was observed between the low‐risk and intermediate‐risk PINK group, but no statistically significant difference was observed in PFS in these two groups (p = 0.201) (Figure 2E,F). The prognosis of the 184 patients with available circulating EBV‐DNA data at initial diagnosis was evaluated for PINK‐E model, and the low‐risk group presented a trend for better OS, but no statistically significant differences were detected in PFS analysis (p = 0.478) or OS (p = 0.057) (Figure 2G,H). Also of concern is the unbalanced population distribution of the three models, with more than 80% of patients classified as low‐risk.
FIGURE 2.
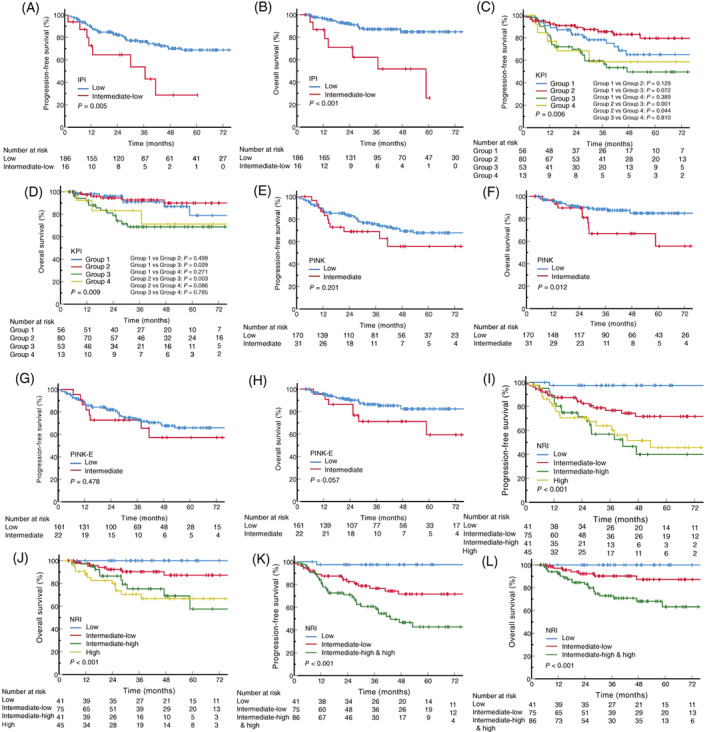
Survival curves stratified by different prognostic models. (A,B) PFS and OS stratified by the IPI model. (C,D) PFS and OS stratified by the KPI model. (E,F) PFS and OS stratified by the PINK model. The one high‐risk patient was excluded from survival analysis. (G,H) PFS and OS stratified by the PINK‐E model. The 184 patients with EBV‐DNA data at initial diagnosis were included, and the one high‐risk patient was excluded from survival analysis. (I,J) PFS and OS stratified by the NRI model. (K,L) PFS and OS stratified by the NRI model after the combination of intermediate‐high‐risk group and high‐risk group. Log‐rank p values are shown. EBV, Epstein–Barr virus; IPI, international prognostic index; KPI, Korean prognostic index; NRI, nomogram‐revised risk index; OS, overall survival; PFS, progression‐free survival; PINK, prognostic index of natural killer lymphoma; PINK‐E, PINK model with circulating EBV‐DNA [Color figure can be viewed at wileyonlinelibrary.com]
For the NRI model, its corresponding PFS (p < 0.001) and OS (p = 0.001) differences among different risk groups are shown in Figure 2I,J. Statistically significant differences were observed in the low‐risk versus intermediate‐low‐risk group (PFS, p = 0.003; OS, p = 0.031) and the intermediate‐low‐risk versus intermediate‐high‐risk group (PFS, p = 0.014; OS, p = 0.027). No survival differences were detected between the intermediate‐high‐risk and the high‐risk group (PFS, p = 0.797; OS, p = 0.673). Thus, the patients in the intermediate‐high and the high‐risk group were combined for analysis. After combination, significant discriminations were also shown between intermediate‐low‐risk group and intermediate‐high−/high‐risk group (PFS, p = 0.007; OS, p = 0.007) (Figure 2K,L). The overall p values were < 0.001 for the three risk groups and the number of patients were 41 (20.3%), 75 (37.1%) and 86 (44.6%), respectively.
