Abstract
Introduction
Numerous studies have been performed assessing optimal treatment regimens for evacuating (retained) products of conception from the uterus, but standardized criteria for diagnosing retained products of conception (RPOC) are still lacking. We aim to provide an overview of diagnostic criteria in current literature, used to diagnose RPOC after induced first‐trimester abortion or early pregnancy loss.
Material and methods
Pubmed, EMBASE, and the Cochrane library were searched systematically up until March 2020 for English articles reporting on induced abortion or early pregnancy loss. Articles not specifying diagnostic criteria used to assess completeness of treatment were excluded, as were conference abstracts, expert opinions, reviews, and case reports. Four elements of diagnostic criteria were described: diagnostic tools, parameters used within these tools, applied cut‐off values, and timing of follow up. Additionally, a meta‐analysis was performed assessing diagnostic qualities of the most often applied diagnostic tool and parameter.
Results
The search strategy yielded 1233 unique articles, of which 248 were included, with a total of 339 517 participants. In the 79 included randomized controlled trials, six diagnostic tools to assess RPOC were identified, combined in 14 ways, with 55 different cut‐off values. In 169 observational studies, seven diagnostic tools were identified, used in 28 combinations, applying 89 different cut‐off values. Transvaginal ultrasonographic measurement of endometrial thickness with a cut‐off value of at least 15 mm indicating RPOC, was used most frequently. In the timing of follow‐up there was great variation, with 55 and 107 different combinations in randomized controlled trials and observational studies, respectively. Assessment of treatment success was scheduled most often around 2 weeks after treatment. Diagnostic qualities of endometrial thickness of 15 mm or more was not adequately assessed.
Conclusions
There is wide variation in the way RPOC are assessed, and the criteria used to define RPOC following induced abortion and early pregnancy loss; ultrasonographic measurement of endometrial thickness, with a cut‐off of 15 mm or more 2 weeks after primary treatment is the most widely used diagnostic approach. A meta‐analysis on diagnostic accuracy of endometrial thickness of 15 mm or more did not lead to solid results. These findings can be a first step to develop a workable standard of establishing RPOC after induced abortion or early pregnancy loss.
Keywords: early pregnancy loss, induced abortion, miscarriage, retained products of conception, review, termination of pregnancy
Abbreviations
- EPL
early pregnancy loss
- ET
endometrial thickness
- RCT
randomized controlled trials
- RPOC
retained products of conception
Key message.
Retained products of conception after induced abortion or early pregnancy loss are detected most often by ultrasonographic endometrial measurement, with a cut‐off of 15 mm or more 2 weeks after primary treatment. A universal definition of suspected retained products of conception requires studies evaluating diagnostic accuracy and effectiveness.
1. INTRODUCTION
“Induced abortion” or “termination of pregnancy,” and “miscarriage” or “early pregnancy loss” (EPL) are very common. Approximately 73 million viable first‐trimester pregnancies are terminated each year, with increasing global numbers. 1 , 2 Furthermore, an estimated 10%–28% of pregnancies end in a miscarriage. 3 , 4 , 5 There are three main treatment options aiming to evacuate products of conception from the uterus; expectant management, surgical treatment using vacuum aspiration or dilatation and curettage, and medical treatment using prostaglandins with possible pre‐treatment with mifepristone. 6 , 7 , 8 , 9 , 10 , 11
Numerous studies have been performed assessing optimal treatment regimens for evacuating (retained) products of conception from the uterus. However, comparison of studies is hampered by heterogeneity of study populations and outcome definitions. This lack of standardization precludes firm conclusions about the most effective treatment regimens. 12 , 13 , 14 A recent systematic review of outcomes reported in randomized controlled trials (RCTs) of miscarriage prevention and treatment, found “efficacy” to be one of the most reported clinical outcomes. This end point was evaluated inconsistently, however, with a large variety of outcome measures and thresholds. 15
“Successful treatment” in induced abortion, is defined as “absence of the need for further intervention,” according to the Medical Abortion Reporting of Efficacy (MARE) guidelines. 16 Fiala et al. suggested modifying this definition to “expulsion of the gestational sac with no need for additional treatment.” 17 Crucial in this respect is that the need for “additional treatment,” common to both these proposed definitions, is invariably influenced by a suspected presence of retained products of conception (RPOC). However, there is still no consensus on how and when to assess and define RPOC. Moreover, the quantity of RPOC necessitating additional treatment is not defined, 18 , 19 , 20 nor is there any integration with clinical symptoms.
Standardized criteria for how to define, and when to evaluate, RPOC are of key importance when comparing treatment strategies for induced abortion or EPL, and they are currently lacking. In this review we report the diagnostic tools used and parameters assessed, including cut‐off values, to diagnose RPOC after induced abortion and EPL.
2. MATERIAL AND METHODS
We conducted a systematic scoping review assessing diagnostic criteria for RPOC following induced abortion and miscarriages or EPL in pregnancies with a gestational age of 14+0 weeks or less. Both articles on termination of viable first‐trimester pregnancies and those describing EPL were included. This review was registered on the PROSPERO International prospective register of systematic reviews (April 24, 2019, ID: CRD42019119081), according to recommended methods for systematic reviews, and reported according to PRISMA guidelines for scoping reviews. 21 At the request of the editorial team we also performed a meta‐analysis, assessing the diagnostic qualities of the most often applied diagnostic tool and parameter.
