Abstract
Purpose
To evaluate the clinical course of idiopathic multifocal choroiditis (MFC) and punctate inner choroidopathy (PIC) and the efficacy and safety of treatment options during pregnancy.
Methods
Patients with MFC or PIC and a pregnancy in 2011–2019 from two academic centres were enrolled. For the most recent pregnancy, data on best‐corrected visual acuity (BCVA) before and after pregnancy, relapse rate in pregnancy and postpartum period and obstetric, maternal and neonatal outcomes were collected. Treatment regimens consisted of a wait‐and‐see regime and an immunosuppressive treatment regime with systemic corticosteroids and/or azathioprine, both combined with intravitreal antivascular endothelial growth factor injections when indicated.
Results
Sixteen women (26 affected eyes) were included. Median Snellen BCVA was 20/19 before pregnancy and 20/18 after delivery. In seven pregnancies a wait‐and‐see regime and in nine pregnancies an immunosuppressive treatment regime was carried out. Fourteen intravitreal anti‐VEGF injections were given in six pregnancies. The relapse rate during pregnancy was 44% and in the postpartum period 31%. Maternal/obstetrical and fetal complications occurred in 31% and 13% of the pregnancies, respectively. Fifteen healthy children were born and one pregnancy ended in a stillbirth in a patient with a complicated obstetrical history. One patient treated with azathioprine developed intrahepatic cholestasis of pregnancy (ICP).
Conclusions
Among women with MFC and PIC BCVA remained stable during pregnancy despite a relapse rate of 44% in pregnancy. No major maternal, obstetric and fetal complications occurred in pregnant patients treated with systemic corticosteroids, azathioprine or intravitreal anti‐VEGF injections, though one patient developed ICP while treated with azathioprine.
Keywords: anti‐VEGF, immunomodulatory therapy, MFC, multifocal choroiditis, pregnancy, punctate inner choroidopathy
Introduction
Idiopathic multifocal choroiditis (MFC) and punctate inner choroidopathy (PIC) are rare types of noninfectious posterior uveitis within the spectrum of white dot syndromes. Both MFC and PIC are characterized by a relapsing inflammatory activity in the choroid in the posterior pole resulting in multiple chorioretinal scars within the temporal vascular arcades. The disease predominantly affects young women with myopia often in their reproductive years (Ahnood et al. 2017). Inflammation, even without symptoms, is thought to trigger the development and reactivation of choroidal neovascularization (CNV), which is considered the most frequent complication in MFC and PIC (Dhingra et al. 2010; Agarwal et al. 2018). CNV demands treatment with intravitreal antivascular endothelial growth factor (VEGF) injections to minimize irreversible retinal damage. There are two viewpoints on how the inflammatory component in MFC and PIC can best be treated. The first treatment strategy is based on the wait‐and‐see principle, where patients are only treated in case of active choroidal inflammation for a relatively short period with periocular, intraocular or systemic corticosteroids. The second treatment strategy is focused on a continuous suppression of the immune system with disease‐modifying antirheumatic drugs (DMARDs), if necessary in combination with systemic corticosteroids, sometimes biologicals are used. As a result, the number of relapses of inflammation and reactivation of CNV is proposed to decline (Turkcuoglu et al. 2011; Goldberg et al. 2014; de Groot et al. 2020).
Pregnancy is well known to be associated with numeral immunological and hormonal changes, favouring the maternal tolerance of the fetus (Chiam & Lim 2014; Grotting & Papaliodis 2017). Most studies regarding noninfectious uveitis demonstrate a decline of disease activity during the second and third trimester of pregnancy with an increase in the relapse rate within the first 6 months postpartum to the prepregnancy situation (Rabiah & Vitale 2003; Kump et al. 2006; Chiam et al. 2013; Verhagen et al. 2017). However, these studies did not specifically investigate patients with MFC and PIC, but noninfectious uveitis in general. Since anti‐VEGF is considered to be potentially teratogenic and embryo‐fetotoxic, treatment with intravitreal anti‐VEGF injections is advised to be avoided in pregnancy, especially in the first trimester (Polizzi & Mahajan 2015; Peracha & Rosenfeld 2016; Fossum et al. 2018). Therefore, it is of utmost importance to prohibit the development or reactivation of CNV during pregnancy in patients with MFC and PIC. Treatment with azathioprine (AZA), a steroid‐sparing immunomodulatory agent, is recognized to be safe in pregnancy though complications have been described (Skorpen et al. 2016). Therefore, whether or not a patient is treated with AZA during pregnancy, is a shared decision of the ophthalmologist, the obstetrician and the patient. Due to the rarity of the disease, and the fact that pregnant women are excluded from medication trials for several reasons (Illamola et al. 2018), literature is scarce regarding different treatment options for MFC and PIC during pregnancy. A few case series have been described for the different treatment options, varying from treatment with intravitreal anti‐VEGF injections, corticosteroid therapy and an observational approach (Sim et al. 2008; Rao et al., 2011; Fossum et al. 2018). This retrospective cohort study aims to contribute to the knowledge concerning the course of MFC and PIC during pregnancy and the postpartum period and to evaluate different treatment options for pregnant women.
