Abstract
Background
Interleukin (IL)‐31 is a cytokine involved in allergic inflammation which induces pruritus across species including dogs. Using recombinant canine IL‐31 we have developed a model of pruritus in the dog to evaluate onset of action and duration of effect of therapeutic drugs.
Objective
To assess the onset of action and duration of effect of lokivetmab (Cytopoint) in the IL‐31‐induced pruritus model.
Animals
Twenty‐four purpose‐bred beagle dogs (neutered males, spayed and intact females) 1.5–4.7 years old and weighing between 6 and14 kg.
Methods and materials
Randomized, blinded, placebo‐controlled studies were designed to evaluate the antipruritic properties of lokivetmab. Laboratory beagle dogs were given either placebo, 0.125, 0.5 or 2.0 mg/kg lokivetmab, subcutaneously. IL‐31 then was administered to evaluate pruritus 3–5 h post‐placebo or ‐lokivetmab administration as well as one, seven, 14, 28, 42 and 56 days post‐dosing. Pruritus was evaluated over a 2 h window in animals by video monitoring and scored using a categorical scoring system.
Results
When animals were given 2.0 mg/kg lokivetmab, a significant reduction in pruritus was observed at 3–4, 4–5 and 3–5 h post‐treatment (P ≤ 0.0001). When animals were given either 0.125, 0.5 or 2 mg/kg lokivetmab, the duration of effect was dose‐dependent and statistically significant for 14, 28 and 42 days, respectively (P ≤ 0.0288).
Conclusion
These data indicate that a single subcutaneous injection of 2 mg/kg lokivetmab produces a significant suppression of pruritus starting 3 h post‐treatment that can be sustained for 42 days.
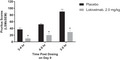
Onset of antipruritic action of lokivetmab in dogs.
Laboratory‐bred beagle dogs were dosed subcutaneously with either placebo or lokivetmab at 2.0 mg/kg on Day (D) 0. Approximately 2.5 h post‐dosing, pruritus was induced using canine interleukin (IL)‐31. Animals were observed for pruritic behaviours for a total of 2 h (3–5 h post‐treatment with placebo or lokivetmab). Observations were presented per hour (3–4 h, 4–5 h and 3–5 h post‐treatment with placebo or lokivetmab). Data are expressed as least square mean (LSM) ± standard error of mean (SEM). Treatment comparisons at each time point were conducted at the two‐sided α = 0.05 significance level (*P < 0.0001).
Résumé
Contexte
L’interleukine (IL)‐31 est une cytokine impliquée dans l’inflammation allergique qui induit le prurit pour toutes les espèces dont le chien. En utilisant un IL‐31 recombinant nous avons développés un modèle de prurit chez le chien pour évaluer le début de l’action et la durée des effets des traitements.
Objectifs
Déterminer le début d’action et la durée de l’effet du lokivetmab (Cytopoint) dans le modèle de prurit induit par IL‐31.
Sujets
Vingt quatre chiens beagle de reproduction (mâles castrés, femelles stérilisées et entières) 1,5 ‐ 4,7 ans et pesant de 6 à 14 kg.
Méthodes
Les études contrôlées contre placebo, en aveugle, randomisées ont été étudiées pour évaluer les propriétés antiprurigineuses du lokivetmab. Les chiens beagle de laboratoire ont été attribués aux goupes placebo, 0.125, 0.5 ou 2.0 mg/kg de lokivetmab en sous‐cutané. L’IL‐31 a ensuite été administrée pour évaluer le prurit 3‐5h après administration de lokivetmab ou de placebo ainsi que un, sept, 14, 38, 42 et 56 jours après dosage. Le prurit était évalué sur une fenêtre de 2h chez les animaux par monitoring vidéo et scoring utilisant un système d’évaluation catégorielle.
Résultats
Quand les sujets ont reçu 2.0 mg/kg de lokivetmab, une diminution significative du prurit était observée à 3‐4, 4‐5 et 3‐5h post traitement (P ≤ 0.0001). Quand les animaux recevaient soit 0.125 soit 0.5 ou 2 mg/kg de lokivetmab, la durée de l’effet était dépendant de la dose et statistiquement significatif, respectivement, pour 14, 28 et 42 jours (P ≤ 0.0288).
