Abstract
IRE1 is an important central regulator of unfolded protein response (UPR) in the endoplasmic reticulum (ER) because of its ability to regulate cell fate as a function of stress sensing. When misfolded proteins accumulated in chondrocytes ER, IRE1 disintegrates with BIP/GRP78 and undergoes dimer/oligomerization and transautophosphorylation. These two processes are mediated through an enzyme activity of IRE1 to activate endoribonuclease and generates XBP1 by unconventional splicing of XBP1 messenger RNA. Thereby promoting the transcription of UPR target genes and apoptosis. The deficiency of inositol‐requiring enzyme 1α (IRE1α) in chondrocytes downregulates prosurvival factors XBP1S and Bcl‐2, which enhances the apoptosis of chondrocytes through increasing proapoptotic factors caspase‐3, p‐JNK, and CHOP. Meanwhile, the activation of IRE1α increases chondrocyte viability and reduces cell apoptosis. However, the understanding of IRE1 responses and cell death fate remains controversial. This review provides updated data about the role IRE1 plays in chondrocytes and new insights about the potential efficacy of IRE1 regulation in cartilage repair and osteoarthritis treatment.
Keywords: apoptosis, chondrocyte, ERS, IRE1, osteoarthritis
IRE1 plays a central regulatory role in endoplasmic reticulum (ER) protein homeostasis, which guide ER stress recovery or cell apoptosis. IRE1 promotes death signalling in the chondrocyte when it combines with HSP47 in response to BiP attachment with misfolded protein. IRE1 can be considered as a potential target to slow down chondrocyte apoptosis and cartilage loss.
1. INTRODUCTION
Osteoarthritis (OA) is a common chronic disease of the joints, which characterized by degeneration of articular cartilage and secondary osteogenesis (Schiraldi et al., 2016). The development of OA affects not only the articular cartilage but also subchondral bone, joint capsule, synovial membrane, and muscle around the joint, which causes severe harm to the whole joint (Barnett, 2018; Hunter & Bierma‐Zeinstra, 2019). The global incidence of OA affects 25% of adults by 2030, and soon will be the largest cause of disability (Wallace et al., 2017). Worthy mention, the current treatment for OA is limited. Mild OA is mostly undergoing physical therapy, drug treatment, such as nonsteroidal antiinflammatory drugs and glucosamine (Cooper et al., 2019; Harrison‐Munoz et al., 2017). Patients with severe OA are at risk of low quality of life, and joint replacement. But, the efficacy of joint replacement is poor and the cost is high (Dieppe, 2011). Hence, a deep understanding of pathomechanisms that involve OA is the only conceivable way to improve medical interventions for curing OA.
Generally, chondrocyte apoptosis is an important process in the occurrence and development of OA (Dai et al., 2018; Hosseinzadeh et al., 2016; Park et al., 2020). The term “apoptosis” was first recognized as a morphologically different type of cell death from normal cell death (Kerr et al., 1972). In normal cell survival, apoptosis is a natural phenomenon that stops the growth and division of cells (Obeng, 2021); Specifically, apoptosis is a process that mediates gene regulation and eventually leads to induce cell death (Obeng, 2021). Apoptosis of eukaryotic cells occurs mainly through exogenous apoptosis pathway mediated by one of the following death receptors, endogenous apoptosis pathway mediated by mitochondria, or apoptosis pathway mediated by endoplasmic reticulum stress (ERS). Nevertheless, ERS has attracted increasing attention (Chen et al., 2008; Galitskii, 2005; Moon et al., 2016). The apoptosis of chondrocyte in OA mediated by ERS has been discussed by several studies which revealed that many extracellular and intracellular factors can promote ERS induced apoptosis (Hwang & Kim, 2015; Lotz et al., 1999; Zhuang et al., 2020). However, there are still many problems to be solved. The mechanism ERS mediates downregulation of inositol‐requiring enzyme 1α (IRE1α) triggering preapoptotic signals particularly in the OA disease remains to be clear. This article mainly focuses on the mechanisms through which IRE1α regulates chondrocyte apoptosis in OA through ERS. The deep understanding of IRE1α‐mediated chondrocyte apoptosis is supposed to demonstrate some interesting options contribute to blockage apoptosis process in the chondrocyte or at least slow down chondrocyte apoptosis and cartilage loss and enhances cartilage repair.
2. ERS AND IRE1
Endoplasmic reticulum (ER) is a largest membranous organelle in eukaryotic cells. It involves in postsynthesis modification and transport of proteins, lipid and steroid synthesis, regulation of Ca2+ balance and secretion, and other cell processes (Schwarz & Blower, 2016). High‐quality protein folding is essential for cell survival and function as well as for normal biophysiology (M. Wang & Kaufman, 2016). ERS can be triggered when proteins become unfolded and/or misfolded and accumulate in ER due to various endogenous and exogenous factors, including hypoxia, hunger, oxidative stress, and protein synthesis overload (Zheng et al., 2019). Initially, ER resumes normal cellular function by increasing the synthesis of molecular chaperones involved in protein folding (Kopp et al., 2019). The suspension of protein translation activates series of signaling pathways that result in unfolded protein response (UPR). UPR is not always effective in the regulation of ER homeostasis (M. Wang & Kaufman, 2016). If ERS persists, it will lead to dysfunction of ER that consequently activates related apoptotic pathways to mediate cell death (Tan et al., 2008).
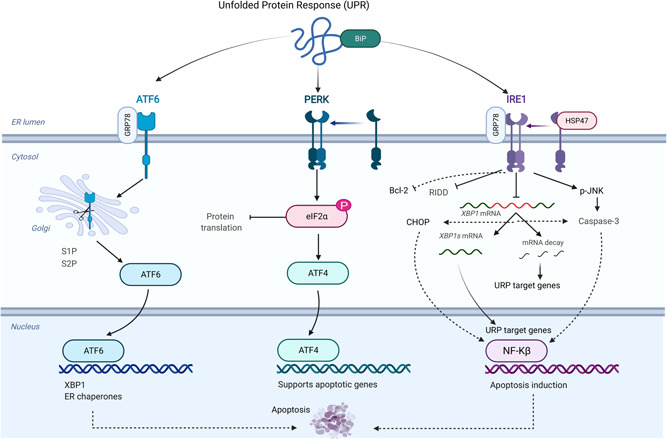
ERS is associated with a variety of pathological changes. Accumulation of unfolded and/or misfolded proteins in the ER lumen can trigger downstream pathways and effector mechanisms, remodeling ER to restore homeostasis (Hetz et al., 2020). This is an adaptive mechanism to deal with protein disorders, and the main response pathway is UPR (Tavernier et al., 2017). UPR has three sensorable branches, which are IRE1, protein kinase R‐like ER kinase (PERK) and activating transcription factor 6 (ATF6) (Bergmann & Molinari, 2018), see Figure 1. After the accumulation of unfolded and/or misfolded proteins in ER lumen, ERS is initiated, and the ER sensor senses the stress signal and disassociates with molecular chaperones. The disintegrated molecular chaperones bind with unfolded and/or misfolded proteins to modify produced proteins and reduce the degree of ERS (Pinkaew et al., 2017). The molecular chaperone systems commonly used in UPR mainly include HSP70 (BIP)/HSP40 (DnaJ proteins), HSP90 (GRP94), calnexin/calreticulin, and protein disulfide isomerases (Fedeles et al., 2015). BIP/GRP78 is a major ER chaperone and one of the most abundant proteins in ER, and is considered to be the main sensor for the activation of UPR (Bakunts et al., 2017), see Figure 2. However, the specific mechanism of BIP/GRP78 as a molecular chaperone and transduction of ERS signal is still unclear. Some scholars believe that unfolded and/or misfolded proteins are recruited to the substrate‐binding domain of BIP/GRP78 to activate ERS sensing under the action of J‐domain chaperone molecule (Behnke et al., 2015; Kopp et al., 2019). Among the three ER transmembrane proteins mediated UPR, IRE1 is the most conserved gene from yeast to human, and it has two subtypes: IRE1α and IRE1β. IRE1α is commonly expressed in most cells and tissues, while IRE1β is limited to gastrointestinal epithelial cells (Y. Liu et al., 2015; Sepulveda et al., 2018). Further, IRE1β acts as a dominant‐negative suppressor of IRE1α and affect how barrier epithelial cells manage the response to stress at the host‐environment interface. Worthy mention, IRE1α plays an important role in chondrocytes, as presented in Figure 1, it involves cell differentiation, extracellular matrix (ECM) production, and the expression of chondrocyte pro‐survival factors XBP1S and Bcl‐2 (Han et al., 2013; Wu et al., 2018). IRE1 is a dual enzyme with both serine/threonine kinase and endoribonuclease activity (Sepulveda et al., 2018). When unfolded and/or misfolded proteins are accumulated in the ER, IRE1 disintegrates with BIP/GRP78 and undergoes dimer/oligomerization and transautophosphorylation through its enzyme activity to activate endoribonuclease activity and generate XBP1 by unconventional splicing of XBP1 messenger RNA (mRNA), thereby promoting the transcription of UPR target genes including BIP, ERDJ4, SEC. 61α, and HERP (Fedeles et al., 2015).