Patients and Methods
For this retrospective multicentre cohort study, patients treated in two tertiary academic centres (the University Medical Center Utrecht, Utrecht, the Netherlands, and the Radboud University Medical Center, Nijmegen, the Netherlands) were included. This study received institutional review board approval from both the University Medical Center Utrecht and the Radboud University Medical Center and was performed in accordance with the tenets of the Declaration of Helsinki regarding research involving human subjects. All participants provided written informed consent.
Study participants
Patients with PIC were included as well as patients with MFC in case they presented with chorioretinal scars within the temporal vascular arcades without other signs of ocular inflammation, including no vasculitis, no papillitis and no cells in the anterior chamber or vitreous. The diagnosis of PIC was made in patients with chorioretinal scars situated exclusively within the temporal vascular arcades. In case patients had chorioretinal scars both within and outside the temporal vascular arcades, the diagnosis of MFC was made. Other frequent causes of posterior uveitis including tuberculosis and sarcoidosis were ruled out, as well as Birdshot chorioretinopathy. Patients with MFC and PIC, who were pregnant between 2011 and 2019, were included.
Data collection
Data were distracted from the medical records for the period between 12 months before pregnancy and 6 months after delivery. In case of multiple pregnancies, only the most recent pregnancy was included. Collected data consisted of maternal, fetal and neonatal variables, ophthalmic information before, during and after pregnancy (best‐corrected visual acuity (BCVA), number of relapses of disease activity), imaging results on the Heidelberg Spectralis® (Heidelberg engineering, Heidelberg, Germany) including spectral domain optical coherence tomography (SD‐OCT) and fluorescein and indocyanine angiography (FA‐ICGA) and information regarding treatment regimens. Treatment regimens were divided into a wait‐and‐see regime and an immunosuppressive treatment regime consisting of treatment with systemic corticosteroids and/or azathioprine. In both treatment regimens, patients with a relapse of disease activity during pregnancy were generally treated with intravitreal anti‐VEGF injections in case of secondary CNV, after careful consideration of the risks for the fetus versus the benefits for the patient, and/or periocular or intravitreal corticosteroid injections in case of active inflammation.
Outcome measures
To evaluate disease activity during pregnancy, we analysed whether a relapse of disease activity occurred before pregnancy, during pregnancy or in the postpartum period. A relapse of disease activity was defined as either the development of new choroidal inflammatory lesions or new CNV, or the growth of pre‐existent choroidal scars due to choroidal inflammation or reactivation of CNV after previously inactive disease confirmed by imaging (SD‐OCT and if available FA‐ICGA). In case of a relapse of disease activity, the fetus’s gestational age in weeks and initiated treatment were points of interest.
To evaluate the efficacy and safety of different treatment regimens in pregnancy, the main outcome measures were the BCVA of the mother before and after pregnancy as well as maternal, fetal and obstetrical complications during the pregnancy and postpartum period. The characteristics and clinical outcomes examined for women with MFC and PIC were maternal age and obstetrical complications including gestational diabetes, gestational hypertension, intrahepatic cholestasis of pregnancy (ICP), delivery before 37 weeks (World Health Organization 2015) and mode of delivery, including caesarean section for ophthalmic indication. Evaluated maternal ocular complications included elevated intraocular pressure, cataract and subretinal bleeding of CNV induced by Valsalva manoeuvre during labour. Evaluated fetal complications were miscarriages, birth defects, still birth, birth weight <2500 g (World Health Organization 2011), early‐onset neonatal sepsis and neonatal hypoglycaemia.
Best‐corrected visual acuity was recorded in Snellen values. Best‐corrected visual acuity was converted to LogMAR (logarithm of the minimum angle of resolution) values in order to perform the Wilcoxon signed‐rank test and patients were case‐wise excluded when data were missing. A p‐value <0.05 was considered significant.
Results
Study participants
Sixteen women (26 affected eyes) with MFC (n = 9) and PIC (n = 7) with a pregnancy between 2011 and 2019 were identified. The median age at the start of the pregnancy was 35 years (range 24–41). Bilateral disease was present in 10/16 women (63%), and 11/16 women (69%) had developed the complication of CNV at any point before the start of pregnancy. In seven patients a wait‐and‐see regime was carried out, and nine patients received immunosuppressive treatment during pregnancy. Immunosuppressive treatment consisted of low‐to‐medium‐dose oral corticosteroids in three patients, azathioprine in one patient and a combination of low‐to‐medium‐dose corticosteroids and azathioprine in the remaining five patients. All patients treated with azathioprine received weight‐based doses varying between 100 and 200 mg/day (Table 1). In advance, an alternative DMARD such as mycophenolate mofetil or methotrexate was switched to azathioprine because of the wish to become pregnant in three patients (Table 2).
