Abstract
Background and purpose
Short‐interval intracortical inhibition by threshold tracking (T‐SICI) has been proposed as a diagnostic tool for amyotrophic lateral sclerosis (ALS) but has not been compared directly with conventional amplitude measurements (A‐SICI). This study compared A‐SICI and T‐SICI for sensitivity and clinical usefulness as biomarkers for ALS.
Methods
In all, 104 consecutive patients referred with suspicion of ALS were prospectively included and were subsequently divided into 62 patients with motor neuron disease (MND) and 42 patient controls (ALS mimics) by clinical follow‐up. T‐SICI and A‐SICI recorded in the first dorsal interosseus muscle (index test) were compared with recordings from 53 age‐matched healthy controls. The reference standard was the Awaji criteria. Clinical scorings, conventional nerve conduction studies and electromyography were also performed on the patients.
Results
Motor neuron disease patients had significantly reduced T‐SICI and A‐SICI compared with the healthy and patient control groups, which were similar. Sensitivity and specificity for discriminating MND patients from patient controls were high (areas under the receiver operating characteristic curves 0.762 and 0.810 for T‐SICI and A‐SICI respectively at 1–3.5 ms). Paradoxically, T‐SICI was most reduced in MND patients with the fewest upper motor neuron (UMN) signs (Spearman ρ = 0.565, p = 4.3 × 10−6).
Conclusions
Amplitude‐based measure of cortical inhibition and T‐SICI are both sensitive measures for the detection of cortical involvement in MND patients and may help early diagnosis of ALS, with T‐SICI most abnormal before UMN signs have developed. The gradation in T‐SICI from pathological facilitation in patients with minimal UMN signs to inhibition in those with the most UMN signs may be due to progressive degeneration of the subset of UMNs experiencing facilitation.
Keywords: amyotrophic lateral sclerosis, conventional TMS, short‐interval intracortical inhibition, transcranial magnetic stimulation, threshold tracking TMS
In 104 patients referred with suspicion of ALS, two methods of recording short‐interval intracortical inhibition (SICI) detected cortical involvement with high sensitivity and specificity. The new method of threshold‐tracking SICI (T‐SICI) was paradoxically most reduced in the patients with fewest upper motor neuron signs, suggesting facilitation in a subset of cortical neurons which subsequently degenerate.
![]()
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a devastating disease, without well‐known aetiology or risk factors [1, 2, 3, 4, 5 and with progressive involvement of upper motor neurons (UMNs) and lower motor neurons (LMNs). The diagnosis is made clinically, and patients are categorized as definite, probable or possible ALS, depending on the distribution of body regions with clinical or electrophysiological signs of LMN degeneration and UMN involvement [6, 7. Patients presenting solely with LMN involvement are classified as progressive muscular atrophy (PMA) and are not considered as ALS according to the revised El‐Escorial [8] or Awaji criteria [6]. Overt or subclinical LMN affection can be reliably detected by routine neurophysiological techniques, notably needle electromyography (EMG). However, there is currently no consensus on the value of electrodiagnostic methods to assess subclinical UMN involvement, so that only UMN clinical signs are accepted in the ALS diagnostic criteria. This may result in delays to diagnosis and commencement of adequate management strategies and recruitment into therapeutic trials.
Single‐pulse transcranial magnetic stimulation (TMS) techniques have low diagnostic sensitivity in ALS [9, 10 unless the F‐wave method [11] or proximal muscle recordings [12] are used. By contrast, abnormalities can be more readily detected by paired‐pulse TMS protocols, such as short‐interval intracortical inhibition (SICI), when a preceding subthreshold conditioning stimulus given at an inter‐stimulus interval (ISI) of 1–5 ms reduces the amplitude of the response to a suprathreshold test stimulus [13]. A reduction of this amplitude‐based measure of cortical inhibition (A‐SICI) was first described in a small cohort of 14 ALS patients [14]. Later, a serial threshold tracking SICI (T‐SICI) methodology was introduced, whereby a target motor evoked potential (MEP) amplitude is tracked and the changes in stimulus intensity in response to subthreshold conditioning stimuli are recorded [15]. This method has been widely used by one group and has been shown to have a high diagnostic utility [10, 16, and it has been advocated as a potential biomarker for ALS [18, 19. However, these findings have never been confirmed by other research groups [20] or compared directly with conventional A‐SICI measurements.
A recent study found that the serial T‐SICI method distorted the relationship between inhibition and ISI [21]. This distortion was avoided by using a parallel T‐SICI method, previously used to assess dependence of SICI on conditioning stimulus intensity [22]. Parallel T‐SICI also estimated inhibition with less variability than serial T‐SICI, although not as little as was achieved by A‐SICI [21].
The clinical diagnostic sensitivity and specificity of A‐SICI and T‐SICI (using the new parallel method) were therefore compared in a cohort of well‐characterized patients referred with the suspicion of ALS, in whom the diagnoses were confirmed or excluded by clinical follow‐up. The findings of T‐SICI and A‐SICI were also correlated with the clinical presentations of the patients, to detect any differences in specificity that might provide insights into the pathophysiology of the disease.
METHODS
Subjects
This study included prospectively consecutive patients referred with the suspicion of ALS in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) criteria [23] and applied the index test and reference standard to all patients at the time of recruitment. None of the patients had been diagnosed with the disease before recruitment; therefore none of the patients was receiving riluzole. All examinations were undertaken at Aarhus University Hospital. Only right‐handed subjects were included. Exclusion criteria were the use of drugs that could affect cortical excitability, history of epilepsy, known dementia and presence of a pacemaker or other metallic biomedical device.
