Abstract
For decades, people have reduced the transmission of pathogens by adding low‐quality hosts to managed environments like agricultural fields. More recently, there has been interest in whether similar ‘dilution effects’ occur in natural disease systems, and whether these effects are eroded as diversity declines. For some pathogens of plants, humans and other animals, the highest‐quality hosts persist when diversity is lost, so that high‐quality hosts dominate low‐diversity communities, resulting in greater pathogen transmission. Meta‐analyses reveal that these natural dilution effects are common. However, studying them remains challenging due to limitations on the ability of researchers to manipulate many disease systems experimentally, difficulties of acquiring data on host quality and confusion about what should and should not be considered a dilution effect. Because dilution effects are widely used in managed disease systems and have been documented in a variety of natural disease systems, their existence should not be considered controversial. Important questions remain about how frequently they occur and under what conditions to expect them. There is also ongoing confusion about their relationships to both pathogen spillover and general biogeographical correlations between diversity and disease, which has resulted in an inconsistent and confusing literature. Progress will require rigorous and creative research.
Keywords: biodiversity, dilution effect, disease ecology, zoonoses, zoonotic
People have used diversity to manage the transmission of pathogens and parasites for many decades. More recently, there has been interest in whether similar “dilution effects” occur in natural disease systems, and whether these effects are eroded as diversity declines. Meta‐analyses suggest that these effects happen often in nature, but many important frontiers remain, including estimating their frequency, considering their effects in multi‐pathogen systems, and distinguishing them from other relationships between diversity and disease.
![]()
INTRODUCTION
A dilution effect occurs when the diversity of an ecological community reduces the transmission of a pathogen. Dilution effects are sometimes referred to as ‘controversial’ (Halliday & Rohr, 2019; Halsey & Miller, 2020; Strauss et al., 2015; Wood et al., 2017). However, these effects have been used for decades to manage the transmission of parasites and pathogens in plants, animals and people, even though our modern conceptualisation only began to develop fairly recently (Keesing et al., 2006; Norman et al., 1999; Ostfeld & Keesing, 2000; Schmidt & Ostfeld, 2001; VanBuskirk & Ostfeld, 1995). Here, we discuss how and where dilution effects and their analogues have been used to manage infectious diseases, in particular by changing the intra‐ or interspecific diversity of a disease system in carefully selected ways. We explore the ecological mechanisms that underlie these effects, and then turn to more recent questions—whether dilution effects occur in natural communities, and if so, whether these effects are impacted by changes to natural biodiversity. We review the evidence for when and how frequently natural dilution effects occur, outline some of the challenges of studying them and describe common mis‐applications of the concepts, as well as important outstanding questions.
MANAGING PATHOGEN TRANSMISSION USING VARIATION WITHIN A SPECIES
Agricultural scientists have recognised for decades that planting different varieties, or cultivars, of the same crop species can reduce the transmission of pathogens (Chin & Wolfe, 1984; Garrett & Mundt, 1999; Leonard, 1969; Mundt, 2002; Smithson & Lenné, 1996), effects supported by a recent meta‐analysis (Gibson & Nguyen, 2021) (Figure 1a). Mixing multiple varieties of a plant species can reduce transmission through a suite of potential pathways (Burdon & Chilvers, 1977; Burdon et al., 2006; Chin & Wolfe, 1984; Mundt, 2002). A large experiment involving rice farmers in Yunnan showed that when individuals of a standard rice strain encountered spores of a rice blast, they did not become infected. Instead, these plants effectively captured the spores without transmitting them onwards, making plants of a more susceptible rice strain planted in the same fields less likely to encounter spores of the blast (Zhu et al., 2000).
FIGURE 1.
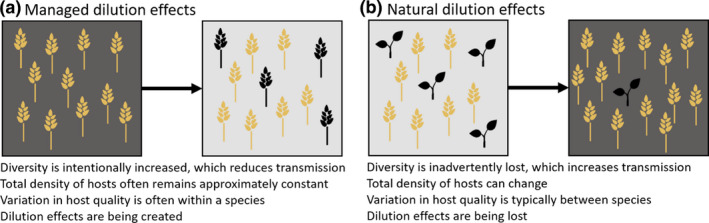
Managed versus natural dilution effects. (a) Managed dilution effects are often created intentionally, by increasing the diversity of strains or species. Low‐quality hosts (black) are added in order to reduce overall transmission of a pathogen, represented by the lighter gray background. (b) In contrast, natural dilution effects are typically eroded inadvertently when low‐quality hosts (black) disappear as biodiversity declines. This results in a relative or absolute increase in the abundance of high‐quality hosts, which leads to an increase in overall transmission, represented by the darker gray background
Underlying the effects of genetic diversity on the transmission of pathogens is intraspecific variation in host quality. Individuals within a species can vary, for example, in their probability of exposure to a pathogen or parasite and their susceptibility to infection (Civitello & Rohr, 2014; Dwyer et al., 1997; Poulin, 2011; Sauer et al., 2019; Warburton & Vonhof, 2018), their probability of transmitting the pathogen or parasite onwards (Cornet et al., 2014; Lloyd‐Smith et al., 2005; Pulkkinen & Ebert, 2004; Siva‐Jothy & Vale, 2021; Stephenson et al., 2017; White et al., 2018), and their attractiveness to vectors such as aphids or mosquitoes (Bruns et al., 2021; Shapiro et al., 2012; Yan et al., 2018). The combined effect of these factors can be that the majority of pathogens or parasites are transmitted by a small number of individuals, sometimes called ‘superspreaders’ (Lloyd‐Smith et al., 2005).
The rice example illustrates how people can use knowledge of variation in host quality among individuals of the same species to reduce the transmission of a parasite or pathogen. The decision about which types of individuals to add is critical (Mundt, 2002; Wolfe, 1985). In plant systems, a common strategy is to add a cultivar that is resistant to the focal pathogen (Burdon et al., 2006). But there is much more to consider, including the effects of the candidate cultivar on a field's microclimate, on the abundance and behaviour of vectors, and on the ultimate yield and profitability of the crop (Boudreau, 2013; Zhu et al., 2000). Broadly speaking, however, the goal is to add individuals that dilute the impact of superspreaders on the overall transmission of the parasite or pathogen. In other words, the goal is to add low‐quality hosts, or ‘superdiluters’.
The connection between intraspecific variation in host quality and the concept of ‘dilution’ has been recognised for some time. For example Mundt (Mundt, 2002) described how mixing plant cultivars in a field can reduce pathogen transmission by spreading out individuals of the more susceptible, disease‐prone cultivar, leading to a ‘dilution of the amount of inoculum’ in the field as a whole. In particular, he highlighted the use of experiments mixing two cultivars to ‘investigate the dilution effect of mixtures on disease’ (Mundt, 2002). In a very different system involving a viral phage that infects two strains of the bacterium Pseudomonas phaseolicola, Dennehy et al. (2007) recognised that one of the strains was trapping the phage, resulting in diluted transmission by the other, which they called an ‘analogue’ of dilution effects in disease ecology. Whether intraspecific diversity can cause a ‘real’ dilution effect or only an analogue could be debated, but the underlying mechanisms are the same (Johnson et al., 2015; Ostfeld & Keesing, 2012), and there is a history of references to dilution effects that arise from intraspecific diversity. For example Civitello and Rohr (2014) pointed out that identifying the differences among individuals within a species that affect transmission could pinpoint mechanisms underlying dilution effects.
MANAGING PATHOGEN TRANSMISSION USING VARIATION BETWEEN SPECIES
Variation in host quality can occur between, in addition to within, species. For a particular pathogen, for example, species can vary in the duration of their infections (Garrido et al., 2021) and their ability to transmit the pathogen onwards (Hersh et al., 2012; Keesing et al., 2012) (Figure 2). For pathogens transmitted among hosts by vectors such as fleas, ticks and mosquitoes, species can vary in their attractiveness to these vectors (Bruns et al., 2021; Shapiro et al., 2012; Yan et al., 2018), in the degree to which vectors survive attempts at a blood meal (Edman et al., 1974; Keesing et al., 2009), and in the ability of vectors to acquire or transmit infections (Figure 2).
FIGURE 2.
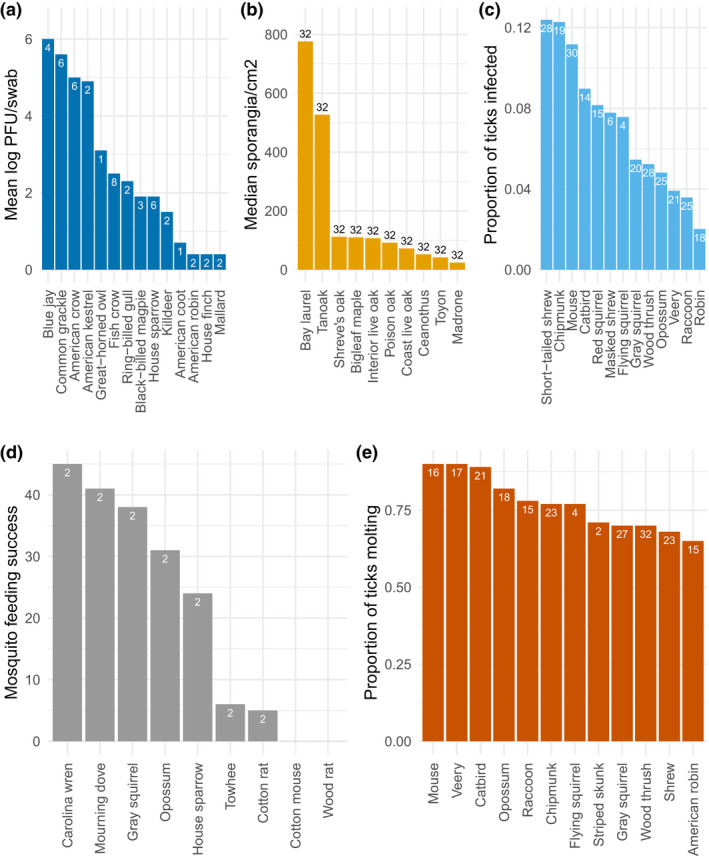
Variation among hosts in different metrics of host quality. Effects of host species on (a) shedding of West Nile virus by birds four days after experimental inoculation (Komar et al., 2003); (b) spore production by Phtyphthorum ramorum, the causative agent of Sudden Oak Death, in woody plants (Rosenthal et al. 2020); (c) the proportion of ticks infected with Anaplasma phagocytophilum, the causative agent of human anaplasmosis, after feeding on mammals and birds (Keesing et al., 2012); (d) feeding success for Culex nigripalpis mosquitoes (Edman et al., 1974); and (e) molting success of blacklegged ticks (Ixodes scapularis) (Brunner et al., 2011). Data in (d) are the mean of eight observations of feeding by 200 female mosquitoes on two individuals of each host species; other sample sizes are indicated on bars. Characterizing the amount of variation across hosts is an important frontier (see main text), particularly how it varies for different types of disease systems, and how those patterns influence dilution effects
This interspecific variation in host quality can be used to manage pathogen transmission. In plant disease systems, the mixing of species reduces diseases caused by a variety of pathogens in both agricultural (Boudreau, 2013) and non‐agricultural settings (Liu et al., 2020). Diversity has also been used to manage vector‐borne diseases of humans. In the early 20th century, an Italian public health specialist suggested that domestic animals could deflect mosquito meals away from humans (reviewed in Service 1991). The World Health Organisation and others have recognised the potential of this ‘zooprophylaxis’ in the management of malaria and other vector‐borne diseases (Ault, 1994; Macdonald, 1957; WHO, 1982). More recently, scientists have recommended using zooprophylaxis while simultaneously treating the added host species with insecticides to further reduce mosquito populations (Kemibala et al., 2020; Morona et al., 2017).
