Abstract
Background
To date, no study has investigated the association between chewing function and related parameters as a function of the degree of dementia using a finer subdivision of the values of the Mini‐Mental State Examination (MMSE).
Objective
This study aimed to investigate the differences in chewing function and related parameters as a function of the degree of dementia.
Methods
An analysis of cross‐sectional data obtained from the OrBiD (Oral Health, Bite Force, and Dementia) pilot study was performed. The participants were stratified into five groups based on the outcomes of the MMSE (no dementia, MMSE 28–30; mild cognitive impairment, MMSE 25–27; mild dementia, MMSE 18–24; moderate dementia, MMSE 10–17; severe dementia, MMSE <10). The chewing efficiency, maximum occlusal force and related parameters (number of supporting zones, number of teeth, Eichner index, tooth/denture status, denture quality, and dental treatment needs) were recorded.
Results
The MMSE groups showed significantly different chewing efficiencies (p = .003, Jonckheere‐Terpstra test) and maximum occlusal forces (p = .003, Jonckheere‐Terpstra test), but the number of supporting zones (p = .055, chi‐square test) and the number of natural teeth (p = .126, chi‐square test) were not different. The Eichner index, tooth/denture status, denture quality and dental treatment need showed no significant associations with the degree of dementia.
Conclusion
An improvement in the usability of the measurement methods for assessing chewing function in people with dementia is needed. Research involving people with dementia is necessary because the nutritional situation often deteriorates rapidly within a multifactorial system, which includes chewing ability and oral health.
Keywords: bite force, cognitive dysfunction, dementia, dentition, mastication, maximum occlusal force
Chewing function as a function of the degree of dementia. Chewing efficiency as the variance of hue (VOH) stratified by the Mini‐Mental State Examination (MMSE) score. Low values indicate good chewing efficiency and vice versa. Maximum occlusal force (kN) stratified by the Mini‐Mental State Examination (MMSE) scores. High values indicate a high maximum occlusal force and vice versa. [noDem, no dementia; mCI, mild cognitive impairment; mDem, mild dementia; modem, moderate dementia; sDem, severe dementia; MMSE, Mini‐Mental State Examination]

1. INTRODUCTION
1.1. Chewing function and maximum occlusal force in general
Chewing function is described subjectively as chewing ability and objectively as chewing efficiency. In general, chewing efficiency is dependent on factors such as age, 1 the number of antagonistic tooth contacts, 2 saliva flow rate, lip function, cheek, tongue, soft palate, 3 reduction in the number of teeth or occlusal surfaces 4 and the quality or type of denture. 5 The maximum occlusal force (also referred to as bite force) provides information about the physiologically possible force of a patient for comminuting a piece of food. It is an important indicator of the functional state and strength of the masticatory muscles, and it can be influenced by various factors, such as masticatory muscles, proprioceptors in the periodontal tissues, temporomandibular joints and subjective perception. 6
1.2. Chewing function and maximum occlusal force in older people and people with dementia
Older people often overestimate their chewing performance. 4 A good self‐reported chewing ability does not necessarily imply a good chewing efficiency or high maximum occlusal force. 7 Age‐related decline in sensorimotor regulation and motor control, as well as changes in structures and biomechanical features, result in impaired mastication, 8 , 9 , 10 which may be worsened by additional age‐related systemic diseases 11 and pathological changes in the brain. 12 Age in general has a heterogeneous effect 9 ; some factors (eg oral mixing ability) show a significant age‐related decrease, whereas others (eg masseter muscle volume), in contrast, do not. 13 , 14
Cognitive impairment or dementia is associated with a reduction in the number of teeth, 15 , 16 , 17 , 18 , 19 the number of pairs of occluding teeth, 20 , 21 or worse self‐reported chewing ability. 19 Additionally, tooth loss and the resulting deficit in chewing function are related to oral health, which is especially impaired in people with cognitive deficits. 22 Therefore, inflammatory processes within the context of microbiological and immunological reactions, such as periodontitis, should also be considered as part of the pathogenesis of cognitive diseases such as Alzheimer's disease. 22 , 23 An association between cognitive impairment or dementia and low chewing efficiency 1 , 24 or a reduced bite force 25 has been observed. Despite evidence of a correlation between cognitive decline/dementia and impaired chewing function, 1 , 24 , 25 , 26 a causal relationship remains to be demonstrated. 27
Additional factors, such as the type of diet, 28 have been investigated for their association with the observed cognitive deficits in animals. 28 , 29 Nutrient intake may also be considered an individual risk factor, and it may explain at least part of the masticatory functions of an individual. 30
1.3. Aim of the study
The aim of this study was to evaluate the associations between chewing efficiency, maximum occlusal force and related parameters as a function of the degree of dementia using a finer subdivision of the values of the Mini‐Mental State Examination (MMSE, 31 ) (Cf. 1 , 20 , 24 , 25 ).
The authors hypothesised that the worsening of dementia is associated with a decrease in chewing function represented by chewing efficiency, maximum occlusal force and deterioration in related parameters (number of supporting zones, number of teeth, Eichner index, tooth and denture status, denture quality and dental treatment need).
2. METHODS
2.1. Study design
The data of the OrBiD (Oral Health, Bite Force and Dementia) pilot study (ClinicalTrials.gov NCT03775772) were analysed. The OrBiD study consists of two parts (Part A: investigation of oral health in people with and without dementia, Part B: Investigation of chewing function as a function of the degree of dementia). Both parts A and B were analysed at two evaluation time points: (a) cross‐sectional analysis with intercohort analysis at baseline, and (b) longitudinal observation after the implementation of interventions. This analysis focuses on the evaluation of cross‐sectional data for chewing function and its association with dementia as part of the OrBiD study.
Participants aged 60 years and older were included, regardless of their cognitive abilities. They were required to not have any acute dental/oral condition (eg pain, abscesses) or problems with their temporomandibular joints or surrounding chewing muscles. At least one antagonistic occlusal contact (including prosthetic restoration) per jaw was required for participation.
The participants were recruited randomly, and their participation in the study was voluntary. Informed consent was obtained. The recruitment took different forms. On the one hand, participants were randomly selected from the patient population of a clinic specialised in gerodontology, among others. On the other hand, participants were randomly selected in cooperating facilities (long‐term care facilities, geronto‐psychiatric facilities) based on the inclusion and exclusion criteria, and informed consent was obtained from their relatives or legal guardians. Afterwards, all recruited participants were stratified into five groups based on the Mini‐Mental State Examination (MMSE), 31 which was conducted during the first examination. The five MMSE groups were as follows: 1—no dementia (noDem, MMSE 28–30), 2—mild cognitive impairment (mCI, MMSE 25–27), 3—mild dementia (mDem, MMSE 18–24), 4—moderate dementia (modDem, MMSE 10–17) and 5—severe dementia (sDem, MMSE <10).