Conclusion
Ces données indiquent qu’une unique injection sous‐cutanée de 2 mg/kg de lokivetmab produit une suppression significative du prurit dès 3h après traitement et persiste pendant 42 jours.
RESUMEN
Introducción
la interleuquina (IL)‐31 es una citoquina involucrada en la inflamación alérgica que induce prurito en todas las especies, incluidos los perros. Utilizando IL‐31 canina recombinante, hemos desarrollado un modelo de prurito en el perro para evaluar el inicio de la acción y la duración del efecto de los fármacos terapéuticos.
Objetivo
evaluar el inicio de la acción y la duración del efecto de lokivetmab (Cytopoint) en el modelo de prurito inducido por IL‐31.
Animales
Veinticuatro perros Beagle de laboratorio (machos castrados, hembras esterilizadas e intactas) de 1,5 a 4,7 años y con un peso de entre 6 y 14 kg.
Métodos
se diseñaron estudios aleatorios, ciegos y controlados con placebo para evaluar las propiedades antipruriginosas del lokivetmab. A los perros Beagle de laboratorio se les administró placebo, 0,125; 0,5 o 2,0 mg/kg de lokivetmab por vía subcutánea. Luego se administró IL‐31 para evaluar el prurito 3‐5 h después de la administración de placebo o lokivetmab, así como a uno, siete, 14, 28, 42 y 56 días después de la dosificación. El prurito se evaluó durante una ventana de 2 h en animales mediante monitorización por vídeo y se puntuó utilizando un sistema de puntuación categórico.
Resultados
cuando se administró a los animales 2,0 mg/kg de lokivetmab, se observó una reducción significativa del prurito a las 3‐4, 4‐5 y 3‐5 h después del tratamiento (P ≤ 0,0001). Cuando se administró a los animales 0,125; 0,5 o 2 mg/kg de lokivetmab la duración del efecto fue dependiente de la dosis y estadísticamente significativa durante 14, 28 y 42 días, respectivamente (P ≤ 0,0288).
Conclusión
estos datos indican que una sola inyección subcutánea de 2 mg/kg de lokivetmab produce una supresión significativa del prurito a partir de las 3 h posteriores al tratamiento que se mantiene durante 42 días.
Zusammenfassung
Hintergrund
Interleukin (IL)‐31 ist ein Zytokin, welches bei der allergischen Entzündung involviert ist und Juckreiz bei allen Spezies wie auch beim Hund auslöst. Wir haben beim Hund ein Juckreiz Modell entwickelt, wobei wir eine rekombinante Form von caninem IL‐31 verwendet haben, um den Wirkungseintritt und die Dauer der Wirkung dieser therapeutischen Medikamente zu evaluieren.
Ziel
Die Beurteilung des Wirkungseintritts und der Wirkungsdauer von Lokivetmab (Cytopoint) beim durch IL‐31 induzierten Juckreizmodell.
Tiere
Vierundzwanzig zu diesem Zweck gezüchtete Beagles (kastrierte Rüden, kastrierte und intakte Hündinnen), im Alter von 1,5 – 4,7 Jahren, mit einem Gewicht zwischen 6 und 14 kg.
Methoden
Es wurden randomisierte, geblindete, Plazebo‐kontrollierte Studien designed, um die juckreizstillenden Eigenschaften von Lokivetmab zu evaluieren. Laborbeagles wurde entweder Plazebo oder 0,125; 0,5 oder 2,0 mg/kg Lokivetmab subkutan verabreicht. IL‐31 wurde verabreicht, um den Juckreiz 3‐5h Post‐Plazebo oder ‐Lokivetmab Verabreichung zu evaluieren, sowie nach einem, sieben, 14, 28, 42 und 56 Tage post‐Administration. Der Juckreiz wurde über ein 2‐stündiges Fenster evaluiert, wobei die Tiere mittels Videoüberwachung und mit Hilfe eines kategorischen Systems bewertet wurden.