The use of interspecific variation to manage transmission has been particularly well‐characterised for Ribeiroia ondatrae, a trematode parasite of wildlife. Cercariae, the middle stage of this parasite's complex life cycle, infect fish and pond‐breeding amphibians, which can suffer severe limb deformities and death (Johnson et al., 2013). Some species of amphibians are far more likely to harbour cercariae than others are (Figure 3c). For example Pacific tree frog tadpoles (Pseudacris regilla) readily acquire the parasite. In contrast, neither American bullfrogs (Lithobates catesbeianus) nor California tiger salamanders (Ambystoma californiense) harbour many parasites at all. Scientists working with Ribeiroia ondatrae stocked artificial ponds with one to four species of amphibians, all of which were hosts for the parasite, but hosts of varying quality (Johnson et al., 2013). When the only amphibians in the pond were Pacific tree frogs, about 25% of the frogs became infected. When other amphibian species were added to the ponds, infection in the tree frogs, and the total amount of infection, dropped by half (Johnson et al., 2013).
FIGURE 3.
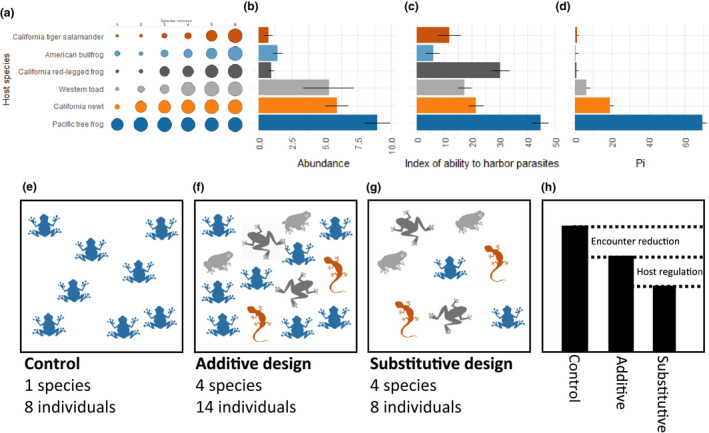
Field (panels a–d) versus laboratory (panels e–h) studies of dilution effects. In field studies, the transmission of parasites or pathogens is often compared among sites that vary in diversity. Studies that include data on underlying causes of differences in overall transmission, such as Johnson et al. (2013), are less common. (a) Patterns of host community composition in 345 wetlands, with the percentage of wetlands represented by the size of the circle. (b) The mean abundance of each species (number per m2) in wetlands where it is present. (c) The ability of each host to harbor parasites, as measured in the laboratory, and (d) an index of each host's contribution to community competency (Pi), which combines information from panels a–c, as described in Johnson et al. (Johnson et al., 2013b), from which the data shown in this figure were extracted. (e–h) Schematic of an experimental approach to studying dilution effects in the laboratory. (e) Transmission in a treatment composed of only high‐quality hosts is compared to (f) transmission in treatments with more species and more individuals (additive design) and (g) transmission in a treatment with more species but an equal number of individuals (substitutive design). (h) The proportion of hosts infected is compared among treatments. In this example, transmission declines when low‐quality hosts are added, which is an example of encounter reduction (see main text), and declines even further when the density of high‐quality hosts also declines, an example of host regulation (see main text). The taxa included in such an experiment might or might not represent natural assemblages
In managing multi‐species disease systems, the decision of which species to add is critical (Boudreau, 2013). Just as with single‐species systems, a common strategy is to add a species that is resistant to the target pathogen. For zooprophylaxis, an effective low‐quality host to add would be one that transmits the focal pathogen or parasite poorly but diverts vector meals away from humans, all while not increasing the abundance of the vector by adding an additional food source (Ault, 1994; Saul, 2003).
A key factor in managing with dilution is whether the system is amenable to a dilution effect in the first place. For example the feasibility of zooprophylaxis for mosquito‐transmitted pathogens depends largely on whether a mosquito species is highly specialised on humans (Ault, 1994). If it is, adding alternative animals to divert mosquito meals is unlikely to be effective because the mosquito will still bite only humans. Even if a system is amenable in theory, the availability of an appropriate low‐quality host is essential in practice. Whether creating a dilution effect will work in a particular system will depend on the net effect of all of the relevant pathways through which low‐quality hosts could affect transmission (Keesing et al., 2006).
DILUTION EFFECTS IN NATURE
Over the last 20 years, attention has focused on whether the patterns that can be made to happen—when someone chooses which organisms are present in a system—ever happen naturally, as diversity changes under natural conditions (Figure 1b). This is a particularly important question because diversity within natural ecosystems is changing rapidly in response to human impacts such as habitat fragmentation, overexploitation, pollution and climate change (Field et al., 2020).
Determining whether dilution effects happen in nature requires understanding the relationship between host quality and the composition of natural ecological communities. For example, outside of the laboratory, Ribeiroia ondatrae parasites live in real ponds. Are there predictable patterns to the distribution of host species among ponds? Johnson et al. (2013) compared patterns of amphibian community composition in 345 wetlands. They found that when ponds had only one species of amphibian living in them, that species was overwhelmingly likely to be a tree frog, Pseudacris regilla (Figure 3a). When ponds had more than one species, the tree frog was always one of those species. Furthermore, the presence of species across diversity levels revealed predictable patterns in which species were present in a pond and which were not. The less diverse ponds contained species that were almost perfect subsets of the species found in the more diverse ponds, a pattern commonly referred to as nestedness (Figure 3a). In addition, both the quality (‘competence’) of the different amphibian species as hosts for the parasite and their abundance within each pond were correlated with their probability of being found at different diversity levels (Figure 3). At least in this disease system, the most competent host for the parasite is found in ponds even when all of the other amphibian species are absent, and the least competent hosts for the pathogen are found only in the most diverse ponds. These patterns have enormous consequences: ponds with high diversity had 78% lower transmission of the parasite (Johnson et al., 2013).
MECHANISMS UNDERLYING DILUTION EFFECTS IN NATURE
The Riberoia disease system provides an example of a natural dilution effect because the presence of naturally high diversity reduces the overall transmission of the parasite (Johnson et al., 2013b). The opposite pattern—when the presence of naturally high diversity increases the overall transmission of a parasite or pathogen—is called an amplification effect (Keesing et al., 2006). More generally, a dilution effect will occur in nature when (1) some species (or strains) are higher‐quality hosts than others, and (2) transmission from those high‐quality hosts is suppressed by naturally high levels of biodiversity (Keesing et al., 2010).
Variation in host quality
Broadly speaking, the first condition required for a dilution effect to occur is always true: there is always at least some variation among species in their ability to harbour a specific pathogen (Casadevall & Pirofski, 2015). At the simplest level, some organisms can become infected by a particular pathogen and some cannot, a distinction that separates ‘hosts’ from ‘non‐hosts’. Non‐hosts represent the extreme end of what is often a range of host quality (e.g. Cronin et al., 2010; Hersh et al., 2012; Johnson et al., 2013b; Keesing et al., 2012; Komar et al., 2003). Furthermore, some pathogens are quite specialised, infecting only one species or strain, while some are more generalised, infecting a wider variety of hosts (Bandín & Dopazo, 2011; Dallas et al., 2017). But regardless of this breadth of host range, there will always be species that fall outside of it.
Host quality cannot be measured solely by asking whether a host gets infected, though. An equally critical aspect is whether an infected host transmits the pathogen onwards, and to what degree. For example, the competence of the bird species that act as hosts for West Nile virus, which causes West Nile virus encephalitis in humans, varies from high for blue jays (Cyanocitta cristata) and grackles (Quiscalus quiscula), to zero for other species (Komar et al., 2003, Figure 2a). In wild plants, the oomycete that causes Sudden Oak Death in forest trees, Phytophthorum ramorum, is transmitted readily by two tree species, bay laurel (Umbellularia californica) and tanoak (Notholithocarpus densiflorus), but little by others, despite the fact that all of these species can be infected (Figure 2b) (Rosenthal et al., 2021).
Though the quantitative measure of a host's ability to transmit is critical, host quality in animals is often approximated using data on seroprevalence, which measures whether an animal's immune system has developed antibodies to a particular pathogen (Becker et al., 2020). The presence of antibodies indicates that the animal has been infected with a pathogen, but not whether that animal can transmit the pathogen onwards, and if so, to what degree. Studies of transmission are much harder to conduct, and therefore much rarer, but they are critically needed in research on the dilution effect. Quantitative explorations of the distribution and magnitude of variation in host quality, similar to those done for superspreaders within species (Lloyd‐Smith et al., 2005), have not been done, in part because appropriate data on host quality are relatively rare.