Figure 1.
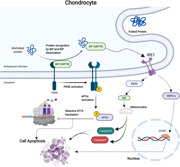
Misfolded protein response mediates IRE1 apoptotic signaling in the chondrocyte. the accumulation of unfolded protein induces the activation of three sensor signals in the chondrocytes (ATF6, PERK, IRE1) that initiate endoplasmic reticulum stress (ERS). The initiation of ERS promotes several signaling pathways, which mainly attempts to reduce ERS and remove unfolded protein. BiP is the main unit separate from IRE1 and move towards unfolded protein and then activates the interaction between GRP78, IRE1, and HSP47 that enhances apoptotic signaling and reduces survival signaling. The activation of PERK induces the phosphorylation of ELF2α that inhibits protein translation and supports apoptosis responses. Further, the interaction between GRP78 and ATF6 in response to the accumulation of unfolded protein blocks the collection of translated protein and promotes apoptosis responses. ATF6, activating transcription factor 6; IRE1, inositol‐requiring enzyme 1; PERK, protein kinase R‐like ER kinase
Figure 2.
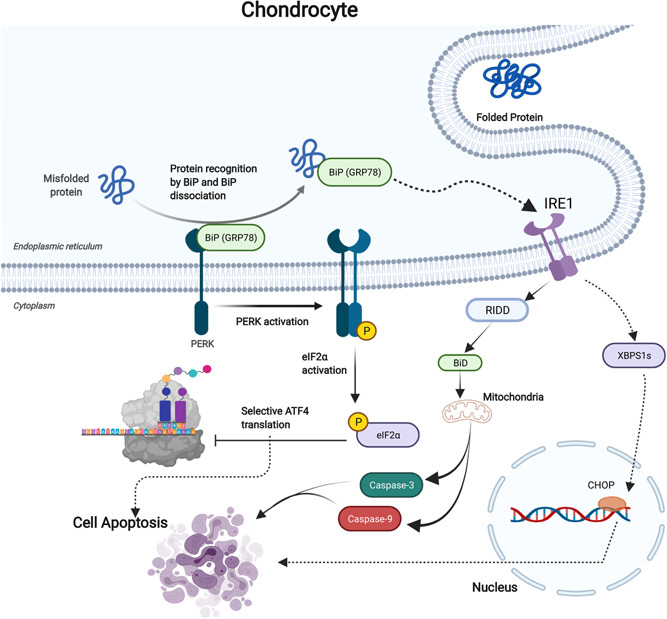
The failure in protein accumulation in the chondrocyte stimulates the formation of the BiP‐ GRP78 complex. This complex promotes PERK signaling to induce elf2α phosphorylation, which activates ATF‐4 to induce comprehensive apoptosis response and inhibiting protein translation. Further, BiP‐GRP78 complex mainly mediates IRE1 activation. IRE1 in this case stimulates several apoptotic signals such as RIDD that induce BiD cleavage that promotes mitochondrial‐dependent apoptosis through caspase‐3 and caspase‐9. The activation of IRE1α dependent apoptosis promotes XBPS1s that mediates proapoptosis signaling (CHOP), which enhances cell apoptosis and inhibits cell survival factors. IRE1, inositol‐requiring enzyme 1; PERK, protein kinase R‐like ER kinase; RIDD, regulated IRE1‐dependent decay
IRE1 is an important central regulator of UPR in the ER because of its ability to regulate cell fate as a function of stress sensing as presented in Figure 2, although the mechanism by which IRE1 regulates these different pathways remains unclear (Lamriben & Hebert, 2018). In the absence of ERS, the chaperone BIP/GRP78 binds to the luminal domain of IRE1, resulting in allosteric inhibition of IRE1 kinase, and thus IRE1 remains inactive (Carrara et al., 2015). However, how IRE1 stimulates endoplasmic ER sensing, it is also unclear. In yeast experiments, some scholars believe that the luminal domain ligand binding slot of IRE1 binds unfolded and/or misfolded proteins to sense ERS, thus leading to allosteric activation of IRE1 (Karagoz et al., 2017; P. Walter & Ron, 2011). In terms of the inactivation of ERS sensor, some scholars believe that under the action of ERDJ4, the cavity domain structure of IRE1 combines with the BIP/GRP78 ATPase domain to make IRE1 allosteric and return to the monomer state (Amin‐Wetzel et al., 2017).
3. IRE1‐RELATED SIGNALING PATHWAYS
IRE1 has been reported to involve several signaling pathways including cell survival and death signaling in various types of cells, see Table 1. Under normal conditions, BIP/GRP78 binds to ERS sensors, namely IRE1, PERK, and ATF6, which are inactive (Carrara et al., 2015). UPR is triggered when there is an increase in unfolded and/or misfolded proteins in the ER lumen, during UPR, BIP/GRP78 and ERS sensors are separated from each other (P. Walter & Ron, 2011). BIP/GRP78 binds unfolded proteins or misfolded proteins to modify unfolded and/or misfolded proteins and can also identify and target proteasomal degradation through ER‐associated degradation mechanism (ERAD) to reduce the effects of ERS (Olzmann et al., 2013). After IRE1 is dissociated from its chaperone protein BIP/GRP78, it is oligomerized and activated by transautophosphorylation, leading to the activation of its kinase and endoribonuclease domains (Lee et al., 2003). Activated IRE1α cleaves a 26‐nucleotide fragment from XBP1 mRNA to produce a transcription factor encoding the XBP1 protein (Yoshida et al., 2001). This transcription factor regulates ERS adaptation by inducing genes involved in protein folding and quality control, such as the expression of ER chaperone genes, adipose genes, and ERAD genes (He et al., 2010). In addition, the endoribonuclease activity of IRE1 is also involved in the degradation of mRNAs, ribosomal RNAs, and microRNAs (Dufey et al., 2020). If the stress factors persist, UPR threshold exceeds and consequently apoptosis process being induced (Sepulveda et al., 2018).