In total, 133 patients were eligible for inclusion and accepted to participate between April 2018 and September 2020 (Figure 1). Of these, 24 patients were excluded because SICI could not be recorded. These patients were more advanced with mostly probable and definite ALS (Figure 1), which is consistent with a previous study [24]. Five patients were excluded because of inconclusive follow‐up diagnosis. The final study cohort of 104 patients comprised two groups: motor neuron disease (MND) patients (n = 62) and patient controls with mimicking disorders (n = 42). Exclusion of mimic disorders by ancillary investigations and disease progression at the clinical follow‐up was required to confirm the MND diagnosis. The categorization of the MND patients according to Awaji criteria [6] was noted at the inclusion. Fifteen patients did not fulfil the ALS criteria and were categorized as PMA when they had LMN signs in at least two regions (n = 5) or as unclassified MND (n = 10) based on the clinical and conventional electrophysiological test results on the examination day. The results were compared with 53 healthy controls.
FIGURE 1.
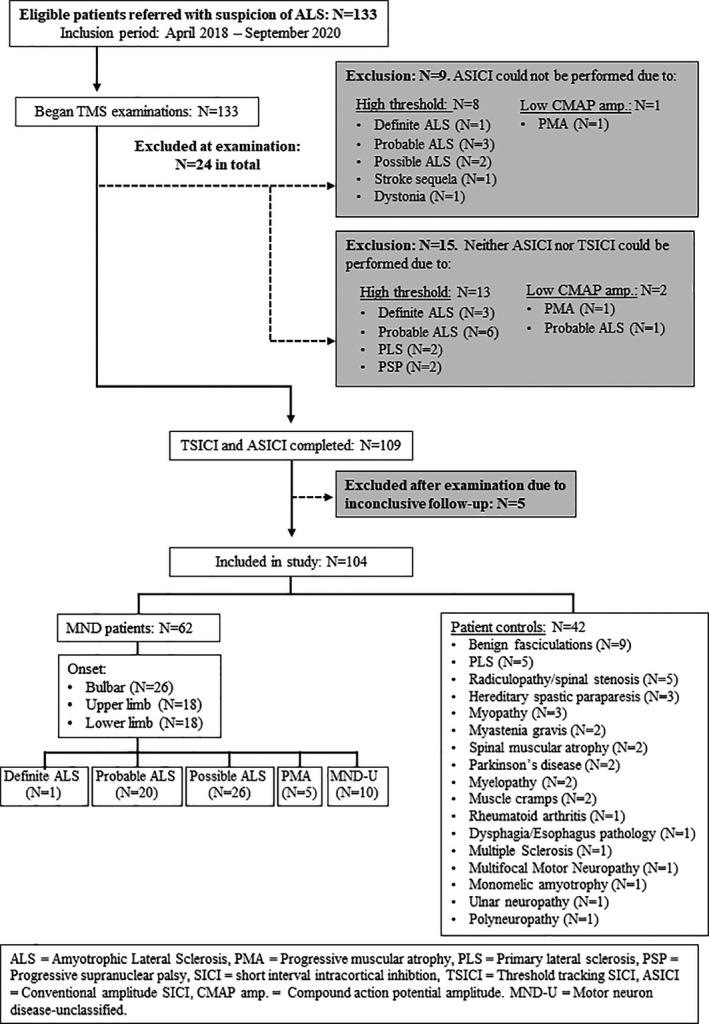
Schematic diagram of patient classification. ALS, amyotrophic lateral sclerosis; A‐SICI, conventional amplitude‐based SICI; CMAP amp., compound action potential amplitude; MND‐U, motor neuron disease unclassified; PLS, primary lateral sclerosis; PMA, progressive muscular atrophy; SICI, short‐interval intracortical inhibition; T‐SICI, threshold tracking SICI
In all patients, a detailed clinical examination was performed and the clinical scores were noted. T‐SICI and A‐SICI recordings, nerve conduction studies and EMG were carried out in all subjects.
All participants gave written informed consent in accordance with the Declaration of Helsinki II. The project was approved by the local ethical committee (case 1‐10‐72‐201‐17).
Clinical scores
The disease duration (in months) from time of symptom onset and the region of onset were noted. UMN involvement was graded using a modified Penn UMN score (UMNS), with higher scores (maximum 27) corresponding to greater disease burden [25, 26. Single points were given for an abnormal jaw‐jerk reflex, palmomental sign and pseudobulbar affect [27]. In the extremities, deep tendon reflexes (biceps, triceps, ankle), pathological reflexes (Hoffman´s and Babinski´s sign and clonus) and spasticity [28] were scored.
Muscle strength using the Medical Research Council (MRC) score for shoulder abduction, elbow flexion, wrist extension, index finger abduction, thumb abduction, hip flexion, knee extension and ankle dorsiflexion was evaluated, yielding a maximum total score of 80. Disease severity was staged using the revised ALS Functional Rating Score (ALSFRS‐R) [29] which yields a maximum score of 48.
Nerve conduction studies and electromyography
Conventional methods were performed according to the department´s routine [30, 31. The only data presented here are the peak‐to‐peak amplitude recorded from the first dorsal interosseus muscle (FDI) and the FDI EMG data. For EMG, the incidence of fibrillation potentials, positive sharp waves and fasciculations at 10 different sites was noted [30] and 20 motor unit potentials were recruited for quantitative analysis.