Suppression of transmission from high‐quality hosts
The second condition necessary for a dilution effect to occur in nature is that transmission from high‐quality hosts is suppressed by naturally high levels of biodiversity. Theoretical explorations, based in epidemiological models, have identified several mechanisms that can lead to this condition (Johnson et al., 2015; Johnson & Thieltges, 2010; Joseph et al., 2013; Keesing et al., 2006). Most simply, the presence of low‐quality hosts (including non‐hosts) in a diverse community can deflect a pathogen, reducing encounters with the hosts most likely to transmit it onwards, a mechanism called ‘encounter reduction’ (Keesing et al., 2006) (Figure 3d–h).
Transmission can also be suppressed by naturally high levels of diversity if there are fewer high‐quality hosts in areas where biodiversity is high. This could happen if, for example, predators or competitors of the most competent hosts are abundant in high‐diversity areas, but decline or disappear in low‐diversity areas. This pathway has been called ‘host regulation’ (Keesing et al., 2006). For host regulation to occur, the taxa present in high‐diversity conditions do not need to be hosts; they simply need to reduce the abundance of high‐quality hosts. This observation makes clear that the selection of a specific community for which to measure diversity is non‐trivial—when non‐host taxa are important in suppressing pathogen transmission, diversity solely within the host community may not adequately capture dilution effects. In studies of plant diseases, host regulation is a well‐known mechanism by which transmission declines in diverse mixtures, and debate has centred around the impacts of other mechanisms that might also operate (Burdon & Chilvers, 1977; Chin & Wolfe, 1984; Knops et al., 1999; Mitchell et al., 2002), a conversation that continues to the present (Liu et al., 2016, 2020; Rottstock et al., 2014). Two recent studies (Liu et al., 2016; Rottstock et al., 2014) found that plots with higher plant species diversity had lower infection with fungal pathogens even when differences in plant density were taken into account.
Although encounter reduction and host regulation are the two mechanisms typically explored empirically, (e.g. Johnson et al., 2013b; Luis et al., 2018; Strauss et al., 2018; Thieltges et al., 2008), other mechanisms have also been proposed in theory and described in at least some disease systems (Johnson & Thieltges, 2010; Keesing et al., 2006). For example, the presence of alternative hosts for vectors like ticks and mosquitoes can deflect vector meals away from high‐quality hosts, and also affect the abundance of vectors (Keesing et al., 2006), mechanisms that underlie zooprophylaxis (Ault, 1994; Kemibala et al., 2020; Saul, 2003). In the Lyme disease system, ticks that feed on some species are less likely to feed successfully (Figure 4) and also less likely to survive over the winter (Brunner et al., 2011; Keesing et al., 2009) (Figure 2e). In aquatic systems, some species consume parasites directly (Johnson & Thieltges, 2010; Thieltges et al., 2008; Venesky et al., 2013).
FIGURE 4.
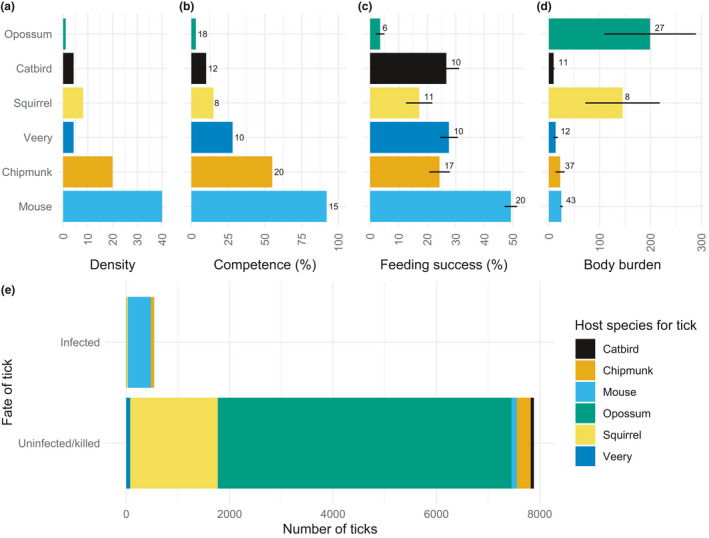
Metrics of host quality for the Lyme disease system in the eastern United States, and their consequences. (a) Average density of each host species per hectare in intact forest in New York state. (b) Mean percentage of uninfected larval ticks that acquire infection after feeding on each species. (c) Percentage (±SE) of ticks attempting to feed on each host species that survive and feed to repletion. (d) Mean (±SE) number of larval ticks on the body of each individual. (e) Number of ticks infected, and uninfected/killed when feeding or attempting to feed, on six common host species. For panels b–d, sample sizes are indicated at ends of bars. Developed from data and calculations in (Keesing et al., 2009)
Critically, multiple mechanisms can operate together in the same disease system. For example, in Johnson's Riberoia experiments in the laboratory, encounter reduction reduced infection by 28% when it was the only pathway for dilution. When both encounter reduction and host regulation were operating, infection went down by 50% (Figure 1c–f). In the Lyme disease system, hosts that are present in high‐diversity communities deflect tick meals away from the most competent hosts, kill many of those ticks through grooming, infect fewer of the ticks that do manage to feed successfully, and cause those fed ticks to survive poorly overwinter (Figure 4a–c) (Brunner et al., 2011; Keesing et al., 2009, 2010).
In both the Riberoia and Lyme disease examples, the mechanisms all operate to decrease transmission when diversity is high, but there are cases in which transmission can decline due to one mechanism while simultaneously increasing due to another. Studying Sin Nombre hantavirus in western North America, for example, Luis et al. (2018) found that viral infection prevalence in the main reservoir host, the deer mouse Peromyscus maniculatus, was reduced in communities with high small‐mammal diversity, a dilution effect. However, this dilution effect was the net result of diversity reducing mouse abundance while simultaneously increasing per capita transmission.
CHALLENGES OF STUDYING DILUTION EFFECTS IN NATURE
Study design
The dilution effect is defined by a change in overall transmission, but transmission is often hard to detect and quantify. As a consequence, scientists studying natural dilution effects typically use proxies for transmission, such as the incidence of a disease (Rosenthal et al. in revision), the presence of antibodies to a pathogen (Suzán et al., 2009), or the density of infected vectors (LoGiudice et al., 2003). No one proxy appears to be appropriate for all disease systems (Keesing et al., 2006), which complicates efforts to arrive at a general understanding.
Studying how transmission changes as diversity changes is also challenging. Ideally, an investigator would experimentally manipulate diversity in a natural disease system, with appropriate controls, and measure the outcomes. Because natural dilution effects are caused by changes in the community of hosts as biodiversity declines, the order in which species are removed in experiments matters (Halliday et al., 2020; Liu et al., 2020; Ostfeld & LoGiudice, 2003). For plants, some researchers have created random assemblages of varying species richness out of a regional pool of native species (e.g. Knops et al., 1999; Mitchell et al., 2002; Rottstock et al., 2014). Whether these random assemblages are realistic enough to draw conclusions about natural dilution effects is an open question. In pioneering work on the Tibetan plateau, Liu et al. (Liu et al., 2018) found that random assemblages underestimated the magnitude of a natural dilution effect, which was evident when species were removed in a realistic order.
Information on the order of species loss is not always available, but perhaps equally important is the empirical challenge of removing species, which is easier for plants than for animals. Fencing can experimentally exclude animal species based on size, as has been done, for example, for size guilds of large mammals in African savannas (Keesing, 1998, 2000; McCauley et al., 2008; Young et al., 1998). Removing entire guilds of large mammals from a savanna leads to a dramatic and sustained increase in the abundance of small mammals, with consequences for the entire ecosystem, including the abundance of flea vectors (Keesing, 2000; Keesing & Young, 2014). But the simultaneous removal of many species, in no particular order, does not shed light on the consequences of biodiversity losses that occur when species are lost non‐randomly or sequentially. Removing individual species, such as a high‐quality host, can be logistically challenging. Brunner et al. (Brunner et al., 2013) reported the results of a field experiment to remove either white‐footed mice (Peromyscus leucopus) or gray squirrels (Sciurus carolinensis) from forest fragments. Despite a massive and prolonged effort to capture and relocate rodents over months, the experiment achieved only modest differences in densities of the focal animals (Brunner et al., 2013). An alternative method of ‘removing’ a key host species is to vaccinate individuals of that species so that they cannot transmit a particular parasite or pathogen (Tsao et al., 2004). This changes their functional role from high‐quality to low‐quality host for the pathogen, though it retains other roles of these individuals, for example as hosts for vectors or competitors of other hosts.
When experimentally manipulating diversity in nature is not possible for logistical, ethical, or financial reasons, scientists studying dilution effects typically do one of three things. If they can, they establish artificial habitats—like aquarium tanks, greenhouse trays, or agricultural plots—in which they can conduct experiments with carefully controlled levels of diversity (Figure 1c–f), as has been done for diseases of amphibians (Johnson et al., 2008), marine invertebrates (Thieltges et al., 2008) and plants (Mitchell et al., 2003; Rottstock et al., 2014) for example. A second strategy is to use correlational approaches, for example by comparing similar habitats that already vary in diversity. Using this approach, an investigator might compare diversity and transmission on islands of varying sizes. Because island size is positively correlated with diversity, the different islands can substitute for diversity levels imposed by the scientist in an experiment (Werden et al., 2014). Alternatively, an investigator might use habitat fragments that have been created by human activity, such as forest fragments, because fragmentation of habitats typically leads to declines in diversity (Allan et al., 2003). Correlational studies are often the only feasible way to study a potential natural dilution effect in the real world. The third option is to build a mathematical model of a disease system and explore its dynamics through the behaviour of the model. An additional consideration is that much of the oft‐claimed controversy about the dilution effect is its ‘generality’ (Civitello et al., 2015a; Halliday & Rohr, 2019; Halliday et al., 2020; Huang et al., 2017; Luis et al., 2018), a concept that no one study, however well‐designed, can resolve.