3.5. Toxicity
Treatment‐emergent adverse events (TEAEs) of the patients are summarized in Table 2. During the treatment, 99.0% of the patients (200/202) experienced TEAEs and most of the TEAEs were grade 1–2. Grade 3–4 TEAEs with incidence rate > 10% were hematological TEAEs: neutropenia (24.8%) and thrombocytopenia (15.3%). As for non‐hematological TEAEs, the incidence of all grades elevated aminotransferase, hyperbilirubinemia and hypofibrinogenemia were 65.8%, 33.7% and 58.4%, respectively, but mostly grade 1–2. Four patients experienced grade 1–2 pancreatitis during chemotherapy. Among the 195 patients who underwent radiotherapy, mucositis/stomatitis occurred frequently with 12.8% of patients experienced grade 3–4 mucositis/stomatitis.
TABLE 2.
Treatment‐emergent adverse events
| All grades | Grade 1–2 | Grade 3–4 | |
|---|---|---|---|
| Hematological adverse events | |||
| Neutropenia | 158 (78.2%) | 108 (53.5%) | 50 (24.8%) |
| Thrombocytopenia | 104 (51.5%) | 73 (36.1%) | 31 (15.3%) |
| Anemia | 123 (60.9%) | 116 (57.4%) | 7 (3.5%) |
| Non‐hematological adverse events | |||
| Nausea or vomiting | 71 (35.1%) | 65 (32.2%) | 6 (3.0%) |
| Elevated aminotransferase | 133 (65.8%) | 126 (62.4%) | 7 (3.5%) |
| Hyperbilirubinemia | 68 (33.7%) | 64 (31.7%) | 4 (2.0%) |
| Hypofibrinogenemia | 118 (58.4%) | 100 (49.5%) | 18 (8.9%) |
| Pancreatitis | 4 (2.0%) | 4 (2.0%) | 0 (0.0%) |
| Mucositis/stomatitis | 176 (90.3%) | 151 (77.4%) | 25 (12.8%) |
Note: Data are shown as number (%). Mucositis/stomatitis were analyzed in 195 patients who received radiotherapy.
The vast majority of TEAEs were manageable and could be resolved by corresponding interventions and proper postponement of chemotherapy. Only four patients discontinued P‐GEMOX chemotherapy because of severe toxicity (grade 4 neutropenia, N = 2; grade 3 vomiting, N = 1; and grade 3 elevated aminotransferase, N = 1) and began radiotherapy according to the physicians’ instructions. The number of P‐GEMOX chemotherapy cycles received by the two patients who discontinued due to neutropenia were two and four, and they achieved CR and PR before discontinuation, respectively. Another patient discontinued therapy due to vomiting after three cycles obtained CR before discontinuation. Lastly, the one patient discontinued therapy due to elevated aminotransferase after four cycles obtained SD before discontinuation. No treatment‐related mortality or new safety signal was observed.
4. DISCUSSION
In this present study, we retrospectively evaluated the efficacy and toxicity of sequential P‐GEMOX and radiotherapy for the treatment of patients with early‐stage ENKTL. Our findings suggested that this CMT showed promising results in both short‐term responses and survival outcomes with moderate AEs. In the analysis of prognostic factors for survival, extensive primary tumor and ECOG‐PS were important clinical parameters correlated with PFS and OS. Further evaluation showed that the NRI model had optimal prognostic efficacy with balanced patient distribution.