Table 1.
The function of IRE1 in different signaling pathways of various cell types
| Cell type | Signaling pathway | Function |
|---|---|---|
| Mesenchymal stem cell | IRE1‐XBP1 signaling way | Involved in osteoblast differentiation through promoting Osx transcription (Tohmonda et al., 2011) |
| Fibroblast | IRE1‐RIDD signaling way | Involved in HCPT‐induced apoptosis of fibroblasts, prevent scar adhesion after knee surgery (X. Li et al., 2016) |
| CD8α + cDCs | IRE1‐XBP1 signaling way | Control ER homeostasis, cell‐to‐cell contact and antigen processing (Osorio et al., 2014) |
| Macrophage | IRE1‐c‐Jun signaling way | Free cholesterol loading of macrophages leads to an apoptotic response that is partially dependent on initiation by activation of IRE1 (F. Li et al., 2008) |
| T cell | IRE1‐XBP1 signaling way | Controlling endoplasmic reticulum stress or targeting IRE1α‐XBP1 signalling may help to restore the metabolic fitness and antitumour capacity of T cells in cancer hosts (Song et al., 2018) |
| LO2 cell | BiP‐IRE1‐CHOP signaling way | Emodin‐induced excessive ROS generation and redox imbalance promoted apoptosis, which was mainly associated with BiP/IRE1α/CHOP signaling‐mediated ER stress and would be enhanced by oxidative stress‐mediated mitochondrial dysfunction (Qiu et al., 2021) |
| Pulmonary artery smooth muscle cells | IRE1‐XBP1 signaling way | IRE1α‐XBP1 pathway is involved in the process of hypoxia‐induced pulmonary vascular remodeling; 4u8c could restrain hypoxia‐induced cell proliferation and migration and reverse the hypoxia‐induced apoptosis arrest, while quercetin excited excessive ERS and the IRE1 pathway in hypoxic PASMCs and promoted apoptosis (Cao et al., 2019) |
| SH‐SY5Y cell | IRE1‐MAMs signaling way | Aβ peptides enhance cytotoxicity and mitochondrial damage in SH‐SY5Y cells by targeting MAMs (Chu et al., 2021) |
| Mouse liver cell | IRE1‐MAMs signaling way | IRE1α deficiency resulted in marked alterations in mitochondrial physiology and energy metabolism under resting conditions (Carreras‐Sureda et al., 2019) |
Abbreviations: IRE1, inositol‐requiring enzyme 1; RIDD, regulated IRE1‐dependent decay; ROS, reactive oxygen species.
Moreover, ER homeostasis is often disrupted by internal and external factors as the aggregation of misfolded proteins in cells, and the ERAD‐related IRE1 signaling pathway is the main quality control mechanism for clearance of misfolded proteins in the ER (Sun et al., 2015). However, the specific molecular mechanism of ERAD in the IRE1 signaling pathway remains to be discussed, and the properties of ERAD degradable substrates need to be further explored. SEL1L‐HRD1 is one of the important members of ERAD in the IRE1 signaling pathway, and it identifies, transplants and degrades some misfolded proteins in the ER (Iida et al., 2011). In the absence of stress, IRE1 interacts with the SEL1L‐HRD1 ERAD complex which is ubiquitinated. ERS reduces ubiquitination of IRE1, resulting in the dissociation of the IRE1α‐ERAD complex, leading to the activation of a series of signaling pathways (Sun et al., 2015). This is consistent with the final results of the binding of BIP to unfolded and/or misfolded proteins leading to the self‐activation of IRE1 to form a dimer. According to the experiments of some scholars, IRE1 is the real substrate of SEL1L‐HRD1 ERAD complex (Sun et al., 2015). IRE1 as the endogenous substrate of ERAD, BIP is essential (Sun et al., 2015). Because the absence of BIP leads to no interaction between IRE1 and ERAD, the IRE1 protein tends to stabilize; If the BIP is in the state of increase, the opposite result will be obtained (Sun et al., 2015).
Worthy mention, the IRE1/XBP1 signaling pathway is an important member of the IRE1‐related signaling pathway, which is not only a part of cellular program that protects chondrocytes' ERS, but also controls cell development and survival (F. Walter et al., 2015). In chronic ERS response, the internal working rhythm of chondrocytes tends to induce apoptosis rather than the UPR to alleviate ERS (Tabas & Ron, 2011). However, the generation of IRE1/XBP1 signaling pathway is mainly due to the interference of internal and external factors, which leads to the activation of IRE1 to adapt to the environment, and then produces a series of reactions. The deletion of XBP1 will lead to functional changes in the cell signaling pathway, but does not affect cell survival. However, it leads to its death in mucosal dendritic cells, demonstrating tissue‐specificity of XBP1 under the action of IRE1 (Tavernier et al., 2017). Among them, the cell death caused by IRE1/XBP1 signal changes could not attribute to activate CHOP or ‐Jun amino terminal kinase (JNK) signaling pathways under ERS, but the ATF4‐dependent adaptive comprehensive stress response may play a major role (Tavernier et al., 2017), see Figure 2. In ER protein homeostasis, IRE1 plays a central regulatory role, guiding ER stress recovery or cell apoptosis. However, the specific role of IRE1 between both mechanisms is still not fully elaborated. Sure, it is well known that IRE1‐BIP is the classical IRE1 silencing binding point. But in a recent paper, Sepulveda et al. (2018) reported that HSP47 could compete with BIP to perform signaling in combination with IRE1 (Shamrock et al., 2021), See Figure 1. Interestingly, HSP47 was previously thought to be associated with collagen transport (Köhler et al., 2020). The new insights put forward by Sepulveda et al. (2018) undoubtedly provide a new idea for researchers studying the IRE1 signaling pathway, and the relevant role of HSP47 and IRE1 is still under study. Some scholars believe that the interaction between HSP47 and IRE1 improves the shear efficiency of XBP1 and provides a guarantee for the homeostasis of proteins in the ER (Lamriben & Hebert, 2018). In addition, HSP47 also regulates protein synthesis through the IRE1‐regulated IRE1‐dependent decay (RIDD) signaling pathway. It blocks the increase of misfolded proteins during ERS; its overexpression enhances the attenuation of mRNA, and then modulates the apoptosis function induced by IRE1 (Lamriben & Hebert, 2018).
However, in the absence of ERS signaling, DNA damage can also activate IRE1α signaling, which is different from classical signaling pathway. It specifically activates RIDD signaling (Chevet et al., 2015). This raises the question that if IRE1 signaling pathway plays a crucial role in ER stress; how does IRE1 be activated in the absence of ERS? Some scholars found that the activation of C‐ABL kinase can cause the self‐phosphorylation of IRE1, and then activate the transduction of IRE1‐dependent RIDD signaling pathway, thus affecting the survival of cells (Dufey et al., 2020). In fibroblasts, even if XBP1 mRNA is cut off after IRE1 activation, the IRE1‐dependent RIDD signaling pathway can still be active to regulate the cell cycle and affect cell survival (Dufey et al., 2020). IRE1 endoribonuclease activity is involved in the regulation of mRNA stability after DNA damage response, affecting cell cycle and apoptosis, which is consistent with the role of IRE1 in nonclassical pathway.