Characterising host quality
Characterising host quality is a major challenge of studying natural dilution effects. Most studies have relied on a handful of metrics of host quality, particularly host abundance and host ‘competence’ (though competence is sometimes measured as the host's susceptibility (e.g. Figure 3c) and sometimes as the host's ability to transmit pathogens (e.g. Figures 2, 4b). Some researchers have subsequently collapsed these metrics into combined indices, for example by calculating the product of a host's abundance and competence (Johnson et al., 2013b; Mitchell et al., 2003) (Figure 3). Across disease systems, however, other metrics of host quality are also important, as has been apparent from theoretical explorations of mechanisms underlying dilution effects (Keesing et al., 2006). For example in a recent experiment, Garrido et al. (Garrido et al., 2021) found that some gerbil species recovered from infection with Mycoplasma bacteria more quickly than others. Additional metrics are appropriate for vector‐borne diseases. Hosts for mosquito‐borne and tick‐borne pathogens can affect vector feeding success (Figures 2d and 4c) as well as the probability that the vector transitions successfully to the next life stage and survives a period of diapause (Figure 2e).
Despite the challenges of measuring host quality in nature, including a more complete set of metrics of host quality can reveal important patterns that might otherwise go undetected. In the Lyme disease system in the northeastern United States, for example, focusing only on variation among host species in abundance (Figure 4a) and competence (Figure 4b) highlights the importance of the white‐footed mouse (Peromyscus leucopus), which is responsible for infecting the most ticks and is thus considered the highest‐quality host (Figure 4e). But taking into account additional metrics, including the feeding success of ticks on each host and the number of ticks each species typically hosts (Figure 4c, d), reveals the importance of low‐quality hosts: gray squirrels (Sciurus carolinensis) and Virginia opossums (Didelphis virginiana) together appear to either kill or prevent infection in far more ticks than mice infect (Figure 4e). Thus, these low‐quality hosts are actually serving a strong protective effect (Keesing et al., 2009).
Including low‐quality hosts
The significance of low‐quality hosts, and of non‐hosts, has received relatively little attention in studies of natural dilution effects but see (Dennehy et al., 2007; Keesing et al., 2009; Venesky et al., 2013), which is ironic given the central importance of low‐quality hosts in managed dilution effects (Burdon et al., 2006; Mundt, 2002; Wolfe, 1985). One cause of the emphasis on high‐quality hosts—and our associated ignorance of low‐quality hosts—arises from the use of methods to identify the sources of blood meals in vectors (Hamer et al., 2009; Kilpatrick et al., 2006; Titcomb et al., 2017). Using these methods, researchers capture vectors such as ticks or mosquitoes that have fed successfully on unknown hosts and then use molecular analysis of blood components to identify the taxon that served as the source of the successful meal. This approach makes estimates of host quality more tractable in the field. But while it can be a suitable method for identifying the source of successful meals, it ignores the unsuccessful ones, thus failing to identify the roles of low‐quality hosts in either killing (Figure 2d) or failing to infect (Figure 4e) vectors.
Accounting for host dynamics
In part because of the challenges inherent in measuring host characteristics in the field, estimates of host quality for various metrics are often assumed to be constant through time, but at least some metrics can vary depending on community context. The most obvious of these is the abundance of hosts, which can vary substantially over time (e.g. Ostfeld et al., 2018). Many studies of natural dilution effects focus on presence/absence data to characterise the diversity of a host community, but ignore changes in the abundance of its constituents as diversity changes through time. Modelling studies have explored the impacts on transmission if the total abundance of hosts changes as diversity changes (Johnson et al., 2015; Mihaljevic et al., 2014), and some studies have documented effects of variation through time in the abundance of important hosts e.g. (Hall et al., 2009). How the abundance of hosts affects managed dilution effects, for example, for zooprophylaxis, has been a major question (Ault, 1994; Kemibala et al., 2020; Saul, 2003). This issue also dominated early conversations about the pathways by which natural dilution effects occur (Begon, 2008; Johnson et al., 2008; Keesing et al., 2010).
Other metrics of host quality might also vary depending on community context, but these have received virtually no attention. For example evidence from several disease systems suggests that the competence of the highest‐quality hosts might actually be lower in highly diverse communities (Kurtenbach et al., 1995; Schauber & Ostfeld, 2002). A frontier for studies of natural dilution effects is collecting and analysing more complete data on host quality, including host abundance, particularly as diversity changes.
UNDERLYING CAUSES OF THE DILUTION EFFECT IN NATURE
What underlies relationships between host quality and ecological resilience that allows some high‐quality hosts to persist in low‐diversity communities from which other species are lost? Several correlational studies have suggested an answer. From a database of 300 disease systems, Plourde et al. (Plourde et al., 2017) considered the traits of the highest‐quality hosts—the ‘reservoir’ hosts that are most responsible for transmitting pathogens—compared to the traits of lower‐quality hosts that transmit at lower rates or perhaps do not transmit at all. They found that mammalian reservoirs tend to have ‘fast’ life histories—they tend to be short‐lived, small‐bodied, and have many offspring early in their lives. These are the very traits that are associated with resilience to disturbance and biodiversity loss. Johnson et al. (2020) found that wild mammals that are becoming less abundant because of human activities host fewer zoonotic viruses, while those that are stable or increasing in abundance host significantly more. It appears that a correlation exists between host quality and fast life‐history traits, which predispose a species to be resilient to disturbance. But how do these life history traits affect host quality?
Determining the connection between host quality and host traits is challenging. Two hypotheses have been proposed. First, short‐lived animal hosts might allocate their immune responses to pathogens differently than longer‐lived hosts do (Martin et al., 2007). Previtali et al. (2012) compared the innate versus adaptive immune responses of four rodent hosts for the pathogen that causes Lyme disease. The hosts, which varied from faster to shorter life‐history strategies, were inoculated with immune challenges in the laboratory. As predicted, the shorter‐lived hosts had more pronounced short‐term immune responses than the longer‐lived hosts, which had stronger adaptive immune responses. These results suggest that, for this one disease system at least, shorter‐lived animal hosts might be vulnerable to pathogens that can circumvent their immune defences, and these shorter‐lived hosts are also the ones that tend to thrive when biodiversity is lost. Gervasi et al. (2017) found this pattern for amphibian hosts of Batrachochytridium dendrobatidis, a chytrid fungus devastating amphibian populations worldwide, and a remarkably similar pattern has been detected in plants. Cronin et al. (Cronin et al., 2010) found that plant hosts that were shorter‐lived, more poorly defended, and contained higher nutrient levels were better reservoirs for a virus that infects wild grasses. More studies like these, in both animal and plant systems, would help us understand how general this pattern is (Albery & Becker, 2021; Valenzuela‐Sánchez et al., 2021).
The second hypothesis for why ecologically resilient species are often high‐quality hosts is that pathogens might adapt to survive best in the hosts they encounter most frequently, and these are likely to be abundant, resilient species (Ostfeld & Keesing, 2000). If hosts are habitats for pathogens, then pathogens that survive and reproduce well in common host‐habitats will leave a larger share of the offspring in the next generation, and natural selection will foster strong associations between pathogens and ecologically resilient hosts. This, too, is difficult to test experimentally, but it is amenable to mathematical modelling.
These adaptive mechanisms would help explain correlations between host quality and ecological resilience. But how strong is the evidence for such a relationship in the first place? Since these mechanisms were first proposed over a decade ago (Keesing et al., 2010), a number of studies have found evidence linking fast‐lived species and various measures of host quality in both plants and animals (Cronin et al., 2010; Downs et al., 2019; Han et al., 2015, 2016b; Huang et al., 2013; Johnson et al., 2012; Ostfeld et al., 2014; Previtali et al., 2012). But much remains to be uncovered (Albery & Becker, 2021; Downs et al., 2019; Valenzuela‐Sánchez et al., 2021). There is counter‐evidence for some metrics (Cooper et al., 2012) and some diseases (Downs et al., 2019). In addition, correlational explorations of species databases must contend with the challenges of uneven sampling, inadequate and incomplete metrics of host quality, and inter‐correlated variables.
LIMITATIONS OF OUR UNDERSTANDING OF DILUTION EFFECTS IN NATURE
Since 2015, multiple meta‐analyses have found that natural dilution effects often occur (Cardinale et al., 2012; Civitello et al., 2015a; Huang et al., 2017; Liu et al., 2020; Magnusson et al., 2020). Approximately 80% of studies in one influential meta‐analysis (Civitello et al., 2015a) detected them, while another found dilution effects in 67% of animal and 85% of plant disease systems (Cardinale et al., 2012). Civitello et al., (2015a) found that dilution effects were equally common for diseases of humans and diseases of wildlife, and for diseases caused by microparsites, for example bacteria and viruses, as for those caused by macroparasites, for example helminths. An analysis of the same underlying data found that dilution effects were equally likely for diseases of plants as for diseases of animals (Huang et al., 2017). For plants, a recent meta‐analysis in non‐agricultural settings (Liu et al., 2020) found strong evidence for dilution effects, with effect sizes that were greater in grasslands than in forests, and for viruses and fungi than for some other types of pathogens.
One challenge for all of these meta‐analyses is which studies to include. For example Halliday et al. (Halliday et al., 2020) synthesised results from four prior meta‐analyses and found that the results became clearer when the underlying studies in the meta‐analyses were first vetted for whether they were appropriate tests of dilution effects. Because true studies of dilution effects address biodiversity loss, Halliday et al. (Halliday et al., 2020) sifted out, for example, studies that compared disease as biodiversity varied along latitudinal gradients. Data from the four prior meta‐analyses (Civitello et al., 2015a; Halliday & Rohr, 2019; Liu et al., 2020; Magnusson et al., 2020) revealed even stronger natural dilution effects when inappropriate studies were excluded (Halliday et al., 2020), a result that was robust to potential misclassification of studies, and the spatial scale at which studies were conducted.