Ergebnisse
Wenn den Tieren 2,0 mg/kg Lokivetmab verabreicht wurde, konnte eine signifikante Reduktion des Pruritus 3‐4, 4‐5 und 3‐5 h nach der Behandlung (P ≤ 0,0001) beobachtet werden. Wenn den Tieren entweder 0,125; 0,5 oder 2 mg/kg Lokivetmab verabreicht wurde, war die Wirkungsdauer Dosis‐abhängig und für 14, 28 bzw 42 Tage statistisch signifikant (P ≤ 0,0288).
Schlussfolgerung
Diese Ergebnisse zeigen, dass eine einzige subkutane Injektion von 2 mg/kg Lokivetmab eine signifikante Unterdrückung des Juckreizes bewirkt, welche 3h nach der Administration beginnt und 42 Tage lang anhält.
要約
背景
インターロイキン (IL)‐31 はアレルギーによる炎症に関与するサイトカインであり、犬を含む様々な種の掻痒を誘発する。我々は、イヌ IL‐31 を用いて、犬の掻痒モデルを開発し、治療薬の作用発現および効果持続時間を評価した。
目的
本研究の目的は、IL‐31誘発性掻痒モデルにおけるロキベトマブ (サイトポイント) の作用発現および効果持続時間を評価することであった。
被験動物
1.5˜4.7歳、体重6˜14kgの24頭の実験ビーグル犬 (去勢済みオス、去勢済みメス、未避妊メス) 。
方法
ロキベトマブの抗掻痒特性を評価するため、無作為化盲検プラセボ対照試験を実施した。実験用ビーグル犬にプラセボ、0.125、0.5または2.0mg/kgのロキベトマブを皮下投与した。その後、IL‐31を投与し、プラセボまたはロキベトマブ投与3˜5時間後、および投与1日後、7日後、14日後、28日後、42日後、56日後の掻痒を評価した。掻痒は、ビデオモニタリングにより動物を2時間評価し、カテゴリカルスコアリングシステムを用いて点数化した。
結果
被験動物に2.0 mg/kgのロキベトマブを投与した場合、掻痒の有意な減少が3‐4、4‐5および3‐5時間後に観察された (P≦0.0001) 。動物に0.125、0.5または2mg/kgのロキベトマブを投与した場合、効果の持続期間はそれぞれ14日、28日および42日で用量依存的であり、統計的に有意であった (P≦0.0288) 。
結論
これらのデータは, 2mg/kgのロキベトマブを単回皮下注射することで, 治療後3時間後から掻痒が有意に抑制され, その効果は42日間持続することを示している。
摘要
背景
白细胞介素(IL)‐31是一种参与过敏性炎症的细胞因子, 可在包括犬等多物种体内诱导瘙痒。使用重组犬IL‐31, 我们开发了犬瘙痒模型, 以评价治疗药物的起效时间和作用持续时间。
目的
评估洛基维特单抗(赛妥敏)在IL‐31诱导瘙痒模型中的起效时间和作用持续时间。
动物
24只专门饲养的比格犬 (去势雄性、切除卵巢和完整雌性) , 1.5‐4.7岁, 体重6‐14 kg。
方法
随机、设盲、安慰剂对照研究旨在评价洛基维特单抗的止痒特性。实验室比格犬经皮下给予安慰剂、0.125、0.5或2.0 mg/kg 洛基维特单抗。然后给予IL‐31,以评价安慰剂或洛基维特单抗给药后3‐5h以及给药后1、7、14、28、42和56天的瘙痒。通过视频监测在2h窗口内评价动物的瘙痒, 并使用分类评分系统进行评分。
结果
当给予动物2.0 mg/kg 洛基维特单抗时, 在给药后3‐4、4‐5和3‐5 h观察到瘙痒显著减轻(P≤0.0001)。当给予动物0.125、0.5或2 mg/kg 洛基维特单抗时, 作用持续时间呈剂量依赖性, 分别为14、28和42天, 具有统计学显著性(P≤0.0288)。
结论
这些数据表明, 2 mg/kg 洛基维特单抗单次皮下注射给药后3h开始显著抑制瘙痒, 并能持续42天。
Resumo
Contexto
A interleucina (IL) ‐31 é uma citocina envolvida na inflamação alérgica que induz prurido em várias espécies, incluindo cães. Utilizando IL‐31 canina recombinante, desenvolvemos um modelo de prurido em cães para avaliar o início da ação e a duração do efeito de drogas terapêuticas.