Transcranial magnetic stimulation
Threshold tracking SICI and A‐SICI measurements were performed in random order. Subjects were seated comfortably in an armchair and asked to relax but keep awake. A figure‐of‐eight coil (Magstim® D70 Remote Coil) connected to two Magstim®200 [2] stimulators was positioned at approximately 4 cm left in the binauricular line from vertex, with the handle pointing 45° to the parasagittal plane. Once the hotspot was located, the outline of the coil was drawn on a swimming cap to enable constant coil positioning and the automated stimulation protocol was initiated. Stimulus delivery and data acquisition were controlled by QtracW software (© University College London) using QTMSG‐12 recording protocols.
The MEPs were measured from the right FDI using disposable surface electrodes placed in a belly‐tendon fashion. The MEP was amplified (1000× gain) and filtered (3 Hz to 3 kHz) using a D440‐2 Channel Isolated Amplifier (Digitimer Ltd). A 50/60 Hz Humbug Noise Eliminator was used to remove line frequency contamination, and the amplified signals were digitized (NI USB‐6251, National Instruments).
Resting motor threshold
Resting motor thresholds (RMTs) for a 200 µV (RMT200) or for a 1 mV (TS1 mV) peak‐to‐peak response were detected by a 4 → 2 → 1 tracking rule, as previously described [21]. RMTs and all further thresholds, whether conditioned or unconditioned, were estimated from the stimuli and responses by weighted logarithmic regression [15, 21.
Threshold tracking SICI and A‐SICI protocols
For T‐SICI the parallel tracking method previously designated T‐SICIp2 was used, in which SICI at each ISI was tracked independently, whilst the A‐SICI protocol was as described previously [21]. For both methods, conditioning stimulus amplitude was set to 70% RMT200 but, whereas in A‐SICI the test stimuli were fixed at TS1 mV, for T‐SICI the test stimuli tracked the 200 µV target. In both methods test‐alone stimuli were delivered after each three conditioning + test combinations, with the ISIs (1, 1.5, 2, 2.5, 3, 3.5, 4, 5 and 7 ms) presented in pseudo‐random order. Each of the nine paired stimuli was delivered 10 times, making a total of 120 stimuli with the test‐alone stimuli.
Data analysis
Recordings were analysed with QtracW software: T‐SICI thresholds were estimated by log regression and A‐SICI amplitudes were averaged as geometric means. A‐SICI amplitudes were normalized, to overcome the ‘floor’ effect, by log conversion and scaled to become comparable with the T‐SICI thresholds, using the relationship found in the healthy controls (see Results) [21]. Mean SICI values were compared between the MND patients and healthy controls as well as between MND patients and patient controls using independent samples t tests. Correlation analyses were made using parametric or non‐parametric tests depending on normality. The ability of a method to discriminate patients from controls was evaluated by determining the area under the receiver operating characteristic (ROC) curve. The age and sex differences between groups were evaluated by analysis of variance tests. p < 0.05 was considered significant.
RESULTS
Patient demographics
Of the 104 patients, 62 patients had disease progression at the clinical follow‐up (up to 2½ years) and were classified as MND patients, whilst in 42 patients, designated patient controls, other diagnoses were given. Of the 62 MND patients, 47 fulfilled the criteria for ALS (definite, 1; probable, 20; possible, 26) whilst 15 patients had only LMN involvement confirmed with EMG at the inclusion. In 42 patients, other diagnoses were given according to the clinical follow‐up (Figure 1). The ages of the three main groups were not significantly different by analysis of variance (mean ± SD: healthy controls 62.9 ± 12.1, patient controls 61.1 ± 15.0, MND patients 66.4 ± 10.3; p = 0.081), but the ratio of males to females was higher in the patient control group (male to female: healthy controls 27/26, patient controls 33/9, MND patients 39/23). The mean disease duration for the MND patients was 11.9 ± 6.4 months, and their mean ALSFRS‐R score was 40.5 ± 4.5.
Relationship between A‐SICI and T‐SICI in healthy controls
Figure 2a shows the relationship between all the A‐SICI and T‐SICI measurements on healthy control subjects, that is, 477 measurements, comprising nine ISIs in 53 subjects. As previously described [21] there is a strong relationship between T‐SICI and log A‐SICI amplitude. This relationship enables the A‐SICI measurements, which exhibit a pronounced ‘floor’ effect (see Figure 2b), to be normalized so that they are comparable to the T‐SICI measurements by A‐SICI‐T = 100 − 18.51 × log 10(A‐SICI/100). These A‐SICI‐T values are compared for 1–3.5 ms means in Figure 2c, and as for the younger healthy subjects in the previous study [21] the A‐SICI distribution is effectively normalized and the A‐SICI‐T values have less variation between subjects than the T‐SICI values.
FIGURE 2.