To our knowledge, this study reported the largest cohort of early‐stage ENKTL patients treated with a specific chemotherapy regimen and radiotherapy. We found that the treatment of sequential P‐GEMOX and radiotherapy achieved an ORR of 96.0% and 83.2% of patients achieving CR. The 3‐year PFS and OS were 74.6% and 85.2%, respectively. The response rates and long‐term survival outcomes were comparable to prior studies for early‐stage ENKTL, including treatments of sequential chemoradiotherapy, CCRT and sandwich chemoradiotherapy. 13 , 22 , 23 , 24 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Additionally, the toxicity of sequential P‐GEMOX and radiotherapy was acceptable and manageable. Our study presented with a similar safety profile compared to CMT based on the P‐GEMOX regimen or other pegaspargase‐containing regimen reported in previous studies for early‐stage ENKTL. 13 , 22 , 23 , 24 , 34 , 35 , 36 , 37 , 38 Compared to CCRT with platinum‐based chemotherapy in clinical trials, relatively lower incidence of severe neutropenia/leukopenia in patients receiving sequential P‐GEMOX and radiotherapy were observed. 31 , 32 , 33 When compared to other recommended pegaspargase‐based regimen, P‐GEMOX showed lower toxicity than AspaMetDex, SMILE (dexamethasone, methotrexate, ifosfamide, pegaspargase, and etoposide) and DDGP (dexamethasone, cisplatin, gemcitabine, pegaspargase) regimen, but the data of those regimens were mainly based on advanced stage or r/r ENKTL patients. 20 , 21 , 24 The summary of efficacy and toxicity data reported in previous studies is presented in Tables S5, S6. The promising effectiveness and moderate toxicity of sequential P‐GEMOX and radiotherapy supports the use of this CMT in localized ENKTL patients.
Sequential chemo radiotherapy has several advantages over CCRT. First, some of the newly diagnosed ENKTL patients present with systemic symptoms such as hyperpyrexia. Chemotherapy is a rapid and effective therapeutic intervention to quickly relieve these symptoms. Second, chemotherapy is more easily accessible than RT, especially in countries, including China, with uneven distribution of medical resources. Third, mucositis and stomatitis caused by RT can negatively affect the patient's nutritional status and limit the dose of chemotherapeutic drugs to be administered safely.
In our study, seven patients experienced PD in the process of P‐GEMOX chemotherapy and approximately one‐quarter of patients relapsed within 3 years. There is still room for improvement in PFS for early‐stage ENKTL patients. In recent years, programmed death protein‐1 (PD‐1) blockade has shown great potential in the treatment of refractory/relapsed ENKTL. 40 , 41 , 42 In a previous study, we demonstrated preliminary efficacy of the combined PD‐1 inhibitor and P‐GEMOX regimen for advanced ENKTL, which achieved an ORR of 88.9% (77.8% CR) with manageable toxicities. 43 Based on these findings, we have initiated a multicenter, phase 3, randomized clinical trial to compare the efficacy of sequential P‐GEMOX and radiotherapy with or without PD‐1 inhibitor in newly diagnosed early‐stage ENKTL patients (ClinicalTrials.gov identifier: NCT04365036).
The invasiveness of primary tumor has been proved to be an important indicator to predict long‐term survival or guide treatment for ENKTL in several studies. In 2005, Kim et al. showed that local tumor invasiveness (LTI) could be regarded as a vital prognostic factor for responses and survival outcomes. 44 Yang et al. constructed a new prognostic nomogram for ENKTL with primary tumor invasion (PTI) included and explored its efficiency in guiding treatment for early‐stage patients. 45 , 46 Recently, this prognostic system was further validated in non‐anthracycline chemotherapy era. 27 We used the definition of extensive primary tumor modified from the study of Li et al. to evaluate primary tumor in our study. 7 Multivariate analysis revealed that extensive primary tumor was one of the two independent prognostic factors for both PFS and OS, which was consistent with previous studies. Imaging evaluation of the primary tumor should be one of the routine examinations in early diagnosis, and it might be one of the stratification factors for treatment decision‐making for ENKTL patients in the future.
Circulating EBV‐DNA is derived from the apoptosis and necrosis of tumor cells in ENKTL patients, and has been observed to have important predictive value in ENKTL patients. 28 , 47 , 48 , 49 However, interestingly, circulating EBV‐DNA was not an independent prognostic factor for survival outcomes in our study. As a potential factor reflecting tumor burden, circulating EBV‐DNA might largely overlap with extensive primary tumor for early‐stage patients who had localized disease. Furthermore, nearly half of the data in our study was lack of actual numerical value of circulating EBV‐DNA, and were recorded only as dichotic variables. Thus, we only divided circulating EBV‐DNA into detected or undetected group, which could not ideally reflect the viral load of the patients. Future studies are warranted to further clarify the optimal cut‐off value of EBV‐DNA.