Objetivo
Avaliar o início de ação e a duração do efeito do lokivetmab (Cytopoint) no modelo de prurido induzido por IL‐31.
Animais
Vinte e quatro cães beagle criados para fins específicos (machos castrados, fêmeas esterilizadas e intactas) com 1,5–4,7 anos de idade e pesando entre 6 e 14 kg.
Métodos
Estudos randomizados, cegos e placebo‐controle foram elaborados para avaliar as propriedades antipruriginosas do lokivetmab. Os cães beagle de laboratório receberam placebo, 0,125, 0,5 ou 2,0 mg/kg de lokivetmab, por via subcutânea. A IL‐31 foi então administrada para avaliar o prurido 3–5 h após a administração de placebo ou lokivetmab, bem como um, sete, 14, 28, 42 e 56 dias após a administração. O prurido dos animais foi avaliado através de monitoramento por vídeo ao longo de uma janela de 2h e pontuado usando um sistema de pontuação categórica.
Resultados
Quando os animais receberam 2,0 mg/kg de lokivetmab, uma redução significativa do prurido foi observada em 3‐4, 4‐5 e 3‐5h após o tratamento (P ≤ 0,0001). Quando os animais receberam 0,125, 0,5 ou 2 mg/kg de lokivetmab, a duração do efeito foi dependente da dose e estatisticamente significativa durante 14, 28 e 42 dias, respetivamente (P ≤ 0,0288).
Conclusão
Esses dados indicam que uma única injeção subcutânea de 2 mg/kg de lokivetmab produz uma supressão significativa do prurido começando 3 horas após o tratamento, que é mantida por 42 dias.
Introduction
Interleukin (IL)‐31 is a cytokine best known for its pruritogenic effects across species. It was discovered back in 2004 by several groups as a cytokine belonging to the glycoprotein 130/ IL‐6 cytokine family and is believed to be produced by T‐helper 2 (Th2) cells after allergen presentation by Langerhans cells. 1 It also has been shown to be secreted by activated macrophages, basophils, eosinophils and keratinocytes after allergen exposure or initiation of Type 2 inflammation within tissues. 2
Interleukin‐31 binds a heterodimeric receptor (IL‐31 receptor A and oncostatin M receptor beta) to activate signal transduction pathways within cells. These receptors can be found on immune cell such as macrophages, mast cells, eosinophils and basophils, as well as keratinocytes and dorsal root ganglia of peripheral neurons. When IL‐31 binds its heterodimeric receptor, signalling pathways such as Janus kinase (JAK)/signal transducer and activator of transcription (STAT), phosphoinositide 3‐kinase (PI3K)/AKT, and different mitogen‐activated protein kinase pathways, such as extracellular signal‐regulated kinase (ERK), p38 and c‐Jun N‐terminal kinase, are activated, leading to biological changes in cells. These changes include immune cell chemotaxis, pro‐inflammatory cytokine and chemokine secretion, pruritic responses in the skin, and skin barrier disruption resulting in part from alterations in cell proliferation, differentiation and barrier protein synthesis. 2
Concentrations of IL‐31 are elevated in pruritic allergic skin conditions and can induce scratching behaviours in a variety of species such as mice, monkeys and dogs. 3 , 4 , 5 , 6 Studies done in laboratory beagle dogs demonstrated that when IL‐31 was given by several routes (intradermally, subcutaneously and intravenously), it induced strong pruritic behaviours within minutes to hours. 7 These findings were used to develop a novel experimental model in dogs to evaluate the antipruritic effects of canine therapeutics. This model was able to determine the onset and duration of action of commonly used treatments for atopic dermatitis (AD) or allergic dermatitis such as oclacitinib (Apoquel, Zoetis; Kalamazoo, MI, USA) and glucocorticoids. 8
Lokivetmab (Cytopoint, Zoetis) is a caninized monoclonal antibody that binds and neutralizes canine IL‐31 and is given as a subcutaneous injection every four to eight weeks. It has been approved for the treatment of clinical signs associated with AD as well as other forms of allergic dermatitis in dogs across the world. In clinical trials, lokivetmab significantly reduced pruritus as early as Day (D)1 and significantly improved the condition of the dogs’ skin as early as D7, the first evaluation days for these end‐points. 9 The aim of the present study was to further characterize the onset of antipruritic action and the duration of effect of a single injection of lokivetmab s.c. in a laboratory model of IL‐31‐induced pruritus in beagle dogs.
