Abstract
Incidence rates of Merkel cell carcinoma (MCC), an uncommon skin cancer with an aggressive disease course, have increased in recent decades. Limited treatment options are available for patients with metastatic MCC (mMCC). Avelumab, an anti‐programmed cell death‐ligand 1 monoclonal antibody, became the first approved treatment for mMCC after the results of the phase 2 JAVELIN Merkel 200 study. Prior to its regulatory approval, an expanded access program (EAP) enabled compassionate use of avelumab in patients with mMCC. Here we report findings from patients enrolled in the EAP in Europe and the Middle East. Efficacy and safety data were provided at the discretion of treating physicians. Between March 2, 2016, and December 22, 2018, 403 requests for avelumab were received from 21 countries, and avelumab was supplied to 335 patients. Most patients (96.7%) received avelumab as second‐line or later treatment. In 150 patients for whom response data were available, the objective response rate was 48.0%, and in responding patients, median duration of treatment was 7.4 months (range, 1.0‐41.7 months). The most common treatment‐related adverse events were infusion‐related reaction (2.4%) and pyrexia (2.1%), and no new safety signals were observed. Overall, results from European and Middle Eastern patients enrolled in this EAP confirm the efficacy and safety of avelumab treatment observed in previous studies in patients with mMCC.
Keywords: avelumab, expanded access program, Merkel cell carcinoma, PD‐L1, second‐line
Short abstract
What's new?
Patients with the aggressive skin cancer Merkel cell carcinoma (MCC) have few treatment options. The first approved treatment for metastatic MCC, the anti‐PD‐L1 monoclonal antibody avelumab, was made available through an expanded access program (EAP) prior to regulatory approval. Here, the authors analyze data from 150 patients in Europe and the Middle East enrolled in that EAP. Avelumab's efficacy and safety in this real‐world population of patients, they found, confirm the findings reported from the JAVELIN Merkel 200 trial.
Abbreviations
- AE
adverse event
- CR
complete response
- EAP
expanded access program
- IRR
infusion‐related reaction
- MCC
Merkel cell carcinoma
- mMCC
metastatic Merkel cell carcinoma
- ORR
objective response rate
- PD
progressive disease
- RECIST 1.1
Response Evaluation Criteria in Solid Tumors version 1.1
- TRAE
treatment‐related adverse event
1. INTRODUCTION
Merkel cell carcinoma (MCC) is an uncommon cancer with an aggressive disease course, which is associated with clonal integration of the Merkel cell polyomavirus, ultraviolet radiation exposure, older age and reduced immune function. 1 , 2 For patients with metastatic MCC (mMCC), treatment options are limited and prognosis is poor, with 5‐year survival rates of approximately 20%. 1 , 3 , 4 , 5 Incidence rates of MCC in Europe have increased in recent decades 6 , 7 , 8 ; approximately 2500 patients are diagnosed with MCC in Europe each year, resulting in approximately 1000 deaths. 9 In addition, incidence rates of MCC in Israel are among the highest in the world, particularly in men. 8 Although chemotherapy has shown antitumor activity in mMCC, responses are rarely durable; the median duration of response with chemotherapy is less than 10 months. 4
Avelumab, an anti‐programmed cell death‐ligand 1 monoclonal antibody, is approved in various countries worldwide as monotherapy for the treatment of mMCC. 10 Avelumab became the first treatment to be approved for mMCC after the results of the phase 2 JAVELIN Merkel 200 trial (NCT02155647). In part A of the trial, which assessed avelumab treatment in patients with progressive disease (PD) after chemotherapy, the objective response rate (ORR) after 3 years of follow‐up in 88 enrolled patients was 33.0%, with 11.4% of patients achieving a complete response (CR), and the median duration of response was 40.5 months. 11 After a minimum follow‐up of 15 months in part B of the trial, which assessed avelumab in 116 patients without prior systemic treatment for metastatic disease, the durable response rate (primary endpoint; response lasting at least 6 months) was 30.2%, and the ORR was 39.7%, including CR in 16.4%. 12
Expanded access programs (EAPs), also termed compassionate use programs, provide access outside of a clinical trial to investigational drugs, biologics and medical devices for patients with unsatisfactory standard‐of‐care treatment options. 13 The EAP described in this report was an ad hoc program that enabled compassionate use of avelumab in patients with mMCC. A summary of results from the global population has been reported previously. 14 To describe region‐specific data, here we report real‐world experience in a subgroup of patients with mMCC who were enrolled in the avelumab EAP in Europe and the Middle East.