4. IRE1‐MEDIATED APOPTOSIS IN CHONDROCYTES
In the past few years, the study of IRE1‐mediated apoptosis has become more extensive. However, the specific mechanism of IRE1 signaling pathway mediated chondrocyte apoptosis remains to be further studied. It is well known that IRE1 is a single transmembrane protein with an intracavitary domain and cytoplasmic domain (CD) that perform different tasks on different sides of the endoplasmic omentum (Adams et al., 2019). Under the ERS condition, cells will initiate the UPR to reduce the stress degree and slow down the damage to cells (Hetz & Papa, 2018). If the rate of unfolded and/or misfolded proteins in the ER is greater than the cellular remission caused by the UPR, then IRE1 initiates apoptotic signaling through a series of signaling pathways. The continuous release of inflammatory factors in OA leads to chronic ER stress, and eventually a large quantity of misfolded proteins accumulates in ER lumen, which then trigger cell apoptosis mainly through the IREI signaling pathway (Zhang et al., 2019).
4.1. IRE1‐tumor necrosis factor receptor‐associated factor 2 (TRAF2)‐apoptotic signal‐regulated kinase 1 (ASK1)‐JNK signaling pathway mediated apoptosis
When ERS occurs, ER homeostasis is disrupted by the folding of overloaded proteins in the ER (Merksamer & Papa, 2010). Dysfunctional ERS can lead to UPR, including activation of IRE1‐dependent signaling pathways that act as ERS transducers. Activated IRE1 binds to TRAF2 through the CD domain, and recruits ASK1 to form an apoptotic complex (Urano et al., 2000). In addition, chronic ERS is transmitted by c‐JNK, and downstream apoptotic molecules such as caspase‐2 and Bax are activated (Urano et al., 2000). When some scholars studied the effect of Tetramethylpyrazine analogue (CSTMP) on the apoptosis of NSCLC A549 cells, they found that CSTMP had obvious antiproliferation effect on A549 cells within the range of 50–150 mm (J. Zhang et al., 2016). Under the action of CSTMP on NSCLC A549 cells, the IRE1 signaling pathway of excessive stress on the ER was activated, and the CD of activated IRE1α was combined with TRAF2 and ASK1 to trigger the activation of JNK pathway (Nishitoh et al., 2002; J. Zhang et al., 2016). The formation of the IRE1A‐TRAF2‐ASK1 complex activates JNK and mediates partial cell death under irreversible ERS (Kanda & Miura, 2004). Besides, mitochondria‐mediated apoptosis is associated with DNA damage. Oligomerization of Bcl‐2 family proapoptotic proteins, such as Bax and Bak, can promote the release of cytochrome C, and then cytochrome C and caspase9 precursors form apoptotic complex to perform apoptosis. Some of the apoptotic proteins of the Bcl‐2 family are also localized in the ER and play corresponding roles under ER stress, including regulating apoptosis (Carpio et al., 2015; Rong & Distelhorst, 2008). This indirectly indicates that ERS‐mediated apoptosis and mitochondria‐mediated apoptosis are not independent of each other. Currently, further studies focus on the mechanism of circ‐RNA in the apoptosis of chondrocyte. Some scholars have reported that in the model of chondrocyte injury induced by interleukin (IL)‐1β, circ‐0114876 can induce the increase of TRAF2 expression, and the inhibition of circ‐0114876 can enhance the activity of chondrocytes, reduce inflammatory response, and reduce cell apoptosis (Q. Wang et al., 2021). However, the specific molecular mechanism of the apoptosis of chondrocytes induced by circ‐RNA in OA induced by IL‐1β is still unclear. Thus, IRE1‐TRAF2 complex could plays a role in the induced apoptosis which associated with circ‐RNA mediate chondrocyte apoptosis through IRE1‐TRAF2‐ASK1‐JNK signaling pathway, but this suggestion needs to be further explored in experiments.
Similarly, many scholars study the molecular mechanism of micro‐RNA to cartilage damage. Some scholars found that miR‐502‐5p levels were significantly downregulated in OA joint tissues and IL‐1β induced chondrocytes (G. Zhang et al., 2016). Further, miR‐502‐5p inhibited the viability of IL‐1β‐induced chondrocyte targeting TRAF2 inhibition, and alleviated IL‐1β‐induced ECM metabolic imbalance and proinflammatory cytokine production (G. Zhang et al., 2016). Altogether, the specific molecular mechanism of miR‐502‐5p's protective effect on IL‐1β‐induced chondrocyte injury through targeting TRAF2 signaling pathway is suggested to be associated with the role of IRE1 signaling pathway in cartilage injury under ERS condition, which needs further study. However, this indirectly indicates that there is an undiscovered mechanism of IRE1‐TRAF2‐ASK1‐JNK signaling pathway in the regulation of chondrocyte apoptosis.
4.2. IRE1‐RIDD signaling pathway mediated apoptosis
As a central regulator of cell fate under ERS, IRE1 integrates information related to injury intensity and duration (L. Liu et al., 2019). RIDD plays an important role in the outcome of cell apoptosis induced by IRE1 overactivation (Tavernier et al., 2017). RIDD can also lead to cell death under severe ERS conditions under the action of IRE1 endonuclease (Hollien et al., 2009). Although this may complement the UPR, which is an adaptive mechanism to reduce the influx of proteins in ER, the RIDD pathway has also been shown to trigger proapoptotic responses (Ghosh et al., 2014). Previous studies have linked the IRE1‐RIDD signaling pathway to inflammation and apoptosis (Lerner et al., 2012). Huang and colleagues found that mRNA degradation of IRE1‐RIDD target genes (such as Bloc1S1 and St3GAL5) in macrophages was associated with apoptosis in cells infected with mycobacterium avium (Huang et al., 2016). In Mycobacterium infected macrophages, the increased production of reactive oxygen species (ROS) stimulates the activation of ERS, and the activation of IRE1‐RIDD leads to the mRNA degradation of RIDD target genes (Go et al., 2019). ROS‐mediated ERS induced apoptosis of macrophages infected with Mycobacterium avium by activating IRE1α‐RIDD (Go et al., 2019). It is well known that the production of ROS is one of the important manifestations of inflammation in the development of OA (Lepetsos & Papavassiliou, 2016). However, it is still unclear whether the production of intra‐articular inflammatory factors can also mediate the apoptosis of chondrocytes caused by IRE1‐RIDD activation, which needs further exploration.
The research on the physiological effects of RIDD is still the tip of the iceberg. Moreover, RIDD mainly works in a case of chronic ERS or deletion of XBP1, and the activation of IRE1‐RIDD appears to be a highly selective process (Tavernier et al., 2017). Some scholars believe that the activation of IRE1‐dependent RIDD plays a pro‐survival role in dendritic cells (Tavernier et al., 2017). This is inconsistent with the cell apoptosis induced by IRE1‐RIDD activation mediated by the production of inflammatory cytokines. This is may be related to the threshold after IRE1 activation. It has been suggested that the over‐active state of IRE1 induces proapoptotic pathways under conditions of irremediable ER stress, but the size of the threshold is still unknown.