Estimates of the frequency of natural dilution effects are also affected by the pool of studies being considered, and deciding on this pool is not trivial. For example, there has almost certainly been selection bias, with some researchers choosing to study disease systems likely to show dilution and others—looking for counter‐examples—favouring systems that likely will not. In addition, not all disease systems are potentially subject to dilution effects (Ostfeld & Keesing, 2012). For example diseases that are transmitted strictly through human‐to‐human transmission—for example sexually transmitted diseases of humans—are not expected to have the underlying ecological dynamics required for dilution. One recent paper suggested a fairly narrow range of applicability for natural dilution effects (Rohr et al., 2020), while providing caveats because of how much uncertainty there is about those boundaries. Determining the range of diseases that could be affected by dilution effects is important. As Rohr et al. (Rohr et al., 2020) point out, the effectiveness of anti‐bacterial compounds is evaluated for a pool of diseases caused by bacterial pathogens, not viral ones; similarly, the frequency of dilution effects should be calculated for diseases to which these effects could apply.
One major constraint on studying dilution effects, including their underlying mechanisms and how commonly they occur in nature, is the politicisation of the topic, both in the literature and by members of the scientific community (Halsey, 2019). Despite the evidence from managed and natural disease systems, the existence of dilution effects is sometimes labelled ‘controversial’. Why this phenomenon has been deemed controversial is a question perhaps best left to sociologists. We suspect, however, that the controversy arises in part from misunderstandings about dilution effects. In particular, there has been a confusing and inappropriate conflation of dilution effects with more general relationships between biodiversity and disease (see Keesing & Ostfeld, 2021). For example, the effect on pathogen transmission of static, innate variation in biodiversity among biogeographical regions is not relevant to natural dilution effects (Halliday et al., 2020; Keesing & Ostfeld, 2021).
Another source of confusion is how dilution effects apply when considering multiple pathogens. The number of pathogens naturally present in an ecosystem is not a function of dilution or amplification effects (Johnson et al., 2013a; Keesing & Ostfeld, 2015, 2021). When they occur, dilution effects operate within a specific disease system caused by a single pathogen (Keesing et al., 2006). Of course, ecological communities typically harbour many pathogens. In pollinator communities in Michigan, for example three RNA viruses co‐occur and infect multiple species of native bees (Fearon & Tibbetts, 2021). For all three pathogens, virus prevalence was lower in more diverse pollinator communities (Fearon & Tibbetts, 2021). These coincident pathogens all demonstrate dilution effects, but dilution effects could occur for some pathogens in an area and not for others, an area of investigation that has received too little attention. The likelihood of pathogen‐specific patterns in a multi‐pathogen system might be affected, for example by whether the different parasites interact in predictable ways with the diversity of hosts. In the Riberioia‐amphibian disease system, parasite diversity itself reduced Riberoia infection, augmenting the effects of host diversity alone, an impact that might arise from within‐host interactions among parasites (Johnson et al., 2013a).
The pace of change in the literature has caused confusion as well. Exploration of the dilution effect has evolved rapidly (e.g. Keesing et al., 2006, 2010; Keesing & Ostfeld, 2021; Ostfeld & Keesing, 2000, 2012), requiring researchers to keep pace with fast‐moving scholarship. For example, almost a decade ago, a meta‐analysis found evidence of a dilution effect for zoonotic diseases from one metric, and not from another, based on their selection of the literature at that time (Salkeld et al., 2013). Subsequent meta‐analyses incorporating more comprehensive literature searches have updated that result (Civitello et al., 2015a, b; Salkeld et al., 2015), demonstrating significant dilution effects for zoonoses. In this case, and more generally, responsible scholarship requires including citations that update earlier conclusions (Editorial, 2017; Penders, 2018).
DIVERSITY AND DISEASE—CONFRONTING CONFUSION
One of the most compelling aspects of natural dilution effects is the connections they establish between the conservation of biological diversity and the health of humans, other animals and plants. But this very connection between diversity and disease has itself been a source of confusion. For example, researchers attempting to address the dilution effect have estimated the diversity of vertebrates in different regions, countries or habitats, and asked whether disease severity is negatively correlated with diversity across these areas (Fernández et al., 2021; Wood et al., 2017). However, dilution effect theory does not predict that regions of the world with higher innate diversity should have lower disease severity, or fewer diseases. If a natural dilution effect is operating in a particular ecological community, the loss of diversity from that community will lead to more transmission of the focal pathogen within that community. This is a narrower claim, and it is driven by our understanding of the basic biology that underlies these phenomena.
Connections between diversity and disease seem amenable to policy and management recommendations, and this too has been a source of confusion. Based on our understanding of natural dilution effects, policies that lead to the conservation of biological diversity should reduce the transmission of many pathogens. Yet such an outcome could be counter‐balanced by increased risk if biodiversity were itself a source of new pathogens. Most newly emerging infectious diseases result from the spillover of pathogens from existing host species to new ones. An implicit assumption of most thinking about spillover has been that diverse communities have more species of hosts, and therefore more kinds of pathogens (Ostfeld & Keesing, 2017). The more kinds of pathogens there are, this thinking goes, the more opportunities there are for one of them to have the particular characteristics that would allow it to spill over into a new host species, including humans (Hosseini et al., 2017; Ostfeld & Keesing, 2017). If this thinking were correct, then biodiversity would be a potentially dangerous source of new pathogens through spillover, while at the same time potentially reducing transmission of endemic pathogens through dilution effects. Should policies, then, focus on conserving diversity to foster dilution, or on reducing diversity to prevent emergence?
A suite of recent studies suggests that this difficult choice is unnecessary. Pathogens that spill over from vertebrate animals into people—causing zoonotic diseases such as covid‐19 and Ebola—are much more likely to originate in specific kinds of animals, specifically Primates, Cetartiodactyls (mostly hooved mammals like deer, sheep, antelopes and cows), Rodents, Chiroptera (bats) and Carnivores (Han et al., 2016a; Johnson et al., 2020; Keesing & Ostfeld, 2021; Mollentze & Streicker, 2020). Which of these is most responsible for new zoonotic pathogens depends on the methods and metrics used, but these five groups consistently emerge as the leading sources (Keesing & Ostfeld, 2021). Within these Orders, the species that serve as hosts of zoonotic diseases are less likely to decline when biodiversity is lost (Johnson et al., 2020; Keesing & Ostfeld, 2021), and more likely to thrive when humans impact natural habitats (Gibb et al., 2020). These lines of evidence suggest an important new synthesis in which specific taxonomic groups are more likely to serve as sources of new pathogens, and specific, identifiable ecological characteristics are correlated with the probability that a species will serve as a high‐quality host. At least for zoonotic diseases, conserving and perhaps restoring, biodiversity should often both reduce the transmission of endemic pathogens and prevent the spillover of new ones (Keesing & Ostfeld, 2021).
LOOKING AHEAD
Our understanding of dilution effects, particularly dilution effects in nature, has progressed rapidly in the past 20 years. Here, we summarise major conclusions and outline some of the most important areas for future research and scholarship.
Dilution effects and their underlying mechanisms are well understood
Dilution effects have been used for many decades to reduce the transmission of pathogens in managed disease systems such as agricultural fields. The existence of these effects is not controversial and their underlying mechanisms are generally well understood. Using dilution effects in managed systems requires careful consideration of which low‐quality hosts are most likely to reduce overall transmission, and whether the net effects of reducing transmission are worth any potential costs, such as the possibility of decreases in crop yield or harvesting efficiency (Smithson & Lenné, 1996). Recent studies of zooprophylaxis suggest that potential costs of adding low‐quality hosts, such as increases in vector abundance, might be mitigated, for example with the use of topical insecticides (Asale et al., 2017; Kemibala et al., 2020).
Our understanding of dilution effects in natural ecosystems has developed more recently. Natural dilution effects arise from basic biological mechanisms that have been explored in theory (Johnson et al., 2015; Joseph et al., 2013; Keesing et al., 2006; Mihaljevic et al., 2014) and in some empirical studies (Johnson et al., 2013b; Liu et al., 2016, 2018; Rottstock et al., 2014), but there is still a great deal to learn. For example, does the pattern of variation in host quality (Figure 2) vary in predictable ways for different metrics (e.g. reservoir competence, vector preference) and across types of disease systems? How do interactions within hosts affect dilution effects in multi‐pathogen systems? How common are positive relationships between ecological resilience and host quality? What are the shapes of these relationships when they do occur, and what are their underlying causes? What are the best metrics for measuring transmission across disease systems? What are the characteristics of natural disease systems that show dilution effects and those that do not, and what does this suggest about whether we might apply our understanding of dilution effects to manage diseases in nature?
Low‐quality hosts deserve greater attention
The literature on disease ecology now includes a variety of examples of plant and animal hosts that proliferate as diversity declines, and these hosts are often likely to transmit pathogens (Gibb et al., 2020; Johnson et al., 2020; Keesing & Ostfeld, 2021). A major frontier is to increase our understanding of the roles of low‐quality hosts, for example those that harbour pathogens but do not transmit them onwards, and those that reduce the survival of feeding vectors. Low‐quality hosts are a major focus in work on managed dilution effects because their characteristics determine whether a given management strategy is likely to be effective. Incorporating this kind of thinking into studies of natural dilution effects could provide important depth to our understanding of the conditions under which such effects are likely to occur.
Dilution effects are distinct from diversity‐disease relationships
Despite confusion in the literature, dilution effects are not synonymous with ‘diversity‐disease relationships’. Dilution effects occur in a given disease system because of specific underlying mechanisms that have been well‐characterised (Halliday et al., 2020; Johnson & Thieltges, 2010; Keesing et al., 2006). In contrast, diversity‐disease relationships are often explored through large‐scale correlations that compare overall disease burdens or incidence in different geographical regions without attention to specific underlying mechanisms (Fernández et al., 2021; Wood et al., 2017). Finding patterns in such studies reveals neither dilution nor amplification effects, though these terms are frequently misapplied. Interpreting a correlation as a dilution or amplification effect requires understanding the biology of the underlying disease system. In our view, it should include determining whether there is variation in host quality and whether community composition changes to favour high‐quality hosts as endemic biodiversity changes.
