Abstract
Background
Because of the increased incidence of multidrug‐resistant (MDR) bacteria, the use of disinfectants over antibiotics has been encouraged. However, the interactions between disinfectants and host local immunity are poorly understood.
Objective
To assess the effects of chlorhexidine digluconate (Chx), with and without selected host defence peptides (HDPs), against MDR Staphylococcus pseudintermedius (MDR‐SP).
Methods and materials
Ten clinical isolates of MDR‐SP were tested, using a modified microbroth dilution method. Four two‐fold dilutions of 2% Chx and 1 μg/mL the HDPs synthetic canine β‐defensin 103 (cBD103) or cathelicidin (cCath) were tested alone or in combination. Colony counts after 5, 15, 30 and 60 min, and a minimum inhibitory concentration (MIC) after 24 h were recorded. Friedman followed by Dunn’s multiple comparison tests with significance of P < 0.05 were used for statistical analysis. Synergy, additivity/neutrality or antagonism were calculated.
Results
Growth was not inhibited by either HDP alone. An MIC of 0.312 μg/mL Chx was achieved for nine of the isolates. One isolate had an MIC of 0.078 μg/mL Chx. A MIC90 (in nine of 10 isolates) of 0.312 µg/mL was seen for Chx in combination with either HDP. Synergy was seen in the combination Chx/cCath used at the highest concentrations of Chx (0.624 µg/mL and 0.312 µg/mL) after 30 and 60 min incubation. Additivity/neutrality was seen for most of the other concentrations and times of incubation.
Conclusions and clinical importance
These results suggest a synergistic/additive effect between Chx and HDPs in dogs. Further studies evaluating the mechanisms behind this effect are needed.
Background – Because of the increased incidence of multidrug‐resistant (MDR) bacteria, the use of disinfectants over antibiotics has been encouraged. However, the interactions between disinfectants and host local immunity are poorly understood. Objective – To assess the effects of chlorhexidine digluconate (Chx), with and without selected host defence peptides (HDPs), against MDR Staphylococcus pseudintermedius (MDR‐SP). Conclusions and clinical importance – These results suggest a synergistic/additive effect between Chx and HDPs in dogs. Further studies evaluating the mechanisms behind this effect are needed.
Résumé
Contexte
En raison de l’augmentation de l’incidence des bactéries multirésistantes (MDR), l’utilisation de désinfectants au lieu d’antibiotiques a été encouragée. Cependant, les interactions entre les désinfectants et l’immunité locale de l’hôte sont peu comprises.
Objectifs
Déterminer les effets de digluconate de chlorhexidine (Chx), avec et sans peptides de défense de l’hôte sélectionnés (HDPs), contre MDR‐SP (Staphylococcus pseudintermedius MDR).
Matériels et méthodes
Dix souches cliniques de MDR‐SP ont été testées, à l’aide d’une méthode de microdilution sur gélose. Quatre dilutions de deux plis de 2% de Chx et 1 μg/mL de HDPs de β‐défensine 103 (cBD103) ou cathelicidine (cCath) ont été testées seul ou associés. Le comptage de colonies après 5, 15, 30 et 60 min, et une concentration minimale inhibitrice (MIC) après 24 h ont été enregistrés. Des tests de Friedman suivis de comparaison multiple de Dunn avec P < 0.05 ont été utilisés pour analyse statistique. Synergie, additivité/neutralité ou antagonisme ont été calculés.
Résultats
Une MIC n’était atteinte pour aucun HDP. Une MIC de 0.312 μg/mL Chx était atteinte pour neufs des souches. Une souche avait une MIC de 0.078 μg/mL Chx. Une MIC90 (pour neuf des 10 souches) de 0.312 µg/mL étaient vues pour Chx en combinaison avec un des HDP. Une synergie était observée pour la combinaison Chx/cCath utilisée aux concentrations les plus élevées de Chx (0.624 µg/mL et 0.312 µg/mL) après 30 et 60 min d’incubation. Une additivité/neutralité était observée pour la plupart des autres concentrations et temps d’incubation.
Conclusions et importance clinique
Ces résultats suggèrent un effet synergique/additif entre Chx et HDPs chez le chien. D’autres études évaluant les mécanismes sous jacents sont nécessaires.