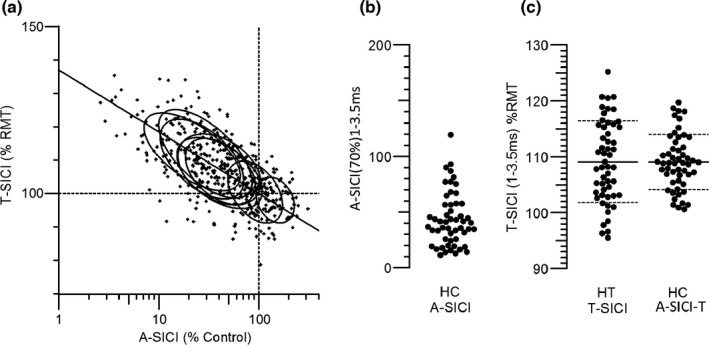
Relationship between A‐SICI and T‐SICI in healthy control subjects. (a) Plot of T‐SICI threshold as a percentage of RMT against log of A‐SICI amplitude as a percentage of RMT1000. Each small cross indicates a measurement on one subject at one of the nine ISIs tested. The ellipses indicate the relationships for each ISI. The line is the best fit straight line through the (100, 100) point to the 477 data points: T‐SICI = 100 − 18.51 × log 10(A‐SICI/100). (b) A‐SICI amplitude, as a percentage of control, averaged between 1 and 3.5 ms, showing the ‘floor’ effect. (c) Comparison between T‐SICI and A‐SICI from 1 to 3.5 ms, after A‐SICI values were normalized to A‐SICI‐T values according to the relationship in (a)
Dependence of T‐SICI, A‐SICI and A‐SICI‐T on ISI for the three groups
Figure 3 shows the mean A‐SICI, A‐SICI‐T and T‐SICI values in MND patients, patient controls and healthy controls. Inhibition is shown in A‐SICI by a reduction of the amplitude but in T‐SICI and A‐SICI‐T by an increase in threshold. There was significantly less inhibition in the MND patients, compared with either control group, for both T‐SICI and A‐SICI, whereas there was no significant difference between the two control groups. The difference between MND patients and controls was less for A‐SICI‐T than for T‐SICI, but the variation in A‐SICI‐T values was less than for T‐SICI as indicated by the coefficients of variation in Figure 4.
FIGURE 3.
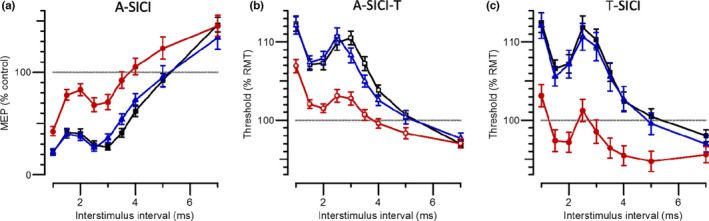
Dependence of SICI measurements on inter‐stimulus interval. (a) A‐SICI plotted as the amplitude of conditioned response as a percentage of control. (b) A‐SICI values normalized to comparable T‐SICI thresholds as a percentage of RMT200. (c) T‐SICI thresholds. Each plot compares the SICI versus ISI relationship for healthy control subjects (black squares), patient controls (blue triangles) and MND patients (red circles), showing geometric means ×/÷ geometric SE in (a) and arithmetic means ± SE in (b) and (c)
FIGURE 4.
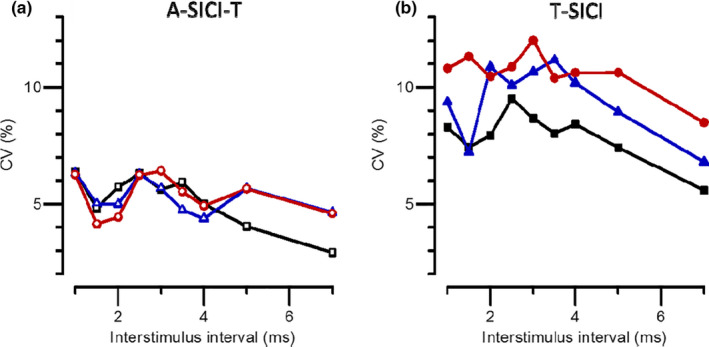
A‐SICI and T‐SICI variability between subjects. Variability is indicated by coefficients of variation (CV, i.e. SD/control threshold) for (a) normalized A‐SICI and (b) T‐SICI measurements. Symbols and colours as in Figure 3: healthy control subjects (black squares), patient controls (blue triangles) and MND patients (red circles)
Discrimination between controls and MND patients by A‐SICI and T‐SICI
Figure 3 shows that both A‐SICI and T‐SICI discriminated clearly between MND patients and both control groups. A better idea of the degree to which individuals were discriminated is given by the examples of mean thresholds from 1 to 3.5 ms in Figure 5. The sensitivity and specificity of the techniques as diagnostic tools is best compared by the areas under ROC curves (ROC AUCs), which are given in Table 1. For SICI averaged from 1 to 3.5 ms, discrimination between patient controls and MND patients can be regarded as ‘good’ (AUCs ~ 0.8) by both methods. However, it is patients with LMN signs but insufficient UMN signs to be classified as ALS for whom a new UMN biomarker would be most useful, and Table 1 (right side) shows that for these the discrimination is even better (AUCs close to 0.9).
FIGURE 5.
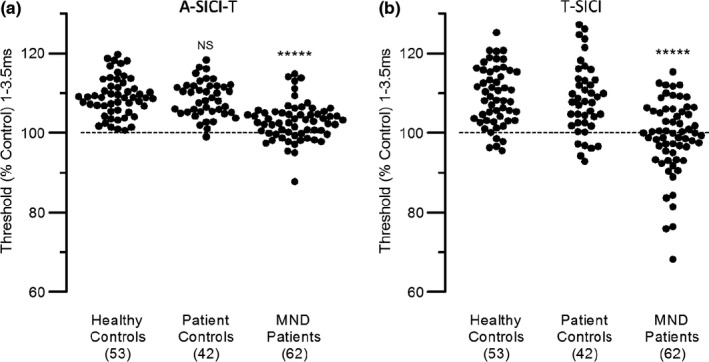
SICI differences between subject groups. SICI averaged from 1 to 3.5 ms compared between healthy controls, patient controls and MND patients. (a) Normalized A‐SICI. (b) T‐SICI. Healthy and patient controls were not significantly different, by t test; asterisks indicate that MND patients were significantly different from controls (p < 0.00001)
TABLE 1.