2.1. Main outcome measures
Four elements of the diagnostic criteria were assessed; (1) type of diagnostic tool, (2) parameter assessed with the diagnostic tool, (3) cut‐off value of parameter, and (4) time of parameter assessment.
2.2. Data sources
Three reviewers CH, SvW, and AC, systematically searched the literature up to March 31, 2020. Pubmed, Embase, and Cochrane Library electronic databases were searched, and reference lists were scanned. Databases of current experimental and observational studies were checked (Current Controlled Trials and ClinicalTrials.gov). All databases were searched using the terms abortion, early pregnancy loss, first trimester and complete or incomplete, all with several extensions to deliver the most comprehensive results possible. The detailed search strategy is provided as Appendix S1.
2.3. Eligibility criteria for selecting articles
All articles describing RCTs or observational studies reporting on interventions for termination of a viable first‐trimester pregnancy or EPL, managed expectantly, medically or surgically, were considered for inclusion. For this review, termination of a viable first‐trimester pregnancy was defined as any treatment, either surgical or medical, aiming to stop the pregnancy and evacuate it from the uterus up to 14 weeks of gestation. EPL is defined as either an early embryonic or fetal demise if ultrasonography showed an embryo with no cardiac activity, or as an anembryonic gestation if ultrasonography showed a gestational sac without development of an embryo, up to 14 weeks of gestation. Articles reporting on women with EPL, established by diagnostics other than ultrasound, or from clinical findings were also considered for inclusion. Articles reporting on women with pregnancies both before and beyond 14 weeks of gestation were included if data pertaining to pregnancies under 14 weeks of gestation could be distinguished, and extracted separately. There was no selection regarding which, if any (in the case of EPL), type of treatment was applied, nor for the year of publication, or the setting where the reported studies were performed. As the presence of RPOC is inversely related to treatment outcome (ie, presence of RPOC implies no successful treatment and vice versa), articles were only included if they either reported on “successful treatment” or “completeness of miscarriage.”
Conference abstracts, expert opinions, reviews, and case reports were excluded, as well as articles not available in English. Articles were excluded following full‐text screening if the diagnostic tools used to assess treatment success were not specified.
2.4. Data collection and analysis
Data were extracted in duplicate by three reviewers (CH, SvW, and AC). Methods of extraction were specified and documented in advance, and a data extraction sheet was developed. Items included study design, year of publication, participants, kind of treatment (and if applicable, control), diagnostic tools and parameters used to assess RPOC and their cut‐off values, and timing of follow up following diagnosis or initiation of treatment. Any disagreement between reviewers was resolved by mutual consensus of all three reviewers.
For the most frequently used diagnostic tool, and its most applied parameter, the use of both the tool and the parameter over time were assessed.
The data were compiled in Microsoft excel 2020 (Microsoft Corporation) for validation and coding. The data were then exported into SPSS version 26 (IBM Corp., released 2019) for analyses. Descriptive statistics were calculated to summarize the data. Frequencies and percentages were used to describe nominal data.
3. RESULTS
The search initially yielded 1233 unique articles. After a first screening based on the title and abstract, 869 articles were excluded for not meeting the predefined inclusion criteria. The remaining 364 articles were included for full‐text screening. For 33 articles, a full‐text was not available from the databases searched, nor through our university library (Radboud University, Nijmegen). Authors of these missing articles were contacted to provide a full‐text of their publication. None of the authors responded, leaving a total of 331 articles to check for eligibility by analyzing the full text. From these, we included 248 articles, 79 reporting RCTs and 169 reporting observational studies meeting the predefined inclusion criteria (included articles are provided in Table S1). The PRISMA flowchart for study selection is shown in Figure 1. The dates, origins, sizes, and study design of the included articles are shown in Table 1.
FIGURE 1.
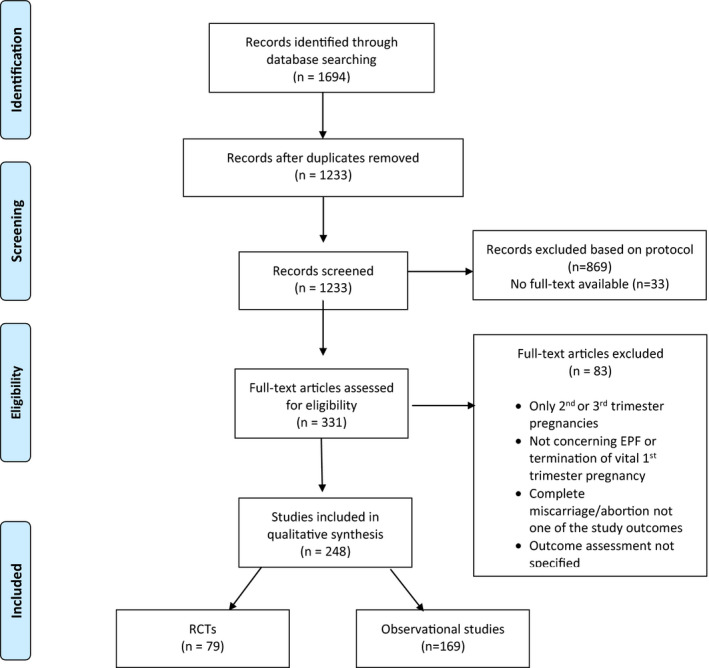
Flowchart for study selection
TABLE 1.