Resumen
Introducción
debido a la mayor incidencia de bacterias multirresistentes (MDR), se ha fomentado el uso de desinfectantes en lugar de antibióticos. Sin embargo, las interacciones entre los desinfectantes y la inmunidad local del huésped son poco conocidas.
Objetivo
evaluar los efectos del digluconato de clorhexidina (Chx), con y sin péptidos de defensa del huésped seleccionados (HDPs), frente a MDR Staphylococcus pseudintermedius (MDR‐SP).
Métodos y materiales
se probaron diez aislados clínicos de MDR‐SP, utilizando un método de dilución de microcaldo modificado. Se probaron cuatro diluciones dobladas de Chx al 2% y 1 μg/ml del HDP β‐defensina 103 canina sintética (cBD103) o catelicidina (cCath), solas o en combinación. Se registraron los recuentos de colonias después de 5, 15, 30 y 60 min, y una concentración inhibitoria mínima (MIC) después de 24 h. Para el análisis estadístico se utilizaron las pruebas de comparación múltiple de Friedman y Dunn significativas para de P <0.05. Se calculó la sinergia, la aditividad/neutralidad o el antagonismo.
Resultados
no se logró una MIC para ninguno de los HDP. Se logró una CMI de 0,312 μg/mL de Chx para nueve de los aislamientos. Un aislado tuvo una CMI de 0,078 μg/mL Chx. Se observó una MIC90 (en nueve de 10 aislamientos) de 0,312 µg/mL para Chx en combinación con HDP. Se observó sinergia en la combinación Chx/cCath usada a las concentraciones más altas de Chx (0,624 µg/ml y 0,312 µg/ml) después de 30 y 60 minutos de incubación. Se observó aditividad/neutralidad para la mayoría de las otras concentraciones y tiempos de incubación.
Conclusiones e importancia clínica
estos resultados sugieren un efecto sinérgico/aditivo entre Chx y HDP en perros. Se necesitan más estudios que evalúen los mecanismos detrás de este efecto.
Zusammenfassung
Hintergrund
Aufgrund der erhöhten Inzidenz von Multidrug‐Resistenzen (MDR) bei Bakterien wird die Verwendung von Desinfektionsmitteln anstelle von Antibiotika gefördert. Über die Interaktionen zwischen den Desinfektionsmitteln und der lokalen Wirtsimmunität ist jedoch wenig bekannt.
Ziel
Eine Erfassung der Wirkunge von Chlorhexidindigluconat (Chx), mit und ohne ausgewählte Wirtsabwehrpeptide (HDPs) gegen MDR Staphylococcus pseudintermedius (MDR‐SP).
Methoden und Materialien
Zehn klinische Isolate von MDR‐SP wurden mittels einer modifizierten Bouillon‐Mikroverdünnungsmethode getestet. Vier zwei‐fache Verdünnungen von 2% igem Chx und 1 µg/mL der HDPs synthetisches canines β‐Defensin 103 (cBD103) oder Cathelicidin (cCath) wurde allein und in Kombination getestet. Koloniezahlen wurden nach 5, 15, 30 und 60 Minuten erfasst und eine minimale Hemmkonzentration (MIC) nach 24h festgehalten. Friedman gefolgt von Dunn´s multiplem Vergleichstest mit einer Signifikanz von P < 0,05 wurde zur statistischen Analyse verwendet. Es wurden Synergie, Additivität/Neutralität oder Antagonismus kalkuliert.
Ergebnisse
Für keine HDP wurde die MIC erreicht. Eine MIC von 0,312 µg/mL Chx wurde für neun der Isolate erreicht. Bei einem Isolat lag die MIC bei 0,078 µg/mL Chx. Eine MIC90 (bei neun von 10 Isolaten) von 0,312 µg/mL wurde für eine Kombination von Chx mit beiden HDPs gesehen. Eine Synergie bestand nach 30 und 60 Minuten Inkubationszeit bei der Kombination Chx/cCath, wenn die höchsten Konzentrationen von Chx (0,624 µg/mL und 0,312 µg/mL) verwendet wurden. Eine Additivität/Neutralität wurde bei den meisten anderen Konzentrationen und Inkubationszeiten gefunden.