Table 1.
Maternal, obstetrical and fetal complications during pregnancy and postpartum period including initiated medical treatment in pregnancy.
| Case | Diagnose | Medical treatment in pregnancy in mg (GA in weeks) | Complications during pregnancy and 6 months postpartum | ||||
|---|---|---|---|---|---|---|---|
| DMARD | Systemic corticosteroids (prednisolone)* | Subconjunctival (SC)/ intravitreal injection (IVI) TA | Anti‐VEGF intravitreal injection | Maternal and obstetrical complications | Fetal complications | ||
| 1 | MFC | AZA 150 | 7.5 | – | – | None | None |
| 2 | PIC | AZA 100 | 7.5–12.5 | – | – | Gestational hypertension and diabetes † | None |
| 3 | PIC | AZA 100 | 17.5–20 | TA SC (8) ‡ | – | None | None |
| 4 | MFC | AZA 200 | 20 | – | RZB (5, 11, 20, 25, 30) | Placental abruption | Intrauterine fetal death § |
| 5 | MFC | AZA 125 | 10 | – | – | Intrahepatic cholestasis | Late preterm birth+LBW ¶ |
| 6 | MFC | – | 10 | – | – | None | None |
| 7 | PIC | AZA 100 | – | – | – | None | None |
| 8 | MFC | – | 5–15 | TA IVI (20) | RZB (30, 37) | Elevated IOP | None |
| 9 | MFC | – | 7.5–12.5 |
TA SC (6) TA IVI (9 + 13) |
– | None | None |
| 10 | MFC | – | – | TA IVI (24) |
½ RZB** (32) 1 RZB (36) |
None | None |
| 11 | PIC | – | – | TA IVI (33) | RZB (33 + 37) | None | None |
| 12 | PIC | – | – | – | RZB (27) | None | None |
| 13 | PIC | – | – | – |
BVZ (2) RZB (20) |
C–section †† | None |
| 14 | MFC | – | – | – | – | None ‡‡ | None ‡‡ |
| 15 | PIC | – | – | – | – | None | None |
| 16 | MFC | – | – | – | – | None ‡‡ | None ‡‡ |
AZA, azathioprine; BVZ, bevacizumab; C‐section, caesarean section; DMARD, disease‐modifying antirheumatic drug; GA, gestational age; IOP, intraocular pressure; LBW, low‐birth weight; MFC, idiopathic multifocal choroiditis; NA, not available; PIC, punctate inner choroidopathy; RZB, ranibizumab; TA, triamcinolone acetonide; VEGF, vascular endothelial growth factor.
The minimum and maximum dose of prednisolone during pregnancy.
Two months prior to the start of the pregnancy, the weight of the patient was 110kg (243 LBS).
Subjective relapse of disease activity. After subconjunctival TA complaints did not improve and a relapse of disease activity was never objectified.
Intrauterine fetal death at a gestational age of 33 weeks due to a placental abruption. Obstetrical history of multiple miscarriages and 2 intrauterine fetal deaths, all prior to the diagnosis of MFC. Histopathology of the three placentas of the stillborn children demonstrated similar abnormalities with unknown origin.
Late preterm birth at GA 36 + 6 with a low‐birth weight of 2240 g.
In consultation with the patient and the partner of the patient, the shared decision was made to administer ½ a dose of ranibizumab at GA of 32 weeks.
Ophthalmic indication for caesarean section due to active choroidal neovascularization with risk of bleeding.
No complete follow‐up of 6 months is available in the postpartum period (case 14 has 5,5 months of follow‐up and case 16 has 4 months of follow‐up).
Table 2.
Summary of the number of relapses of disease activity in the 12 months before pregnancy, during pregnancy and in the postpartum period.