2.2. Measurements
All evaluations were performed by a single investigator. Socio‐demographic data (age, sex) and the MMSE scores (maximum score 30 – no dementia) 31 were recorded. O ral functional capacity, 32 which consists of therapeutic capability and oral hygiene ability (levels: 1—normal to 2—slightly reduced, 3—greatly reduced and 4—none) and self‐responsibility (levels: normal, reduced and none), was used to assess the resilience capacity level (determined by the highest value of one of the three parameters) of the participants from a multifactorial perspective. Independence or the need for care (Activities of Daily Living) was assessed using the Barthel index 33 (maximum score 100—no need of care). The handgrip strength (kg) was measured with high validity using the JAMAR® dynamometer. 34 , 35 Body mass index (BMI, kg/m2) was calculated. 36 Additionally, the Mini Nutritional Assessment (MNA, maximum score 30) was used to classify nutritional status (24–30 points: normal nutritional state, 17–23.5–points: risk for malnutrition, <17 points: poor nutritional status/malnutrition). 37
The number of supporting zones (ie two opposing pairs of teeth in occlusal contact (premolars and molars right and left) was recorded (categories: 0 none – 4 four support zones). The number of natural teeth was also recorded. The Eichner index 38 was used to classify the remaining dentition (categories: A: intermaxillary contact in all occlusal supporting zones, B: intermaxillary contact, not in all occlusal supporting zones; and C: no intermaxillary contact). The teeth and denture statuses were categorised into five (1—fully dentate, no dentures or fixed dentures, 2—partially dentate, fixed dentures, 3—partially dentate, removable denture, 4—partially dentate, no dentures available and 5—edentulous, removable dentures). The combination ‘edentulous, no dentures available’ was not necessary because of the inclusion criteria. The presence of dentures (categories: no denture available/used, removable denture, fixed denture, combination of removable and fixed dentures) and the type of denture (categories: complete denture, model cast prosthesis, temporary denture/moulded clamp, temporary denture, denture/precision attachment, telescopic denture, hybrid denture) were recorded separately for the upper and lower jaws. Denture quality was assessed (very good: no defects; no deviations from the ideal; good: acceptable quality, small deviations, corrections chairside possible; moderate: slight defects, correction by a dental technician necessary; poor: major defects, replacement required). 39 The dental treatment needs (assessment of the necessity and urgency of dental treatment) based on the dental examination, dental status, oral hygiene findings, and tooth and denture status (objective or relative treatment need: not necessary, immediate or acute, or plannable) were recorded. 32
The maximum occlusal force (in Newton, N) was recorded according to the procedure described in the literature, 40 using the occlusal force meter GM 10® (Morita, Nagano Keiki, Higashimagome, Ohta‐ku, Tokyo, Japan). The measurement (three times for each side of the jaw) for the region of the first molar or the closest area (dentures inserted, if available) was taken by applying the maximum possible jaw closing force. The peak value of the maximum occlusal force for all measurements was analysed.
Chewing efficiency was determined using the colour‐mixing ability test by Schimmel et al., 41 where the participants were chewing gums (Hue‐check Gum®, Orophys GmbH); this was a subjective visual assessment that used 5‐step ordinal scale (SA): SA1—chewing gum not mixed, impressions of cups or folded once; SA2—large parts of chewing gum unmixed; SA3—bolus slightly mixed, but bits of unmixed original colour; SA4—bolus well mixed, but colour not uniform; SA5—bolus perfectly mixed with uniform colour. An optoelectronic analysis was used to determine the ratio of the unmixed fraction of the chewing gum to the total pixel number in a fixed‐size template (variance of hue (VOH)) (ViewGum®, www.dhal.com). 42 The inadequate mixing of colours, which represents poor chewing efficiency, results in high VOH and vice versa. 41 , 42
2.3. Ethical considerations
The study was approved by the Cantonal Ethics Committee (CEC) of Zurich (KEK‐ZH 2017‐00363). All participants and legal guardians provided written informed consent.
2.4. Statistical considerations
Since the data for the endpoints of interest for the entire OrBiD study (OrBiD pilot study [ClinicalTrials.gov, NCT03775772] were for two study arms for the cross‐sectional and longitudinal components) could not be obtained, the sample size was not based on power calculations. The sample size was chosen within the same range used by comparable studies. 43 , 44 We assumed that a minimum sample size of 120 (24 participants [12 experimental and 12 controls] in each of the five MMSE groups) would allow us to obtain the first assessments of the influence of the mental status (cross‐sectional analysis) and the interventions related to the outcome variables (longitudinal analysis). Accordingly, the participants were recruited using stratified random sampling.
Descriptive statistics were used where indicated. Quantitative characteristics are presented as median, range, mean and SD. The qualitative characteristics were evaluated as absolute and relative frequencies. Pearson's chi‐squared test was used to determine the statistically significant differences between the expected and observed frequencies of the categories of the variables. The Kruskal–Wallis test was used to determine the differences in the central tendencies of several independent samples. The Jonckheere‐Terpstra test was used the same way as the Kruskal–Wallis test, with considerations of the MMSE score‐based grouping in this study. The p‐values of all statistical tests were interpreted. The significance level was set to α = .05. The statistical tests were not performed for variables with extremely low expected values. The statistical analysis was conducted using IBM SPSS version 23.0.
3. RESULTS
A total of 135 participants (mean age 81.7 ± 9.4 years, female n = 89, 65.9%) belonging to five MMSE groups were included for the analysis. The number of participants analysed was higher than the targeted 120 participants, as drop‐outs resulted in additional recruitment. This was carried out at the times of the drop‐outs to ensure an adequate number of participants for the longitudinal part of the OrBiD study (not analysed here).
Cognitive impairment increased significantly with age (p = .001; Jonckheere‐Terpstra test). The proportion of women in the MMSE group increased with age and cognitive impairment. This reflects a longer life expectancy 45 and a higher morbidity rate related to dementia in women. 46 This indicates an asymmetrical gender distribution in long‐term care facilities involved in the recruitment process of people with dementia. The MMSE groups differed based on all parameters of the oral functional capacity and the resilience capacity level (deterioration with an increase in dementia) (Table 1). Statistically significant differences were observed between the Barthel indexes (adjusted re‐tests: overall p = .001, significance in pairs in all MMSE groups after alpha adjustment p = .001, except between MMSE group noDem and mDem) and handgrip strengths (p = .001, Jonckheere‐Terpstra test). An increase in the number of participants who are malnourished or at risk of malnutrition with an increase in the prevalence of cognitive impairment has been observed (BMI, MNA) (Table 1).
TABLE 1.