The discrimination of short‐interval intracortical inhibition patient controls and patients
| ISI (ms) | Patient controls (42) vs. MND patients (62) | Patient controls (42) vs. PMA + MND‐U (15) | ||
|---|---|---|---|---|
| T‐SICI | A‐SICI | T‐SICI | A‐SICI | |
| 1.0 | 0.725 | 0.706 | 0.785 | 0.817 |
| 1.5 | 0.728 | 0.808 | 0.795 | 0.859 |
| 2.0 | 0.742 | 0.820 | 0.859 | 0.854 |
| 2.5 | 0.728 | 0.805 | 0.871 | 0.873 |
| 3.0 | 0.746 | 0.757 | 0.864 | 0.863 |
| 3.5 | 0.727 | 0.735 | 0.814 | 0.805 |
| 4 | 0.686 | 0.704 | 0.800 | 0.776 |
| 5 | 0.626 | 0.630 | 0.759 | 0.689 |
| 7 | 0.547 | 0.569 | 0.618 | 0.646 |
| 1–7 | 0.737 | 0.732 | 0.851 | 0.829 |
| 1–3.5 | 0.762 | 0.810 | 0.867 | 0.890 |
| 2–3 | 0.754 | 0.820 | 0.881 | 0.878 |
Areas under the receiver operating characteristic curves comparing patient controls with all motor neuron disease (MND) patients and MND patients not classified as amyotrophic lateral sclerosis, that is, progressive muscular atrophy (PMA) and unclassified MND patients (MND‐U).
The bold is the highest of all ISIs and ISI combinations tested.
Abbreviations: A‐SICI, short‐interval intracortical inhibition measured by amplitude changes; ISI, inter‐stimulus interval; T‐SICI, short‐interval intracortical inhibition measured by threshold tracking.
Nature of the change in SICI in MND patients and relationship to UMN signs
The most profound abnormality in SICI was the facilitation recorded by T‐SICI in MND patients with few UMN signs (Figure 6a). In contrast, patients with the most pronounced UMN signs had almost normal SICI. A‐SICI changes were not so strongly related to UMNS (Figure 6b), but comparison of the differences in SICI from healthy controls, as a function of ISI, indicated that a similar time course of relative facilitation was detected by both techniques (Figure 6c). This time course, with a single peak at 3 ms, was very different from what might have been expected from a simple loss of inhibition.
FIGURE 6.
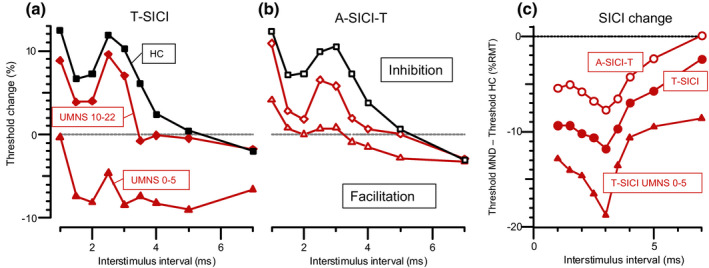
SICI facilitation in MND patients. (a) T‐SICI as a function of ISI plotted separately for patients with high and low UMN scores, compared with healthy controls (HC), showing facilitation rather than inhibition in patients with UMNS from 0 to 5 (triangles), but almost normal inhibition in patients with UMNS from 10 to 22 (diamonds). (b) Normalized A‐SICI data plotted similarly, showing less facilitation. (c) SICI differences from healthy controls, showing similar time courses of relative facilitation. T‐SICI and A‐SICI‐T data for all MND patients in circles, and T‐SICI data for low UMNS patients in triangles
The relationship between SICI measurements and UMNS is explored in more detail in Figure 7, where the MND patients have been divided into five UMNS subgroups. Mean SICI from 1 to 3.5 ms shows a progressive change with UMNS that is paradoxical, in the sense that SICI is most abnormal in the MND patients with the lowest UMNS and becomes more normal as the UMNS gets worse, that is, higher. The correlation between these two variables is highly significant (Spearman ρ = 0.565, p = 4.3 × 10−6), and even stronger for the peak of facilitation at 3 ms (ρ = 0.634, p = 1.5 × 10−7). On the other hand, normalized A‐SICI behaves rather differently (Figure 7b). The correlation with UMNS is much weaker (ρ = 0.388, p = 0.0021), but whereas T‐SICI may be normal in the high UMNS patients there is still an abnormality in A‐SICI‐T. It is notable that these conspicuous abnormalities in SICI are not accompanied by any significant changes in resting motor thresholds (Figure 7c,d), so that conditioning stimuli (at 70% RMT200 for T‐SICI and A‐SICI) are similar for all these recordings.
FIGURE 7.