2. MATERIALS AND METHODS
Participation in the avelumab EAP was based on an initial, individual patient screening and approval process; all patients provided written informed consent before enrollment. The EAP did not require a protocol. However, to enable participation of French patients through the Temporary Authorization for Use designation, the EAP was registered with ClinicalTrials.gov (NCT03089658). Patients were selected if they had measurable MCC based on Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) and PD after 1 or more prior line of chemotherapy (ie, received avelumab as second‐line or later therapy) or were ineligible to receive chemotherapy in the metastatic setting (ie, received avelumab as first‐line therapy). Eligibility criteria permitted inclusion of patients with an Eastern Cooperative Oncology Group performance status between 0 and 3 who had treated brain metastases (without steroid use) that were not progressing or who were potentially immunocompromised. Patients were evaluated individually by the sponsor according to their medical history, including those with immunosuppressive conditions or HIV infection and those receiving immunosuppressive medications. Data for all immunocompromised patients who participated in the EAP were summarized in a previous report. 14 All patients were excluded from participation in any ongoing clinical trial for MCC. An online portal was implemented in May 2017 that processed EAP requests and collated responses.
Patients received avelumab 10 mg/kg via 1‐hour intravenous infusion every 2 weeks, including premedication with antihistamine and acetaminophen (paracetamol), to mitigate infusion‐related reactions (IRRs), as described in the summary of product characteristics. 10 Treatment with avelumab (and resupply by the sponsor) continued until confirmed PD, significant clinical deterioration, unacceptable toxicity, or withdrawal of consent; continuation of avelumab in patients with radiological PD was permitted in the absence of significant clinical deterioration on a case‐by‐case basis and per assessment by the treating physician of potential clinical benefit.
Disease monitoring was performed according to local clinical practice. Assessments included best overall response according to RECIST 1.1 and duration of treatment/drug supply in responding patients, as well as safety/tolerability. It was recommended within the EAP protocol that patients underwent a first radiological evaluation (computed tomography scan of known disease sites) to assess response or PD within 8 to 12 weeks of starting avelumab and that a second radiological assessment was performed within 4 to 8 weeks for confirmation. All adverse events (AEs; including serious and nonserious AEs) were reported by treating physicians to a global safety database (Global Patient Safety, the healthcare business of Merck KGaA, Darmstadt, Germany) and also to the local health unit and ethics committee when avelumab was resupplied or at disease progression or death. IRRs were identified using a prespecified list of Medical Dictionary for Regulatory Activities terms and managed per established guidance for avelumab. 10 Immune‐related AEs were identified based on medical review. Patient data, including response and safety, were summarized using descriptive statistics.
3. RESULTS
Between March 2, 2016, and December 22, 2018, 403 requests for avelumab were received from 21 countries in Europe and the Middle East (Table S1). In total, 367 requests were approved, 8 were withdrawn prior to approval and 28 were rejected prior to approval for reasons that included incorrect diagnosis, no appropriate previous therapy and insufficient information provided; 32 were withdrawn after approval but prior to supply. Avelumab was supplied to 335 patients. Median age in approved patients was 71.6 years (range, 28.0‐95.0 years), and 67.3% were male. Most patients (96.7%) received avelumab as second‐line or later treatment (Table 1). After regulatory approval of avelumab in various countries, enrollment in the EAP was closed on December 31, 2018.
TABLE 1.