Socio‐demographic characteristics, oral functional capacity, geriatric characteristics and nutritional characteristics for the entire cohort (n = 135) and stratified by the MMSE (Mini‐Mental State Examination) outcomes (no dementia (noDem), mild cognitive impairment (mCI), mild dementia (mDem), moderate dementia (modDem), severe dementia (sDem))
| Total, n = 135 | noDem, MMSE 28–30 (n = 26) | mCI, MMSE 25–27 (n = 29) | mDem, MMSE 18–24 (n = 27) | modDem, MMSE 10–17 (n = 28) | sDem, MMSE <10 (n = 25) | |
|---|---|---|---|---|---|---|
| MMSE | ||||||
| Median (range) | 21 (0–30) | 29 (28–30) | 27 (25–27) | 21 (18–24) | 13 (10–17) | 4 (0–9) |
| Age (years) | ||||||
| Median (range) | 83 (61–99) | 75 (62–92) | 80 (61–95) | 86 (65–95) | 88 (61–99) | 87 (67–97) |
| Sex (n/%) | ||||||
| Female | 89/65.9 | 13/50.0 | 18/62.1 | 20/74.1 | 20/71.4 | 18/72.0 |
| Oral functional capacity | ||||||
| Therapeutic capability (n/%) | ||||||
| Normal | 35/25.9 | 20/76.9 | 14/48.3 | 1/3.7 | 0/0 | 0/0 |
| Slightly reduced | 33/24.4 | 6/23.1 | 15/51.7 | 9/33.3 | 2/7.1 | 1/4.0 |
| Greatly reduced | 44/32.6 | 0/0 | 0/0 | 16/59.3 | 20/71.4 | 8/32.0 |
| None | 23/17.0 | 0/0 | 0/0 | 1/3.7 | 6/21.4 | 16/64.0 |
| Oral hygiene ability (n/%) | ||||||
| Normal | 23/17.0 | 16/61.5 | 6/20.7 | 1/3.7 | 0/0 | 0/0 |
| Slightly reduced | 37/27.4 | 9/34.6 | 19/65.5 | 7/25.9 | 2/7.1 | 0/0 |
| Greatly reduced | 48/35.6 | 1/3.8 | 4/13.8 | 16/59.3 | 19/67.9 | 8/32.0 |
| None | 27/20.0 | 0/0 | 0/0 | 3/11.1 | 7/25.0 | 17/68.0 |
| Self‐responsibility (n/%) | ||||||
| Normal | 47/34.8 | 24/92.3 | 21/72.4 | 2/7.4 | 0/0 | 0/0 |
| Reduced | 32/23.7 | 2/7.7 | 8/27.6 | 11/40.7 | 11/39.3 | 0/0 |
| None | 56/41.5 | 0/0 | 0/0 | 14/51.9 | 17/60.7 | 25/100 |
| Resilience capacity level (RCL) (n/%) | ||||||
| RCL 1 | 20/14.8 | 14/53.8 | 5/17.2 | 1/3.7 | 0/0 | 0/0 |
| RCL 2 | 30/22.2 | 11/42.3 | 18/62.1 | 1/3.7 | 0/0 | 0/0 |
| RCL 3 | 29/21.5 | 1/3.8 | 6/20.7 | 11/40.7 | 11/39.3 | 0/0 |
| RCL 4 | 56/41.5 | 0/0 | 0/0 | 14/51.9 | 17/60.7 | 25/100 |
| Geriatric items | ||||||
| Barthel index | (n = 123) | (n = 26) | (n = 29) | (n = 27) | (n = 22) | (n = 19) |
| Median (range) | 95 (0–100) | 100 | 100 (30–100) | 90 (25–100) | 30 (10–100) | 20 (0–65) |
| Handgrip strength (kg) | (n = 115) | (n = 25) | (n = 25) | (n = 24) | (n = 27) | (n = 14) |
| Median (range) | 16 (1–50) | 22 (12–50) | 20 (6–45) | 12 (3–38) | 12 (2–26) | 6.5 (1–16) |
| Nutritional items | ||||||
| Body Mass Index (BMI) | (n = 123) | (n = 26) | (n = 29) | (n = 26) | (n = 21) | (n = 21) |
| Median (range) | 23.0 (14–38.3) | 26.3 (18.2–38.3) | 25.1 (18.7–37.1) | 22.8 (17−37.1) | 20.3 (16.7–32) | 21.5 (14–28.2) |
| Mini Nutritional Assessment (MNA) | ||||||
| Normal | 51/41.1 | 21/80.8 | 18/62.1 | 9/34.6 | 3/13.6 | 0/0 |
| At risk | 56/45.2 | 3/11.5 | 11/37.9 | 15/57.7 | 15/68.2 | 12/57.1 |
| Malnourished | 17/13.7 | 2/7.7 | 0/0 | 2/7.7 | 4/18.2 | 9/42.9 |
3.1. Chewing function
The MMSE groups differed significantly based on chewing efficiency (VOH) (p = .003, Jonckheere‐Terpstra test). With the increase in dementia, a deterioration in chewing efficiency (ie an increase in VOH values) was observed in the modDem and sDem groups (Figure 1A).
FIGURE 1.
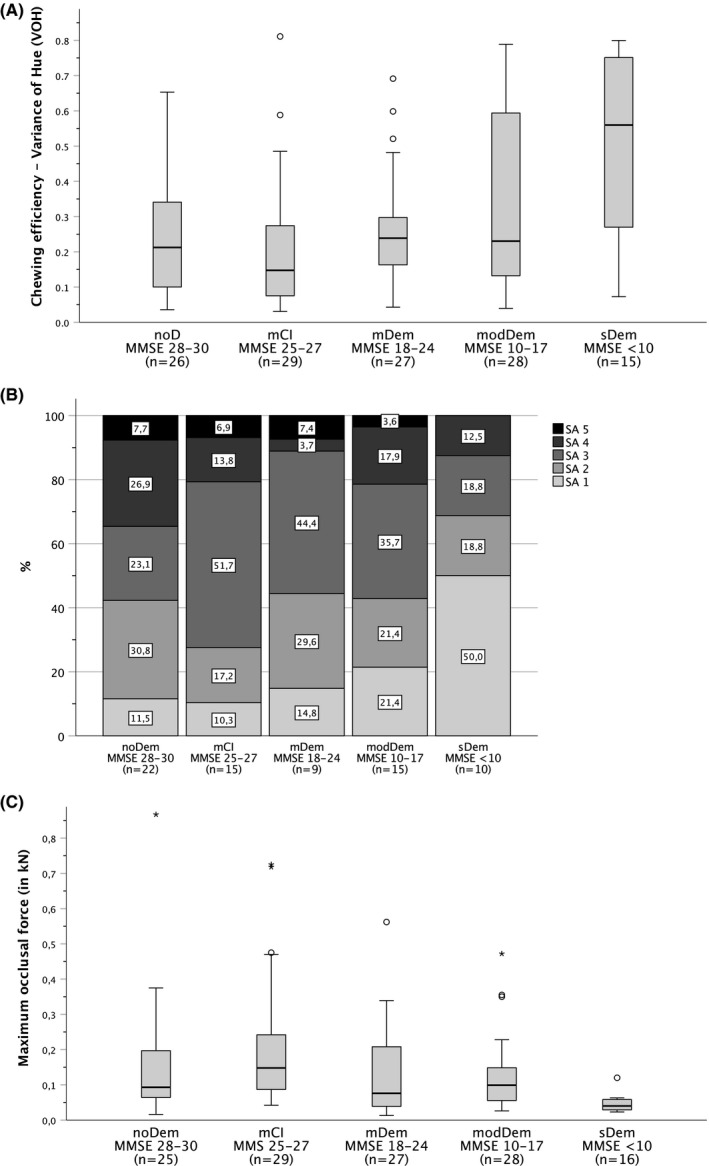
Chewing function as a function of the degree of dementia. (A) Chewing efficiency as the variance of hue (VOH) stratified by the Mini‐Mental State Examination (MMSE) score. Low values indicate good chewing efficiency and vice versa. [noDem, no dementia; mCI, mild cognitive impairment; mDem, mild dementia; modem, moderate dementia; sDem, severe dementia; MMSE, Mini‐Mental State Examination]. (B) Chewing efficiency was assessed using the Subjective Assessment (SA) Scale of the Mini‐Mental State Examination (MMSE). SA 1 and SA 2 indicate difficulties in chewing. Chewing efficiency increases with an increase in the SA scale from SA 1 to SA 5. [SA1—chewing gum not mixed, impressions of cups or folded once; SA2—large parts of chewing gum unmixed; SA3—bolus slightly mixed, but bits of unmixed original colour; SA4—bolus well mixed, but colour not uniform; SA5—bolus perfectly mixed with uniform colour]. [noDem—no dementia, mCI—mild cognitive impairment, mDem—mild dementia, modem—moderate dementia, sDem—severe dementia]. (C) Maximum occlusal force (kN) stratified by the Mini‐Mental State Examination (MMSE) scores. High values indicate a high maximum occlusal force and vice versa. [noDem, no dementia; mCI, mild cognitive impairment; mDem, mild dementia; modem, moderate dementia; sDem, severe dementia]
Within the mCI group, the lowest proportion of participants were unable to chew their food properly (mCI ‐ SA 1 and SA 2: n = 8, 27.5%). A total of 78.8% (n = 11) of the participants with sDem who were able to perform the test (n = 16, 64% of all participants in the sDem group) could not chew sufficiently (Figure 1B).