Study characteristics
| Study characteristics | RCTs (N = 79) | Observational studies (N = 169) | |
|---|---|---|---|
| Year of publication | 1970–1979 | 1 (1.3%) | 13 (7.7%) |
| 1980–1989 | 4 (5.1%) | 17 (10.1%) | |
| 1990–1999 | 15 (19.0%) | 29 (17.2%) | |
| 2000–2009 | 27 (34.2%) | 61 (36.1%) | |
| 2010–2019 | 32 (40.5%) | 49 (28.9%) | |
| Continent | Africa | 6 (7.6%) | 5 (3.0%) |
| Asia | 34 (43.0%) | 41 (24.3%) | |
| Oceania | 2 (2.5%) | 6 (3.6%) | |
| Europe | 20 (25.3%) | 65 (38.5%) | |
| North America | 11 (13.9%) | 42(24.8%) | |
| South America | 0 | 2 (1.2%) | |
| Multiple | 6 (7.6%) | 8 (4.7%) | |
| Sample size | 1–50 | 10 (12.7%) | 32 (18.9%) |
| 51–100 | 19 (24.1%) | 37 (21.9%) | |
| 101–200 | 24 (30.4%) | 33 (19.5%) | |
| 201–500 | 13 (16.5%) | 34 (20.1%) | |
| 501–1000 | 7 (8.9%) | 13 (7.7%) | |
| >1000 | 6 (7.6%) | 20 (11.8%) | |
Abbreviation: RCT, randomized controlled trial.
3.1. Randomized controlled trials
In the 79 RCTs included, six different diagnostic tools were used to assess or rule out the presence of RPOC, namely: clinical features (patient history, clinical interpretation, visual inspection of tissue lost), laboratory testing (human chorionic gonadotrophin (hCG) measurement), ultrasonographic imaging, and a miscellaneous set of tools summarized as “other.” In total, 14 different combinations of diagnostic tools, measuring different parameters, were found using up to 55 different cut‐off values. An overview of all parameters with their cut‐off values is provided in Table S2.
Ultrasonography was used to asses RPOC in 83.5% (66/79) of RCTs. Total endometrial thickness (ET), presence of gestational sac, and presence of intrauterine remains were used most frequently (36.4% [24/66], 22.7% [15/66], and 19.7% [13/66], respectively). The diagnostic tools and their parameters are shown in Table 2.
TABLE 2.
Diagnostic tools for evaluating the presence of retained products of conception with their four most frequently used parameters
| Diagnostic tool | Parameter | RCTs (N = 79) | Observational studies (N = 169) |
|---|---|---|---|
| Ultrasound | 66/79 (83.5%) | 124 (73.4%) | |
| Endometrial thickness | 24 (36.4%) | 31 (25.0%) | |
| Presence of gestational sac | 15 (22.7%) | 22 (17.7%) | |
| Intrauterine remains | 13 (19.7%) | 15 (12.1%) | |
| Uterus not empty | 2 (3.0%) | 15 (12.1%) | |
| Clinical interpretation | 32 (40.5%) | 78 (46.2%) | |
| Not specified | 14 (43.8%) | 32 (41.0%) | |
| Bleeding | 10 (31.3%) | 32 (41.0%) | |
| Uterine size | 3 (9.4%) | 12 (15.4%) | |
| Expulsion of POC | 5 (15.6%) | 4 (5.1%) | |
| Human chorionic gonadotropin | 22 (27.8%) | 59 (34.9%) | |
| Not negative | 5 (22.7%) | 22 (37.3%) | |
| Not specified | 5 (22.7%) | 12 (20.3%) | |
| Not <10 IU/L | 3 (13.6%) | 2 (3.4%) | |
| Decrease less than 50% | 3 (13.6%) | 4 (6.8%) | |
| No decrease | 2 (9.1%) | 7 (11.9%) | |
| Visual inspection of tissue lost | 3 (3.8%) | 9 (5.3%) | |
| Not specified | 2 (66.7%) | 3 (33.3%) | |
| No (complete) expulsion of POC | 1 (33.3%) | n.a. | |
| Examination of POC by staff | n.a. | 3 (33.3%) | |
| No tissue documented as POC | n.a. | 2 (22.2%) | |
| Pathologic/histologic examination of tissue | 0 (0%) | 7 (4.1%) | |
| POC histologically confirmed | n.a. | 5 (71.4%) | |
| Tissue obtained at curettage | n.a. | 2 (28.6%) | |
| Patients’ history | 4 (5.1%) | 5 (3.0%) | |
| Not specified | 2 (50.0%) | 4 (80.0%) | |
| No passage of POC | 1 (25.0%) | n.a. | |
| Persistence of heavy bleeding | 1 (25.0%) | n.a. | |
| Contractions, abdominal pain, bleeding | n.a. | 1 (20.0%) | |
| Other | 3 (3.8%) | 15 (8.9%) | |
| Not specified | 2 (66.7%) | 5 (33.3%) | |
| Ongoing pregnancy | 1 (33.3%) | n.a. | |
| No complete abortion | n.a. | 2 (13.3%) | |
| Other | n.a. | 2 (13.3%) |
Abbreviations: IU/L, international units per litre; n.a., not applicable; POC, products of conception; RCT, randomized controlled trial.