Currently, the optimal prognostic model for ENKTL remains undefined. Several studies have constructed new prognostic models that presented with better predictive efficiency than the traditional IPI score. 27 , 28 , 29 However, the construction of these models was based on patients with all stages of the disease, and the prognostic value for early‐stage ENKTL needs to be further validated. The IPI was first proposed for patients with aggressive non‐Hodgkin's lymphoma. 26 Thus, it might not apply for early‐stage ENKTL, which was a distinct subtype of non‐Hodgkin lymphoma presenting with localized disease. KPI, PINK and PINK‐E contain risk factors that are not applicable or barely applicable for early‐stage patients. These reasons could explain why those models did not apply for the patients in our study. 27 , 28 , 29 Those models might perform well for advanced‐stage ENKTL patients. In comparison, the NRI model showed optimal patient distribution and predictive ability for prognosis. The primary tumor invasiveness and ECOG‐PS were important risk‐stratified factors in our study. Besides, the other three unfavorable risk factors (age >60 years, stage II and elevated LDH) also showed a trend for the distinction of survival. Based on the findings of our study, the NRI model is preferred for early‐stage patients treated with sequential P‐GEMOX and radiotherapy, however, verification is still needed for patients receiving other CMT.
In addition to prognostic models containing clinical features, the molecular cytogenetic characterization of ENKTL has been investigated to establish molecular models. Xiong et al. reported three molecular subtypes of ENKTL based on genomic alternations that have potential value in guiding targeting therapy. 50 Recently, our team identified a seven‐single‐nucleotide polymorphism‐based signature that could predict the survival outcomes of ENKTL patients and might help guide clinical decision‐making. 51 Future studies focusing on genetic features of ENKTL could improve the accuracy of prognostic prediction and help to obtain better understandings of this disease.
There were several limitations in our study. First, the retrospective nature of this study might introduce selection bias. Second, the number of patients in several subgroups such as ECOG‐PS ≥ 2 and non‐UADT involvement, was relatively small and might have affected the results of prognostic analysis. Third, the study population was from China, which could only represent the features of ENKTL in this endemic region. Further studies are needed to validate the effectiveness and safety profile of applying sequential P‐GEMOX and radiotherapy in larger sample size and multiple patient populations.
In conclusion, this multicenter retrospective study illustrated that sequential P‐GEMOX chemotherapy and radiotherapy was associated with promising efficacy and manageable toxicity, and could be considered as a valid treatment option for patients with early‐stage ENKTL.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
PATIENT CONSENT
Written informed consent was given by all the included participants.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
This work was supported by grants from the Special Support Program of Sun Yat‐sen University Cancer Center (PT19020401), the Science and Technology Planning Project of Guangzhou, China (202002030205), and the Clinical Oncology Foundation of Chinese Society of Clinical Oncology (Y‐XD2019‐124).
Zhang Y, Ma S, Cai J, et al. Sequential P‐GEMOX and radiotherapy for early‐stage extranodal natural killer/T‐cell lymphoma: A multicenter study. Am J Hematol. 2021;96(11):1481-1490. doi: 10.1002/ajh.26335
Yuchen Zhang, Shuyun Ma, Jun Cai, Yu Yang, and Hongmei Jing contributed equally.
Funding information Chinese Society of Clinical Oncology, Grant/Award Number: Y‐XD2019‐124; Guangdong Science and Technology Department, Grant/Award Number: 202002030205; Sun Yat‐sen University Cancer Center, Grant/Award Number: PT19020401
Contributor Information
Huiqiang Huang, Email: huanghq@sysucc.org.cn.