5. PROBLEMS TO BE SOLVED AND NEW PROSPECTS
Certainly, IRE1 signaling pathway mediates ERS induced cell apoptosis. As known, OA development is inseparable from the apoptosis of chondrocytes, but the exact mechanism that initiates chondrocyte apoptosis is still uncertain. At present, several in‐depth studies have been conducted on IRE1 signaling pathway and the relation of this pathway with chondrocyte apoptosis (Wu et al., 2018). However, the blocking of chondrocyte apoptosis and enhancing cartilage regeneration through direct blocking of IRE1 pathway have not been studied neither in vivo nor in vitro. These kinds of studies could significantly contribute to develop advance clinical studies leading to the delay of the development of OA. Further, the study of cartilage formation in the IRE1 knock out mice compared to overexpressed mice can provide new understanding of the role IRE1 could play in the cartilage regeneration or OA development. Furthermore, there are many problems to be considered in the mechanism of IRE1 signaling pathway mediating chondrocyte apoptosis such as the relation of series of internal signal transduction to offset the interference of external factors or internal factors leading to induce UPR and ERS in the chondrocyte. The question we are trying to address here, the boundary between the UPR that cells tolerate and that induces intense apoptosis. Up to date the level of UPR chondrocyte can tolerate is unknown. We believe that continues accumulation of UPR increases the stress that triggers cell apoptosis but temporary accumulation, cells can tolerate with different ways. However, what extent does stress factor cause IRE1‐mediated ERS‐related apoptosis is still unclear. We suggest that using cells with different mutation models that express different levels of UPR could explore new findings provide clear answer on what doses and levels of UPR can stimulate cell apoptosis. Second, although IRE1 is the most conserved transmembrane protein receptor in ER and also an important part of ERS‐related apoptosis, it's unclear whether some other ER transmembrane protein receptors, such as PERK and ATF6 play the same important role in chondrocyte apoptosis in combination with IRE1 or there is another uncertain signal induces IRE1. The factors specially trigger ERS‐related apoptosis initiation by IRE1, PERK, and ATF6 remain to be studies. Using animal models with specific knock down of related genes could explore clear understanding for ESR‐related initiators. Finally, IRE1 is an important receptor of ER transmembrane protein, which plays an important role in physiological, pathological and other mechanisms of action. Theoretically, blocking IRE1 can slow down the apoptosis of chondrocytes and cartilage degeneration, meanwhile it could delay the development of OA but what we need to consider is the optimal condition to block IRE1 in the treatment of OA. Researchers should consider that IRE1 is also an important component of ER physiology which is supposed to modulate the function of several genes that participate in the chondrocyte fate.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Rongxiang Huang and Murad Alahdal; data curation: R.H and Murad Alahdal; writing original draft preparation: Murad Alahdal; writing review and editing: Zhang Hui, Duan Li; visualization: Murad Alahdal; supervision: M.A, W.L and Wang Daping; project administration: Murad Alahdal; funding acquisition: Murad Alahdal. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
This work has been supported by International Science and Technology Cooperation Project of Guangdong Province 2019, China (1035043); National Natural Science Foundation of China (No. 81772394; No. 81972116; No. 81972085); a key clinical discipline of Guangdong Province, Orthopaedics (No. 2000005).
Huang, R. , Hui, Z. , Wei, S. , Li, D. , Li, W. , Daping, W. , & Alahdal, M. (2022). IRE1 Signaling Regulates Chondrocyte Apoptosis and Death Fate in the Osteoarthritis. J Cell Physiol, 237, 118–127. 10.1002/jcp.30537
Rongxiang Huang and Murad Alahdal contributed equally in this work.
Contributor Information
Wencui Li, Email: 13923750767@163.com.
Wang Daping, Email: wangdp@mail.sustech.edu.cn.
Murad Alahdal, Email: muadalahdal@zju.edu.cn.
DATA AVAILABILITY STATEMENT
The authors confirm that this article does not include any experimental data but the sources collected were included in the list of references.
REFERENCES
- Adams, C. J. , Kopp, M. C. , Larburu, N. , Nowak, P. R. , & Ali, M. M. U. (2019). Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Frontiers in Molecular Biosciences, 6, 11. 10.3389/fmolb.2019.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin‐Wetzel, N. , Saunders, R. A. , Kamphuis, M. J. , Rato, C. , Preissler, S. , Harding, H. P. , & Ron, D. (2017). A J‐protein co‐chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell, 171(7), 1625–1637. e1613. 10.1016/j.cell.2017.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakunts, A. , Orsi, A. , Vitale, M. , Cattaneo, A. , Lari, F. , Tadè, L. , Sitia, R. , Raimondi, A. , Bachi, A. , & van Anken, E. (2017). Ratiometric sensing of BiP‐client versus BiP levels by the unfolded protein response determines its signaling amplitude. eLife, 6, 10.7554/eLife.27518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, R. (2018). Osteoarthritis. Lancet, 391(10134), 1985. 10.1016/S0140-6736(18)31064-X [DOI] [PubMed] [Google Scholar]
- Behnke, J. , Feige, M. J. , & Hendershot, L. M. (2015). BiP and its nucleotide exchange factors Grp170 and Sil1: Mechanisms of action and biological functions. Journal of Molecular Biology, 427(7), 1589–1608. 10.1016/j.jmb.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, T. J. , & Molinari, M. (2018). Three branches to rule them all? UPR signalling in response to chemically versus misfolded proteins‐induced ER stress. Biology of the Cell, 110(9), 197–204. 10.1111/boc.201800029 [DOI] [PubMed] [Google Scholar]
- Cao, X. , He, Y. , Li, X. , Xu, Y. , & Liu, X. (2019). The IRE1alpha‐XBP1 pathway function in hypoxia‐induced pulmonary vascular remodeling, is upregulated by quercetin, inhibits apoptosis and partially reverses the effect of quercetin in PASMCs. American Journal of Translational Research, 11(2), 641–654. [PMC free article] [PubMed] [Google Scholar]