Baseline characteristics
| Characteristic | Approved patients (N = 367) |
|---|---|
| Age | |
| Median (range), y | 71.6 (28.0‐95.0) |
| <65 y, n (%) | 82 (22.3) |
| ≥65 y, n (%) | 285 (77.7) |
| Sex, n (%) | |
| Female | 119 (32.4) |
| Male | 247 (67.3) |
| Data missing | 1 (0.3) |
| ECOG PS, n (%) | |
| 0 | 123 (33.5) |
| 1 | 131 (35.7) |
| 2 | 19 (5.2) |
| 3 | 7 (1.9) |
| Data missing | 87 (23.7) |
| Line of therapy, n (%) | |
| 1 | 12 (3.3) |
| ≥2 | 355 (96.7) |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
The data cutoff was March 22, 2019. Of 335 patients for whom avelumab was supplied, data for responses were available from 150 patients (44.8%). The ORR in 150 response‐evaluable patients was 48.0%, comprising CR in 38 patients (25.3%) and partial response in 34 patients (22.7%); best overall response was stable disease in 37 patients (24.7%) (Table 2). Using the denominator of 335 patients in the total population, the ORR was 21.5%. Images showing tumor changes with avelumab treatment are provided in Figures 1 and 2 and Figures S1 and S2. Because resupply of avelumab depended on clinical benefit, duration of avelumab treatment (ie, duration of drug supply) was assessed as a surrogate for duration of response/clinical benefit. Median treatment duration at data cutoff in responding patients was 7.4 months, and the longest treatment duration was 41.7 months.
TABLE 2.
Physician‐reported responses in evaluable patients
| Response parameter a | Response‐evaluable patients (N = 150) |
|---|---|
| ORR, % | 48.0 |
| DCR, % b | 72.7 |
| Confirmed BOR, n (%) | |
| CR | 38 (25.3) |
| PR | 34 (22.7) |
| SD | 37 (24.7) |
| PD c | 41 (27.3) |
| Duration of treatment in patients with response d | |
| Median (range), mo | 7.4 (1.0‐41.7) |
Abbreviations: BOR, best overall response; CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Response was reported according to the treating physician's assessment of follow‐up scans at the time of resupply.
Among patients treated for a minimum of 3 months with available data.
Patients with PD or adverse events that required treatment discontinuation within the first 90 days were never resupplied with drug and did not have a follow‐up response evaluation; thus, these values may be underreported.
Duration of avelumab treatment/drug supply is reported as a surrogate for duration of response or clinical benefit.
FIGURE 1.
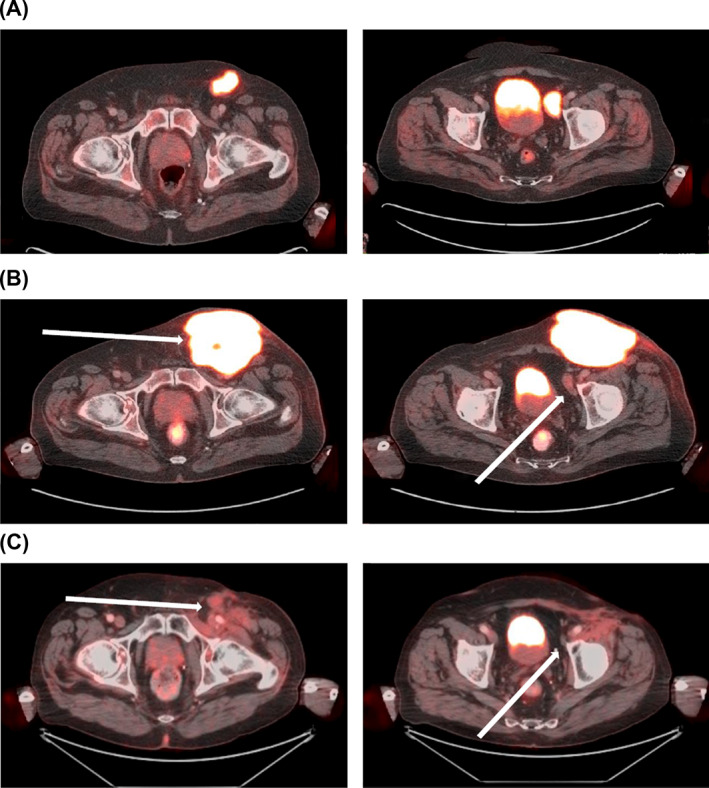
Clinical response with avelumab and radiotherapy after disease progression with avelumab. Positron emission tomography‐computed tomography scans of a 72‐year‐old male patient with metastatic Merkel cell carcinoma (MCPyV status unknown), chronic arterial fibrillation and paraneoplastic Lambert‐Eaton myasthenic syndrome, and a history of endocarditis with mitral valve replacement: (A) at baseline (January 2018); (B) disease progression after 7 cycles of avelumab (May 2018); and (C) clinical response after 15 cycles of avelumab and concomitant radiotherapy (50 Gy at 2 Gy/fraction) (September 2018). At the last follow‐up (January 2021), the patient had an ongoing partial response after 45 cycles of avelumab. Images were provided by Prof Bechter
FIGURE 2.