Qingqing Cai, Email: caiqq@sysucc.org.cn.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Liu W, Liu J, Song Y, et al. Mortality of lymphoma and myeloma in China, 2004‐2017: an observational study. J Hematol Oncol. 2019;12(1):22. 10.1186/s13045-019-0706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai Q, Cai J, Fang Y, Young KH. Epstein‐Barr virus‐positive natural killer/T‐cell lymphoma. Front Oncol. 2019;9:386. 10.3389/fonc.2019.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim TM, Lee SY, Jeon YK, et al. Clinical heterogeneity of extranodal NK/T‐cell lymphoma, nasal type: a national survey of the Korean cancer study group. Ann Oncol. 2008;19(8):1477‐1484. 10.1093/annonc/mdn147 [DOI] [PubMed] [Google Scholar]
- 4. Tian XP, Cai J, Ma SY, et al. BRD2 induces drug resistance through activation of the RasGRP1/Ras/ERK signaling pathway in adult T‐cell lymphoblastic lymphoma. Cancer Commun. 2020;40(6):245‐259. 10.1002/cac2.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheung MM, Chan JK, Lau WH, Ngan RK, Foo WW. Early stage nasal NK/T‐cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 2002;54(1):182‐190. 10.1016/s0360-3016(02)02916-4 [DOI] [PubMed] [Google Scholar]
- 6. Young KH. Intensive chemotherapy and sequential hematopoietic stem cell transplantation: is it necessary for high‐risk T‐cell lymphoblastic lymphoma? Cancer Commun. 2021;41(3):273‐274. 10.1002/cac2.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y‐X, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T‐cell lymphoma. J Clin Oncol. 2006;24(1):181‐189. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network . Guidelines: T‐Cell Lymphomas. Version 1.2021 ‐ October 5, 2020. [DOI] [PubMed]
- 9. Kim SJ, Yoon SE, Kim WS. Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review. J Hematol Oncol. 2018;11(1):140. 10.1186/s13045-018-0687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T‐cell lymphoma, nasal type. Blood. 2018;131(23):2528‐2540. 10.1182/blood-2017-12-791418 [DOI] [PubMed] [Google Scholar]
- 11. Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T‐cell lymphoma. Ann Oncol. 2018;29(1):256‐263. 10.1093/annonc/mdx684 [DOI] [PubMed] [Google Scholar]
- 12. Lunning M, Pamer E, Maragulia J, et al. Modified SMILE (mSMILE) is active in the treatment of Extranodal natural killer/T‐cell lymphoma: a single center US experience. Clin Lymphoma Myeloma Leuk. 2014;14:S143‐S144. [Google Scholar]
- 13. Bi XW, Xia Y, Zhang WW, et al. Radiotherapy and PGEMOX/GELOX regimen improved prognosis in elderly patients with early‐stage extranodal NK/T‐cell lymphoma. Ann Hematol. 2015;94(9):1525‐1533. 10.1007/s00277-015-2395-y [DOI] [PubMed] [Google Scholar]
- 14. Dong LH, Zhang LJ, Wang WJ, et al. Sequential DICE combined with l‐asparaginase chemotherapy followed by involved field radiation in newly diagnosed, stage IE to IIE, nasal and extranodal NK/T‐cell lymphoma. Leuk Lymphoma. 2016;57(7):1600‐1606. 10.3109/10428194.2015.1108415 [DOI] [PubMed] [Google Scholar]
- 15. Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P‐glycoprotein in previously untreated extranodal NK/T‐cell lymphoma, nasal type. Am J Hematol. 2008;83(10):795‐799. 10.1002/ajh.21256 [DOI] [PubMed] [Google Scholar]
- 16. Nam YS, Im KI, Kim N, et al. Down‐regulation of intracellular reactive oxygen species attenuates P‐glycoprotein‐associated chemoresistance in Epstein‐Barr virus‐positive NK/T‐cell lymphoma. Am J Transl Res. 2019;11(3):1359‐1373. [PMC free article] [PubMed] [Google Scholar]