| Case | 12 months before pregnancy | During pregnancy | 6 months postpartum | ||||
|---|---|---|---|---|---|---|---|
| Systemic CS* | DMARD | Relapses | Systemic CS* | DMARD | Relapses (GA) | Relapses | |
| 1 | + | MMF → AZA | 0 | + | AZA | 0 | 1 (24) |
| 2 | +/− | − | 1 | + | AZA | 0 | 0 |
| 3 | +/− | MMF → AZA | 2 | + | AZA | 0 | 0 |
| 4 | +/− | MMF → MTX → AZA | 1 | + | AZA | 1 (5) † | 0 |
| 5 | + | AZA | 0 | + | AZA | 0 | 0 |
| 6 | +/− | − | 1 | + | − | 0 | 1 (26) |
| 7 | +/− | AZA ‡ | 1 | − | AZA | 0 | 0 |
| 8 | NA § | NA § | NA § | + | − | 1 (20) † | 1 (11) |
| 9 | +/− | MMF → AZA ¶ | 1 | + | − | 1 (6) † | 0 |
| 10 | − | − | 1 | − | − | 2 (24 + 32) | 0 |
| 11 | NA § | NA § | NA § | − | − | 1 (33) | 0 |
| 12 | − | − | 0 | − | − | 1 (27) | 0 |
| 13 | NA § | NA § | NA § | − | − | 2 (9 + 20) | 0 |
| 14 | − | − | 0 | − | − | 0 | 1 (6)** |
| 15 | − | − | 0 | − | − | 0 | 2 (3 + 20) |
| 16 | − | − | 1 | − | − | 0 | 0** |
AZA, azathioprine; CS, corticosteroids; DMARD, disease‐modifying antirheumatic drug; GA, gestational age; MMF, mycophenolate mofetil; MTX, methotrexate; NA, not available.
Treatment with systemic corticosteroids: maintenance dose of prednisolone (+), prednisolone in tapering schedule (+/−), no prednisolone (−).
A relapse of disease activity occurred while treated with prednisolone. At the time of a relapse of disease activity, the doses were 20 mg (case 4), 15 mg (case 8) and 7.5 mg (case 9).
Azathioprine was started 5 months prior to pregnancy.
No information was present concerning the period before pregnancy. Case 8 was referred to the UMCU Utrecht at GA of 5 weeks, case 11 presented for the first time with symptoms during the third trimester of pregnancy and case 13 was referred to the Radboud UMC at GA of 9 weeks.
Azathioprine was discontinued prior to the start of the pregnancy due to intolerance (elevated liver enzymes).
No complete follow‐up of 6 months is available in the postpartum period (case 14 has 5.5 months of follow‐up and case 16 has 4 months of follow‐up).
Relapse during pregnancy and postpartum period
The number of relapses in the 12 months prior to pregnancy, during pregnancy and postpartum period are demonstrated in Table 2. Figure 1 illustrates an example of a case with relapses of disease activity during pregnancy and in the postpartum period. The percentage of patients with relapse‐free survival over the time course of 40 weeks after conception is demonstrated in Fig. 2A. In 7/16 pregnancies (44%), a relapse of disease activity occurred, demanding for prompt treatment. When analysing the treatment regimens separately, in 3/9 (33%) pregnancies with patients receiving an immunosuppressive treatment regime and in 4/7 (57%) of the pregnancies with patients receiving a wait‐and‐see regime, a relapse of disease activity was observed (Table 2, Fig. 2B). In 5/16 (31%) pregnancies, a relapse of disease activity occurred in the postpartum period, though in two pregnancies no complete follow‐up of 6 months was available (Table 2).
Figure 1.
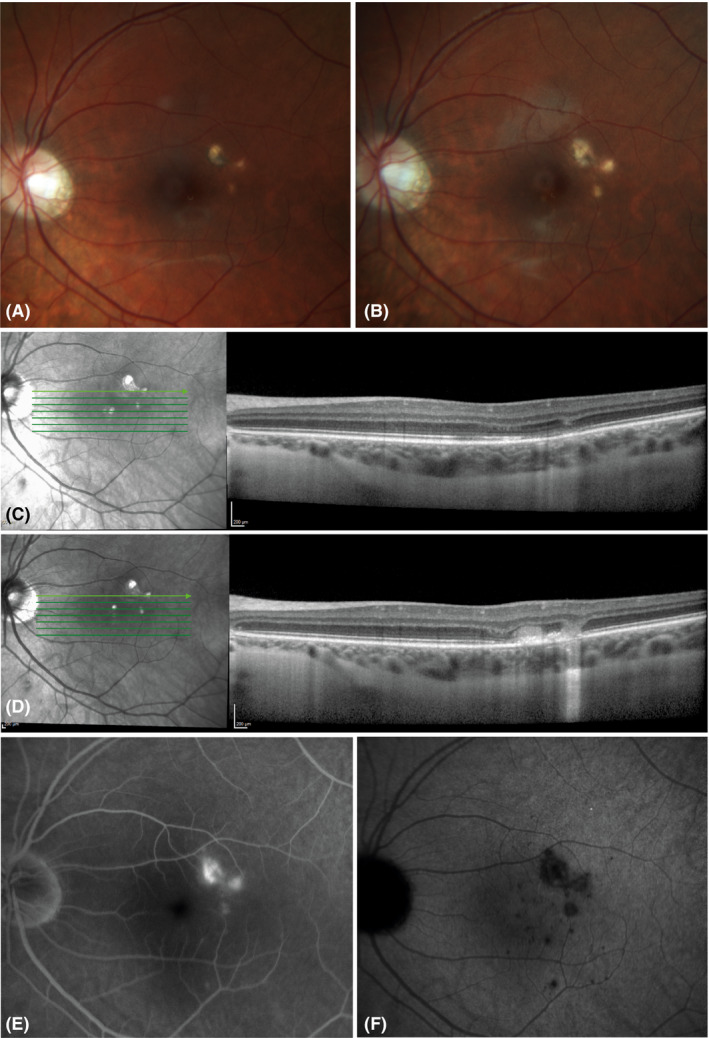
Disease activity in pregnancy and postpartum period in case 8. (A, B) Colour fundus pictures taken at gestational age (GA) of 5 weeks (A) and 11 weeks postpartum (B) showing growth of central choroidal lesions during pregnancy and the postpartum period. (C, D) Optical coherence tomography scans on the Heidelberg Spectralis® with eye‐tracker function. (C) No disease activity at GA 10 weeks. (D) A relapse of disease activity at GA of 20 weeks. (E, F) A relapse of disease activity at 11 weeks postpartum. Fluorescein (E) and indocyanine green (F) pictures at 20 min showing (E) leakage of active CNV and (F) dark choroidal lesions with blurred boundaries indicating inflammatory activity.