The effects of biodiversity loss on pathogen emergence and subsequent transmission are much more similar than previously recognised
A decade ago, it seemed possible that areas that were high in endemic biodiversity were likely to be the richest sources of new pathogens of humans, and hence of new zoonotic diseases (Keesing et al., 2010). This expectation arose from the simple logic that more species must mean more pathogens, more pathogens must mean more zoonotic pathogens, and more zoonotic pathogens must mean greater risk to humans (Ostfeld & Keesing, 2017). Recent research, however, has demonstrated that this logic is not supported by the evidence. Some taxonomic groups are far more likely to be sources of zoonotic pathogens, and those taxa tend to thrive when biodiversity is lost (Gibb et al., 2020; Johnson et al., 2020; Keesing & Ostfeld, 2021). We have much left to learn about the relationship between biodiversity change and the emergence of pathogens. Important questions include how biodiversity, and its loss, affect the emergence of pathogens of non‐human hosts; how we can effectively determine whether hosts can actually transmit pathogens, as opposed to simply becoming infected with them (Becker et al., 2020); and how to manage our behaviour and use of landscapes to minimise spillover events (Plowright et al., 2021).
CONCLUSION
The COVID‐19 pandemic has highlighted the global importance—and challenge—of understanding the ecology of infectious diseases. The impacts of diversity on the emergence and transmission of pathogens have never been more relevant. Acknowledging what we have learned about dilution effects in nature over the past 20 years is critically important, as is understanding their similarities and differences to the dilution effects that operate in managed disease systems like agricultural fields. Perhaps most importantly, it is time to tackle the big questions of the next decade while moving on from those that have been addressed in the past one.
AUTHOR STATEMENT
FK wrote the first draft of the manuscript, and both authors contributed substantially to editing.
ACKNOWLEDGEMENT
This work was supported by a grant from the US National Science Foundation to FK (OPUS #1948419).
Keesing, F. & Ostfeld, R.S. (2021) Dilution effects in disease ecology. Ecology Letters, 24, 2490–2505. 10.1111/ele.13875
DATA AVAILABILITY STATEMENT
No new data are presented in the manuscript.
REFERENCES
- Albery, G.F. & Becker, D.J. (2021). Fast‐lived hosts and zoonotic risk. Trends in Parasitology, 37(2), 117–129. [DOI] [PubMed] [Google Scholar]
- Allan, B.F. , Keesing, F. & Ostfeld, R.S. (2003) Effect of forest fragmentation on Lyme disease risk. Conservation Biology, 17, 267–272. [Google Scholar]
- Asale, A. , Duchateau, L. , Devleesschauwer, B. , Huisman, G. & Yewhalaw, D. (2017) Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): a systematic review. Infectious Diseases of Poverty, 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault, S.K. (1994) Environmental management: a re‐emerging vector control strategy. American Journal of Tropical Medicine and Hygiene, 50, 35–49. [DOI] [PubMed] [Google Scholar]
- Bandín, I. & Dopazo, C.P. (2011) Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Veterinary Research, 42, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, D.J. , Seifert, S.N. & Carlson, C.J. (2020) Beyond infection: integrating competence into reservoir host prediction. Trends in Ecology & Evolution, 35, 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begon, M. (2008) Effects of host diversity on disease dynamics. In: Ostfeld, R.S. , Keesing, F. & Eviner, V.T. ), (Eds.) Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton, NJ: Princeton University Press, pp. 12–29. [Google Scholar]
- Boudreau, M.A. (2013) Diseases in intercropping systems. Annual Review of Phytopathology, 51, 499–519. [DOI] [PubMed] [Google Scholar]
- Brunner, J.L. , Cheney, L. , Keesing, F. , Killilea, M. , Logiudice, K. , Previtali, A. et al. (2011) Molting success of Ixodes scapularis varies among individual blood meal hosts and species. Journal of Medical Entomology, 48(4), 860–866. [DOI] [PubMed] [Google Scholar]
- Brunner, J.L. , Duerr, S. , Keesing, F. , Killilea, M. , Vuong, H. & Ostfeld, R.S. (2013) An experimental test of competition among mice, chipmunks, and squirrels in deciduous forest fragments. PLoS One, 8(6), e66798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns, E. , Pierce, L. , Antonovics, J. & Hood, M. (2021) Vector preference and heterogeneity in host sex ratio can affect pathogen spread in natural plant populations. Ecology, 102, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon, J.J. & Chilvers, G.A. (1977) The effect of barley mildew on barley and wheat competition in mixtures. Australian Journal of Botany, 25, 59–65. [Google Scholar]
- Burdon, J.J. , Thrall, P.H. & Ericson, L. (2006) The current and future dynamics of disease in plant communities. Annual Review of Phytopathology, 44, 19–39. [DOI] [PubMed] [Google Scholar]
- Cardinale, B.J. , Duffy, J.E. , Gonzalez, A. , Hooper, D.U. , Perrings, C. , Venail, P. et al. (2012) Biodiversity loss and its impact on humanity. Nature, 486, 59–67. [DOI] [PubMed] [Google Scholar]
- Casadevall, A. & Pirofski, L.A. (2015) What is a host? Incorporating the microbiota into the damage‐response framework. Infection and Immunity, 83, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, K.M. & Wolfe, M.S. (1984) The spread of Erysiphe graminis f. sp. hordei in mixtures of barley varieties. Plant Pathology, 33, 89–100. [Google Scholar]
- Civitello, D.J. , Cohen, J. , Fatima, H. , Halstead, N.T. , Liriano, J. , McMahon, T.A. et al. (2015a) Biodiversity inhibits parasites: broad evidence for the dilution effect. Proceedings of the National Academy of Sciences, 112, 8667–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello, D.J. , Cohen, J. , Fatima, H. , Halstead, N.T. , McMahon, T.A. , Ortega, C.N. et al. (2015b). Reply to Salkeld et al.: Diversity‐disease patterns are robust to study design, selection criteria, and publication bias. Proceedings of the National Academy of Sciences, 112(46), E6262. 10.1073/pnas.1518473112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello, D.J. & Rohr, J.R. (2014) Disentangling the effects of exposure and susceptibility on transmission of the zoonotic parasite Schistosoma mansoni . Journal of Animal Ecology, 83, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Cooper, N. , Kamilar, J.M. & Nunn, C.L. (2012) Host longevity and parasite species richness in mammals. PLoS One, 7, e42190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet, S. , Bichet, C. , Larcombe, S. , Faivre, B. & Sorci, G. (2014) Impact of host nutritional status on infection dynamics and parasite virulence in a bird‐malaria system. Journal of Animal Ecology, 83, 256–265. [DOI] [PubMed] [Google Scholar]
- Cronin, J.P. , Welsh, M.E. , Dekkers, M.G. , Abercrombie, S.T. & Mitchell, C.E. (2010) Host physiological phenotype explains pathogen reservoir potential. Ecology Letters, 13, 1221–1232. [DOI] [PubMed] [Google Scholar]
- Dallas, T. , Huang, S. , Nunn, C. , Park, A.W. & Drake, J.M. (2017) Estimating parasite host range. Proceedings of the Royal Society B: Biological Sciences, 284(1861), 20171250– 10.1098/rspb.2017.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy, J.J. , Friedenberg, N.A. , Yang, Y.W. & Turner, P.E. (2007) Virus population extinction via ecological traps. Ecology Letters, 10, 230–240. [DOI] [PubMed] [Google Scholar]
- Downs, C.J. , Schoenle, L.A. , Han, B.A. , Harrison, J. & Martin, L.B. (2019) Scaling of host competence. Trends in Parasitology, 35, 182–192. [DOI] [PubMed] [Google Scholar]
- Dwyer, G. , Elkinton, J.S. & Buonaccorsi, J.P. (1997) Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. American Naturalist, 150, 685–707. [DOI] [PubMed] [Google Scholar]
- Editorial , (2017) Neutral citation is poor scholarship. Nature Genetics, 49, 1559. [DOI] [PubMed] [Google Scholar]
- Edman, J.D. , Webber, L.A. & Schmidt, A.A. (1974) Effect of host defenses on the feeding pattern of Culex nigripalpis when offered a choice of blood sources. Journal of Parasitology, 60, 874–883. [PubMed] [Google Scholar]
- Fearon, M.L. & Tibbetts, E.A. (2021) Pollinator community species richness dilutes prevalence of multiple viruses within multiple host species. Ecology, 102, e03305. [DOI] [PubMed] [Google Scholar]
- Fernández, D. , Giné‐Vázquez, I. , Liu, I. , Yucel, R. , Nai Ruscone, M. , Morena, M. et al. (2021) Are environmental pollution and biodiversity levels associated to the spread and mortality of COVID‐19? A four‐month global analysis. Environmental Pollution, 271, 116326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. , Tilman, D. , DeFries, R. , Montgomery, D.R. , Gleick, P. , Frumkin, H. et al. (2020) A changing planet. In: Myers, S. & Frumkin, H. (Eds.) Planetary health: protecting nature to protect ourselves. Washington: Island Press, pp. 71–110. [Google Scholar]
- Garrett, K.A. & Mundt, C.C. (1999) Epidemiology in mixed host populations. Phytopathology, 89, 984–990. [DOI] [PubMed] [Google Scholar]
- Garrido, M. , Halle, S. , Flatau, R. , Cohen, C. , Navarro‐Castilla, Á. , Barja, I. et al. (2021) The dilution effect behind the scenes: testing the underlying assumptions of its mechanisms through quantifying the long‐term dynamics and effects of a pathogen in multiple host species. Proceedings of the Royal Society B‐Biological Sciences, 288, 20210773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi, S.S. , Stephens, P.R. , Hua, J. , Searle, C.L. , Xie, G.Y. , Urbina, J. et al. (2017) Linking ecology and epidemiology to understand predictors of multi‐host responses to an emerging pathogen, the amphibian chytrid fungus. PLoS One, 12, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb, R. , Redding, D.W. , Chin, K.Q. , Donnelly, C.A. , Blackburn, T.M. , Newbold, T. et al. (2020) Zoonotic host diversity increases in human‐dominated ecosystems. Nature, 584, 398–402. [DOI] [PubMed] [Google Scholar]