To aid clarity, the parameters presented are restricted to the four most commonly used. Hence, the total amount of articles reporting a particular diagnostic tool do not always correlate with the total number of articles applying one of the four relevant parameters.
Cut‐off values of ET defining RPOC varied from 5 mm or more to 30 mm or more. A cut‐off of 15 mm or more defining RPOC was used most often, in 66.7% (16/24) of RCTs reporting ET. A cut‐off value for ultrasonographic imaging was not specified in 18.2% (12/66) of articles. An overview of the ultrasonographic parameters and their cut‐off values is shown in Table 3.
TABLE 3.
Ultrasonographic parameters for evaluating retained products of conception with their cut‐off values
| Ultrasonographic parameter | Cut‐off value | RCTs (N = 79) | Observational studies (N = 169) |
|---|---|---|---|
| Endometrial thickness | 24 (30.4%) | 31 (18.3%) | |
| ≥5 mm | 1 (4.2%) | 1 (3.2%) | |
| ≥8 mm | 1 (4.2%) | 2 (6.5%) | |
| ≥10 mm | 2 (8.3%) | 4 (12.9%) | |
| ≥12 mm | n.a. | 1 (3.2%) | |
| ≥14 mm | n.a. | 1 (3.2%) | |
| ≥15 mm | 16 (66.7%) | 18 (58.1%) | |
| ≥20 mm | n.a. | 1 (3.2%) | |
| ≥30 mm | 4 (16.7%) | 3 (9.7%) | |
| Gestational sac | 15 (19.0%) | 22 (13.0%) | |
| Present or absent | 15 (100%) | 22 (100%) | |
| Intrauterine remains | 12 (15.2%) | 15 (8.9%) | |
| Present or absent | 10 (83.3%) | 12 (80%) | |
| >2 cm | n.a. | 1 (6.7%) | |
| >15 mm | n.a. | 2 (13.3%) | |
| Cavity surface area ≥6 cm2 | 1 (8.3%) | n.a. | |
| Intrauterine dimension ≥11 cm2 | 1 (8.3%) | n.a. | |
| Uterus not empty | 2 (2.5%) | 15 (8.9%) | |
| Yes or no | 2 (100%) | 15 (100%) |
Abbreviations: n.a., not available; RCT, randomized controlled trial.
In the 79 RCTs, a total of 54 different follow‐up regimens were specified. A complete overview of all regimens of follow up can be found in Table S3. Follow up was performed most often (in 32.9% or 26/79 articles) at 2 weeks after treatment, as shown in Table 4.
TABLE 4.
Three most applied moments of follow up, assessing presence of retained products of conception
| RCTs (N = 79) | Observational studies (N = 169) | |
|---|---|---|
| Moment of follow‐up | ||
| 1 week | 11 (13.9%) | 29 (17.2%) |
| 2 weeks | 26 (32.9%) | 59 (34.9%) |
| 1 and 2 weeks | 22 (27.8%) | 33 (19.5%) |
| Not specified | 0 (0%) | 5 (3.0%) |
Abbreviation: RCT, randomized controlled trial.
The three most applied moments of follow up are mentioned here, therefore the numbers in this table do not add up to total number of included articles.
3.2. Observational studies
In these 169 articles we identified seven diagnostic tools used to assess the presence of RPOC. These were the same as identified for RCTs with one additional tool: pathologic/histologic examination of tissue lost. A total of 28 different combinations of diagnostic tools measuring different parameters were used, with 89 different cut‐off values. A complete overview of all parameters with their cut‐off values can be found in Table S4.
In keeping with the RCTs, ultrasonography was used most often to assess RPOC, in 73.4% (124/169) of articles, and ET was the ultrasonic parameter reported most often (in 25.0% or 31/124 articles) followed by presence of the gestational sac, intrauterine remains, and a so‐called empty uterus (17.7% [22/124], 12.1% [15/124], and 12.1% [15/124], respectively). A cut‐off of 15 mm or more defining RPOC was most commonly used, being reported in 58.1% (18/31) of articles assessing ET. A cut‐off value for ultrasonographic imaging was not specified in 25.4% (43/169) of articles. An overview of the ultrasonographic parameters and their cut‐off values is shown in Table 3.
In the 169 included articles, 107 different regimens of follow up were used. A complete overview is shown in Table S5. Follow up was again performed most often at 2 weeks after treatment, in 34.9% of articles (59/169) (see Table 4).