Figure 2.
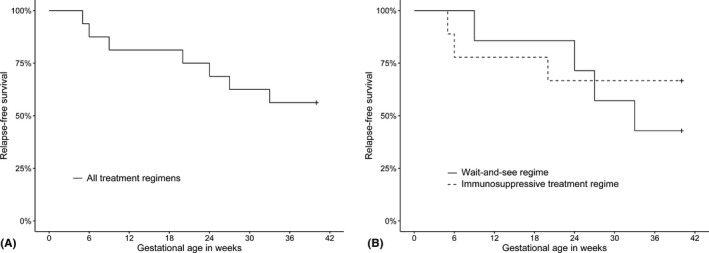
Relapse‐free survival during pregnancy. (A) Demonstrates the relapse‐free survival during pregnancy of all treatment regimens. (B) Demonstrates the relapse‐free survival during pregnancy split out in treatment regimens. The continuing line represents the patients treated with a wait‐and‐see regime, the dashed line represents the patients treated with an immunosuppressive treatment regime with systemic corticosteroids and/or azathioprine.
Intravitreal anti‐VEGF injections in pregnancy
A total number of 14 intravitreal anti‐VEGF injections were given in six pregnancies. Thirteen intravitreal anti‐VEGF injections with ranibizumab and one intravitreal injection with bevacizumab were administered. In general, intravitreal anti‐VEGF injections were not given in the first trimester except for one patient. In this case, the shared decision was made to continue treatment with intravitreal anti‐VEGF injections in the first trimester to preserve vision despite the pregnancy (Table 1).
Visual acuity
Best‐corrected visual acuity prior to pregnancy and after delivery was available for 13 pregnancies (20 eyes). The median Snellen BCVA measured in the visit prior to the start of pregnancy was 20/19. This is not significantly different from the median Snellen BCVA of 20/18 measured in the first consult after delivery (Table 3). When evaluating both treatment regimens separately, no significant difference was observed (wait‐and‐see regime p = 0.78, immunosuppressive treatment p = 0.58).
Table 3.
Summary of visual functioning before pregnancy and after delivery of 20 eyes*.
|
Before pregnancy Median (range) |
After delivery Median (range) |
p‐value | |
|---|---|---|---|
| LogMAR BCVA |
−0.02 (−0.15 to 1.30) |
−0.04 (−0.18 to 1.78) |
0.86 † |
| Snellen BCVA |
20/19 (20/14–20/400) |
20/18 (20/13–20/1200) |
NA |
| Time to consult in weeks ‡ |
4 (0–25) |
8 (2–15) |
NA |
BCVA, best‐corrected visual acuity; NA, not applicable.
Missing data prior to pregnancy for 6 eyes.
Wilcoxon signed‐rank test.
The time in weeks between the moment of evaluation of BCVA prior to the start of pregnancy and after delivery.
Maternal, obstetric and fetal outcomes
A total of 16 pregnancies resulted in the birth of 15 healthy children. Maternal/obstetrical complications occurred in 5/16 (31%) pregnancies. One patient developed an elevated intraocular pressure following an intravitreal corticosteroid injection. Nonocular complications included a placental abruption in one patient, gestational hypertension and diabetes in one patient and ICP in one patient. Moreover, in one patient an elective caesarean section was performed because of an active CNV with increased risk of subretinal bleeding during Valsalva manoeuvre (Table 1). Fetal complications occurred in 2/16 (13%) of the unborn children. One pregnancy resulted in a stillbirth at a gestational age of 33 weeks due to a placental abruption and one infant was born late preterm with a birth weight of 2240 g but was otherwise healthy. None of the infants had birth defects or suffered from complications in the neonatal period (Table 1).