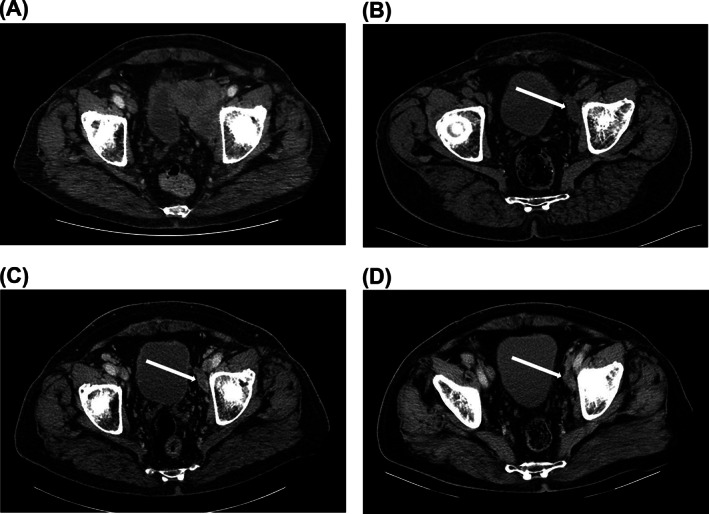
Durable partial response with avelumab. Computed tomography scans of a 68‐year‐old male patient with MCPyV+ metastatic Merkel cell carcinoma (A) with disease progression after treatment with chemotherapy (May 2017), (B) who subsequently achieved a partial response after 6 months of avelumab treatment (October 2017), which was maintained after (C) 15 months (August 2018) and (D) 27 months of avelumab treatment (July 2019). Treatment was stopped in September 2019, and the patient had an ongoing partial response with no evidence of recurrent disease at the last follow‐up (January 2020). Images were provided by Prof Samimi. MCPyV+, Merkel cell polyomavirus positive
The most frequent treatment‐related AEs (TRAEs) reported by physicians were IRR (single preferred term; n = 8 [2.4%]), pyrexia (n = 7 [2.1%]), dyspnea (n = 3 [0.9%]) and chills (n = 3 [0.9%]) (Table 3); the most frequently reported immune‐related TRAE was hypothyroidism (n = 2 [0.6%]). Images of rash observed after avelumab treatment are shown in Figure 3. Safety events were likely to have been underreported as a result of data being reported at the discretion of the treating physician at the time of avelumab resupply, and because evaluable data were not available in many patients after the initial 3‐month supply.
TABLE 3.