3.3. Use of ultrasound over time
The proportion of studies using ultrasound, with or without ET measurement, to diagnose RPOC following induced abortion or EPL over time is summarized in Table 5. The use of ultrasound rose from 50% and 35% in RCTs and observational studies, respectively, during the 1980s to over 85% after the year 2000, regardless of study design. Measurement of ET was rarely used before 2000. The reporting of ET in RCTs evaluating induced abortion or EPL has risen in the last two decades, but is still reported in fewer than half such studies published.
TABLE 5.
Number of articles using ultrasound, and endometrial thickness (ET) measurement, to diagnose retained products of conception following early pregnancy loss or termination of pregnancy up to 14 weeks of gestation, per decade of article publication
| Year of publication | Number of publications | Number reporting ultrasound | Number reporting ET | Number reporting cut‐off value ET 15 mm | |
|---|---|---|---|---|---|
| RCTs (N = 79) | <1979 | 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| 1980–1989 | 4 | 2 (50%) | 0 (0%) | 0 (0%) | |
| 1990–1999 | 15 | 12 (80%) | 1 (6.67%) | 1 (100%) | |
| 2000–2009 | 27 | 24 (88.89%) | 8 (29.6%) | 5 (62.5%) | |
| 2010–2019 | 32 | 28 (87.5%) | 15 (46.9%) | 11 (73.3%) | |
| Observational studies (N = 169) | <1979 | 13 | 0 (0%) | 0 (0%) | 0 (0%) |
| 1980–1989 | 17 | 6 (35.3%) | 0 (0%) | 0 (0%) | |
| 1990–1999 | 29 | 22 (75.9%) | 1 (3.4%) | 0 (0%) | |
| 2000–2009 | 61 | 54 (88.5%) | 17 (27.9%) | (70.6%) | |
| 2010–2019 | 49 | 42 (85.7%) | 14 (28.6%) | 8 (57.1%) |
Abbreviations: ET, endometrial thickness; RCT, randomized controlled trial.
Between 2010 and 2019, 46% of RCTs and 28.6% of observational studies reported measurement of ET as assessment for RPOC. More than half of these articles use the cut‐off value of 15 mm.
3.4. Publications with no full‐text available
In an attempt to mitigate any bias arising from missing data, we checked the abstracts of the 33 articles without full‐text availability, for data varying significantly from available full‐text articles. However, only eight of these abstracts mentioned which diagnostic tool was used to determine the presence of RPOC, and no new diagnostic tools, or parameters were found (Table S6).
3.5. Additional analysis of the most frequently used diagnostic criterion and follow up
In order to make a statement about the applicability of ET of 15 mm as a possible standard for diagnosing RPOC, a meta‐analysis of articles reporting on this outcome was performed, as requested by the editorial team. There we aimed to evaluate the test accuracy of ET of 15 mm compared with the risk of need of curettage or other surgical treatment, and risk of uterine infection.
Unfortunately, our additional literature search did not yield any suitable articles, reporting a diagnostic accuracy of ET of 15 mm or less. Additionally, none of the articles included in our scoping review reported whether the women who developed an infection had an ultrasonographic ET of 15 mm or less. It was therefore not possible to evaluate the test accuracy on this matter.
Only three articles using ET of 15 mm as the cut‐off for RPOC gave information on whether the assessment of complete or incomplete miscarriage or abortion was correct. A diagnostic test accuracy evaluation, unfortunately with only these three articles, showed a high specificity of 94% or more, and a sensitivity of 75% up to 100% (Figure 2). Two of these articles used histology to confirm RPOC, the other used ultrasonographic assessment, because revision of the ultrasound at first follow up showed the intrauterine echogenic structures were measured incorrectly.
FIGURE 2.

Forest plot of diagnostic accuracy of endometrial thickness 15 mm for retained products of conception
In 11 articles using ET of 15 mm as the cut‐off for RPOC the mean time of vaginal bleeding was reported, ranging from 4 to 16 days, with an overall mean of 9 days after diagnosis or treatment. A distinction between participants with an ET above or below 15 mm was not made.
4. DISCUSSION
This systematic scoping review reveals wide variation in literature assessing the presence of RPOC after induced abortion and treatment of EPL up to 14 weeks of gestation. The most frequently used diagnostic tool in both RCTs and observational series was ultrasonography. Transvaginal ultrasonographic measurement of ET was the most often used ultrasonic parameter, although a wide range of cut‐off values was reported. Despite the observed large variation, in both RCTs and observational studies, a threshold of 15 mm or more to define RPOC was used most often, in over 50% of studies. Timing of assessment for RPOC in both RCTs and observational studies also varied widely. Assessment at 2 weeks after induced abortion, or diagnosis and management of EPL, was used most often, in almost one‐third of studies.
To our knowledge, this review is the first providing a scoping overview of the way RPOC after induced abortion or EPL is diagnosed. We included both RCTs and observational studies to maximize the data available for a comprehensive synthesis of the current literature.