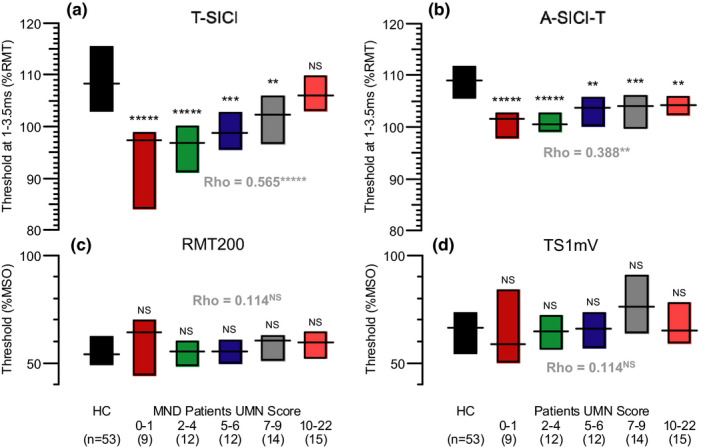
Paradoxical relationship between SICI and UMN score in MND patients. (a) Mean T‐SICI from 1 to 3.5 ms comparing healthy controls with progressive changes in UMN score in subgroups of MND patients, with the most abnormal SICI found in patients with the fewest UMN signs. (b) Corresponding changes in normalized A‐SICI. (c) RMT200 values for the same recordings as in (a), showing no significant differences. (d) TS1 mV values for the recordings in (b). In each plot, horizontal lines are medians and bars indicate interquartile ranges. Asterisks indicate significant differences from healthy controls by Mann–Whitney U test: NS, p > 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; *****p < 0.00001. Spearman's rho values also shown for correlations between UMNS and SICI or RMT for all 62 MND patients
Correlation between SICI measurements and LMN findings in MND patients
Threshold tracking SICI also showed paradoxical correlations with several LMN‐dominated measures of motor neuron degeneration. These included MRC sum score (ρ = −0.36, p = 0.0045), fibrillation potentials/positive sharp waves (e.g., at 2–3 ms, ρ = 0.489, p = 0.00026) and FDI force in the right hand, evaluated with the MRC score (ρ = −0.366, p = 0.0036). There was no significant correlation between T‐SICI parameters and motor amplitude, fasciculations or motor unit potential parameters. As with the UMN measures, LMN correlations with A‐SICI were less significant: A‐SICI‐T did not correlate with the MRC score or any other EMG or nerve conduction studies measures. There was no significant correlation between T‐SICI or A‐SICI and ALSFRS‐R.
DISCUSSION
This study is the first to compare threshold tracking and conventional SICI methods in a substantial cohort of patients referred for suspicion of ALS or other MNDs. Following our previous comparison between three SICI methods in healthy controls [21] it was suspected that our method of assessing SICI by conventional amplitude measurements (A‐SICI) would provide less variable measurements and therefore be better at revealing UMN dysfunction in the patients than our parallel threshold tracking method (T‐SICI). In the event, the results have proved more complicated and more interesting than merely revealing differences in discrimination. They have shown that the two methods are affected differently by the cortical degeneration in MND patients, with different susceptibilities, and that the methods are not only good candidates for use as diagnostic biomarkers in suspected ALS but provide new insights into the likely pathophysiological mechanisms involved.
The T‐SICI protocol used in this study differs from the one used in the previous papers on T‐SICI and ALS. Whereas the former studies used a serial tracking protocol in which ISIs were increased progressively from 1 to 7 ms and only the changes in threshold were tracked, a parallel protocol was used in which thresholds at each ISI were tracked independently in parallel. This followed the finding that serial tracking results in threshold estimates that are too dependent on the previous one, so that the profile of SICI versus ISI depends strongly on the initial settings and on the direction of change of ISI [21]. Parallel tracking is therefore better suited to resolving the ISI profiles of SICI abnormality in MND, as illustrated in Figure 6c.
Threshold tracking SICI and A‐SICI differences and the pathophysiology of MNDs
In healthy subjects there is a consistent relationship between the two different SICI measurements as inhibition varies with ISI [21] and this is illustrated in Figure 2. In MND, however, this simple logarithmic relationship breaks down, and T‐SICI exhibits a greater reduction in SICI than expected from the relationship with A‐SICI which is most pronounced in patients with the fewest UMN symptoms (Figures 6 and 7) and therefore most probably in the earliest stages of the disease. Our new data provide compelling reasons for concluding that this discrepancy between the two SICI measurements is because the dominant abnormality in these patients is a pathological facilitation rather than a reduction in inhibition. In the first place, the threshold changes measured by T‐SICI in the low UMNS patients is a net facilitation, almost as great as the inhibition in healthy controls (Figure 6a). Secondly, the differences in SICI between patients and controls, whether measured by T‐SICI or A‐SICI, show a single peak at 3 ms (Figure 6c) rather than peaks at 1 and 2.5 ms as in normal SICI. The similarity in time course of the threshold changes in Figure 6c indicates that a similar mechanism underlies the abnormalities in all the MND patients. The changes in SICI in these newly diagnosed MND patients appear similar to those described in de novo Parkinson's patients by Shirota et al. [32] They used a triple stimulation technique to separate the contributions of short‐interval cortical facilitation and SICI and concluded that the underlying SICI was unchanged, whilst an exaggerated facilitation around the second peak (i.e., at 3 ms) caused an apparent loss of inhibition.
A striking feature of the facilitation is the strong positive correlation between T‐SICI and the modified Penn UMN score (Figure 7a), indicating that the pathological facilitation is reduced as corticospinal neurons degenerate. If loss of SICI was a simple sign of UMN damage, then one would have expected a negative correlation between T‐SICI and UMNS. Instead, patients with little clinical evidence of UMN damage showed the greatest drop in SICI, whilst patients with marked UMN signs had T‐SICI that was normal in extent and time course (Figure 6a). The resting motor thresholds did not differ significantly between healthy controls and patients and there was no correlation between RMT200 and UMNS, so that conditioning stimuli were much the same (70% of RMT200) for all T‐SICI and A‐SICI recordings and all the differences were due to differences in the test stimuli. Comparing A‐SICI and T‐SICI for the low UMNS patients, this means that there was a subset of cortical motor neurons that experienced sufficient facilitation to generate a 200 μV MEP with a stimulus well below RMT200. On the other hand, the stronger test stimuli used for A‐SICI recruited neurons with much less facilitation, so that on average there was a small net inhibition. Comparing T‐SICI between patients with increasing UMN scores (Figure 7a), the simplest explanation for the apparently paradoxical relationship is that the cortical motor neurons experiencing most facilitation progressively degenerate (consistent with an excitotoxic mechanism), whilst those experiencing inhibition survive. The changes in A‐SICI are less pronounced since the stronger stimuli used sample a larger proportion of motor neurons.