Materials and methods
Animal care and ethics
A group of 24 purpose‐bred beagles were used to complete the onset of duration study (eight neutered males, 15 spayed females, one intact female; 2–3 years old weighing 7.2–14.4 kg; originally from Marshall BioResources; North Rose, NY, USA). The duration of effect study was performed in a different set of 24 purpose‐bred beagle dogs (10 neutered males, 14 spayed females; 1.5–4.7 years old weighing 6.3–14.8 kg; originally from Marshall BioResources or Ridglan Farms, Inc.; Mt. Horeb, WI, USA). All dogs for both studies were maintained and used as part of an in‐house colony whose pruritic behavioural responses to exogenous IL‐31 were extensively characterized in previous studies.
All animal procedures were performed following site‐specific, local and national Animal Health IACUC guidance to assure compliance with the Animal Welfare Act, Regulations 9 CFR parts 1, 2 and 3, and with the Guide for the Care and Use of Laboratory Animals, issued by the ILAR Commission of Life Sciences, National Academy Press (Washington, DC, USA, 1996). Water and a diet (based on caloric needs) of Royal Canin Adult Medium (Royal Canin; Aimargues, France) (onset of action study) or Purina Lab diet #5007 (Purina; St Louis, MI, USA) (duration of effect study) were available. Dogs were not fasted before placebo or IL‐31 dosing.
Test article
Refrigerated sterile‐filtered stock solutions of vehicle (placebo) and lokivetmab were brought up to room temperature and aliquoted at volumes matched to the D0 body weights of the study animals. Each dog received a single injection of 1 mL per 10 kg body weight of 20, 5 or 1.25 mg/mL of lokivetmab s.c. for doses of 2, 0.5 or 0.125 mg/kg, respectively.
Study design
Onset of action study. This study was run after the approval of lokivetmab to characterize onset of action of lokivetmab, and hypothesis testing was conducted at the two‐sided P ≤ 0.05 level of significance. A blinded, randomized, placebo‐controlled study was conducted using laboratory beagle dogs. Twenty‐four dogs (n = 12 per treatment) were given either placebo or 2.0 mg/kg lokivetmab s.c. Dogs were randomized to treatments according to a randomized complete block design with blocking based on historical pruritus (based on the average of three challenge assessments before study start) and pen location. Blocks then were randomized to two batches (cohorts for pruritus assessment) of three blocks. They were then challenged with IL‐31 (2.5 µg/kg) to induce pruritus before lokivetmab or placebo administration on D–7 and 2.5 h post‐lokivetmab or ‐placebo administration on D0. Pruritus was scored between 0.5 h and 2.5 h post‐IL‐31 administration on each of those days. Dogs were evaluated for pruritus between 3 and 5 h post‐placebo or ‐lokivetmab administration to evaluate the onset of action of lokivetmab.
Duration of effect study. This study was run to investigate the duration of effect in the development of lokivetmab. Hypothesis testing was conducted at the two‐sided P ≤ 0.10 level of significance because greater risk at this phase of the programme was acceptable. A blinded, randomized, placebo‐controlled study was conducted using laboratory beagle dogs. Twenty‐four dogs (n = 6 per treatment) were given either placebo or 0.125, 0.5 or 2.0 mg/kg lokivetmab s.c. Dogs were randomized to treatments according to a randomized complete block design, with blocking based on historical pruritus (based on the average of three challenge assessments before study start) and pen location. Blocks then were randomized to two batches (cohorts for pruritus assessment), one containing eight and the second four blocks. They then were challenged with IL‐31 (1.75 µg/kg) to induce pruritus before lokivetmab or placebo administration on D–7, and challenged with IL‐31 again on D1, D7, D14, D28, D42 and D56 post‐lokivetmab or ‐placebo administration to evaluate the duration of antipruritic effect of the monoclonal antibody (mAb). Pruritus was scored between 0.5 h and 2.5 h post‐IL‐31 administration on each of those days.