The maximum occlusal force differed significantly across the five MMSE groups (p = .003; Jonckheere‐Terpstra test). Because of the small number of participants in the sDem group (n = 7 of 25 participants) who were able to perform the test, the analysis of the maximum occlusal force in groups 1 (noDem) to 4 (modDem) was conducted, and a statistically significant difference was not observed (p = .106, Jonckheere‐Terpstra test) (Figure 1C).
3.2. Chewing function influencing factors
The MMSE groups did not differ based on the number of supporting zones (p = .055, chi‐square test) and the number of present natural teeth (p = .126, chi‐square test) (Table 2).
TABLE 2.
Factors influencing chewing function
| noDem, MMSE 28–30 (n = 26) | mCI, MMSE 25–27 (n = 29) | mDem, MMSE 18–24 (n = 27) | modDem, MMSE 10–17 (n = 28) | sDem, MMSE <10 (n = 25) | |
|---|---|---|---|---|---|
| Number of supporting zones | |||||
| Median (range) | 4 (2–4) | 4 (0–4) | 4 (0–4) | 4 (0–4) | 4 (1–4) |
| Number of natural teeth | |||||
| Related to 28 teeth | |||||
| Median (Range) | 13 (3–28) | 16 (2–28) | 22 (4–28) | 19 (4–27) | 19 (2–28) |
| Related to 32 teeth | |||||
| Median (Range) | 13 (3–28) | 17 (2–28) | 22 (4–29) | 19 (4–28) | 19 (2–32) |
| Presence of denture [n/%] | |||||
| Upper jaw | |||||
| No denture available/used | 2/7.7 | 5/17.2 | 4/14.8 | 3/10.7 | 8/32.0 |
| Removable denture | 19/73.1 | 14/48.3 | 8/29.6 | 12/42.9 | 10/40.0 |
| Fixed denture | 4/15.4 | 10/34.5 | 15/55.6 | 13/46.4 | 7/28.0 |
| Combination of removable and fixed | 1/3.8 | 0/0 | 0/0 | 0/0 | 0/0 |
| Lower jaw | |||||
| No denture available/used | 4/15.4 | 10/34.5 | 4/14.8 | 8/28.6 | 10/40.0 |
| Removable denture | 15/57.7 | 8/27.6 | 10/37.0 | 5/17.9 | 6/24.0 |
| Fixed denture | 6/23.1 | 9/31.0 | 13/48.1 | 14/50.0 | 9/36.0 |
| Combination of removable and fixed | 1/3.8 | 2/6.9 | 0/0 | 1/3.6 | 0/0 |
| Type of denture [n/%] | |||||
| Upper jaw | (n = 20) | (n = 14) | (n = 8) | (n = 12) | (n = 10) |
| Complete denture | 6/30 | 2/14.3 | 1/12.5 | 5/41.7 | 7/70 |
| Model cast prosthesis | 5/25 | 8/57.1 | 3/37.5 | 4/33.3 | 1/10 |
| Temporary denture moulded clamp | 2/10 | 0/0 | 0/0 | 0/0 | 0/0 |
| Temporary denture | 4/20 | 1/7.1 | 1/12.5 | 2/16.7 | 1/10 |
| Denture with precision attachment (telescopic denture, hybrid denture) | 3/15 | 3/31.4 | 3/37.5 | 1/8.3 | 1/10 |
| Lower jaw | (n = 16) | (n = 10) | (n = 10) | (n = 6) | (n = 6) |
| Complete denture | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Model cast prosthesis | 9/56.3 | 5/50 | 4/40 | 3/50 | 1/16.7 |
| Temporary denture moulded clamp | 3/18.8 | 0/0 | 0/0 | 0/0 | 1/16.7 |
| Temporary denture | 1/6.3 | 1/10 | 1/10 | 1/16.7 | 1/16.7 |
| Denture with precision attachment (telescopic denture, hybrid denture) | 3/18.8 | 4/40 | 5/50 | 2/33.3 | 3/50 |
| Denture quality (n/%) | |||||
| (n = 22) | (n = 15) | (n = 10) | (n = 13) | (n = 10) | |
| Very good | 10/45.5 | 5/33.3 | 1/10 | 0/0 | 1/10 |
| Good | 8/36.4 | 6/40 | 4/40 | 5/38.5 | 6/60 |
| Moderate | 2/9.1 | 3/20 | 4/40 | 7/53.8 | 1/10 |
| Poor | 2/9.1 | 1/6.7 | 1/10 | 1/7.7 | 2/20 |
| Treatment need | |||||
| Theoretical‐objective | |||||
| No treatment necessary | 11/42.3 | 7/24.1 | 3/11.1 | 3/10.7 | 1/4.0 |
| Emergency treatment necessary | 0/0 | 0/0 | 1/3.7 | 0/0 | 0/0 |
| Treatment plannable | 15/57.7 | 22/75.9 | 23/85.2 | 25/89.3 | 24/96.0 |
| Relativised‐objective | |||||
| No treatment necessary | 11/42.3 | 12/41.4 | 13/48.1 | 21/75.0 | 22/88.0 |
| Emergency treatment necessary | 0/0 | 0/0 | 1/3.7 | 0/0 | 0/0 |
| Treatment plannable | 15/57.7 | 17/58.6 | 13/48.1 | 7/25.0 | 3/12.0 |
[noDem—no dementia, mCI—mild cognitive impairment, mDem—mild dementia, modem—moderate dementia, sDem—severe dementia].
The greatest proportion of participants with intermaxillary contact in all occlusal support zones (Eichner index A) was found in the mDem group. The participants in the noDem and mCI groups had the highest proportion of intermaxillary contact, but not in all occlusal supporting zones (Eichner index B). The sDem group had the greatest proportion of participants with no intermaxillary contacts (Eichner index C) (Figure 2).
FIGURE 2.