Our results confirm an earlier observation on patients with SOD‐1 mutations that T‐SICI can be abolished before signs of ALS develop [33]. They indicate that the reason for the high sensitivity of T‐SICI in detecting early UMN dysfunction is because threshold measurements can best reveal when only a few cortical motor neurons are affected.
Threshold tracking SICI and A‐SICI as diagnostic biomarkers for ALS
Threshold tracking SICI has been shown to be reduced in ALS in several studies and has been proposed as a diagnostic [10, 16, 18, 34, 35 and prognostic [24] biomarker for ALS. However, a comparison with conventional A‐SICI has been lacking. Taking the MND group of patients as a whole, it was found that the A‐SICI method produces less variable SICI measurements in all three groups of subjects than the T‐SICI method (Figure 4), and it provided slightly better discrimination between the MND patients and patient controls (Table 1). However, as diagnostic biomarkers of UMN pathology in MND patients that did not fall into any ALS category, both SICI methods provided similarly excellent discrimination from the patient controls.
There has been frequent discussion in the literature as to how broad the definition of ALS should be [18] and the most widely accepted criteria have changed with time [6, 8. The ALS criteria have been simplified in a recent proposal, where TMS has been proposed as supportive evidence of UMN dysfunction [36]. Our results strongly support the contention that the requirement for a positive diagnosis that there should be clinical UMN and LMN dysfunction in different body regions is too restrictive. Loss of T‐SICI or A‐SICI, even in the absence of clinical UMN signs, should be accepted as supporting evidence of UMN pathology, to allow earlier diagnosis and increased recruitment into therapeutic trials, especially in the early stages of the disease when treatments are likely to be most effective [10].
CONCLUSION
It has been found that both A‐SICI and T‐SICI are sensitive early indicators of UMN dysfunction in patients referred for suspicion of ALS, with T‐SICI most abnormal before UMN signs have developed. Arguments that SICI should be accepted as an aid to the early diagnosis of ALS are therefore strongly supported [10, 18, 19, 35. On the other hand, since T‐SICI becomes more normal as signs of disease progression increase, it may not be suitable for monitoring potential ALS treatments.
CONFLICT OF INTERESTS
H. Bostock and J. Howells receive from UCL a share of the royalties for sales of their QtracW software used in this study. Other authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Hatice Tankisi: Conceptualization (lead); data curation (lead); formal analysis (equal); funding acquisition (lead); investigation (lead); methodology (equal); project administration (lead); resources (lead); writing—original draft (lead); writing—review and editing (equal). Christina S.‐Z. Nielsen: Conceptualization (supporting); funding acquisition (supporting); investigation (supporting); writing—review and editing (supporting). James Howells: Formal analysis (supporting); methodology (supporting); software (supporting); writing—original draft (supporting); writing—review and editing (supporting). Bülent Cengiz: Conceptualization (supporting); investigation (supporting); methodology (supporting); writing—review and editing (supporting). Gintaute Samusyte: Formal analysis (supporting); methodology (supporting); writing—original draft (supporting); writing—review and editing (supporting). Martin Koltzenburg: Methodology (supporting); software (supporting); writing—original draft (supporting); writing—review and editing (supporting). Jakob U. Blicher: Conceptualization (supporting); methodology (supporting); writing—review and editing (supporting). Anette T Møller: Conceptualization (supporting); writing—review and editing (supporting). Kirsten Pugdahl: Conceptualization (supporting); project administration (supporting); writing—review and editing (supporting). Anders Fuglsang‐Frederiksen: Conceptualization (supporting); funding acquisition (supporting); writing—review and editing (supporting). Mamede A de Carvalho: Conceptualization (supporting); writing—original draft (supporting); writing—review and editing (supporting). Hugh Bostock: Conceptualization (equal); data curation (equal); formal analysis (lead); software (lead); supervision (lead); writing—original draft (lead); writing—review and editing (lead).
ACKNOWLEDGEMENTS
The authors are grateful to Professor J.C. Rothwell (Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, London) and to an anonymous EJoN reviewer for advice on interpretation of the results.