- Carpio, M. A. , Michaud, M. , Zhou, W. , Fisher, J. K. , Walensky, L. D. , & Katz, S. G. (2015). BCL‐2 family member BOK promotes apoptosis in response to endoplasmic reticulum stress. Proceedings of the National Academy of Sciences of the United States of America, 112(23), 7201–7206. 10.1073/pnas.1421063112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara, M. , Prischi, F. , Nowak, P. R. , Kopp, M. C. , & Ali, M. M. (2015). Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. eLife, 4, 10.7554/eLife.03522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras‐Sureda, A. , Jaña, F. , Urra, H. , Durand, S. , Mortenson, D. E. , Sagredo, A. , Bustos, G. , Hazari, Y. , Ramos‐Fernández, E. , Sassano, M. L. , Pihán, P. , van Vliet, A. R. , González‐Quiroz, M. , Torres, A. K. , Tapia‐Rojas, C. , Kerkhofs, M. , Vicente, R. , Kaufman, R. J. , Inestrosa, N. C. , … Hetz, C. (2019). Non‐canonical function of IRE1alpha determines mitochondria‐associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nature Cell Biology, 21(6), 755–767. 10.1038/s41556-019-0329-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. , Guo, Y. B. , Liu, S. L. , Zheng, D. X. , & Liu, Y. X. (2008). [Construction and expression of anti‐tumor necrosis factor related apoptosis‐inducing ligand receptor death receptor 5 chimeric antibody in eukaryotic cells]. Zhongguo yi Xue Ke Xue Yuan Xue Bao, 30(6), 690–695. [PubMed] [Google Scholar]
- Chevet, E. , Hetz, C. , & Samali, A. (2015). Endoplasmic reticulum stress‐activated cell reprogramming in oncogenesis. Cancer Discovery, 5(6), 586–597. 10.1158/2159-8290.CD-14-1490 [DOI] [PubMed] [Google Scholar]
- Chu, B. , Li, M. , Cao, X. , Li, R. , Jin, S. , Yang, H. , Xu, L. , Wang, P. , & Bi, J. (2021). IRE1alpha‐XBP1 Affects the mitochondrial function of Abeta25‐35‐treated SH‐SY5Y cells by regulating mitochondria‐associated endoplasmic reticulum membranes. Frontiers in Cellular Neuroscience, 15, 614556. 10.3389/fncel.2021.614556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, C. , Chapurlat, R. , Al‐Daghri, N. , Herrero‐Beaumont, G. , Bruyère, O. , Rannou, F. , Roth, R. , Uebelhart, D. , & Reginster, J. Y. (2019). Safety of oral non‐selective non‐steroidal anti‐inflammatory drugs in osteoarthritis: What does the literature say? Drugs & Aging, 36(Suppl 1), 15–24. 10.1007/s40266-019-00660-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, M. , Sui, B. , Xue, Y. , Liu, X. , & Sun, J. (2018). Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials, 180, 91–103. 10.1016/j.biomaterials.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Dieppe, P. (2011). Who should have a joint replacement? A plea for more 'phronesis'. Osteoarthritis and Cartilage, 19(2), 145–146. 10.1016/j.joca.2010.08.018 [DOI] [PubMed] [Google Scholar]
- Dufey, E. , Bravo‐San Pedro, J. M. , Eggers, C. , González‐Quiroz, M. , Urra, H. , Sagredo, A. I. , Sepulveda, D. , Pihán, P. , Carreras‐Sureda, A. , Hazari, Y. , Sagredo, E. A. , Gutierrez, D. , Valls, C. , Papaioannou, A. , Acosta‐Alvear, D. , Campos, G. , Domingos, P. M. , Pedeux, R. , Chevet, E. , … Hetz, C. (2020). Genotoxic stress triggers the activation of IRE1alpha‐dependent RNA decay to modulate the DNA damage response. Nature Communications, 11(1), 2401. 10.1038/s41467-020-15694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedeles, S. V. , So, J. S. , Shrikhande, A. , Lee, S. H. , Gallagher, A. R. , Barkauskas, C. E. , Somlo, S. , & Lee, A. H. (2015). Sec. 63 and Xbp1 regulate IRE1alpha activity and polycystic disease severity. Journal of Clinical Investigation, 125(5), 1955–1967. 10.1172/JCI78863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitskii, V. A. (2005). [The origin of eukaryotic cells and origination of apoptosis]. TSITOLOGIIA, 47(2), 103–120. [PubMed] [Google Scholar]
- Ghosh, R. , Wang, L. , Wang, E. S. , Perera, B. G. , Igbaria, A. , Morita, S. , Prado, K. , Thamsen, M. , Caswell, D. , Macias, H. , Weiberth, K. F. , Gliedt, M. J. , Alavi, M. V. , Hari, S. B. , Mitra, A. K. , Bhhatarai, B. , Schürer, S. C. , Snapp, E. L. , Gould, D. B. , … Papa, F. R. (2014). Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell, 158(3), 534–548. 10.1016/j.cell.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go, D. , Lee, J. , Choi, J. A. , Cho, S. N. , Kim, S. H. , Son, S. H. , & Song, C. H. (2019). Reactive oxygen species‐mediated endoplasmic reticulum stress response induces apoptosis of Mycobacterium avium‐infected macrophages by activating regulated IRE1‐dependent decay pathway. Cellular Microbiology, 21(12), e13094. 10.1111/cmi.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X. , Zhou, J. , Zhang, P. , Song, F. , Jiang, R. , Li, M. , Xia, F. , & Guo, F. J. (2013). IRE1α dissociates with BiP and inhibits ER stress‐mediated apoptosis in cartilage development. Cellular Signalling, 25(11), 2136–2146. 10.1016/j.cellsig.2013.06.011 [DOI] [PubMed] [Google Scholar]
- Harrison‐Munoz, S. , Rojas‐Briones, V. , & Irarrazaval, S. (2017). Is glucosamine effective for osteoarthritis? Medwave, 17(Suppl1), e6867. 10.5867/medwave.2017.6867 [DOI] [PubMed] [Google Scholar]
- He, Y. , Sun, S. , Sha, H. , Liu, Z. , Yang, L. , Xue, Z. , Chen, H. , & Qi, L. (2010). Emerging roles for XBP1, a sUPeR transcription factor. Gene Expression, 15(1), 13–25. 10.3727/105221610x12819686555051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz, C. , & Papa, F. R. (2018). The unfolded protein response and cell fate control. Molecular Cell, 69(2), 169–181. 10.1016/j.molcel.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Hetz, C. , Zhang, K. , & Kaufman, R. J. (2020). Mechanisms, regulation and functions of the unfolded protein response. Nature Reviews Molecular Cell Biology, 21(8), 421–438. 10.1038/s41580-020-0250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien, J. , Lin, J. H. , Li, H. , Stevens, N. , Walter, P. , & Weissman, J. S. (2009). Regulated Ire1‐dependent decay of messenger RNAs in mammalian cells. Journal of Cell Biology, 186(3), 323–331. 10.1083/jcb.200903014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh, A. , Kamrava, S. K. , Joghataei, M. T. , Darabi, R. , Shakeri‐Zadeh, A. , Shahriari, M. , Reiter, R. J. , Ghaznavi, H. , & Mehrzadi, S. (2016). Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. Journal of Pineal Research, 61(4), 411–425. 10.1111/jpi.12362 [DOI] [PubMed] [Google Scholar]
- Huang, M. , Xu, A. , Wu, X. , Zhang, Y. , Guo, Y. , Guo, F. , Pan, Z. , & Kong, L. (2016). Japanese encephalitis virus induces apoptosis by the IRE1/JNK pathway of ER stress response in BHK‐21 cells. Archives of Virology, 161(3), 699–703. 10.1007/s00705-015-2715-5 [DOI] [PubMed] [Google Scholar]
- Hunter, D. J. , & Bierma‐Zeinstra, S. (2019). Osteoarthritis. Lancet, 393(10182), 1745–1759. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- Hwang, H. S. , & Kim, H. A. (2015). Chondrocyte apoptosis in the pathogenesis of osteoarthritis. International Journal of Molecular Sciences, 16(11), 26035–26054. 10.3390/ijms161125943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida, Y. , Fujimori, T. , Okawa, K. , Nagata, K. , Wada, I. , & Hosokawa, N. (2011). SEL1L protein critically determines the stability of the HRD1‐SEL1L endoplasmic reticulum‐associated degradation (ERAD) complex to optimize the degradation kinetics of ERAD substrates. Journal of Biological Chemistry, 286(19), 16929–16939. 10.1074/jbc.M110.215871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, H. , & Miura, M. (2004). Regulatory roles of JNK in programmed cell death. Journal of Biochemistry, 136(1), 1–6. 10.1093/jb/mvh098 [DOI] [PubMed] [Google Scholar]