- Gibson, A.K. & Nguyen, A.E. (2021) Does genetic diversity protect host populations from parasites? A meta‐analysis across natural and agricultural systems. Evolution Letters, 5, 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, T.L. , Ruiz, M.O. , Hamer, G.L. , Brawn, J.D. , Kitron, U.D. , Hayes, D.B. et al. (2009) Host selection by Culex pipiens mosquitoes and West Nile virus amplification. American Journal of Tropical Medicine and Hygiene, 80, 268–278. [PubMed] [Google Scholar]
- Hall, S.R. , Becker, C.R. , Simonis, J.L. , Duffy, M.A. , Tessier, A.J. & Cáceres, C.E. (2009) Friendly competition: evidence for a dilution effect among competitors in a planktonic host‐parasite system. Ecology, 90, 791–801. [DOI] [PubMed] [Google Scholar]
- Halliday, F.W. & Rohr, J.R. (2019) Measuring the shape of the biodiversity‐disease relationship across systems reveals new findings and key gaps. Nature Communications, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday, F.W. , Rohr, J.R. & Laine, A.‐L. (2020) Biodiversity loss underlies the dilution effect of biodiversity. Ecology Letters, 23, 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey, S. (2019) Defuse the dilution effect debate. Nature Ecology & Evolution, 3, 145–146. [DOI] [PubMed] [Google Scholar]
- Halsey, S. & Miller, J.R. (2020) Maintenance of Borrelia burgdorferi among vertebrate hosts: a test of dilution effect mechanisms. Ecosphere, 11, e03048. [Google Scholar]
- Han, B.A. , Kramer, A.M. & Drake, J.M. (2016a) Global patterns of zoonotic disease in mammals. Trends in Parasitology, 32, 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, B.A. , Schmidt, J.P. , Alexander, L.W. , Bowden, S.E. , Hayman, D.T.S. & Drake, J.M. (2016b) Undiscovered bat hosts of filoviruses. PLoS Neglected Tropical Diseases, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, B.A. , Schmidt, J.P. , Bowden, S.E. & Drake, J.M. (2015) Rodent reservoirs of future zoonotic diseases. Proceedings of the National Academy of Sciences, 2015, 201501598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh, M.H. , Tibbetts, M. , Strauss, M. , Ostfeld, R.S. & Keesing, F. (2012) Reservoir competence of wildlife host species for Babesia microti. Emerging Infectious Diseases, 18(12), 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, P.R. , Mills, J.N. , Prieur‐Richard, A.‐H. , Ezenwa, V.O. , Bailly, X. , Rizzoli, A. et al. (2017) Does the impact of biodiversity differ between emerging and endemic pathogens? The need to separate the concepts of hazard and risk. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1722), 20160129. 10.1098/rstb.2016.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z.Y.X. , de Boer, W.F. , van Langevelde, F. , Olson, V. , Blackburn, T.M. & Prins, H.H.T. (2013) Species’ life‐history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS One, 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z.Y.X. , Yu, Y. , van Langevelde, F. & de Boer, W.F. (2017) Does the dilution effect generally occur in animal diseases? Parasitology, 144, 823–826. [DOI] [PubMed] [Google Scholar]
- Johnson, C.K. , Hitchens, P.L. , Pandit, P.S. , Rushmore, J. , Evans, T.S. , Young, C.C.W. et al. (2020) Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proceedings of the Royal Society B: Biological Sciences, 287(1924), 20192736. 10.1098/rspb.2019.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Hartson, R.B. , Larson, D.J. & Sutherland, D.R. (2008) Diversity and disease: community structure drives parasite transmission and host fitness. Ecology Letters, 11, 1017–1026. [DOI] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Ostfeld, R.S. & Keesing, F. (2015) Frontiers in research on biodiversity and disease. Ecology Letters, 18, 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Preston, D.L. , Hoverman, J.T. & LaFonte, B.E. (2013a) Host and parasite diversity jointly control disease risk in complex communities. Proceedings of the National Academy of Sciences, 110, 16916–16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Preston, D.L. , Hoverman, J.T. & Richgels, K.L.D. (2013b) Biodiversity decreases disease through predictable changes in host community competence. Nature, 494, 230–233. [DOI] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Rohr, J.R. , Hoverman, J.T. , Kellermanns, E. , Bowerman, J. & Lunde, K.B. (2012) Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecology Letters, 15, 235–242. [DOI] [PubMed] [Google Scholar]
- Johnson, P.T.J. & Thieltges, D.W. (2010) Diversity, decoys and the dilution effect: how ecological communities affect disease risk. Journal of Experimental Biology, 213, 961–970. [DOI] [PubMed] [Google Scholar]
- Joseph, M.B. , Mihaljevic, J.R. , Orlofske, S. & Paull, S.H. (2013) Does life history mediate changing disease risk when communities disassemble? Ecology Letters, 16, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Keesing, F. (1998) Impacts of ungulates on the demography and diversity of small mammals in central Kenya. Oecologia, 116, 381–389. [DOI] [PubMed] [Google Scholar]
- Keesing, F. (2000) Cryptic consumers and the ecology of an African savanna. BioScience, 50, 205–215. [Google Scholar]
- Keesing, F. , Belden, L.K. , Daszak, P. , Dobson, A. , Harvell, C.D. , Holt, R.D. et al. (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. , Brunner, J. , Duerr, S. , Killilea, M. , LoGiudice, K. , Schmidt, K. et al. (2009) Hosts as ecological traps for the vector of Lyme disease. Proceedings of the Royal Society B, 276, 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. , Hersh, M.H. , Tibbetts, M. , McHenry, D.J. , Duerr, S. , Brunner, J. et al. (2012) Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerging Infectious Diseases, 18(12), 2013–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. , Holt, R.D. & Ostfeld, R.S. (2006) Effects of species diversity on disease risk. Ecology Letters, 9, 485–498. [DOI] [PubMed] [Google Scholar]
- Keesing, F. & Ostfeld, R.S. (2015) Ecology. Is biodiversity good for your health? Science (80), 349, 235–236. [DOI] [PubMed] [Google Scholar]
- Keesing, F. & Ostfeld, R.S. (2021) Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proceedings of the National Academy of Sciences, 118, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. & Young, T.P. (2014) Cascading consequences of the loss of large mammals in an African savanna. BioScience, 64, 487–495. [Google Scholar]
- Kemibala, E.E. , Mafra‐Neto, A. , Dekker, T. , Saroli, J. , Silva, R. , Philbert, A. et al. (2020) A zooprophylaxis strategy using l‐lactic acid (Abate) to divert host‐seeking malaria vectors from human host to treated non‐host animals. Malaria Journal, 19, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick, A.M. , Daszak, P. , Jones, M.J. , Marra, P.P. & Kramer, L.D. (2006) Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B: Biological Sciences, 273, 2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops, J. , Tilman, D. , Haddad, N.M. , Naeem, S. , Mitchell, C.E. , Haarstad, J. et al. (1999) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecology Letters, 2, 286–293. [DOI] [PubMed] [Google Scholar]
- Komar, N. , Langevin, S. , Hinten, S. , Nemeth, N. , Edwards, E. , Hettler, D. et al. (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infectious Diseases, 9, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach, K. , Kampen, H. , Dizij, A. , Arndt, S. , Seitz, H.M. , Schaible, U.E. et al. (1995) Infestation of rodents with larval Ixodes ricinus (Acari: Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s.l. in German woodlands. Journal of Medical Entomology, 32, 807–817. [DOI] [PubMed] [Google Scholar]
- Leonard, K.J. (1969) Factors affecting rates of stem rust increase in mixed plantings of susceptible and resistant oat varieties. Phytopathology, 59, 1845–1850. [Google Scholar]
- Liu, X. , Chen, F. , Lyu, S. , Sun, D. & Zhou, S. (2018) Random species loss underestimates dilution effects of host diversity on foliar fungal diseases under fertilization. Ecology and Evolution, 8(3), 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Chen, L. , Liu, M. , García‐Guzmán, G. , Gilbert, G.S. & Zhou, S. (2020) Dilution effect of plant diversity on infectious diseases: latitudinal trend and biological context dependence. Oikos, 129, 457–465. [Google Scholar]
- Liu, X. , Lyu, S. , Zhou, S. & Bradshaw, C.J.A. (2016) Warming and fertilization alter the dilution effect of host diversity on disease severity. Ecology, 97, 1680–1689. [DOI] [PubMed] [Google Scholar]
- Lloyd‐Smith, J.O. , Schreiber, S.J. , Kopp, P.E. & Getz, W.M. (2005) Superspreading and the effect of individual variation on disease emergence. Nature, 438, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice, K. , Ostfeld, R. , Schmidt, K. & Keesing, F. (2003) The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences, 100, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis, A.D. , Kuenzi, A.J. & Mills, J.N. (2018) Species diversity concurrently dilutes and amplifies transmission in a zoonotic host–pathogen system through competing mechanisms. Proceedings of the National Academy of Sciences, 115, 7979–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, G. (1957) The theory of control. The epidemiology and control of malaria. London: Oxford University Press, pp. 121–128. [Google Scholar]
- Magnusson, M. , Fischhoff, I.R. , Ecke, F. , Hörnfeldt, B. & Ostfeld, R.S. (2020) Effect of spatial scale and latitude on diversity–disease relationships. Ecology, 101, e02955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L.B. , Weil, Z.M. & Nelson, R.J. (2007) Immune defense and reproductive pace of life in Peromyscus mice. Ecology, 88, 2516–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley, D.J. , Keesing, F. , Young, T. & Dittmar, K. (2008) Effects of the removal of large herbivores on fleas of small mammals. Journal of Vector Ecology, 33, 263–268. [DOI] [PubMed] [Google Scholar]
- Mihaljevic, J.R. , Joseph, M.B. , Orlofske, S.A. & Paull, S.H. (2014) The scaling of host density with richness affects the direction, shape, and detectability of diversity‐disease relationships. PLoS One, 9, e97812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, C.E. , Reich, P.B. , Tilman, D. & Groth, J.V. (2003) Effects of elevated CO2, nitrogen deposition, and decreased species diversity on foliar fungal plant disease. Global Change Biology, 9, 438–451. [Google Scholar]