Schlussfolgerungen und klinische Bedeutung
Diese Ergebnisse weisen auf eine synergistische/additive Wirkung zwischen Chx und HDPs bei Hunden hin. Weitere Studien, die die Mechanismen hinter diesem Effekt evaluieren, werden benötigt.
要約
背景
多剤耐性 (MDR) 菌の発生率増加に伴い、抗生物質よりも消毒薬の使用が推奨されている。ただし、消毒薬と宿主の局所免疫との相互作用については十分に理解されていない。
目的
本研究の目的は、MDR Staphylococcus pseudintermedius(MDR‐SP) に対するジグルコン酸クロルヘキシジン (Chx) の効果を、選択的宿主防御ペプチド (HDP) の有無で評価することであった。
材料と方法
MDR‐SPの臨床分離株10株を修正微量液体希釈法を用いて試験した。 2%Chxおよび1μg/ mLHDP合成イヌβ‐ディフェンシン103(cBD103) またはカテリシジン (cCath) の2倍希釈液を単独または併用して試験した。 5、15、30、60分後のコロニー数、および24時間後の最小発育阻止濃度 (MIC) を記録した。統計解析には、Friedman法とDunnの多重比較法を用い、P < 0.05の有意差で解析した。相乗効果、相加性/中立性、拮抗性を算出した。
結果
どちらのHDPでもMICは達成されなかった。 9株のChx分離株のMICは0.312μg/ mLであった。 1株のChx分離株のMICは0.078μg/ mLであった。ChxといずれかのHDPとの併用では、MIC90(10分離株のうち9株) が0.312 µg / mLとなった。ChxとCathの組み合わせでは、Chxの最高濃度 (0.624µg/mLおよび0.312µg/mL) で30分および60分培養したところ, 相乗効果が認められた。その他の濃度と時間ではほとんどの場合、相加性/中立性が認められた。
結論と臨床的重要性
これらの結果は、犬のChxとHDPの間の相乗/相加効果を示唆している。この効果の背後にあるメカニズムを評価するさらなる研究が必要である。
摘要
背景
由于多重耐药(MDR)细菌的发生率增加, 鼓励使用消毒剂而不是抗生素。然而, 消毒剂与宿主局部免疫之间的相互作用知之甚少。
目的
评估有和无选定的宿主防御肽(HDPs)的葡萄糖酸氯己定(Chx)对MDR假中间型葡萄球菌(MDR‐SP)的作用。
方法和材料
使用改良的微量肉汤稀释法检测10株MDR‐SP临床分离株。单独或联合检测4份2%Chx和1 μg/mL HDP合成犬β‐防御素103(cBD103)或抗菌肽(cCath)的2倍稀释液。记录5、15、30和60 min后的菌落计数, 以及24h后的最小抑菌浓度(MIC)。采用Friedman后进行Dunn多重比较检验进行统计分析, 显著性为P<0.05。计算协同作用、相加作用/中性或拮抗作用。
结果
两种HDP均未达到MIC。其中9株分离株的MIC为0.312μg/mL Chx。1株分离株的MIC为0.078μg/mL Chx。Chx联合任一HDP的MIC90(10个分离株中的9个) 为0.312µg/mL。孵育30min和60min后, 在Chx最高浓度 (0.624µg/mL和0.312µg/mL) 下使用的Chx/cCath组合中观察到协同作用。在大多数其他浓度和孵育时间中观察到相加/中性。
结论和临床重要性
这些结果表明Chx和HDPs在犬中具有协同/累加效应。需要进一步研究评价这种效应背后的机制。
Resumo
Contexto
Devido ao aumento da incidência de bactérias multirresistentes (MDR), a utilização de desinfectantes ao invés de antibióticos tem sido encorajada. Entretanto, as interações entre os desinfectantes e a imunidade local do hospedeiro não são bem compreendidas.
Objetivo
Avaliar os efeitos do digluconato de clorexidine (Chx) com e sem peptídeos de defesa selecionados (HDPs), contra Staphylococcus pseudintermedius MDR (MDR‐SP).