Physician‐reported TRAEs with avelumab
| Patients with TRAEs, n (%) a | Treated patients (N = 335) | ||
|---|---|---|---|
| Nonserious events | Serious events | Total events | |
| Infusion‐related TRAEs b | |||
| Infusion‐related reaction c | 7 (2.1) | 1 (0.3) | 8 (2.4) |
| Pyrexia | 5 (1.5) | 2 (0.6) | 7 (2.1) |
| Chills | 1 (0.3) | 2 (0.6) | 3 (0.9) |
| Asthenia | 1 (0.3) | 1 (0.3) | 2 (0.6) |
| Tachycardia | 0 | 2 (0.6) | 2 (0.6) |
| Abdominal pain | 1 (0.3) | 0 | 1 (0.3) |
| Anaphylactic reaction | 1 (0.3) | 0 | 1 (0.3) |
| Back pain | 1 (0.3) | 0 | 1 (0.3) |
| Chest pain | 1 (0.3) | 0 | 1 (0.3) |
| Cough | 0 | 1 (0.3) | 1 (0.3) |
| Hypersensitivity | 0 | 1 (0.3) | 1 (0.3) |
| Oxygen saturation decreased | 0 | 1 (0.3) | 1 (0.3) |
| Tremor | 0 | 1 (0.3) | 1 (0.3) |
| Vomiting | 1 (0.3) | 0 | 1 (0.3) |
| Immune‐related TRAEs d | |||
| Hypothyroidism | 1 (0.3) | 1 (0.3) | 2 (0.6) |
| Blood creatine phosphokinase increased | 1 (0.3) | 0 | 1 (0.3) |
| Facial paralysis | 0 | 1 (0.3) | 1 (0.3) |
| Myasthenia gravis | 0 | 1 (0.3) | 1 (0.3) |
| Liver disorder | 0 | 1 (0.3) | 1 (0.3) |
| Myositis | 1 (0.3) | 0 | 1 (0.3) |
| Pneumonitis | 0 | 1 (0.3) | 1 (0.3) |
| Other TRAEs | |||
| Dyspnea | 0 | 3 (0.9) | 3 (0.9) |
| Decreased appetite | 2 (0.6) | 0 | 2 (0.6) |
| Rash | 2 (0.6) | 0 | 2 (0.6) |
| Acute myocardial infarction | 0 | 1 (0.3) | 1 (0.3) |
| Alanine aminotransferase increased | 1 (0.3) | 0 | 1 (0.3) |
| Anemia | 0 | 1 (0.3) | 1 (0.3) |
| Arthralgia | 1 (0.3) | 0 | 1 (0.3) |
| Aspartate aminotransferase increased | 1 (0.3) | 0 | 1 (0.3) |
| Autoimmune colitis | 0 | 1 (0.3) | 1 (0.3) |
| Colitis | 0 | 1 (0.3) | 1 (0.3) |
| Eczema | 0 | 1 (0.3) | 1 (0.3) |
| Edema peripheral | 0 | 1 (0.3) | 1 (0.3) |
| Fatigue | 1 (0.3) | 0 | 1 (0.3) |
| Hepatic enzyme increased | 1 (0.3) | 0 | 1 (0.3) |
| Hepatitis | 1 (0.3) | 0 | 1 (0.3) |
| Hyperglycemia | 0 | 1 (0.3) | 1 (0.3) |
| Hyperthyroidism | 1 (0.3) | 0 | 1 (0.3) |
| Metastases to meninges | 0 | 1 (0.3) | 1 (0.3) |
| Nausea | 1 (0.3) | 0 | 1 (0.3) |
| Neutropenia | 0 | 1 (0.3) | 1 (0.3) |
| Pulmonary fibrosis | 0 | 1 (0.3) | 1 (0.3) |
| Rectal abscess | 0 | 1 (0.3) | 1 (0.3) |
| Urinary tract infection | 0 | 1 (0.3) | 1 (0.3) |
| Vertigo | 1 (0.3) | 0 | 1 (0.3) |
Abbreviation: TRAE, treatment‐related adverse event.
The table shows preferred terms for TRAEs obtained from the safety database, including unsolicited cumulative events provided by the treating physician. Overall safety events may have been underreported in this ad hoc program. Serious events refer to adverse events that were considered life‐threatening, required inpatient hospitalization or prolonged existing hospitalization, resulted in persistent or significant disability/incapacity, resulted in death, or were otherwise considered medically important.
Infusion‐related reaction adverse events based on a prespecified list of Medical Dictionary for Regulatory Activities preferred terms.
Based on the single Medical Dictionary for Regulatory Activities preferred term for infusion‐related reaction.
Immune‐related adverse event based on medical review.
FIGURE 3.