Tankisi H, Nielsen CS‐Z, Howells J, et al. Early diagnosis of amyotrophic lateral sclerosis by threshold tracking and conventional transcranial magnetic stimulation. Eur J Neurol. 2021;28:3030–3039. 10.1111/ene.15010
Funding information
This work was supported by the Lundbeck Foundation (grant R290‐2018‐751), and the Independent Research Fund Denmark (grant numbers 7025‐00066A and 9039‐00272B).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mejzini R, Flynn LL, Pitout IL, et al. ALS genetics, mechanisms, and therapeutics: where are we now? Front Neurosci. 2019;13:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun J, Carrero JJ, Zagai U, et al. Blood biomarkers and prognosis of amyotrophic lateral sclerosis. Eur J Neurol. 2020;27(11):2125‐2133. [DOI] [PubMed] [Google Scholar]
- 3. Mariosa D, Kamel F, Bellocco R, et al. Antidiabetics, statins and the risk of amyotrophic lateral sclerosis. Eur J Neurol. 2020;27(6):1010‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petimar J, O'Reilly É, Adami HO, et al. Coffee, tea, and caffeine intake and amyotrophic lateral sclerosis mortality in a pooled analysis of eight prospective cohort studies. Eur J Neurol. 2019;26(3):468‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thouvenot E, Demattei C, Lehmann S, et al. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. Eur J Neurol. 2020;27(2):251‐257. [DOI] [PubMed] [Google Scholar]
- 6. de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119:497‐503. [DOI] [PubMed] [Google Scholar]
- 7. Masrori P, Van Damme P. Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol. 2020;27(10):1918‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293‐299. [DOI] [PubMed] [Google Scholar]
- 9. de Carvalho M, Swash M. Sensitivity of electrophysiological tests for upper and lower motor neuron dysfunction in ALS: a six‐month longitudinal study. Muscle Nerve. 2010;41:208‐211. [DOI] [PubMed] [Google Scholar]
- 10. Menon P, Geevasinga N, Yiannikas C, et al. Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: a prospective study. Lancet Neurol. 2015;14:478‐484. [DOI] [PubMed] [Google Scholar]
- 11. Di Lazzaro V, Oliviero A, Profice P, et al. The diagnostic value of motor evoked potentials. Clin Neurophysiol. 1999;110(7):1297‐1307. [DOI] [PubMed] [Google Scholar]
- 12. Truffert A, Rösler KM, Magistris MR. Amyotrophic lateral sclerosis versus cervical spondylotic myelopathy: a study using transcranial magnetic stimulation with recordings from the trapezius and limb muscles. Clin Neurophysiol. 2000;111(6):1031‐1038. [DOI] [PubMed] [Google Scholar]
- 13. Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziemann U, Winter M, Reimers CD, et al. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology. 1997;49:1292‐1298. [DOI] [PubMed] [Google Scholar]
- 15. Fisher RJ, Nakamura Y, Bestmann S, et al. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240. e8. [DOI] [PubMed] [Google Scholar]
- 16. Vucic S, Kiernan MC. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain. 2006;129:2436‐2446. [DOI] [PubMed] [Google Scholar]
- 17. Vucic S, Kiernan MC. Utility of transcranial magnetic stimulation in delineating amyotrophic lateral sclerosis pathophysiology. Handb Clin Neurol. 2013;116:561‐575. [DOI] [PubMed] [Google Scholar]
- 18. Vucic S, van den Bos M, Menon P, et al. Utility of threshold tracking transcranial magnetic stimulation in ALS. Clin Neurophysiol Practice. 2018;3:164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vucic S, Kiernan MC. Transcranial magnetic stimulation for the assessment of neurodegenerative disease. Neurotherapeutics. 2017;14:91e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Carvalho M. Electrodiagnosis of amyotrophic lateral sclerosis: a review of existing guidelines. J Clin Neurophysiol. 2020;37:294‐298. [DOI] [PubMed] [Google Scholar]
- 21. Tankisi H, Cengiz B, Howells J, et al. Short‐interval intracortical inhibition as a function of inter‐stimulus interval: three methods compared. Brain Stim. 2021;14:22‐32. [DOI] [PubMed] [Google Scholar]
- 22. Samusyte G, Bostock H, Rothwell J, Koltzenburg M. Short‐interval intracortical inhibition: comparison between conventional and threshold tracking techniques. Brain Stim. 2018;11:806‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dharmadasa T, Howells J, Matamala JM, et al. Cortical inexcitability defines an adverse clinical profile in amyotrophic lateral sclerosis. Eur J Neurol. 2021;28(1):90‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woo JH, Wang S, Melhem ER, et al. Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PLoS One. 2014;9:e105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinn C, Edmundson C, Dahodwala N, Elman L. Reliable and efficient scale to assess upper motor neuron disease burden in amyotrophic lateral sclerosis. Muscle Nerve. 2020;61:508‐511. [DOI] [PubMed] [Google Scholar]
- 27. Moore SR, Gresham LS, Bromberg MB, et al. A self report measure of affective lability. J Neurol Neurosurg Psychiatry. 1997;63:89‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bohannon R, Smith M. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206‐207. [DOI] [PubMed] [Google Scholar]
- 29. Cedarbaum J, Stambler N, Malta E, et al. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–. [DOI] [PubMed] [Google Scholar]
- 30. Tankisi H, Pugdahl K, Johnsen B, Fuglsang‐Frederiksen A. Correlations of nerve conduction measures in axonal and demyelinating polyneuropathies. Clin Neurophysiol. 2007;118:2383‐2392. [DOI] [PubMed] [Google Scholar]
- 31. Tankisi H, Pugdahl K, Beniczky S, et al. Evidence‐based recommendations for examination and diagnostic strategies of polyneuropathy electrodiagnosis. Clin Neurophysiol Pract. 2019;4:214‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shirota Y, Ohminami S, Tsutsumi R, et al. Increased facilitation of the primary cortex in de novo Parkinson’s disease. Parkinsonism Rel Disord. 2019;66:125‐129. [DOI] [PubMed] [Google Scholar]
- 33. Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 34. Vucic S, Cheah BC, Yiannikas C, Kiernan MC. Cortical excitability distinguishes ALS from mimic disorders. Clin Neurophysiol. 2011;122:1860‐1866. [DOI] [PubMed] [Google Scholar]
- 35. van den Bos MAJ, Higashihara M, Geevasinga N, et al. Pathophysiological associations of transcallosal dysfunction in ALS. Eur J Neurol. 2021;28(4):1172‐1180. [DOI] [PubMed] [Google Scholar]
- 36. Shefner JM, Al‐Chalabi A, Baker MR, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020;131:1975‐1978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