- 17. Ando M, Sugimoto K, Kitoh T, et al. Selective apoptosis of natural killer‐cell tumours by l‐asparaginase. Br J Haematol. 2005;130(6):860‐868. 10.1111/j.1365-2141.2005.05694.x [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Bi XW, Zhu YJ, et al. IL‐2Rα up‐regulation is mediated by latent membrane protein 1 and promotes lymphomagenesis and chemotherapy resistance in natural killer/T‐cell lymphoma. Cancer Commun. 2018;38(1):62. 10.1186/s40880-018-0334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaccard A, Gachard N, Marin B, et al. Efficacy of L‐asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T‐cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834‐1839. 10.1182/blood-2010-09-307454 [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T‐cell lymphoma, nasal type: the NK‐cell tumor study group study. J Clin Oncol. 2011;29(33):4410‐4416. 10.1200/jco.2011.35.6287 [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Zhang L, Liu X, et al. Efficacy and survival in newly diagnosed advanced Extranodal natural killer/T‐cell lymphoma: a randomized, controlled, multicenter and open‐Labled study with Ddgp regimen versus SMILE regimen. Blood. 2019;134. 10.1182/blood-2019-127811 31076441 [DOI] [Google Scholar]
- 22. Wang J‐H, Wang H, Wang Y‐J, et al. Analysis of the efficacy and safety of a combined gemcitabine, oxaliplatin and pegaspargase regimen for NK/T‐cell lymphoma. Oncotarget. 2016;7(23):35412‐35422. 10.18632/oncotarget.8643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan G, Huang H‐Q, Wang X, et al. P‐Gemox regimen (Pegaspargase, gemcitabine, oxaliplatin) for extranodal natural killer cell lymphoma: 10 years’ real‐world clinical experience from China. Blood. 2018;132. 10.1182/blood-2018-99-116551 29866817 [DOI] [Google Scholar]
- 24. Huang H‐Q, Yan G, Su H, et al. Clinical outcome of an multicentre, randomized, phase II clinical trial for patients with extranodal NK/T cell lymphoma treated by P‐Gemox or Aspametdex. Blood. 2019;134. 10.1182/blood-2019-123478 31076441 [DOI] [Google Scholar]
- 25. Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2015;92(1):11‐31. 10.1016/j.ijrobp.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 26. A predictive model for aggressive non‐Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987‐994. 10.1056/NEJM199309303291402 [DOI] [PubMed] [Google Scholar]
- 27. Chen SY, Yang Y, Qi SN, et al. Validation of nomogram‐revised risk index and comparison with other models for extranodal nasal‐type NK/T‐cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision‐making. Leukemia. 2021;35(1):130‐142. 10.1038/s41375-020-0791-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non‐anthracycline‐based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389‐400. 10.1016/s1470-2045(15)00533-1 [DOI] [PubMed] [Google Scholar]
- 29. Lee J, Suh C, Park YH, et al. Extranodal natural killer T‐cell lymphoma, nasal‐type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612‐618. [DOI] [PubMed] [Google Scholar]
- 30. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T‐cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027‐6032. 10.1200/JCO.2009.23.8592 [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T‐cell lymphoma: Japan clinical oncology group study JCOG0211. J Clin Oncol. 2009;27(33):5594‐5600. 10.1200/JCO.2009.23.8295 [DOI] [PubMed] [Google Scholar]
- 33. Kim SJ, Yang DH, Kim JS, et al. Concurrent chemoradiotherapy followed by L‐asparaginase‐containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08‐01 phase II study. Ann Hematol. 2014;93(11):1895‐1901. 10.1007/s00277-014-2137-6 [DOI] [PubMed] [Google Scholar]
- 34. Wang L, Wang ZH, Chen XQ, et al. First‐line combination of gemcitabine, oxaliplatin, and L‐asparaginase (GELOX) followed by involved‐field radiation therapy for patients with stage IE/IIE extranodal natural killer/T‐cell lymphoma. Cancer. 2013;119(2):348‐355. 10.1002/cncr.27752 [DOI] [PubMed] [Google Scholar]
- 35. Wang L, Wang Z‐H, Chen X‐Q, Wang K‐F, Huang H‐Q, Xia Z‐J. First‐line combination of GELOX followed by radiation therapy for patients with stage IE/IIE ENKTL: an updated analysis with long‐term follow‐up. Oncol Lett. 2015;10(2):1036‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei W, Wu P, Li L, Zhang ZH. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P‐GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal‐type, extranodal natural killer/T‐cell lymphoma. Hematology. 2017;22(6):320‐329. 10.1080/10245332.2016.1264163 [DOI] [PubMed] [Google Scholar]