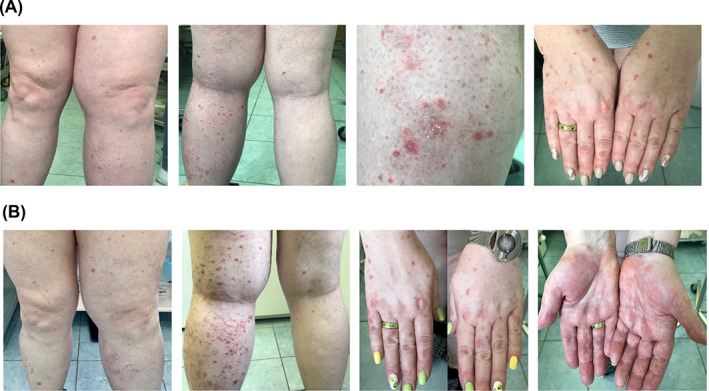
Treatment‐related rash with avelumab. Images of a female patient (born 1964) with MCPyV+ metastatic Merkel cell carcinoma with rash related to avelumab treatment. (A) Grade 1 rash observed after 8 cycles of avelumab; (B) Grade 2 rash observed on lower extremities and grade 1 palmar erythema observed after 9 cycles of avelumab. Images were provided by Dr Orlova. MCPyV+, Merkel cell polyomavirus positive
4. DISCUSSION
Before the approval of avelumab (in 2017), no treatment for mMCC had been approved by the European Commission or other regulatory authority; the most recently published European guidelines (in 2015) recommend enrollment in clinical trials for patients with mMCC. 3 To our knowledge, the avelumab MCC EAP represents the only EAP established for this rare and aggressive tumor, and it addressed an urgent, unmet medical need in patients who had limited options for treatment.
Clinical benefits seen with avelumab in European and Middle Eastern patients were consistent with the overall findings in the global EAP. The physician‐reported ORR (per RECIST 1.1) in 150 response‐evaluable patients in this European and Middle Eastern population, most of whom received avelumab as second/later‐line treatment, was 48.0%, compared to 46.7% in the global population. 14 However, many patients were not evaluable for response, meaning that the proportion of all European and Middle Eastern patients who had an objective response was 21.5%. Thus, the “true” ORR is within the range of 21.5% to 48.0%. In comparison, the ORR among all patients enrolled in part A of JAVELIN Merkel 200 (intention‐to‐treat analysis) was 33.0%. 11 The most frequently reported TRAEs in this patient population in the EAP were IRR, pyrexia, chills and dyspnea, and no new safety signals were identified.
Data from this EAP are limited because of the challenges of collecting data from a real‐world population compared to a clinical trial. For example, some countries placed restrictions on the availability of patient data, which reduced the number of response‐evaluable patients. In the European and Middle Eastern patients examined, 55.2% (n = 185 of total 335) were not evaluable for response compared to 51.4% in the overall global population (n = 254 of total 494). 14 Furthermore, results may have been underreported because safety and efficacy observations were reported at the discretion of the treating physician. Reporting may also have been affected by the resupply of avelumab by the sponsor being dependent on determination of clinical benefit by the treating physician. Additionally, patients with PD according to RECIST 1.1 or an AE that led to discontinuation of avelumab within the initial 90 days of treatment were not resupplied with avelumab and thus did not have additional response evaluations. The avelumab EAP included patients with mMCC who were considered to be potentially immunocompromised; antitumor activity in this subgroup of patients was reported previously for the global population 14 and was not analyzed in the subgroup reported here.
In conclusion, the efficacy and safety of avelumab seen in a real‐world population of patients in Europe and the Middle East, including patients who were not eligible for chemotherapy or participation in a clinical trial, support previously reported findings from the JAVELIN Merkel 200 trial.