Thirty‐three articles selected for full‐text screening, comprising 10% of all selected articles after abstract screening, were not available, despite maximum effort to obtain them. To ensure this did not lead to a bias, we checked the abstracts and found no new diagnostic tools or parameters. Five non‐English articles were excluded, none of these had defined “successful treatment,” “complete abortion,” or RPOC in their abstracts. The included data from 248 studies incorporate 339 517 patients. Exclusions and missing data exerted no substantial influence on the outcomes of our review.
As this is a scoping review, our aim was to provide an overview of diagnostic assessment for RPOC and the criteria or thresholds used for each diagnostic tool, rather than to ascribe greater validity to the selection of particular modes of diagnostic assessment according to methodological quality of the overall conduct of the study. We therefore did not assess methodological quality nor quality of describing and reporting outcomes, as this is most often not performed for scoping reviews, 21 and is deemed not relevant in the most recent PRISMA statement regarding these reviews. 22 We included observational studies in addition to higher quality RCTs to maximize the available data. The choice of diagnostic tools and variation in parameters or cut‐off values for diagnosing RPOC did not appear to differ between these two different methodological approaches to study design.
As “successful treatment” in cases of induced abortion of viable pregnancy or EPL has been defined as the absence of need for further intervention, it appears crucial to define clear‐cut, reproducible parameters that indicate that no further intervention is needed. 15 , 16 , 17 The presence or absence of RPOC is pivotal to this decision, and so, accurate and reproducible identification of RPOC from a consensus‐based threshold is crucial. Unrecognized and untreated RPOC can lead to both short‐term and long‐term complications such as persistent or heavy bleeding, and infection. However, standard treatment of any type or amount of RPOC also comes with a risk of complications, such as infection, bleeding, and intrauterine adhesions. 23 The World Health Organization advises surgical or medical treatment with misoprostol. 19 Surgical treatment bears the additional risk of cervical injury, intrauterine adhesions, or uterine perforation, whereas expectant management may increase the time to a next pregnancy, and medical treatment is not always effective. 10 Hysteroscopy may appear to be simple and safe, but is not always a feasible option worldwide. 24 As a result, RPOC need to be correctly identified in a timely fashion and, when necessary, treated in a safe and efficacious way. Therefore, a straightforward and workable definition of RPOC is essential.
The number of articles reporting on, and therefore using, ultrasound has been steadily rising since its first use in the 1990s. This review shows that ET, with a cut‐off value of 15 mm or more measured by transvaginal ultrasound, is used most often to establish the suspicion or diagnosis of RPOC. Several studies claim ultrasonographic measurement of ET to be a useful diagnostic tool, being both highly reliable to distinguish between complete and incomplete spontaneous miscarriages, and to predict which patients are more likely to require dilatation and curettage, and have unplanned visits to the emergency department. 25 , 26 Furthermore, it was recently found that ultrasonographic measurements of endometrial pattern and thickness could serve as objective criteria in the management of early medical abortions. 27 Ultrasonographic evaluation is known to have a higher sensitivity compared with clinical estimation. 28 , 29 , 30 On the other hand, these studies either used a different cut‐off value for ET, did not verify the actual presence of RPOC, or both. Other studies state that ET should only be used when combined with serum β‐hCG and/or clinical assessment. 31 , 32 Rørbye et al. 33 even state that ET and β‐hCG values are not useful in predicting late (>2 weeks) failure after medical abortion.
To evaluate the diagnostic accuracy of ET we aimed to perform a diagnostic meta‐analysis. However, as this analysis was based on three articles, only reporting on false negatives (the need for curettage with ET of <15 mm) the question is to what extent our findings are a reliable representation of the test properties, as there appears to be a verification bias.
These findings might explain why the use of ET for assessing RPOC is not mentioned in several (inter)national guidelines, and is not consistently used in recent prospective trials. 34 , 35 , 36 , 37 The only study regarding termination of first‐trimester viable pregnancy with a protocol available on clinicaltrials.gov uses ultrasonographic assessment, but does not specify a cut‐off value for presence of RPOC. 38
Follow up was most often undertaken at 2 weeks after treatment, although with variation in timing. Intuitively, the longer the follow‐up interval after treatment start, the higher the rate of complete miscarriages will be. 39 In this respect, the MisoREST trial showed that ultrasonic assessment at 6 weeks after treatment start leads to a complete miscarriage in up to 85% of women suspected of RPOC (ET of >10 mm)at assessment at 2 weeks. 40 The agreed timing of assessment following treatment to achieve optimal patient outcomes in terms of morbidity and minimizing the need for medical or surgical re‐intervention remains to be determined. As treatment of RPOC comes with the potential risk of complications, it is essential for future research to investigate if, how, and when RPOC should be treated.
As shown by this review, there is a large variability in diagnostic criteria used to identify RPOC, which could be due to the lack of consensus on this topic. The “guidance for developing guidelines” in 2010 recommended using the Delphi method for guidelines. 41 Therefore, we believe final consensus regarding diagnostic criteria for establishing RPOC should be obtained using the Delphi method among a group of experts on this topic. Although this review emphasizes the need for consensus, it remains unclear what diagnostic criteria would be most suited for use in clinical practice, with regards to accuracy and applicability. A specific Pubmed search into these aspects of ultrasound criteria did not identify any primary diagnostic accuracy studies to inform a test accuracy meta‐analysis. This subject should therefore also be the focus of future research.