- Karagoz, G. E. , Acosta‐Alvear, D. , Nguyen, H. T. , Lee, C. P. , Chu, F. , & Walter, P. (2017). An unfolded protein‐induced conformational switch activates mammalian IRE1. eLife, 6, 10.7554/eLife.30700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, J. F. , Wyllie, A. H. , & Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide‐ranging implications in tissue kinetics. British Journal of Cancer, 26(4), 239–257. 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, M. C. , Larburu, N. , Durairaj, V. , Adams, C. J. , & Ali, M. M. U. (2019). UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nature Structural & Molecular Biology, 26(11), 1053–1062. 10.1038/s41594-019-0324-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler, A. , Mörgelin, M. , Gebauer, J. M. , Öcal, S. , Imhof, T. , Koch, M. , Nagata, K. , Paulsson, M. , Aumailley, M. , Baumann, U. , Zaucke, F. , & Sengle, G. (2020). New specific HSP47 functions in collagen subfamily chaperoning. FASEB Journal, 34(9), 12040–12052. 10.1096/fj.202000570R [DOI] [PubMed] [Google Scholar]
- Lamriben, L. , & Hebert, D. N. (2018). Activating and repressing IRE1alpha: The Hsp47 and BiP Tug of war. Molecular Cell, 69(2), 159–160. 10.1016/j.molcel.2017.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A. H. , Iwakoshi, N. N. , & Glimcher, L. H. (2003). XBP‐1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and Cellular Biology, 23(21), 7448–7459. 10.1128/mcb.23.21.7448-7459.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepetsos, P. , & Papavassiliou, A. G. (2016). ROS/oxidative stress signaling in osteoarthritis. Biochimica et Biophysica Acta/General Subjects, 1862(4), 576–591. 10.1016/j.bbadis.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Lerner, A. G. , Upton, J. P. , Praveen, P. V. , Ghosh, R. , Nakagawa, Y. , Igbaria, A. , Shen, S. , Nguyen, V. , Backes, B. J. , Heiman, M. , Heintz, N. , Greengard, P. , Hui, S. , Tang, Q. , Trusina, A. , Oakes, S. A. , & Papa, F. R. (2012). IRE1alpha induces thioredoxin‐interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metabolism, 16(2), 250–264. 10.1016/j.cmet.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Guo, Y. , Sun, S. , Jiang, X. , Tang, B. , Wang, Q. , & Wang, L. (2008). Free cholesterol‐induced macrophage apoptosis is mediated by inositol‐requiring enzyme 1 alpha‐regulated activation of Jun N‐terminal kinase. Acta Biochim Biophys Sin (Shanghai), 40(3), 226–234. 10.1111/j.1745-7270.2008.00396.x [DOI] [PubMed] [Google Scholar]
- Li, X. , Sun, Y. , Chen, H. , Zhu, G. , Liang, Y. , Wang, Q. , Wang, J. , & Yan, L. (2016). Hydroxycamptothecin induces apoptosis of fibroblasts and prevents intraarticular scar adhesion in rabbits by activating the IRE‐1 signal pathway. European Journal of Pharmacology, 781, 139–147. 10.1016/j.ejphar.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Zhao, M. , Jin, X. , Ney, G. , Yang, K. B. , Peng, F. , Cao, J. , Iwawaki, T. , Del Valle, J. , Chen, X. , & Li, Q. (2019). Adaptive endoplasmic reticulum stress signalling via IRE1alpha‐XBP1 preserves self‐renewal of haematopoietic and pre‐leukaemic stem cells. Nature Cell Biology, 21(3), 328–337. 10.1038/s41556-019-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Shao, M. , Wu, Y. , Yan, C. , Jiang, S. , Liu, J. , Dai, J. , Yang, L. , Li, J. , Jia, W. , Rui, L. , & Liu, Y. (2015). Role for the endoplasmic reticulum stress sensor IRE1alpha in liver regenerative responses. Journal of Hepatology, 62(3), 590–598. 10.1016/j.jhep.2014.10.022 [DOI] [PubMed] [Google Scholar]
- Lotz, M. , Hashimoto, S. , & Kühn, K. (1999). Mechanisms of chondrocyte apoptosis. Osteoarthritis and Cartilage, 7(4), 389–391. 10.1053/joca.1998.0220 [DOI] [PubMed] [Google Scholar]
- Merksamer, P. I. , & Papa, F. R. (2010). The UPR and cell fate at a glance. Journal of Cell Science, 123(Pt 7), 1003–1006. 10.1242/jcs.035832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, H. S. , Kim, B. , Gwak, H. , Suh, D. H. , & Song, Y. S. (2016). Autophagy and protein kinase RNA‐like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2 alpha kinase (eIF2alpha) pathway protect ovarian cancer cells from metformin‐induced apoptosis. Molecular Carcinogenesis, 55(4), 346–356. 10.1002/mc.22284 [DOI] [PubMed] [Google Scholar]
- Nishitoh, H. , Matsuzawa, A. , Tobiume, K. , Saegusa, K. , Takeda, K. , Inoue, K. , Hori, S. , Kakizuka, A. , & Ichijo, H. (2002). ASK1 is essential for endoplasmic reticulum stress‐induced neuronal cell death triggered by expanded polyglutamine repeats. Genes and Development, 16(11), 1345–1355. 10.1101/gad.992302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng, E. (2021). Apoptosis (programmed cell death) and its signals—A review. Brazilian Journal of Biology, 81(4), 1133–1143. 10.1590/1519-6984.228437 [DOI] [PubMed] [Google Scholar]
- Olzmann, J. A. , Kopito, R. R. , & Christianson, J. C. (2013). The mammalian endoplasmic reticulum‐associated degradation system. Cold Spring Harbor Perspectives in Biology, 5(9):a013185. 10.1101/cshperspect.a013185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio, F. , Tavernier, S. J. , Hoffmann, E. , Saeys, Y. , Martens, L. , Vetters, J. , Delrue, I. , De Rycke, R. , Parthoens, E. , Pouliot, P. , Iwawaki, T. , Janssens, S. , & Lambrecht, B. N. (2014). The unfolded‐protein‐response sensor IRE‐1alpha regulates the function of CD8alpha + dendritic cells. Nature Immunology, 15(3), 248–257. 10.1038/ni.2808 [DOI] [PubMed] [Google Scholar]
- Park, D. R., Kim, J. , Kim, G. M. , Lee, H. , Kim, M. , Hwang, D. , Lee, H. , Kim, H. S. , Kim, W. , Park, M. C. , Shim, H. , & Lee, S. Y. (2020). Osteoclast‐associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation. Nature Communications, 11(1), 4343. 10.1038/s41467-020-18208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkaew, D. , Chattopadhyay, A. , King, M. D. , Chunhacha, P. , Liu, Z. , Stevenson, H. L. , Chen, Y. , Sinthujaroen, P. , McDougal, O. M. , & Fujise, K. (2017). Fortilin binds IRE1alpha and prevents ER stress from signaling apoptotic cell death. Nature Communications, 8(1), 18. 10.1038/s41467-017-00029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, L. Z. , Yue, L. X. , Ni, Y. H. , Zhou, W. , Huang, C. S. , Deng, H. F. , Wang, N. N. , Liu, H. , Liu, X. , Zhou, Y. Q. , Xiao, C. R. , Wang, Y. G. , & Gao, Y. (2021). Emodin‐induced oxidative inhibition of mitochondrial function assists BiP/IRE1alpha/CHOP signaling‐mediated ER‐Related apoptosis. Oxidative Medicine and Cellular Longevity, 2021, 8865813. 10.1155/2021/8865813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. , & Distelhorst, C. W. (2008). Bcl‐2 protein family members: Versatile regulators of calcium signaling in cell survival and apoptosis. Annual Review of Physiology, 70, 73–91. 10.1146/annurev.physiol.70.021507.105852 [DOI] [PubMed] [Google Scholar]