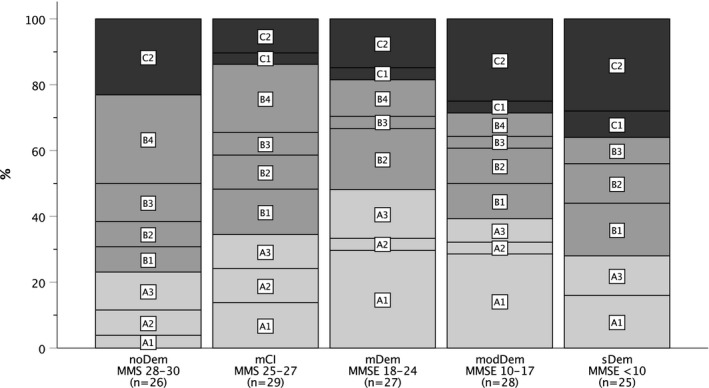
Eichner indexes stratified by MMSE (Mini‐Mental State Examination) scores. [noDem—no dementia, mCI—mild cognitive impairment, mDem—mild dementia, modem—moderate dementia, sDem—severe dementia] (Eichner Index categories/subcategories: A—Intermaxillary contact in all occlusal supporting zones: A1, both jaws fully dentate, individual teeth decayed, but can be restored; A2, one jaw dentate, one jaw with interdental gaps; A3, both jaws with interdental gaps; B—Intermaxillary contact, not in all occlusal supporting zones: B1, in three supporting zones; B2, in two supporting zones; B3, in one supporting zone; B4, occlusal contact outside the supporting zones; C—No intermaxillary contact: C1, residual teeth in both jaws without antagonistic contact; C2, one jaw edentulous, residual teeth in the other jaw; C3, both jaws edentulous (not applicable due to the eligibility criteria))
Most participants who were edentulous in one jaw and had removable dentures were found in the noDem and sDem groups. No participant was edentulous in the lower jaw. Due to the inclusion criteria, no subject was edentulous in either jaw or had no prosthetic restoration in the edentulous jaw. The proportion of fully edentulous participants with no or fixed dentures was approximately 10% in all the groups (except group mDem in the lower jaw 18.5%). Most of the participants were partially edentulous, although the proportion of participants without dentures in the upper jaw was lower, except for the participants with sDem. The participants with noDem and mCI were more likely to have removable dentures in the upper jaw if they were partially edentulous. In contrast, participants with mDem and modDem tended to have fixed dentures. The greater proportion of participants in all MMSE groups (except mDem) was partially edentulous but had no prosthetic restoration in the lower jaw than in the upper jaw. The fixed and removable dentures in the lower and upper jaws in partially edentulous participants were similarly distributed (Figure 3).
FIGURE 3.
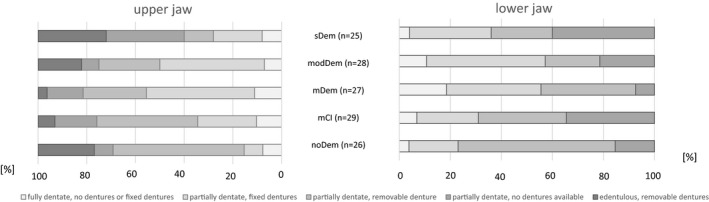
Tooth and denture statuses separated for the upper and lower jaws for the MMSE (Mini‐Mental State Examination) groups. [noDem—no dementia, mCI—mild cognitive impairment, mDem—mild dementia, modem—moderate dementia, sDem—severe dementia]
Denture quality in participants with noDem was predominantly assessed as very good and good (very good, n = 10, 45.5%; good, n = 8, 36.4%). With the increase in cognitive impairment (except for the MMSE group, sDem), the proportion of dentures rated as moderate increased (Table 2).
The theoretical objective treatment needs showed that treatment was predominantly plannable in all the MMSE groups. With the increase in dementia, this proportion is increasing. The relativised objective treatment should be based on considerations of the oral functional capacity of the participants. Here, it was observed that the treatment needs change, compared with the theoretical objective needs, with the worsening of dementia and the deterioration in resilience (increase in the resilience capacity level). The number of necessary plannable treatments decreases with an increase in the proportion of participants for whom no treatment is considered necessary (Table 2).
4. DISCUSSION
4.1. Study limitations
The present study has several limitations, which may have caused a bias.
The MMSE 31 is an easy‐to‐use and time‐efficient screening instrument for assessing cognitive impairment. Nevertheless, participants with mCI or mDem may already be familiar with the MMSE questions from medical examinations. 47 Due to time‐consuming alternatives, as for example the Montreal Cognitive Assessment Test, 48 which is more sensitive in mCI, and the non‐availability of a geriatric psychiatrist to perform this extensive screening, this bias could not be ruled out.
The investigator and participants were non‐blinded (bias especially for the investigator and participants with noDem, mCI and mDem). These biases could not be prevented for organisational and structural reasons (eg availability of study personnel, assignment to intervention groups for longitudinal observation).
Although various associations between chewing function and dementia have been described in the literature, 1 , 19 , 24 , 25 , 49 the measurement methods (eg mixing ability test), and their reliability for people with dementia should be questioned, as previously described in the literature 49 and observed by the investigator in the study. In this study, only the participants who were able to perform the measurements satisfactorily (decision based on investigators’ judgement of participants’ ability to follow simple instructions) were included. Therefore, bias by the investigator, as well as overrated positive results, cannot be ruled out, since there are currently no measurement methods adapted to people with dementia.
Since the maximum occlusal force varies, depending on the position of the teeth in the oral cavity 50 and the region of the measurement, 51 the measurements in this study were recorded according to the procedure described in the literature 40 (measurement at region of the first molar or the closest area (dentures inserted, if available)) using the occlusal force meter GM 10® to enable comparability with other studies. It should be assumed that the natural capacity of the masticatory muscle is greater than that depicted by the values indicated by the Occlusal Force Meter GM 10®, since not all participants may have applied their maximum possible bite force during measurement, for example, due to fear of injury and pain, among others. To rule out this bias, the investigator explained the measurement method to each participant, attempted to meet their fears and then asked for maximum occlusal force. If they had concerns, a test measurement was taken to get used to the device. Nevertheless, the device is frequently used because of its ease of application. 52 , 53
Due to the few participants who were able to conduct the measurements for chewing function, this study cannot make a conclusive statement about the influencing factors of chewing function. Furthermore, it is not possible to determine whether the general age‐related changes in the brain influence motor function and cognition 8 , 54 , 55 or specific changes due to dementia 56 are the causes of the MMSE group‐dependent differences in masticatory function, due to the lack of a longitudinal study component.