- 37. Xu PP, Xiong J, Cheng S, et al. A phase II study of methotrexate, etoposide, dexamethasone and Pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage IE to IIE Extranodal natural‐killer/T‐cell lymphoma, Nasal‐type. EBioMedicine. 2017;25:41‐49. 10.1016/j.ebiom.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei C, Cao X, Zhang W, et al. Combined gemcitabine, cisplatin, dexamethasone, methotrexate, and pegaspargase (GDP‐ML) for patients with newly diagnosed extranodal natural killer/T cell lymphoma, nasal type: a single arm, single center, prospective phase 2 study. Ann Hematol. 2020;99(12):2801‐2809. 10.1007/s00277-020-04036-z [DOI] [PubMed] [Google Scholar]
- 39. Tian S, Li R, Wang T, et al. Gemcitabine, dexamethasone, and cisplatin (GDP) chemotherapy with sandwiched radiotherapy in the treatment of newly diagnosed stage IE/IIE extranodal natural killer/T‐cell lymphoma, nasal type. Cancer Med. 2019;8(7):3349‐3358. 10.1002/cam4.2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T‐cell lymphoma failing l‐asparaginase. Blood. 2017;129(17):2437‐2442. 10.1182/blood-2016-12-756841 [DOI] [PubMed] [Google Scholar]
- 41. Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T‐cell lymphoma. J Hematol Oncol. 2018;11(1):15. 10.1186/s13045-018-0559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li JY, Tao R, Fan L, et al. Sintilimab for relapsed/refractory (r/r) extranodal NK/T cell lymphoma (ENKTL): extended follow‐up on the multicenter, single‐arm phase II trail (ORIENT‐4). Meeting abstract. J Clin Oncol. 2020;38(15):2. [Google Scholar]
- 43. Cai J, Liu PP, Huang HQ, et al. Combination of anti‐PD‐1 antibody with P‐GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma. Article. Signal Transduct Target Ther. 2020;5(1):9. 10.1038/s41392-020-00331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim TM, Park YH, Lee S‐Y, et al. Local tumor invasiveness is more predictive of survival than international prognostic index in stage I(E)/II(E) extranodal NK/T‐cell lymphoma, nasal type. Blood. 2005;106(12):3785‐3790. [DOI] [PubMed] [Google Scholar]
- 45. Yang Y, Zhang YJ, Zhu Y, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T‐cell lymphoma, nasal‐type: a multicenter study. Leukemia. 2015;29(7):1571‐1577. 10.1038/leu.2015.44 [DOI] [PubMed] [Google Scholar]
- 46. Yang Y, Zhu Y, Cao JZ, et al. Risk‐adapted therapy for early‐stage extranodal nasal‐type NK/T‐cell lymphoma: analysis from a multicenter study. Blood. 2015;126(12):1424‐1432. 10.1182/blood-2015-04-639336 [DOI] [PubMed] [Google Scholar]
- 47. Kim HS, Kim KH, Kim KH, et al. Whole blood Epstein‐Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T‐cell lymphoma. Leuk Lymphoma. 2009;50(5):757‐763. 10.1080/10428190902803669 [DOI] [PubMed] [Google Scholar]
- 48. Suzuki R, Yamaguchi M, Izutsu K, et al. Prospective measurement of Epstein‐Barr virus‐DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T‐cell lymphoma, nasal type. Blood. 2011;118(23):6018‐6022. 10.1182/blood-2011-05-354142 [DOI] [PubMed] [Google Scholar]
- 49. Wang ZY, Liu QF, Wang H, et al. Clinical implications of plasma Epstein‐Barr virus DNA in early‐stage extranodal nasal‐type NK/T‐cell lymphoma patients receiving primary radiotherapy. Blood. 2012;120(10):2003‐2010. 10.1182/blood-2012-06-435024 [DOI] [PubMed] [Google Scholar]
- 50. Xiong J, Cui BW, Wang N, et al. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell. 2020;37(3):403‐419. 10.1016/j.ccell.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 51. Tian XP, Ma SY, Young KH, et al. A composite single‐nucleotide polymorphism prediction signature for extranodal natural killer/T‐cell lymphoma. Blood. 2021;138(6):452‐463. 10.1182/blood.2020010637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