Métodos e materiais
Os isolados clínicos de MDR‐SP foram testados, utilizando um método de microdiluição em caldo modificado. Quatro diluições de Chx e 1 μg/mL dos HPDs sintéticos β‐defensina 103 (cBD103) ou catelicidina (cCath) foram testados isoladamente ou em associação. Registrou‐se as contagens de colônias após 5, 15, 30 e 60 minutos e as concentrações inibitórias mínimas (MIC) após 24 horas. Os testes de comparação múltiplos de Friedman seguido por Dunn com significância de P < 0,05 foram utilizados para a análise estatística. Sinergia, aditividade/neutralidade ou antagonismo foram calculados.
Resultados
O MIC não foi alcançado para nenhum HDP. Um MIC de 0,312 μg/mL Chx foi alcançado para nove dos isolados. Um isolado teve um MIC de 0,078 μg/mLpara Chx. Um MIC90 (em nove de 10 isolados) de 0,312 µg/mL foi observado para Chx em combinação com qualquer HDP. Observou‐se sinergia na combinação Chx cCath usada nas concentrações mais altas de Chx (0,624 µg/mL e 0,312 µg/mL) após 30 e 60 min de incubação. Aditividade/neutralidade foi observada para a maioria das outras concentrações e tempos de incubação.
Conclusões e importância clínica
Esses resultados sugerem um efeito sinérgico/aditivo entre Chx e HDPs em cães. Mais estudos avaliando os mecanismos por trás desse efeito são necessários.
Introduction
The incidence of antimicrobial resistance has increased over the past few years, with multidrug‐resistant (MDR) organisms being commonly isolated in clinical practice. 1 , 2 In particular, the isolation of MDR Staphylococcus pseudintermedius (SP) is becoming a common finding in veterinary dermatological practice. 1 , 2
Unfortunately, the rate of discovery of new antibiotics has plateaued, leaving – in some cases – topical antimicrobials as the only viable option to treat such infections. Among others, chlorhexidine digluconate (Chx), a biguanide compound, has been used successfully as antimicrobial agent against many Gram‐positive and Gram‐negative bacteria, yeasts, moulds and viruses. 3 , 4 Its mechanism of action (MoA) is not completely understood, yet it seems related to its ability to bind and interfere with bacterial membranes. 5 This strong binding induces structural modifications leading to a leakage of the intracellular components. 5 In veterinary medicine, Chx is used widely in clinical dermatological practice (in concentrations between 0.5% and 4%) as sole or adjuvant therapy for cutaneous bacterial infections. 6
Although extremely effective, the wide use of Chx has increased the awareness of a potential decrease in sensitivity among bacterial isolates, especially in human medicine. 7 , 8 , 9 Because of such risk and the lack of new antibiotics, increased attention has been focused on ways to increase the efficacy of commonly used antimicrobials. This goal has been achieved combining anti‐inflammatory medications to antibiotics, 10 using plant extracts to stimulate the local immune response, 11 , 12 or testing host defence peptides (HDPs) with commonly used antimicrobials. 13 , 14 In particular, Chx has shown a synergistic effect with the HDP human β‐defensin (BD)3 against oral bacteria. 13
Host defence peptides are an important component of the local innate immunity against microbes. In dogs, several BDs have been identified in epithelial tissues including skin, lungs and urogenital tract. 15 , 16 , 17 , 18 , 19 Among the HDPs studied in dogs, BD103 and cathelicidin (Cath) have the highest antimicrobial activity against a wide range of bacteria. 15 , 16 , 20 Alterations in HDP production and/or secretion have been imputed as the potential reasons why some dogs and people may be more affected by bacterial infections. This theory has been somewhat reinforced by a study showing how skin washes from atopic dogs have a reduced antimicrobial activity compared with skin washes collected from healthy dogs. 21 Furthermore, another study 22 showed how HDPs are more adherent to the surface of atopic skin compared with healthy skin, potentially reducing the concentration of readily available HDPs.
Although Chx is used worldwide as a topical antimicrobial, its antimicrobial interaction with cutaneous innate immune defences is unknown. To answer this question, this study was designed to assess the effects of Chx, at minimal inhibitory concentration (MICs) and sub‐MIC, with and without selected HDPs, against clinical isolates of MDR‐SP.