4.2. Discussion of the results and comparability with other studies
As described before in the literature, in this study, significant differences in chewing efficiency 1 , 19 , 24 , 49 and maximum occlusal force 25 were observed in association with degrees of dementia (MMSE groups) when considering all MMSE groups. However, most of the previous studies 1 , 19 , 24 , 49 were not comparable to the present study. The reasons for this lie in differences in the study population (different living conditions, age and cognitive impairment 1 , 24 ), in the measurement methods (assessment of chewing function subjectively using self‐assessment 19 ), or differences in the median MMSE values of the study population. 49
An analysation excluding the sDem group (low number of measurements) showed that there was no difference in maximum occlusal force between the MMSE groups, while chewing efficiency decreased with the increase in dementia (assumption of the first part of the initial hypothesis). This proves that high occlusal forces may not imply high chewing efficiency and vice versa since maximum occlusal force does not provide any information about the efficiency of the process because different factors influence the maximum occlusal force 6 and chewing efficiency. 1 , 2 , 3 , 4
Whereas, the number of supporting zones, number of natural teeth, Eichner index, tooth/denture status, denture quality, and dental treatment needs were not significantly associated with the degree of dementia (rejection of the second part of the initial hypothesis). Lexomboon et al. 19 stated that as long as older patients do not have difficulties in chewing hard food, factors, such as using natural teeth or dentures, may not play a significant role in cognitive impairment, which confirms our results (no difference in the number of teeth between different degrees of dementia). Nevertheless, the findings of the current study do not corroborate the findings of other previous studies, which show that the number of teeth, 1 , 20 number of missing teeth 57 , 58 , 59 , 60 , 61 or pairs of antagonistic teeth (ie supporting zones) 20 , 21 is significantly associated with chewing ability 7 or cognitive impairment or dementia 20 , 21 or an increased risk of dementia. 57 , 58 , 59 , 60 , 61 This may be attributed to the long‐term adaptation of the participants suffering from tooth loss and the associated chewing impairment. 27
Consequently, the study could not find evidence that the dental and oral status, as part of the ‘brain‐stomatognathic axis’, affects masticatory function in people with cognitive impairment or dementia. Referring to the top‐down regulation in the concept of the ‘brain‐stomatognathic axis’, 27 the authors concluded from their findings that cognitive influences or dementia, within the context of alterations in the brain, have a greater impact on masticatory performance than structural influences (eg tooth loss). 10 The brain's sensorimotor cortex responds to changes in oral function. 8 Oral pathological conditions, rehabilitation, oromotor training or prosthetic treatment influence the brain 11 , 27 , 62 , 63 through neuroplasticity. 63 , 64 Therefore, more emphasis should be placed on the comprehensive and structured rehabilitation of function (eg training or relearning of oral motor tasks for optimising masticatory performance in dental prosthesis wearers 11 ) than on the restoration of anatomic structures.
4.3. Importance and meaning of study outcomes
The concept of the ‘brain‐stomatognathic axis’ assumes that both the stomatognathic system and the brain/cognitive conditions contribute collaboratively to oral sensorimotor function. 27 , 65 On the one hand, a top‐down control from the brain to the stomatognathic system (eg control of coordination of motions) has been established. A causal relationship between a decrease in cognition and a chewing dysfunction 66 has been demonstrated in animal research. 67 , 68 , 69 , 70 On the other hand, it can be assumed that input from the stomatognathic system influences the brain. Tooth loss 71 , 72 , 73 and the resulting masticatory deficits 22 , 23 , 74 , 75 , 76 , 77 are due to clinical and epidemiological risk factors, discovered through animal studies, for the decline in cognitive functions.
This results in the importance of an integrative assessment of the brain and the stomatognathic system in geriatric and special needs patients to understand the clinical findings and transfer them to clinical practice. 78 The present study may therefore contribute to basic research on chewing function and its association with cognitive status and dementia due to its study design (assessment of MMSE, adequate and direct chewing function measurements, data on dental and prosthetic status).
4.4. Future research needs
Based on the findings and limitations of this study, the authors suggest that future research should be multidisciplinary, 27 and it should focus on long‐term studies that include a mandatory assessment of the stomatognathic system, cognitive ability or degree of dementia, an adequate assessment of chewing efficiency and maximum occlusal force, and an assessment of the brain and other physiological and non‐physiological aspects.
Because of the complexity of the masticatory system, correlations with other factors not localised in the oral cavity are obvious. The ‘brain‐stomatognathic axis’ concept should therefore be extended to include other possible influencing factors (physiological/non‐physiological, mechanical/non‐mechanical). Therefore, longitudinal studies should be adjusted to consider nutritional status, eating habits and general physical conditions 705), as well as microbiological, immunological, hormonal and behavioural factors. 22 , 23 , 27 In addition, lack of appetite, medications and social factors (eg loneliness) should not be disregarded as influencing factors as well.
An improvement in the applicability of the measurement methods for assessing chewing function in people with dementia is fundamental for future research. 49
CONFLICT OF INTEREST
The whole study was supported by Alzheimer Schweiz, altaDent and GABA. The funding sources were not involved in the study design, collection of the data, analysation and interpretation, writing of the article or its submission for publication. The authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
JJ contributed to conceptualisation and design, methodology, writing—original draft, visualisation and project administration. WH contributed to methodology and writing—review and editing (critically revising the manuscript). IN contributed to conceptualisation and design, methodology, writing—review and editing, supervision and project administration.
ACKNOWLEDGEMENTS
The authors would like to thank Alzheimer Schweiz and altaDent™ for the financial and GABA for material support of the study. Open Access Funding provided by Universitat Zurich.
Jockusch J, Hopfenmüller W, Nitschke I. Chewing function and related parameters as a function of the degree of dementia: Is there a link between the brain and the mouth? J Oral Rehabil. 2021;48:1160–1172. 10.1111/joor.13231
DATA AVAILABILITY STATEMENT
Data are available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Kimura Y, Ogawa H, Yoshihara A, et al. Evaluation of chewing ability and its relationship with activities of daily living, depression, cognitive status and food intake in the community‐dwelling elderly. Geriatr Gerontol Int. 2013;13(3):718‐725. [DOI] [PubMed] [Google Scholar]
- 2. Bourdiol P, Mioche L. Correlations between functional and occlusal tooth‐surface areas and food texture during natural chewing sequences in humans. Arch Oral Biol. 2000;45(8):691‐699. [DOI] [PubMed] [Google Scholar]
- 3. Ikebe K, Matsuda K‐I, Morii K, Furuya‐Yoshinaka M, Nokubi T, Renner RP. Association of masticatory performance with age, posterior occlusal contacts, occlusal force, and salivary flow in older adults. Int J Prosthodont. 2006;19(5):475–481. [PubMed] [Google Scholar]
- 4. Millwood J, Heath MR. Food choice by older people: the use of semi‐structured interviews with open and closed questions. Gerodontology. 2000;17(1):25‐32. [DOI] [PubMed] [Google Scholar]