- Mitchell, C.E. , Tilman, D. & Groth, J.V. (2002) Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology, 83, 1713–1726. [Google Scholar]
- Mollentze, N. & Streicker, D.G. (2020) Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proceedings of the National Academy of Sciences, 117, 9423–9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona, D. , Mazigo, H.D. , Kimaro, E.E. & Kweka, E.J. (2017) Zooprophylaxis: a strategy for effective delivery of endectocides for vector control. Journal of Transmitted Diseases and Immunity, 01, 3–5. [Google Scholar]
- Mundt, C.C. (2002) Use of multiline cultivars and cultivar mixtures for disease management. Annual Review of Phytopathology, 40, 381–410. [DOI] [PubMed] [Google Scholar]
- Norman, R. , Bowers, R.G. , Begon, M. & Hudson, P.J. (1999) Persistence of tick‐borne virus in the presence of multiple host species: Tick reservoirs and parasite mediated competition. Journal of Theoretical Biology, 200, 111–118. [DOI] [PubMed] [Google Scholar]
- Ostfeld, R.S. & Keesing, F. (2000) The function of biodiversity in the ecology of vector‐borne zoonotic diseases. Canadian Journal of Zoology, 78, 2061–2078. [Google Scholar]
- Ostfeld, R.S. & Keesing, F. (2012) Effects of host diversity on infectious disease. Annual Review of Ecology Evolution and Systematics, 43, 157–182. [Google Scholar]
- Ostfeld, R.S. & Keesing, F. (2017) Is biodiversity bad for your health? Ecosphere, 8, e01676. [Google Scholar]
- Ostfeld, R.S. , Levi, T. , Jolles, A.E. , Martin, L.B. , Hosseini, P.R. & Keesing, F. (2014) Life history and demographic drivers of reservoir competence for three tick‐borne zoonotic pathogens. PLoS One, 9, e107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld, R.S. , Levi, T. , Keesing, F. , Oggenfuss, K. & Canham, C.D. (2018) Tick‐borne disease risk in a forest food web. Ecology, 99, 1562–1573. [DOI] [PubMed] [Google Scholar]
- Ostfeld, R.S. & LoGiudice, K. (2003) Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology, 84, 1421–1427. [Google Scholar]
- Penders, B. (2018) Ten simple rules for responsible referencing. PLoS Computational Biology, 14, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plourde, B.T. , Burgess, T.L. , Eskew, E.A. , Roth, T.M. , Stephenson, N. & Foley, J.E. (2017) Are disease reservoirs special? Taxonomic and life history characteristics. PLoS One, 12(7), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright, R.K. , Reaser, J.K. , Locke, H. , Woodley, S.J. , Patz, J.A. , Becker, D.J. et al. (2021) Land use‐induced spillover: a call to action to safeguard environmental, animal, and human health. The Lancet Planetary Health, 5(4), e237–e245. 10.1016/S2542-5196(21)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin, R. (2011) Evolutionary ecology of parasites. Second: Princeton University Press, Princeton, NJ. [Google Scholar]
- Previtali, M.A. , Ostfeld, R.S. , Keesing, F. , Jolles, A.E. , Hanselmann, R. & Martin, L.B. (2012) Relationship between pace of life and immune responses in wild rodents. Oikos, 121, 1483–1492. [Google Scholar]
- Pulkkinen, K. & Ebert, D. (2004) Host starvation decreases parasite load and mean host size in experimental populations. Ecology, 85, 823–833. [Google Scholar]
- Rohr, J.R. , Civitello, D.J. , Halliday, F.W. , Hudson, P.J. , Lafferty, K.D. , Wood, C.L. et al. (2020) Towards common ground in the biodiversity‐disease debate. Nature Ecology & Evolution, 4, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, L. , Fajardo, S.N. & Rizzo, D.M. (2021). Sporulation potential of Phytophthora ramorum differs among common California plant species in the Big Sur region. Plant Disease, PDIS03200485RE. 10.1094/PDIS-03-20-0485-RE [DOI] [PubMed] [Google Scholar]
- Rottstock, T. , Joshi, J. , Kummer, V. & Fischer, M. (2014) Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology, 95, 1907–1917. [DOI] [PubMed] [Google Scholar]
- Salkeld, D.J. , Padgett, K.A. & Jones, J.H. (2013) A meta‐analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecology Letters, 16, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld, D.J. , Padgett, K.A. , Jones, J.H. & Antolin, M.F. (2015) Public health perspective on patterns of biodiversity and zoonotic disease. Proceedings of the National Academy of Sciences, 112, 201517640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer, E.L. , Trejo, N. , Hoverman, J.T. & Rohr, J.R. (2019) Behavioural fever reduces ranaviral infection in toads. Functional Ecology, 33, 2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul, A. (2003) Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malaria Journal, 2, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber, E.M. & Ostfeld, R.S. (2002) Modeling the effects of reservoir competence decay and demographic turnover in Lyme disease ecology. Ecological Applications, 12, 1142–1162. [Google Scholar]
- Schmidt, K.A. & Ostfeld, R.S. (2001) Biodiversity and the dilution effect in disease ecology. Ecology, 82, 609–619. [Google Scholar]
- Service, M.W. (1991) Agricultural development and arthropod‐borne diseases: a review. Revista de Saude Publica, 25, 165–178. [DOI] [PubMed] [Google Scholar]
- Shapiro, L. , De Moraes, C.M. , Stephenson, A.G. & Mescher, M.C. (2012) Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecology Letters, 15, 1430–1438. [DOI] [PubMed] [Google Scholar]
- Siva‐Jothy, J.A. & Vale, P.F. (2021) Dissecting genetic and sex‐specific sources of host heterogeneity in pathogen shedding and spread. PLoS Path, 17, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson, J.B. & Lenné, J.M. (1996) Varietal mixtures: a viable strategy for sustainable productivity in subsistence agriculture. The Annals of Applied Biology, 128, 127–158. [Google Scholar]
- Stephenson, J.F. , Young, K.A. , Fox, J. , Jokela, J. , Cable, J. & Perkins, S.E. (2017) Host heterogeneity affects both parasite transmission to and fitness on subsequent hosts. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, A.T. , Bowling, A.M. , Duffy, M.A. , Cáceres, C.E. & Hall, S.R. (2018) Linking host traits, interactions with competitors and disease: Mechanistic foundations for disease dilution. Functional Ecology, 32, 1271–1279. [Google Scholar]
- Strauss, A.T. , Civitello, D.J. , Caceres, C.E. & Hall, S.R. (2015) Success, failure and ambiguity of the dilution effect among competitors. Ecology Letters, 18, 916–926. [DOI] [PubMed] [Google Scholar]
- Suzán, G. , Marcé, E. , Giermakowski, J.T. , Mills, J.N. , Ceballos, G. , Ostfeld, R.S. et al. (2009) Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PLoS One, 4, e5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieltges, D.W. , Bordalo, M.D. , Caballero Hernández, A. , Prinz, K. & Jensen, K.T. (2008) Ambient fauna impairs parasite transmission in a marine parasite‐host system. Parasitology, 135, 1111–1116. [DOI] [PubMed] [Google Scholar]
- Titcomb, G. , Allan, B.F. , Ainsworth, T. , Henson, L. , Hedlund, T. , Pringle, R.M. et al. (2017) Interacting effects of wildlife loss and climate on ticks and tick‐borne disease. Proceedings of the Royal Society B: Biological Sciences, 284(1862), 20170475– 10.1098/rspb.2017.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, J.I. , Wootton, J.T. , Bunikis, J. , Luna, M.G. , Fish, D. & Barbour, A.G. (2004) An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proceedings of the National Academy of Sciences, 101, 18159–18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela‐Sánchez, A. , Wilber, M.Q. , Canessa, S. , Bacigalupe, L.D. , Muths, E. , Schmidt, B.R. et al. (2021) Why disease ecology needs life‐history theory: a host perspective. Ecology Letters, 24, 876–890. [DOI] [PubMed] [Google Scholar]
- VanBuskirk, J. & Ostfeld, R.S. (1995) Controlling Lyme disease by modifying the density and species composition of tick hosts. Ecological Applications, 5, 1133–1140. [Google Scholar]
- Venesky, M.D. , Liu, X. , Sauer, E. & Rohr, J. (2013) Linking manipulative experiments to field data to test the dilution effect. Journal of Animal Ecology, 83(3), 557–565. [DOI] [PubMed] [Google Scholar]
- Warburton, E.M. & Vonhof, M.J. (2018) From individual heterogeneity to population‐level overdispersion: quantifying the relative roles of host exposure and parasite establishment in driving aggregated helminth distributions. International Journal for Parasitology, 48, 309–318. [DOI] [PubMed] [Google Scholar]
- Werden, L. , Barker, I.K. , Bowman, J. , Gonzales, E.K. , Leighton, P.A. , Lindsay, L.R. et al. (2014) Geography, deer, and host biodiversity shape the pattern of Lyme disease emergence in the thousand islands archipelago of Ontario, Canada. PLoS One, 9, e85640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, L.A. , Forester, J.D. & Craft, M.E. (2018) Covariation between the physiological and behavioral components of pathogen transmission: host heterogeneity determines epidemic outcomes. Oikos, 127, 538–552. [Google Scholar]
- Wolfe, M.S. (1985) The current status and prospects of multiline cultivars and variety mixtures for disease resistance. Annual Review of Phytopathology, 23, 251–273. [Google Scholar]
- Wood, C.L. , Mcinturff, A. , Young, H.S. , Kim, D. & Lafferty, K.D. (2017) Human infectious disease burdens decrease with urbanization but not with biodiversity. Philosophical Transactions of the Royal Society of London. Series B, 372, 20160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (1982) Manual on environmental management. 284. [Google Scholar]
- Yan, J. , Broggi, J. , Martínez‐de la Puente, J. , Gutiérrez‐López, R. , Gangoso, L. , Soriguer, R. et al. (2018) Does bird metabolic rate influence mosquito feeding preference? Parasites and Vectors, 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, T.P. , Okello, B. , Kinyua, D. & Palmer, T.M. (1998) KLEE: a long‐term multi‐species herbivore exclusion experiment in Laikipia, Kenya. African Journal of Range & Forage Science, 104, 92–104. [Google Scholar]
- Zhu, Y. , Chen, H. , Fan, J. , Wang, Y. , Li, Y. , Chen, J. et al. (2000) Genetic diversity and disease control in rice. Nature, 406, 718–722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data are presented in the manuscript.