Induction of pruritus and video surveillance and pruritus scoring
On each scheduled day of pruritus measurements, dogs were transferred to video rooms and placed in free‐standing, single housed pens (approximately 90 cm × 180 cm), each equipped with ceiling‐mounted cameras (Multicam Digital Surveillance System, RMISS Inc.; Wilmington, DE, USA) that digitally recorded the animals for real‐time observation and/or viewing of recordings via computer links. Animals were acclimated ≥ 1 h before initiation of any video observation period for pruritus assessment. For each observation period, four dogs were evaluated for 2 h (recordings started ˜ 15–20 min post‐challenge with IL‐31‐) in real time by one observer using split‐screen monitors. Video observers were scientists trained to observe and score pruritic behaviours in dogs. There was one observer for every four dogs, and each observer watched and scored their four dogs for the duration of the study. Observers were blinded to treatment. Categorical “yes/no” decisions were made at discrete 1 min intervals with regard to whether at least one pruritic behaviour was displayed by the study animals. Displays of pruritic behaviour such as licking/chewing of paws, flank and/or anal regions, scratching of flanks or neck, floor pawing, head‐shaking and scooting of their bottom across the cage flooring were registered with a “yes” response. The cumulative number of “yes” determinations made within each observation period provided the pruritus score.
Statistical methods
Pruritic score data for onset and duration of action studies were analysed using general mixed linear models. In both studies pre‐treatment pruritic scores at D–7 were used as a covariate in the statistical models and least squares means (LSM) were used as estimates of the treatment means. If the covariate was not significant (P < α) it was dropped from the final model.
Onset of action. Scores for first hour, second hour and for the total 2 h period were analysed using a model with the fixed effect of treatment, and random effects of batch, block within batch and error. Treatment comparisons were conducted using the two‐sided α = 0.05 significance level. The pre‐treatment (D–7) covariate used was time‐matched (first hour, second hour or total).
Duration of effect. Scores for the total 2 h period were analysed using a repeated measures model with fixed effects of treatment, time and treatment‐by‐time, and random effects of batch, block within batch, the interaction between block and treatment within batch (animal term), and error. Treatment comparisons were conducted within time points using the two‐sided α = 0.10 significance level.
Results
Evaluation of the onset of action of lokivetmab demonstrated that a single dose (2 mg/kg s.c.) significantly reduced pruritic activity in a canine model of IL‐31‐induced pruritus 3–4 h post‐dosing (P < 0.0001). The LSM pruritic scores [± standard error of measurement (SEM)] were 38 ± 4.4 for placebo‐treated animals compared to 9 ± 2.6 for lokivetmab‐treated animals. Pruritus also was significantly reduced 4–5 h post‐dosing (51 ± 2.2 for placebo‐ versus 20 ± 5.4 for lokivetmab‐treated animals; P < 0.0001) or for the full 2 h observation window of 3–5 h post‐administration with antibody (90 ± 6.2 for placebo‐ versus 29 ± 7.4 for lokivetmab‐treated animals; P < 0.0001.) See Figure 1 for results.
Figure 1.

Onset of antipruritic action of lokivetmab in dogs.
Laboratory‐bred beagle dogs were dosed subcutaneously with either placebo or lokivetmab at 2.0 mg/kg on Day (D) 0. Approximately 2.5 h post‐dosing, pruritus was induced using canine interleukin (IL)‐31. Animals were observed for pruritic behaviours for a total of 2 h (3–5 h post‐treatment with placebo or lokivetmab). Observations were presented per hour (3–4 h, 4–5 h and 3–5 h post‐treatment with placebo or lokivetmab). Data are expressed as least square mean (LSM) ± standard error of mean (SEM). Treatment comparisons at each time point were conducted at the two‐sided α = 0.05 significance level (*P < 0.0001).