CONFLICT OF INTEREST
Paolo Antonio Ascierto has provided a consulting or advisory role for 4SC, Alkermes, Array BioPharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, EMD Serono, Eisai, Idera, Immunocore, Incyte, MedImmune, Italfarmaco, Lunaphore, Merck & Co, Nektar, Nouscom, Novartis, OncoSec Medical, Pfizer, Pierre Fabre, Regeneron, Roche, Sandoz, Sanofi, Seagen, Sun Pharma, Syndax and Ultimovacs; has received research funding from Array BioPharma, Bristol Myers Squibb, Roche and Sanofi; and has received reimbursement for travel expenses from Merck Sharp & Dohme. Kristina Orlova has provided a consulting or advisory role for Novartis, Bristol Myers Squibb, EMD Serono, Merck Sharp & Dohme and Roche/Genentech; has received research funding from Novartis; and has received reimbursement for travel expenses from Novartis and EMD Serono. Giovanni Grignani has received honoraria and provided a consulting or advisory role for Bayer, Eisai, Eli Lilly, the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis and PharmaMar. Monika Dudzisz‐Śledź has received honoraria for lectures from Bristol Myers Squibb, the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis, Pierre Fabre and Sanofi; received honoraria for participating in advisory meetings from the healthcare business of Merck KGaA, Darmstadt, Germany, and Novartis; and received financial support for conference attendance from Novartis. Vanna Chiarion Sileni has provided a consulting or advisory role for Bristol Myers Squibb, Merck d.o.o., Beograd, Serbia, an affiliate of Merck KGaA, Darmstadt, Germany, Merck Sharp & Dohme, Novartis, Pierre Fabre and Roche; has provided speaker's services for Bristol Myers Squibb, Novartis and Pierre Fabre; and has received reimbursement for travel expenses from Bristol Myers Squibb. Nicola Fazio has received honoraria from Advanced Accelerator Applications, Ipsen, the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis and Sanofi; has provided a consulting or advisory role for Advanced Accelerator Applications, Ipsen, EMD Serono, Merck Sharp & Dohme, Novartis, Pfizer and Wren Laboratories; has received research funding from EMD Serono, Ipsen, Merck Sharp & Dohme and Novartis; and reports other relationships with Il Pensiero Scientifico Editore and Springer. Mahtab Samimi has provided speakers services for Janssen and has received reimbursement for travel expenses from Abbvie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma and Merck Sharp & Dohme. Laurent Mortier has received research funding and reimbursement for travel expenses from and provided a consultancy or advisory role for Bristol Myers Squibb, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck Sharp & Dohme and Novartis. Christoffer Gebhardt has provided a consulting or advisory role for and/or received honoraria and travel support from Amgen, Almirall, Bristol Myers Squibb, Immunocore, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche, Sun Pharma and Sanofi Genzyme. Oliver Bechter has provided a consulting or advisory role for Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre and Sanofi; and has received reimbursement for travel expenses from Bristol Myers Squibb and Merck Sharp & Dohme. Ana Arance has received honoraria, research funding, and reimbursement for travel expenses from, and provided a consulting or advisory role and speaker's services for, Bristol Myers Squibb, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche and Sanofi; and has received research funding from Amgen. Elena Benincasa is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany. Lenka Kostkova is an employee of Merck spol. s r.o., Bratislava, Slovakia, an affiliate of Merck KGaA, Darmstadt, Germany. Nuno Costa is an employee of Pfizer. Paul Lorigan has received honoraria and reimbursement for travel expenses from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, Nektar, NeraCare, Novartis, Oncology Education Canada, Pierre Fabre and Roche, and has received research funding from Bristol Myers Squibb. Eyal Fenig, Nora Kramkimel and Neil Steven declare no conflict of interest.
ETHICS STATEMENT
The EAP was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization Guideline for Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each center. All patients provided written informed consent before entering the EAP (ClinicalTrials.gov NCT03089658).
Supporting information
Table S1. Summary of requests for avelumab from patients enrolled in the avelumab mMCC EAP in Europe and the Middle East.
Figure S1. Complete response with avelumab.
Figure S2. Complete response with avelumab after initial disease progression.
ACKNOWLEDGMENTS
The authors thank all the patients and their families, as well as the investigators who participated in the global avelumab EAP for MCC. Medical writing support and editorial support were provided by ClinicalThinking and funded by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945), as part of an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany and Pfizer.
Ascierto PA, Orlova K, Grignani G, et al. Avelumab expanded access program in metastatic Merkel cell carcinoma: Efficacy and safety findings from patients in Europe and the Middle East. Int. J. Cancer. 2021;149(11):1926‐1934. 10.1002/ijc.33746
[Correction added after first online publication on August 14, 2021: One of the author affiliations has been updated.]
Funding information The healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945), as part of an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer
DATA AVAILABILITY STATEMENT
For all new products or new indications approved in both the European Union and the United States after January 1, 2014, the healthcare business of Merck KGaA, Darmstadt, Germany, will share patient‐level and study‐level data after deidentification, as well as redacted study protocols and clinical study reports from clinical trials in patients. These data will be shared with qualified scientific and medical researchers, upon researcher's request, as necessary for conducting legitimate research. Such requests must be submitted in writing to the company's data‐sharing portal. More information can be found at https://www.merckgroup.com/en/research/our‐approach‐to‐research‐and‐development/healthcare/clinical‐trials/commitment‐responsible‐data‐sharing.html. Where the healthcare business of Merck KGaA, Darmstadt, Germany, has a coresearch, codevelopment, or comarketing/copromotion agreement or where the product has been out‐licensed, it is recognized that the responsibility for disclosure may be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany, will endeavor to gain agreement to share data in response to requests.