Discussion
In this study, we report that in 44% of the pregnancies a relapse of disease activity occurred demanding for prompt treatment. Remarkable was the higher relapse rate in the women with a wait‐and‐see regime compared to the women with an immunosuppressive treatment regime, though due to the small number of patients no direct conclusions can be drawn from this observation. Despite these relapses of disease activity, the median BCVA remained stable throughout the pregnancy and no difference was observed between treatment regimens. Surprisingly, a considerable proportion of the relapses of disease activity occurred in the third trimester of pregnancy whereas most studies regarding uveitis in general, report a decline in uveitis activity in the second and third trimester (Rabiah & Vitale 2003; Kump et al. 2006; Chiam et al. 2013).
We report that none of the infants had birth defects and the majority of the children were healthy term infants. The reported rate of the different maternal, obstetrical and fetal complications does not seem notably higher than the overall complication rate in the general population. (Wilcox et al. 1988; Beck et al. 2010; Ammon Avalos, Galindo & Li 2012; Blencowe et al. 2012; Gillon et al. 2014; Williamson & Geenes 2014; Zhu & Zhang 2016; Eades, Cameron & Evans 2017; Shen et al. 2017; Magnus et al. 2019; Smith & Rood 2020). One pregnancy ended in a stillbirth due to a placental abruption. The obstetrical history of this patient before this pregnancy was complicated and stated four miscarriages and two stillborn children with unknown cause all prior to the diagnosis of MFC.
Literature on the use of intravitreal anti‐VEGF injections during pregnancy is scarce. The signal protein VEGF plays an important role in the embryo implantation and the development of the lungs, kidneys and central nervous system of the fetus (Peracha & Rosenfeld 2016). Keeping this in mind, it is reasonable that anti‐VEGF can potentially harm the fetus particularly during the development of the vital organs in the first trimester. One publication summarizes 20 cases of pregnant women who were treated with intravitreal anti‐VEGF injections (18 with bevacizumab and two with ranibizumab) during pregnancy. In three pregnancies a miscarriage was observed, all treated with bevacizumab within 5 weeks of gestational age (GA) (Polizzi & Mahajan 2015). To understand the difference between ranibizumab on the one hand, and aflibercept and bevacizumab on the other hand, one should take the pharmacological differences into account. Firstly, ranibizumab has the shortest half‐life and therefore is most rapidly cleared when entering the systemic circulation. Due to the short period of drug exposure in ranibizumab, it affects the plasma level of free VEGF molecules for the shortest time period (Avery et al. 2014; Peracha & Rosenfeld 2016). Secondly, aflibercept has the highest affinity with VEGF and therefore leads to the most dramatic suppression of free VEGF molecules in the plasma (Avery et al. 2014). Thirdly, ranibizumab does not have a Fc fragment and therefore is not expected to be able to bind to the neonatal Fc receptor. It is proposed that this receptor is important for transporting the antibodies (f.e. bevacizumab) over the placental barrier to the fetus (Krohne, Holz & Meyer 2016). Based on this theory, ranibizumab will not reach the fetal circulation, for it will not pass the placental barrier, in contrast to bevacizumab and aflibercept.
Treatment with steroid‐sparing immunomodulatory therapy in pregnancy is contraindicated for some agents including mycophenolate mofetil and methotrexate. On the contrary, no increase in the rate of miscarriages and congenital malformations is observed in pregnant patients treated with azathioprine, cyclosporine, tacrolimus and anti‐tumour necrosis factor inhibitors and thus treatment with these agents is considered to be safe in pregnancy (Skorpen et al. 2016). Literature regarding the influence of azathioprine on the prevalence of low‐birth weight, preterm birth and small for gestational age is conflicting (Saavedra et al. 2015; Plauborg, Hansen & Garne 2016). In our study, one patient treated with azathioprine developed the complication of ICP and spontaneously delivered preterm an infant with a low‐birth weight. Two case reports documented a similar finding in patients treated with azathioprine for Vogt–Koyanagi–Harada disease and Crohn’s disease (Ingolotti et al. 2019; Lauterbach et al. 2020).
This retrospective study comes with several limitations. Firstly, the number of patients was small and more importantly, limited to patients treated in tertiary academic centres. As a result, it is possible that the real‐life relapse rate is lower than the reported relapse rate due to selection bias. Secondly, we report that the median BCVA remained stable throughout follow‐up despite multiple relapses. Though, for a more thorough evaluation of the visual function in these patients, central visual field tests should be performed and evaluated before and after pregnancy to monitor the number and size of the scotomas due to the chorioretinal scars. Thirdly, it is possible that minor relapses of disease activity are missed during pregnancy since fluorescein and indocyanine green angiography is preferably not performed during pregnancy, though can be performed in urgent cases. Both fluorescein and indocyanine green are classified as a category C drug by the Food and Drug Administration indicating that it is unknown to what extent it will harm the fetus. Though indocyanine green has proven to be harmless for nonophthalmic indications, many ophthalmologists remain reluctant to use it (Fineman 2001).