5. CONCLUSION
There is a large variability in diagnostic criteria used to identify RPOC after termination of first‐trimester viable pregnancy or EPL at gestational age of less than 14 weeks, regarding diagnostic tool, parameter, cut‐off values, and time of assessment. At present, ultrasonographic measurement of the ET with a cut‐off of 15 mm or more at 2 weeks or more after primary treatment to detect RPOC is the most widely used diagnostic approach. A meta‐analysis on diagnostic accuracy did not lead to trustworthy results. There is a lack of consensus concerning the diagnostics used for RPOC; future research should aim to provide evidence for the best diagnostic criteria for RPOC.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
ME, MS, SC, and CH contributed to the conception and design of the review. CH, SvW, and AC contributed to the acquisition and analysis of data. CH drafted the manuscript and ME, MS, SC, SvW, and JC critically revised the manuscript.
Supporting information
Appendix S1. Detailed search strategy
Table S1. Included articles
Table S2. Complete overview of reported diagnostic tools, primary outcome parameters, and their cut‐off values in randomized controlled trials
Table S3. Timing of follow up in randomized controlled trials
Table S4. Complete overview of reported diagnostic tools, primary outcome parameters, and their cut‐off values in observational studies
Table S5. Timing of follow up in observational studies
Table S6. Information retrieved from articles with no full‐text available
Hamel CC, van Wessel S, Carnegy A, et al. Diagnostic criteria for retained products of conception—A scoping review. Acta Obstet Gynecol Scand. 2021;100:2135–2143. 10.1111/aogs.14229
REFERENCES
- 1. Sedgh G, Bearak J, Singh S, et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet. 2016;388:258‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guttmacher Intstitute . Induced Abortion Worldwide Fact Sheet. New York: Guttmacher Intstitute; 2016. [Google Scholar]
- 3. Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res Part A Clin Mol Teratol. 2012;94:417‐423. [DOI] [PubMed] [Google Scholar]
- 4. Buck Louis GM, Sapra KJ, Schisterman EF, et al. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: the LIFE Study. Fertil Steril. 2016;106:180‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage—results from a UK‐population‐based case‐control study. BJOG. 2007;114:170‐186. [DOI] [PubMed] [Google Scholar]
- 6. Allen R, O’Brien BM. Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol. 2009;2:159‐168. [PMC free article] [PubMed] [Google Scholar]
- 7. Soulat C, Gelly M. Immediate compliations of surgical abortion. J Gynecol Obstet Biol Reprod. 2006;35:157‐162. [DOI] [PubMed] [Google Scholar]
- 8. Niinimäki M, Jouppila P, Martikainen H, Talvensaari‐Mattila A. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367‐372. [DOI] [PubMed] [Google Scholar]
- 9. Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta‐analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long‐term reproductive outcome. Hum Reprod Update. 2014;20:262‐278. [DOI] [PubMed] [Google Scholar]
- 10. Lemmers M, Verschoor MAC, Hooker AB, et al. Dilatation and curettage increases the risk of subsequent preterm birth: a systematic review and meta‐analysis. Hum Reprod. 2016;31:34‐45. [DOI] [PubMed] [Google Scholar]
- 11. Hamel C, Coppus S, van den Berg J, et al. Mifepristone followed by misoprostol compared with placebo followed by misoprostol as medical treatment for early pregnancy loss (the Triple M trial): a double‐blind placebo‐controlled randomised trial. EClinicalMedicine. 2021;32:100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Den Berg J, Gordon BBM, Snijders MPML, Vandenbussche FPHA, Coppus SFPJ. The added value of mifepristone to non‐surgical treatment regimens for uterine evacuation in case of early pregnancy failure: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol. 2015;195:18‐26. [DOI] [PubMed] [Google Scholar]
- 13. Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JPA. Expectant, medical, or surgical management of first‐trimester miscarriage: a meta‐analysis. Obstet Gynecol. 2005;105:1104‐1113. [DOI] [PubMed] [Google Scholar]
- 14. Lemmers M, Verschoor MAC, Kim BV, et al. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2019;6:CD002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith P, Dhillon‐Smith R, O’Toole E, Cooper N, Coomarasamy A, Clark T. Outcomes in prevention and management of miscarriage trials: a systematic review. BJOG. 2019;126:176‐189. [DOI] [PubMed] [Google Scholar]
- 16. Creinin MD, Chen MJ. Medical abortion reporting of efficacy: the MARE guidelines. Contraception. 2016;94:97‐103. [DOI] [PubMed] [Google Scholar]
- 17. Fiala C, Cameron S, Bombas T, et al. Outcome of first trimester medical termination of pregnancy: definitions and management. Eur J Contracept Reprod Heal Care. 2018;23:451‐457. [DOI] [PubMed] [Google Scholar]