- 5. Boven G, Raghoebar G, Vissink A, Meijer H. Improving masticatory performance, bite force, nutritional state and patient's satisfaction with implant overdentures: a systematic review of the literature. J Oral Rehabil. 2015;42(3):220‐233. [DOI] [PubMed] [Google Scholar]
- 6. Thompson D, Throckmorton G, Buschang P. The effects of isometric exercise on maximum voluntary bite forces and jaw muscle strength and endurance. J Oral Rehabil. 2001;28(10):909‐917. [DOI] [PubMed] [Google Scholar]
- 7. Carlsson GE, Lindquist LW. Ten‐year longitudinal study of masticatory function in edentulous patients treated with fixed complete dentures on osseointegrated implants. Int J Prosthodont. 1994;7(5):448‐453. [PubMed] [Google Scholar]
- 8. Avivi‐Arber L, Sessle BJ. Jaw sensorimotor control in healthy adults and effects of ageing. J Oral Rehabil. 2018;45(1):50‐80. 10.1111/joor.12554 [DOI] [PubMed] [Google Scholar]
- 9. Peyron MA, Woda A, Bourdiol P, Hennequin M. Age‐related changes in mastication. J Oral Rehabil. 2017;44(4):299‐312. 10.1111/joor.12478 [DOI] [PubMed] [Google Scholar]
- 10. Morita K, Tsuka H, Kato K, et al. Factors related to masticatory performance in healthy elderly individuals. J Prosthodont Res. 2018;62(4):432‐435. 10.1016/j.jpor.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 11. Kumar A, Kothari M, Grigoriadis A, Trulsson M, Svensson P. Bite or brain: implication of sensorimotor regulation and neuroplasticity in oral rehabilitation procedures. J Oral Rehabil. 2018;45(4):323‐333. 10.1111/joor.12603 [DOI] [PubMed] [Google Scholar]
- 12. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721‐733. 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin CS, Wu CY, Wu SY, et al. Age‐ and sex‐related differences in masseter size and its role in oral functions. J Am Dent Assoc. 2017;148(9):644‐653. 10.1016/j.adaj.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 14. Lin CS, Wu CY, Wang DH, et al. Brain signatures associated with swallowing efficiency in older people. Exp Gerontol. 2019;115:1‐8. 10.1016/j.exger.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 15. Park H, Suk S‐H, Cheong J‐S, et al. Tooth loss may predict poor cognitive function in community‐dwelling adults without dementia or stroke: the PRESENT project. J Korean Med Sci. 2013;28(10):1518‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saito Y, Sugawara N, Yasui‐Furukori N, Takahashi I, Nakaji S, Kimura H. Cognitive function and number of teeth in a community‐dwelling population in Japan. Ann Gen Psychiatry. 2013;12(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nilsson H, Berglund J, Renvert S. Tooth loss and cognitive functions among older adults. Acta Odontol Scand. 2014;72(8):639‐644. [DOI] [PubMed] [Google Scholar]
- 18. Peres MA, Bastos JL, Watt RG, Xavier AJ, Barbato PR, D’Orsi E. Tooth loss is associated with severe cognitive impairment among older people: findings from a population‐based study in Brazil. Aging Ment Health. 2015;19(10):876‐884. [DOI] [PubMed] [Google Scholar]
- 19. Lexomboon D, Trulsson M, Wårdh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60(10):1951‐1956. [DOI] [PubMed] [Google Scholar]
- 20. Delwel S, Maier AB, Parvaneh D, Meijers J, Scherder EJ, Lobbezoo F. Chewing efficiency, global cognitive functioning, and dentition: a cross‐sectional observational study in older people with mild cognitive impairment or mild to moderate dementia. Front Aging Neurosci. 2020;12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardoso MG, Diniz‐Freitas M, Vázquez P, Cerqueiro S, Diz P, Limeres J. Relationship between functional masticatory units and cognitive impairment in elderly persons. J Oral Rehabil. 2019;46(5):417‐423. [DOI] [PubMed] [Google Scholar]
- 22. Tada A, Miura H. Association between mastication and cognitive status: a systematic review. Arch Gerontol Geriatr. 2017;70:44‐53. 10.1016/j.archger.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 23. Tonsekar PP, Jiang SS, Yue G. Periodontal disease, tooth loss and dementia: is there a link? A systematic review. Gerodontology. 2017;34(2):151‐163. 10.1111/ger.12261 [DOI] [PubMed] [Google Scholar]
- 24. Kim EK, Lee SK, Choi YH, et al. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch Gerontol Geriatr. 2017;70:209‐213. [DOI] [PubMed] [Google Scholar]
- 25. Shin HE, Cho MJ, Amano A, Song KB, Choi YH. Association between mastication‐related factors and the prevalence of dementia in Korean elderly women visiting senior centres. Gerodontology. 2020;37(2):177‐184. [DOI] [PubMed] [Google Scholar]
- 26. Weijenberg RA, Scherder EJ, Lobbezoo F. Mastication for the mind–the relationship between mastication and cognition in ageing and dementia. Neurosci Biobehav Rev. 2011;35(3):483‐497. 10.1016/j.neubiorev.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 27. Lin CS. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr. 2018;18(1):5. 10.1186/s12877-017-0693-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kida K, Tsuji T, Tanaka S, Kogo M. Zinc deficiency with reduced mastication impairs spatial memory in young adult mice. Physiol Behav. 2015;152(Pt A):231‐237. 10.1016/j.physbeh.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 29. Kondo H, Kurahashi M, Mori D, et al. Hippocampus‐dependent spatial memory impairment due to molar tooth loss is ameliorated by an enriched environment. Arch Oral Biol. 2016;61:1‐7. 10.1016/j.archoralbio.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 30. Taniguchi Y, Shinkai S, Nishi M, et al. Nutritional biomarkers and subsequent cognitive decline among community‐dwelling older Japanese: a prospective study. J Gerontol A Biol Sci Med Sci. 2014;69(10):1276‐1283. 10.1093/gerona/glt286 [DOI] [PubMed] [Google Scholar]
- 31. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 32. Nitschke I, Kunze J, Hopfenmüller W, Reiber T. Die zahnmedizinische funktionelle Kapazität–ein Instrument in der Gerostomatologie. Quintessenz. 2012;63(2):207‐210. [Google Scholar]
- 33. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 34. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222‐226. [DOI] [PubMed] [Google Scholar]
- 35. Abizanda P, Navarro JL, García‐Tomás MI, López‐Jiménez E, Martínez‐Sánchez E, Paterna G. Validity and usefulness of hand‐held dynamometry for measuring muscle strength in community‐dwelling older persons. Arch Gerontol Geriatr. 2012;54(1):21‐27. [DOI] [PubMed] [Google Scholar]
- 36. Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25(6–7):329‐343. [DOI] [PubMed] [Google Scholar]
- 37. Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short‐form mini‐nutritional assessment (MNA‐SF). J Gerontol A Biol Sci Med Sci. 2001;56(6):M366‐M372. [DOI] [PubMed] [Google Scholar]
- 38. Eichner K. Über eine Gruppeneinteilung der Lückengebisse für die Prothetik. Dtsch Zahnarztl Z. 1955;10:1831‐1834. [Google Scholar]
- 39. Marxkors R. Kriterien für die zahnärztliche Prothetik. Partielle Prothese Studienhandbuch des Projektes: Qualitätssicherung in der Zahnmedizin Würzburg, Germany: Gesellschaft für Strahlen‐und Umweltforschung und Technologie; 1988:25‐26.
- 40. Varga S, Spalj S, Lapter Varga M, Anic Milosevic S, Mestrovic S, Slaj M. Maximum voluntary molar bite force in subjects with normal occlusion. Eur J Orthod. 2011;33(4):427‐433. [DOI] [PubMed] [Google Scholar]
- 41. Schimmel M, Christou P, Herrmann F, Müller F. A two‐colour chewing gum test for masticatory efficiency: development of different assessment methods. J Oral Rehabil. 2007;34(9):671‐678. [DOI] [PubMed] [Google Scholar]
- 42. Schimmel M, Christou P, Miyazaki H, Halazonetis D, Herrmann FR, Müller F. A novel colourimetric technique to assess chewing function using two‐coloured specimens: validation and application. J Dent. 2015;43(8):955‐964. [DOI] [PubMed] [Google Scholar]
- 43. Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Malekmahmoodi M, Shamsi M, Roozbahani N, Moradzadeh R. A randomized controlled trial of an educational intervention to promote oral and dental health of patients with type 2 diabetes mellitus. BMC Public Health. 2020;20(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. World Health Organization . Global Health Observatory (GHO) data. Women and health. Female mortality and causes of death. Female life expectancy, 2021. https://www.who.int/gho/women_and_health/mortality/life_expectancy_text/en/#:~:text=Women%20generally%20live%20longer%20than,differences%20between%20men%20and%20women (Accessed 03.02.21).