Despite the limitations of this study, this is the first study of this extent to report on the course of disease and different treatment options in pregnancy specifically for patients with MFC and PIC. We expect that the current data are of great value for clinicians dealing with this blinding disease and could be helpful in the process of shared decision‐making.
In conclusion, we report that BCVA remained stable during pregnancy despite a considerable relapse rate of 44% in pregnancy. Overall, no major maternal, obstetrical and fetal complications were observed in patients treated with corticosteroids, azathioprine or intravitreal anti‐VEGF injections, though one patient developed ICP while treated with azathioprine. We emphasize no conclusions can be drawn based on these results, due to the small number of patients. The optimal medical treatment of MFC and PIC during pregnancy should be established by the ophthalmologist together with the obstetrician considering the health of the unborn child and the risk of loss of visual function in the mother. Moreover, at all times medical treatment should be established within the concept op shared decision‐making.
Funding/Support: F.P.Fischer‐Stichting; Achtersloot 212‐C, 3401 NZ IJsselstein, the Netherlands. Foundation Beheer het Schild; Wolfhezerweg 101, 6874 AD Wolfheze, the Netherlands. Landelijke Stichting voor Blinden en Slechtzienden (LSBS); Galvanistraat 1, 6716 AE Ede, the Netherlands. Rotterdamse Stichting Blindenbelangen (RBS); Schiekade 77, 3033 BE Rotterdam, the Netherlands. Stichting Louise Rottinghuis Fonds; Ringenum 6, 9934 PM Delfzijl, the Netherlands. Oogfonds; Churchilllaan 11, 3527 GV Utrecht, the Netherlands
The corresponding author is a member of the Dutch Ophthalmological Societies
References
- Agarwal A, Invernizzi A, Singh RB et al. (2018): An update on inflammatory choroidal neovascularization: epidemiology, multimodal imaging, and management. J Ophthalmic Inflamm Infect 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnood D, Madhusudhan S, Tsaloumas MD, Waheed NK, Keane PA & Denniston AK (2017): Punctate inner choroidopathy: a review. Surv Ophthalmol 62: 113–126. [DOI] [PubMed] [Google Scholar]
- Ammon Avalos L, Galindo C & Li DK (2012): A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res Part A ‐ Clin Mol Teratol 94: 417–423. [DOI] [PubMed] [Google Scholar]
- Avery RL, Castellarin AA, Steinle NC et al. (2014): Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular amd. Br J Ophthalmol 98: 1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L et al. (2010): The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ et al. (2012): National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379: 2162–2172. [DOI] [PubMed] [Google Scholar]
- Chiam NPY, Hall AJH, Stawell RJ, Busija L & Lim LLP (2013): The course of uveitis in pregnancy and postpartum. Br J Ophthalmol 97: 1284–1288. [DOI] [PubMed] [Google Scholar]
- Chiam NPY & Lim LLP (2014): Uveitis and gender: the course of uveitis in pregnancy. J Ophthalmol 2014: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot EL, ten Dam‐van Loon NH, de Boer JH & Ossewaarde‐van Norel J (2020): The efficacy of corticosteroid‐sparing immunomodulatory therapy in treating patients with central multifocal choroiditis. Acta Ophthalmol 98: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra N, Kelly S, Majid MA, Bailey CB & Dick AD (2010): Inflammatory choroidal neovascular membrane in posterior uveitis‐pathogenesis and treatment. Indian J Ophthalmol 58: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eades CE, Cameron DM & Evans JMM (2017): Prevalence of gestational diabetes mellitus in Europe: a meta‐analysis. Diabetes Res Clin Pract 129: 173–181. [DOI] [PubMed] [Google Scholar]
- Fineman MS (2001): Safety of indocyanine green angiography during pregnancy. Arch Ophthalmol 119: 353. [DOI] [PubMed] [Google Scholar]
- Fossum P, Couret C, Briend B, Weber M & Lagarce L (2018): Safety of intravitreal injection of ranibizumab in early pregnancy: a series of three cases. Eye 32: 830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon TER, Pels A, von Dadelszen P, MacDonell K & Magee LA (2014): Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One 9: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg NR, Lyu T, Moshier E, Godbold J & Jabs DA (2014): Success with single‐agent immunosuppression for multifocal choroidopathies. Am J Ophthalmol 158: 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götestam Skorpen C, Hoeltzenbein M, Tincani A et al. (2016): The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 75: 795–810. [DOI] [PubMed] [Google Scholar]