Methods and materials
Staphylococcal isolates
Ten clinical isolates of MDR‐SP were tested (see Table S1 in Supporting information). The isolates were identified and collected by the clinical microbiology laboratory at the authors’ (DS, KL) institution. Staphylococcal isolates were obtained from skin cultures and processed for routine bacterial culture and sensitivity as per Clinical and Laboratory Standards Institute (CLSI) guidelines. 23 Isolates were identified based on biochemical reactions, using the Trek Sensititre (TREK Diagnostic Systems Inc.; Cleveland, OH, USA) automated system and confirmed via matrix‐assisted laser desorption/ionisation time‐of‐flight (MALDI‐TOF) mass spectroscopic analysis. Likewise, the MRSP status of the isolates was confirmed via agglutination test for the penicillin bind protein 2a (PBP2a) and confirmed via mecA gene analysis. The MIC was measured by the same system using CLSI guidelines. 23 Although a specific definition of MDR is not present for S. pseudintermedius, isolates were defined as MDR if resistant to at least three classes of antibiotic, based on published guidelines for S. aureus (SA). 24 The American Type Culture Collection (ATCC) S. pseudintermedius (ATCC# 49444) strain was tested as an experimental internal control.
Peptide preparation
For this study two HDPs (cBD103 and cCath) were synthesised (Peptide Protein Research Ltd; Fareham, UK) and tested. Each peptide was prepared as described previously. 15 , 16 , 18 , 20 One milligram of each lyophilised peptide was diluted in 10 mM 0.01%acetic acid to generate a stock concentration of 4 mg/mL; the acetic acid activates the bonds between the cysteine residues without adverse effects on fungi or other bacteria. 15 , 16 , 25 , 26 Each peptide was further diluted (1:2,000) with 10 mM sodium phosphate buffer (SPB) at pH 7.4.
Minimal inhibitory concentration (MIC) assay
For each assay, clinical isolates, and the internal control (ATCC# 49444), were subcultured on nutrient media agar (Himedia; Maharashtra‐Mumbai, India) at 37°C for 24 h. Colonies then were collected and suspended in sterile Nutrient broth (Himedia) to achieve an optical density equal to 0.5 McFarland Standard [˜1 x 108 colony forming units (cfu)/mL] using a Sensititre nephelometer (ThermoFisher Scientific; Waltham, MA, USA). The bacterial suspension then was diluted in sterile SPB (1:100) to obtain a concentration of ˜1 x 106 cfu/mL. All assays were performed in duplicate using sterile 96 well polystyrene round‐bottomed plates (Costar, Corning Inc.; Corning, NY, USA) using the broth microdilution method adapted from CLSI, as reported previously. 20 The experimental error was accepted if falling within the doubling dilution.
Four two‐fold serial dilutions [1:32,000 (0.624 µg/mL), 1:64,000 (0.312 µg/mL), 1:128,000 (0.156 µg/mL), and 1:256,000 (0.078 µg/mL)] of a 2% Chx solution (Sigma‐Aldrich; St Louis, MO, USA) were made. Each HDP (cBD103 and cCath) was tested alone at a concentration of 1 µg/mL. Each concentration of Chx also was tested alone or in combination with 1 µg/mL synthetic HDP (cBD103 or cCath) in SPB. The concentrations of Chx to be tested were based on MIC data derived from preliminary data (data not shown) and previous studies on SA and SP. 27 , 28 , 29 , 30 Likewise, the concentration of 1 µg/mL synthetic HDP was based on previous studies on the amount of HDPs secreted in skin washes from healthy and atopic dogs, 22 and the MIC/MBC of both HDPs tested. 15 , 16 , 19 , 20 Each well contained 50 µL antimicrobial (Chx or HDP), 50 µL inoculum (bacteria in SPB), and 50 µL SPB. To test the interactions between Chx and each HDP, 50 µL inoculum was added to 50 µL HDP and 50 µL Chx. As described previously, 20 the inoculum with or without the antimicrobial(s) was incubated for 2 h in the absence of broth. Once all the samples were collected for the time–kill experiments (see MBC assays section below), 50 µL Nutrient broth (Himedia) was added to each well. Negative control wells contained 50 µL Nutrient broth (Himedia) without bacteria and 100 µL SPB, whereas positive control wells contained 50 µL inoculum and 100 µL SPB. The plates were incubated at 35°C for 18–20 h and the MIC recorded. The MIC was defined as the lowest concentration of antimicrobial with no visible pellets.