- 46. Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences between women and men in incidence rates of dementia and Alzheimer's disease. J Alzheimers Dis. 2018;64(4):1077‐1083. 10.3233/JAD-180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gross AL, Chu N, Anderson L, Glymour MM, Jones RN, Diseases CAM. Do people with Alzheimer’s disease improve with repeated testing? Unpacking the role of content and context in retest effects. Age Ageing. 2018;47(6):866‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak‐Szabela A, Kędziora‐Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini‐Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta‐analysis. Psychiatr Pol. 2016;50(5):1039‐1052. 10.12740/PP/45368 [DOI] [PubMed] [Google Scholar]
- 49. Weijenberg R, Lobbezoo F, Visscher C, Scherder E. Oral mixing ability and cognition in elderly persons with dementia: a cross‐sectional study. J Oral Rehabil. 2015;42(7):481‐486. [DOI] [PubMed] [Google Scholar]
- 50. Tortopidis D, Lyons MF, Baxendle RH, Gilmour WH. The variability of bite force measurements between sessions, in different positions within the dental arch. J Oral Rehabil. 1998;25:681‐686. [DOI] [PubMed] [Google Scholar]
- 51. Lujan‐Climent M, Martinez‐Gomis J, Palau S, Ayuso‐Montero R, Salsench J, Peraire M. Influence of static and dynamic occlusal characteristics and muscle force on masticatory performance in dentate adults. Eur J Oral Sci. 2008;116(3):229‐236. 10.1111/j.1600-0722.2008.00530.x [DOI] [PubMed] [Google Scholar]
- 52. Carlsson GE. Bite force and chewing efficiency. In: Kawamura Y, ed. Physiology of Mastication, Frontiers of Oral Physiology. Vol. 1. Karger, 1974:265‐292. [DOI] [PubMed] [Google Scholar]
- 53. Choy E, Kydd LW. Bite force duration: a diagnostic procedure for mandibular dysfunction. J Prosthet Dent. 1988;60:365‐368. [DOI] [PubMed] [Google Scholar]
- 54. Bernard JA, Seidler RD. Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 2014;42:193‐207. 10.1016/j.neubiorev.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62‐75. 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 56. Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172‐186. 10.1038/jcbfm.2015.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Batty G‐D, Li Q, Huxley R, et al. Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the action in diabetes and vascular disease: Preterax and Diamicron modified‐release controlled evaluation (ADVANCE) trial. Eur Psychiatry. 2013;28(1):49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaye EK, Valencia A, Baba N, Spiro A 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58(4):713‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paganini‐Hill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: the leisure world cohort study. J Am Geriatr Soc. 2012;60(8):1556‐1563. [DOI] [PubMed] [Google Scholar]
- 60. Tsakos G, Watt RG, Rouxel PL, de Oliveira C, Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc. 2015;63(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association between self‐reported dental health status and onset of dementia: a 4‐year prospective cohort study of older Japanese adults from the Aichi Gerontological evaluation study (AGES) project. Psychosom Med. 2012;74(3):241‐248. [DOI] [PubMed] [Google Scholar]
- 62. Henderson LA, Peck CC, Petersen ET, et al. Chronic pain: lost inhibition? J Neurosci. 2013;33(17):7574‐7582. 10.1523/JNEUROSCI.0174-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Luraschi J, Korgaonkar MS, Whittle T, Schimmel M, Müller F, Klineberg I. Neuroplasticity in the adaptation to prosthodontic treatment. J Orofac Pain. 2013;27(3):206‐216. 10.11607/jop.1097 [DOI] [PubMed] [Google Scholar]
- 64. Holland JH. Adaptation in Natural and Artificial Systems: An Introductory Analysis with Applications to Biology, Control, and Artificial Intelligence. MIT Press; 1992. [Google Scholar]
- 65. Lin CS, Yeung AWK. What do we learn from brain imaging? A primer for the dentists who want to know more about the association between the brain and human stomatognathic functions. J Oral Rehabil. 2020;47(5):659‐671. 10.1111/joor.12935 [DOI] [PubMed] [Google Scholar]
- 66. Pascual‐Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377‐401. 10.1146/annurev.neuro.27.070203.144216 [DOI] [PubMed] [Google Scholar]
- 67. Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11(1):57‐91. 10.1177/10454411000110010401 [DOI] [PubMed] [Google Scholar]
- 68. Sessle BJ, Adachi K, Avivi‐Arber L, et al. Neuroplasticity of face primary motor cortex control of orofacial movements. Arch Oral Biol. 2007;52(4):334‐337. 10.1016/j.archoralbio.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 69. Kubo KY, Murabayashi C, Kotachi M, et al. Tooth loss early in life suppresses neurogenesis and synaptophysin expression in the hippocampus and impairs learning in mice. Arch Oral Biol. 2017;74:21‐27. 10.1016/j.archoralbio.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 70. Fukushima‐Nakayama Y, Ono T, Hayashi M, et al. Reduced mastication impairs memory function. J Dent Res. 2017;96(9):1058‐1066. 10.1177/0022034517708771 [DOI] [PubMed] [Google Scholar]
- 71. Cerutti‐Kopplin D, Feine J, Padilha DM, et al. Tooth loss increases the risk of diminished cognitive function: a systematic review and meta‐analysis. JDR Clin Trans Res. 2016;1(1):10‐19. 10.1177/2380084416633102 [DOI] [PubMed] [Google Scholar]
- 72. Chen H, Iinuma M, Onozuka M, Kubo KY. Chewing maintains hippocampus‐dependent cognitive function. Int J Med Sci. 2015;12(6):502‐509. 10.7150/ijms.11911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Okamoto N, Morikawa M, Amano N, Yanagi M, Takasawa S, Kurumatani N. Effects of tooth loss and the Apolipoprotein E ɛ4 allele on mild memory impairment in the Fujiwara‐kyo Study of Japan: a nested case‐control study. J Alzheimers Dis. 2017;55(2):575‐583. 10.3233/JAD-160638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu B, Fillenbaum GG, Plassman BL, Guo L. Association between oral health and cognitive status: a systematic review. J Am Geriatr Soc. 2016;64(4):739‐751. 10.1111/jgs.14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Delwel S, Binnekade TT, Perez RS, Hertogh CM, Scherder EJ, Lobbezoo F. Oral health and orofacial pain in older people with dementia: a systematic review with focus on dental hard tissues. Clin Oral Investig. 2017;21(1):17‐32. 10.1007/s00784-016-1934-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Teixeira FB, Pereira Fernandes Lde M, Noronha PA, et al. Masticatory deficiency as a risk factor for cognitive dysfunction. Int J Med Sci. 2014;11(2):209‐214. 10.7150/ijms.6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Azuma K, Zhou Q, Niwa M, Kubo KY. Association between mastication, the hippocampus, and the HPA axis: a comprehensive review. Int J Mol Sci. 2017;18(8):1687. 10.3390/ijms18081687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin CS. Functional adaptation of oromotor functions and aging: a focused review of the evidence from brain neuroimaging research. Front Aging Neurosci. 2020;11:354. 10.3389/fnagi.2019.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request due to privacy/ethical restrictions.