- Schiraldi, C. , Stellavato, A. , de Novellis, F. , La Gatta, A. , & De Rosa, M. (2016). Hyaluronan viscosupplementation: State of the art and insight into the novel cooperative hybrid complexes based on high and low molecular weight HA of potential interest in osteoarthritis treatment. Clinical Cases in Mineral and Bone Metabolism, 13(1), 36–37. 10.11138/ccmbm/2016.13.1.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, D. S. , & Blower, M. D. (2016). The endoplasmic reticulum: Structure, function and response to cellular signaling. Cellular and Molecular Life Science, 73(1), 79–94. 10.1007/s00018-015-2052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda, D. , Rojas‐Rivera, D. , Rodríguez, D. A. , Groenendyk, J. , Köhler, A. , Lebeaupin, C. , Ito, S. , Urra, H. , Carreras‐Sureda, A. , Hazari, Y. , Vasseur‐Cognet, M. , Ali, M. , Chevet, E. , Campos, G. , Godoy, P. , Vaisar, T. , Bailly‐Maitre, B. , Nagata, K. , Michalak, M. , … Hetz, C. (2018). Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1alpha. Molecular Cell, 69(2), 238–252. 10.1016/j.molcel.2017.12.028. e237. [DOI] [PubMed] [Google Scholar]
- Shamrock, A. G. , Donnally, I. C. , & Varacallo, M. (2021). Lumbar Spondylolysis And Spondylolisthesis. In StatPearls. Treasure Island (FL). [PubMed]
- Song, M., Sandoval, T. A. , Chae, C. S. , Chopra, S. , Tan, C. , Rutkowski, M. R. , Raundhal, M. , Chaurio, R. A. , Payne, K. K. , Konrad, C. , Bettigole, S. E. , Shin, H. R. , Crowley, M. , Cerliani, J. P. , Kossenkov, A. V. , Motorykin, I. , Zhang, S. , Manfredi, G. , Zamarin, D. , … Cubillos‐Ruiz, J. R. (2018). IRE1alpha‐XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature, 562(7727), 423–428. 10.1038/s41586-018-0597-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S. , Shi, G. , Sha, H. , Ji, Y. , Han, X. , Shu, X. , Ma, H. , Inoue, T. , Gao, B. , Kim, H. , Bu, P. , Guber, R. D. , Shen, X. , Lee, A. H. , Iwawaki, T. , Paton, A. W. , Paton, J. C. , Fang, D. , Tsai, B. , … Qi, L. (2015). IRE1alpha is an endogenous substrate of endoplasmic‐reticulum‐associated degradation. Nature Cell Biology, 17(12), 1546–1555. 10.1038/ncb3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas, I. , & Ron, D. (2011). Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature Cell Biology, 13(3), 184–190. 10.1038/ncb0311-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Y. Y. , Zhou, H. Y. , Wang, Z. Q. , & Chen, S. D. (2008). Endoplasmic reticulum stress contributes to the cell death induced by UCH‐L1 inhibitor. Molecular and Cellular Biochemistry, 318(1‐2), 109–115. 10.1007/s11010-008-9862-x [DOI] [PubMed] [Google Scholar]
- Tavernier, S. J. , Osorio, F. , Vandersarren, L. , Vetters, J. , Vanlangenakker, N. , Van Isterdael, G. , Vergote, K. , De Rycke, R. , Parthoens, E. , van de Laar, L. , Iwawaki, T. , Del Valle, J. R. , Hu, C. C. , Lambrecht, B. N. , & Janssens, S. (2017). Regulated IRE1‐dependent mRNA decay sets the threshold for dendritic cell survival. Nature Cell Biology, 19(6), 698–710. 10.1038/ncb3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmonda, T. , Miyauchi, Y. , Ghosh, R. , Yoda, M. , Uchikawa, S. , Takito, J. , Morioka, H. , Nakamura, M. , Iwawaki, T. , Chiba, K. , Toyama, Y. , Urano, F. , & Horiuchi, K. (2011). The IRE1alpha‐XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Reports, 12(5), 451–457. 10.1038/embor.2011.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano, F. , Wang, X. , Bertolotti, A. , Zhang, Y. , Chung, P. , Harding, H. P. , & Ron, D. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science, 287(5453), 664–666. 10.1126/science.287.5453.664 [DOI] [PubMed] [Google Scholar]
- Wallace, I. J. , Worthington, S. , Felson, D. T. , Jurmain, R. D. , Wren, K. T. , Maijanen, H. , Woods, R. J. , & Lieberman, D. E. (2017). Knee osteoarthritis has doubled in prevalence since the mid‐20th century. Proceedings of the National Academy of Sciences of the United States of America, 114(35), 9332–9336. 10.1073/pnas.1703856114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, F. , Schmid, J. , Dussmann, H. , Concannon, C. G. , & Prehn, J. H. (2015). Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival. Cell Death and Differentiation, 22(9), 1502–1516. 10.1038/cdd.2014.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, P. , & Ron, D. (2011). The unfolded protein response: From stress pathway to homeostatic regulation. Science, 334(6059), 1081–1086. 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- Wang, M. , & Kaufman, R. J. (2016). Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature, 529(7586), 326–335. 10.1038/nature17041 [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Luo, S. , Yang, J. , Li, J. , Huan, S. , She, G. , & Zha, Z. (2021). Circ_0114876 promoted IL‐1beta‐induced chondrocyte injury by targeting miR‐671/TRAF2 axis. Biotechnology Letters, 43(4), 791–802. 10.1007/s10529-020-03070-1 [DOI] [PubMed] [Google Scholar]
- Wu, L. , Liu, H. , Li, L. , Xu, D. , Gao, Y. , Guan, Y. , & Chen, Q. (2018). 5,7,3',4′‐Tetramethoxyflavone protects chondrocytes from ER stress‐induced apoptosis through regulation of the IRE1α pathway. Connective Tissue Research, 59(2), 157–166. 10.1080/03008207.2017.1321639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H. , Matsui, T. , Yamamoto, A. , Okada, T. , & Mori, K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 107(7), 881–891. 10.1016/s0092-8674(01)00611-0 [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Sun, Y. , Wang, Y. , Liu, R. , Bao, Y. , & Li, Q. (2016). MiR‐502‐5p inhibits IL‐1beta‐induced chondrocyte injury by targeting TRAF2. Cellular Immunology, 302, 50–57. 10.1016/j.cellimm.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Liang, Y. , Lin, Y. , Liu, Y. , YouYou, & Yin, W. (2016). IRE1alpha‐TRAF2‐ASK1 pathway is involved in CSTMP‐induced apoptosis and ER stress in human non‐small cell lung cancer A549 cells. Biomedicine & pharmacotherapy = Biomédecine & pharmacothérapie, 82, 281–289. 10.1016/j.biopha.2016.04.050 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Xing, R. , Huang, Z. , Zhang, N. , Zhang, L. , Li, X. , & Wang, P. (2019). Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediators of Inflammation, 2019, 2165918. 10.1155/2019/2165918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. , Shang, Y. , Tao, J. , Zhang, J. , & Sha, B. (2019). Endoplasmic reticulum stress signaling pathways: Activation and diseases. Current Protein & Peptide Science, 20(9), 935–943. 10.2174/1389203720666190621103145 [DOI] [PubMed] [Google Scholar]
- Zhuang, C. , Ni, S. , Yang, Z.‐c , & Liu, R.‐p (2020). Oxidative stress induces chondrocyte apoptosis through caspase‐dependent and caspase‐independent mitochondrial pathways and the antioxidant mechanism of angelica sinensis polysaccharide. Oxidative Medicine and Cellular Longevity, 2020, 3240820. 10.1155/2020/3240820 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that this article does not include any experimental data but the sources collected were included in the list of references.