- Grotting LA & Papaliodis GN (2017): A review of the course and treatment of non‐infectious uveitis during pregnancy. Semin Ophthalmol 32: 75–81. [DOI] [PubMed] [Google Scholar]
- Illamola SM, Bucci‐Rechtweg C, Costantine MM, Tsilou E, Sherwin CM & Zajicek A (2018): Inclusion of pregnant and breastfeeding women in research – efforts and initiatives. Br J Clin Pharmacol 84: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolotti M, Schlaen BA, Roig Melo‐Granados EA, García HR & Partida JAA (2019): Azathioprine during the first trimester of pregnancy in a patient with Vogt‐Koyanagi‐Harada disease: a multimodal imaging follow‐up study. Am J Case Rep 20: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne TU, Holz FG & Meyer CH (2016): Essentials in Ophthalmology. In: Stahl A (ed.). Anti‐angiogenic therapy in ophthalmology. Cham: Springer International Publishing; 139–148. [Google Scholar]
- Kump LI, Cervantes‐Castañeda RA, Androudi SN, Foster CS & Christen WG (2006): Patterns of exacerbations of chronic non‐infectious uveitis in pregnancy and puerperium. Ocul Immunol Inflamm 14: 99–104. [DOI] [PubMed] [Google Scholar]
- Lauterbach R, Linder R, Vitner D & Solt I (2020): Azathioprine‐induced cholestasis of pregnancy‐A new insight on azathioprine safety in pregnancy. Eur J Obstet Gynecol Reprod Biol 250: 271–272. [DOI] [PubMed] [Google Scholar]
- Magnus MC, Wilcox AJ, Morken NH, Weinberg CR & Håberg SE (2019): Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ 364: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracha ZH & Rosenfeld PJ (2016): Anti‐vascular endothelial growth factor therapy in pregnancy: what we know, what we don’t know, and what we don’t know we don’t know. Retina 36: 1413–1417. [DOI] [PubMed] [Google Scholar]
- Plauborg AV, Hansen AV & Garne E (2016): Use of azathioprine and corticosteroids during pregnancy and birth outcome in women diagnosed with inflammatory bowel disease. Birth Defects Res Part A ‐ Clin Mol Teratol 106: 494–499. [DOI] [PubMed] [Google Scholar]
- Polizzi S & Mahajan VB (2015): Intravitreal anti‐VEGF injections in pregnancy: case series and review of literature. J Ocul Pharmacol Ther 31: 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiah PK & Vitale AT (2003): Noninfectious uveitis and pregnancy. Am J Ophthalmol 136: 91–98. [DOI] [PubMed] [Google Scholar]
- Rao VG, Rao GS & Narkhede NS (2011): Flare up of choroiditis and choroidal neovasculazation associated with punctate inner choroidopathy during early pregnancy. Indian J Ophthalmol 59: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra MÁ, Sánchez A, Morales S, Ángeles U & Jara LJ (2015): Azathioprine during pregnancy in systemic lupus erythematosus patients is not associated with poor fetal outcome. Clin Rheumatol 34: 1211–1216. [DOI] [PubMed] [Google Scholar]
- Shen M, Smith GN, Rodger M, White RR, Walker MC & Wen SW (2017): Comparison of risk factors and outcomes of gestational hypertension and pre‐eclampsia. PLoS One 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim DA, Sheth HG, Kaines A & Tufail A (2008): Punctate inner choroidopathy‐associated choroidal neovascular membranes during pregnancy. Eye 22: 725–727. [DOI] [PubMed] [Google Scholar]
- Smith DD & Rood KM (2020): Intrahepatic cholestasis of pregnancy. Clin Obstet Gynecol 63: 134–151. [DOI] [PubMed] [Google Scholar]
- Turkcuoglu P, Chang PY, Rentiya ZS et al. (2011): Mycophenolate mofetil and fundus autofluorescence in the management of recurrent punctate inner choroidopathy. Ocul Immunol Inflamm 19: 286–292. [DOI] [PubMed] [Google Scholar]
- Verhagen FH, Braakenburg AM, Kremer T, Drylewicz J, Rothova A & de Boer JH (2017): Reduced number of relapses of human leucocyte antigen‐B27‐associated uveitis during pregnancy. Acta Ophthalmol 95: e798–e799. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Conner JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong GE & Nisula BC (1988): Incidence of early loss of pregnancy. N Engl J Med 319: 189–194. [DOI] [PubMed] [Google Scholar]
- Williamson C & Geenes V (2014): Intrahepatic cholestasis of pregnancy. Obstet Gynecol 124: 120–133. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011): Guidelines on Optimal Feeding of Low Birth‐Weight Infants in Low‐and Middle‐Income Countries. Geneva: WHO; 16–45. [PubMed] [Google Scholar]
- World Health Organization (2015): WHO recommendations on interventions to improve preterm birth outcomes. [PubMed]
- Zhu Y & Zhang C (2016): Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