- 18. Royal College of Obstetricians and Gynaecologists . The Care of Women Requesting Induced Abortion (Evidence‐Based Clinical Guideline Number 7). London: Royal College of Obstetricians and Gynaecologists; 2011. [Google Scholar]
- 19. World Health Organization . Clinical Practice Handbook for Safe Abortion. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 20. Faúndes A. The combination of mifepristone and misoprostol for the termination of pregnancy. Int J Gynecol Obstet. 2011;115:1‐4. [DOI] [PubMed] [Google Scholar]
- 21. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91‐108. [DOI] [PubMed] [Google Scholar]
- 22. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169:467. [DOI] [PubMed] [Google Scholar]
- 23. Hooker AB, Aydin H, Brölmann HAM, Huirne JAF. Long‐term complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156‐164.e2. [DOI] [PubMed] [Google Scholar]
- 24. Golan A, Dishi M, Shalev A, Keidar R, Ginath S, Sagiv R. Operative hysteroscopy to remove retained products of conception: novel treatment of an old problem. J Minim Invasive Gynecol. 2011;18:100‐103. [DOI] [PubMed] [Google Scholar]
- 25. Rulin MC, Bornstein SG, Campbell JD. The reliability of ultrasonography in the management of spontaneous abortion, clinically thought to be complete: a prospective study. Am J Obstet Gynecol. 1993;168:12‐15. [DOI] [PubMed] [Google Scholar]
- 26. Lavecchia M, Klam S, Abenhaim HA. Effect of uterine cavity sonographic measurements on medical management failure in women with early pregnancy loss. J Ultrasound Med. 2016;35:1705‐1710. [DOI] [PubMed] [Google Scholar]
- 27. Tzeng CR, Hwang JL, Au HK, Chien LW. Sonographic patterns of the endometrium in assessment of medical abortion outcomes. Contraception. 2013;88:153‐159. [DOI] [PubMed] [Google Scholar]
- 28. Shalev J, Royburt M, Fite G, et al. Sonographic evaluation of the puerperal uterus: correlation with manual examination. Gynecol Obstet Invest. 2002;53:38‐41. [DOI] [PubMed] [Google Scholar]
- 29. Wong SF, Lam MH, Ho LC. Transvaginal sonography in the detection of retained products of conception after first‐trimester spontaneous abortion. J Clin Ultrasound. 2002;30:428‐432. [DOI] [PubMed] [Google Scholar]
- 30. Ben‐Ami I, Schneider D, Maymon R, Vaknin Z, Herman A, Halperin R. Sonographic versus clinical evaluation as predictors of residual trophoblastic tissue. Hum Reprod. 2005;20:1107‐1111. [DOI] [PubMed] [Google Scholar]
- 31. Haimov‐Kochman R, Arbel R, Sciaky‐Tamir Y, Brzezinski A, Laufer N, Yagel S. Risk factors for unsuccessful medical abortion with mifepristone and misoprostol. Acta Obstet Gynecol Scand. 2007;86:462‐466. [DOI] [PubMed] [Google Scholar]
- 32. El‐Baradie SMY, El‐Said MH, Ragab WS, Elssery KM, Mahmoud M. Endometrial thickness and serum β‐hCG as predictors of the effectiveness of oral misoprostol in early pregnancy failure. J Obstet Gynaecol Canada. 2008;30:877‐881. [DOI] [PubMed] [Google Scholar]
- 33. Rørbye C, Nørgaard M, Nilas L. Prediction of late failure after medical abortion from serial beta‐hCG measurements and ultrasonography. Hum Reprod. 2004;19:85–89. 10.1093/humrep/deh041 [DOI] [PubMed] [Google Scholar]
- 34. Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161‐2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsia JK, Lohr PA, Taylor J, Creinin MD. Medical abortion with mifepristone and vaginal misoprostol between 64 and 70 days’ gestation. Contraception. 2019;100:178‐181. [DOI] [PubMed] [Google Scholar]
- 36. ACOG . The American College of Obstetricians and Gynaecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;124:1258‐1267. [DOI] [PubMed] [Google Scholar]
- 37. NICE guideline . Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management. London: National Institute for Health and Care Excellence; 2019. [Google Scholar]
- 38. Grossman D. Mail order mifepristone study. 2019; https://clinicaltrials.gov/ct2/show/NCT03913104. Accessed January 18, 2021.
- 39. Mizrachi Y, Tamayev L, Shemer O, Kleiner I, Bar J, Sagiv R. Early versus delayed follow‐up after misoprostol treatment for early pregnancy loss. Reprod Biomed Online. 2019;39:155‐160. [DOI] [PubMed] [Google Scholar]
- 40. Lemmers M, Verschoor MAC, Oude Rengerink K, et al. MisoREST: surgical versus expectant management in women with an incomplete evacuation of the uterus after misoprostol treatment for miscarriage: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;211:83‐89. [DOI] [PubMed] [Google Scholar]
- 41. Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Medicine. 2010;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Detailed search strategy
Table S1. Included articles
Table S2. Complete overview of reported diagnostic tools, primary outcome parameters, and their cut‐off values in randomized controlled trials
Table S3. Timing of follow up in randomized controlled trials
Table S4. Complete overview of reported diagnostic tools, primary outcome parameters, and their cut‐off values in observational studies
Table S5. Timing of follow up in observational studies
Table S6. Information retrieved from articles with no full‐text available