Results from the duration of effect study showed that a significant reduction in pruritus was observed to D14 for a dose of 0.125 mg/kg (LSM pruritic scores ± SEM of 91 ± 5.1 for placebo versus 51 ± 5.1 for 0.125 mg/kg lokivetmab; P < 0.0001), to D28 for a dose of 0.5 mg/kg (81 ± 8 for placebo versus 55 ± 8 for 0.5 mg/kg lokivetmab, P = 0.0288) and to D42 for a dose of 2.0 mg/kg (90 ± 8.7 for placebo versus 61 ± 8.7 for 2 mg/kg lokivetmab, P = 0.0245). No significant differences were seen on D56 for any of the doses tested. See Figure 2 for results.
Figure 2.
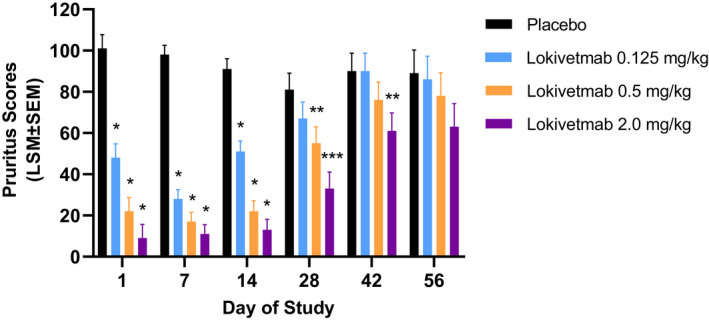
Duration of the antipruritic effect of lokivetmab in dogs.
Laboratory‐bred beagle dogs were dosed subcutaneously with either placebo or one of three different lokivetmab concentrations (0.125, 0.5 or 2 mg/kg) on Day (D)0. Pruritus was induced on D1, D7, D14, D28, D42 and D56 using canine interleukin (IL)‐31, and animals were observed for pruritic behaviours for a total of 2 h post‐IL‐31 challenge. Data are expressed as least square mean (LSM) ± standard error of mean (SEM). Treatment comparisons were conducted at the two‐sided α = 0.10 significance level (*P < 0.0001; **P ≤ 0.028; ***P = 0.0003).
Discussion
Lokivetmab (Cytopoint) is a caninized monoclonal antibody that binds and neutralizes canine IL‐31. Its ability to control/treat clinical signs associated with AD confirms that IL‐31 is a key mediator in canine AD. 9 IL‐31 has been shown to elicit pruritic responses in multiple species 3 , 4 , 5 , 6 and induce several pro‐inflammatory cytokines from a variety of immune cells implicated in allergic skin disease. 10 , 11 Intravenous administration of canine IL‐31 to beagle dogs results in a rapid, transient induction of a variety of pruritic phenotypes including itching, scratching and headshaking. 8 This phenotypic response to IL‐31 was used to establish a canine model of IL‐31‐induced pruritus 8 and allowed us to characterize the onset and dose/duration of action of lokivetmab in this model of pruritus in beagle dogs.
By comparing the results of laboratory model studies to clinical field studies it is possible to determine the translatability of model studies to the clinical setting. Lokivetmab is an approved therapy to control clinical signs of itch and inflammation due to allergic dermatitis or AD in the United States, and as such, was tested in a dose‐determination, randomized, blinded, placebo‐controlled field study. 9 That field study demonstrated that administration of lokivetmab at doses of 0.125, 0.5 or 2.0 mg/kg s.c. resulted in a statistically significant (P < 0.05) reduction in LSM values for owner‐assessed pruritus relative to placebo for as long as 21, 35 or 49 days, respectively. Those results are in general correlation with the efficacy observed in the present study where statistically significant (P < 0.05) reduction in pruritic behaviour relative to placebo in the laboratory model was observed for 14, 28 or 42 days for 0.125, 0.5 or 2.0 mg/kg doses, respectively. These results, taken together, bring relevance of the efficacy observed in the model to the efficacy observed in the field and suggest that this laboratory beagle dog IL‐31‐mediated pruritic model may be an attractive translational model for evaluation of IL‐31 inhibitors. One critical component in applying this model for the development of inhibitors is the assurance that the binding kinetics and specificity of the test article for the canine homologue is similar to that of target species protein. Another important consideration is the pharmacokinetic properties of the test article in canine species relative to the target species.