REFERENCES
- 1. Schadendorf D, Lebbe C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53‐69. [DOI] [PubMed] [Google Scholar]
- 2. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebbe C, Becker JC, Grob JJ, et al. European Dermatology Forum (EDF), the European Association of Dermato‐Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of Merkel cell carcinoma. European consensus‐based interdisciplinary guideline. Eur J Cancer. 2015;51:2396‐2403. [DOI] [PubMed] [Google Scholar]
- 4. NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. V1. 2020. https://www.nccn.org/professionals/physician_gls/pdf/mcc_blocks.pdf. Accessed July 16, 2020.
- 5. Prieto Muñoz I, Pardo Masferrer J, Olivera Vegas J, Medina Montalvo MS, Jover Díaz R, Pérez Casas AM. Merkel cell carcinoma from 2008 to 2012: reaching a new level of understanding. Cancer Treat Rev. 2013;39:421‐429. [DOI] [PubMed] [Google Scholar]
- 6. Reichgelt BA, Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population‐based study of 808 cases in 1993–2007. Eur J Cancer. 2011;47:579‐585. [DOI] [PubMed] [Google Scholar]
- 7. Zaar O, Gillstedt M, Lindelöf B, Wennberg‐Larkö A‐M, Paoli J. Merkel cell carcinoma incidence is increasing in Sweden. J Eur Acad Dermatol Venereol. 2016;30:1708‐1713. [DOI] [PubMed] [Google Scholar]
- 8. Stang A, Becker JC, Nghiem P, Ferlay J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: an international assessment. Eur J Cancer. 2018;94:47‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becker JC, Stang A, Hausen AZ, et al. Epidemiology, biology and therapy of Merkel cell carcinoma: conclusions from the EU project IMMOMEC. Cancer Immunol Immunother. 2018;67:341‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bavencio (avelumab) summary of product characteristics. Darmstadt, Germany: Merck KGaA; 2020. [Google Scholar]
- 11. D'Angelo SP, Bhatia S, Brohl AS, et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long‐term data and biomarker analyses from the single‐arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer. 2020;8:e000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Angelo S, Lebbé C, Mortier L, et al. First‐line avelumab treatment in patients with metastatic Merkel cell carcinoma: primary analysis after ≥15 months of follow‐up from JAVELIN Merkel 200, a registrational phase 2 trial [abstract P362]. J Immunother Cancer. 2019;7:282.31694725 [Google Scholar]
- 13. Iudicello A, Alberghini L, Benini G, Mosconi P. Expanded access programme: looking for a common definition. Trials. 2016;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker JW, Lebbé C, Grignani G, et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: experience from a global expanded access program. J Immunother Cancer. 2020;8:e000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of requests for avelumab from patients enrolled in the avelumab mMCC EAP in Europe and the Middle East.
Figure S1. Complete response with avelumab.
Figure S2. Complete response with avelumab after initial disease progression.
Data Availability Statement
For all new products or new indications approved in both the European Union and the United States after January 1, 2014, the healthcare business of Merck KGaA, Darmstadt, Germany, will share patient‐level and study‐level data after deidentification, as well as redacted study protocols and clinical study reports from clinical trials in patients. These data will be shared with qualified scientific and medical researchers, upon researcher's request, as necessary for conducting legitimate research. Such requests must be submitted in writing to the company's data‐sharing portal. More information can be found at https://www.merckgroup.com/en/research/our‐approach‐to‐research‐and‐development/healthcare/clinical‐trials/commitment‐responsible‐data‐sharing.html. Where the healthcare business of Merck KGaA, Darmstadt, Germany, has a coresearch, codevelopment, or comarketing/copromotion agreement or where the product has been out‐licensed, it is recognized that the responsibility for disclosure may be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany, will endeavor to gain agreement to share data in response to requests.