There are multiple challenges associated with evaluating the onset of activity in the clinical setting that primarily are consequences of the difficulty in monitoring client‐owned animals on an hourly basis for rapid onset molecules and behavioural changes associated with a visit to the veterinarian. To help establish what the predicted onset of action may be in the clinical setting, we evaluated multiple time points shortly post‐administration of 2 mg/kg lokivetmab s.c. in the model. This experiment demonstrated a rapid onset of activity (within 3 h) of subcutaneous lokivetmab administration. In accordance with the model efficacy data observed in this study, clinical field trials demonstrated statistically significant reduction in client‐observed pruritus at the first time point evaluated, one day following a single 2 mg/kg s.c. administration. Results from the duration of effect laboratory study support the translatability of effects seen in the IL‐31‐induced pruritus model to effects seen in a clinical setting. These data further indicate that a single subcutaneous injection of lokivetmab (2 mg/kg) produces a significant suppression of pruritus starting 3 h post‐treatment with lokivetmab and is sustained for 42 days.
Acknowledgements
The authors would like to thank Katie Christiansen, Kaila Zuehlk and Michelle Aleo for their assistance in scoring pruritus.
Sources of Funding: This study was self‐funded.
Conflicts of Interest: All authors are employees of Zoetis Inc.
References
- 1. McCandless EE, Rugg CA, Fici GJ et al. Allergen‐induced production of IL‐31 by canine Th2 cells and identification of immune, skin, and neuronal target cells. Vet Immunol Immunopathol 2014; 157: 42–48. [DOI] [PubMed] [Google Scholar]
- 2. Bağci IS, Ruzicka T. IL‐31: A new key player in dermatology and beyond. J Allergy Clin Immunol 2018; 141: 858–866. [DOI] [PubMed] [Google Scholar]
- 3. Arai I, Tsuji M, Takeda H et al. A single dose of interleukin‐31 (IL‐31) causes continuous itch‐associated scratching behaviour in mice. Exp Dermatol 2013; 22: 669–671. [DOI] [PubMed] [Google Scholar]
- 4. Gangemi S, Quartuccio S, Casciaro M et al. Interleukin 31 and skin diseases: A systematic review. Allergy Asthma Proc 2017; 38: 401–408. [DOI] [PubMed] [Google Scholar]
- 5. Lewis KE, Holdren MS, Maurer MF et al. Interleukin (IL) 31 induces in cynomolgus monkeys a rapid and intense itch response that can be inhibited by an IL‐31 neutralizing antibody. J Eur Acad Dermatol Venereol 2017; 31: 142–150. [DOI] [PubMed] [Google Scholar]
- 6. Furue M, Yamamura K, Kido‐Nakahara M et al. Emerging role of interleukin‐31 and interleukin‐31 receptor in pruritus in atopic dermatitis. Allergy 2018; 73: 29–36. [DOI] [PubMed] [Google Scholar]
- 7. Gonzales AJ, Humphrey WR, Messamore JE et al. Interleukin‐31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol 2013; 24: 48–53: e11–2. [DOI] [PubMed] [Google Scholar]
- 8. Gonzales AJ, Fleck TJ, Humphrey WR et al. IL‐31‐induced pruritus in dogs: a novel experimental model to evaluate anti‐pruritic effects of canine therapeutics. Vet Dermatol 2016; 27: 34–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michels GM, Ramsey DS, Walsh KF et al. A blinded, randomized, placebo‐controlled, dose determination trial of lokivetmab (ZTS‐00103289), a caninized, anti‐canine IL‐31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet Dermatol 2016; 27: 478–e129. [DOI] [PubMed] [Google Scholar]
- 10. Cheung PF‐Y, Wong C‐K, Ho AW‐Y et al. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL‐31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol 2010; 22: 453–467. [DOI] [PubMed] [Google Scholar]
- 11. Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)‐31 induces pro‐inflammatory cytokines in human monocytes and macrophages following stimulation with staphylococcal exotoxins. Allergy 2010; 65: 712–721. [DOI] [PubMed] [Google Scholar]