Minimal bactericidal concentration (MBC) assays
After 5, 15, 30 and 60 min incubation, 1 µL bacterial suspension (Speed Streaks, Hardy Diagnostics; Santa Maria, CA, USA) at each tested dilution and controls, after energetic stirring, was plated in duplicate,on nutrient media agar and incubated at 35°C for 24 h. The MBC was defined as the lowest dilution at which micro‐organisms were no longer viable on subculture.
Time‐kill method
The median log10 values of the individual colony counts of bacteria recovered from each antimicrobial concentration at each time point (5, 15, 30 and 60 min) were collected and represented graphically. The time–kill method was used and adapted to calculate the interaction between Chx and HDPs. Using this method, 31 synergy was defined as a 2‐log10 decrease in colony count at each time point by the combination compared with the colony count of the most active single agent (Chx). Additivity or indifference was defined as 1‐log10 decrease in colony count at each time point by the combination compared with the colony count of the most active single agent (Chx). Antagonism was defined as a 2‐log10 increase in colony count at each time point by the combination compared with the colony count of the most active agent alone (Chx).
Statistical analysis
Statistical analysis was applied to the MBC data only, comparing the combination of Chx with HDPs and Chx alone at the same concentration of Chx and the same time points. The collected data first were tested for normal distribution using the Shapiro–Wilks test (α = 0.05). Then, Friedman’s test was performed to evaluate the behaviour of each data variable in each group at each time point. If statistically significant, a Dunn’s Multiple Comparison Test was performed as post hoc analysis. A P‐value of ≤0.05 was considered statistically significant. All statistical comparisons were performed using prism v6 (GraphPad Software Inc.; La Jolla, CA, USA).
Results
MIC assays
After 24 h of incubation, Chx alone showed an MIC of 0.312 µg/mL in nine of 10 isolates (MIC90) with a single isolate (ID: 276) having an MIC of <0.078 µg/mL. Likewise, an MIC90 of 0.312 µg/mL was achieved when the bacteria were incubated with the combination of Chx and cBD103. More precisely, MICs were achieved of 0.312 µg/mL in six isolates, 0.156 µg/mL in two isolates, and 0.624 µg/mL in one isolate (ID: 42400) and <0.078 µg/mL in another (ID: 276). When bacteria were incubated with Chx and cCath in combination, an MIC90 was achieved at a concentration of 0.312 µg/mL Chx; notwithstanding this, three isolates had an MIC of 0.156 µg/mL and one (ID: 276) had an MIC of <0.078 µg/mL. However, the wells containing the HDPs alone showed growth for each isolate without reaching an MIC. The negative controls showed no bacterial growth, while the positive controls showed growth for each isolate. The ATCC strain had an MIC of <0.078 µg/mL for the Chx alone or in combination with either HDP, although an MIC was not reached with the HDPs alone.
MBC assays
After 30 and 60 min of incubation with Chx alone, an MBC of 0.624 µg/mL was found in only one isolate (ID: 41781). An MBC was not achieved for any other clinical isolates and any time point. The wells containing the HDPs alone showed growth for most of the clinical isolates tested without reaching an MBC; one isolate (ID: 41781) reached an MBC after 5 min of incubation with cBD103, while a second isolate (ID: 349) reached an MBC at 60 min of incubation. As far as cCath, only one isolate (ID: 46046) reached an MBC after 60 min of incubation. The MBCs for the combinations of Chx and cBD103, and of Chx and cCath varied among isolates, different concentrations of Chx, and time points.
When the colonies recovered from the combination of Chx and cBD103 were compared to the colonies recovered from Chx alone, a significant decrease in colony count was observed at 15 min (0.624 µg/mL; P = 0.04) and 60 min (0.312 µg/mL; P = 0.03). However, when the colonies recovered from the combination of Chx and cCath were compared to the colonies recovered from Chx alone, a significant decrease in colony count was observed in multiple concentrations at multiple time points except after 15 min of incubation (0.15625 µg/mL; P = 0.09) (Figure 1). Like the MIC, the negative controls showed no bacterial growth, while the positive controls showed growth for each isolate. After 60 min of incubation with Chx alone, the ATCC strain had an MBC of 0.321 µg/mL. However, an MBC of <0.078 µg/mL was achieved for bacteria incubated for 15 min in the combination of Chx and cBD103, and for 60 min in the combination of Chx and cCath.
Figure 1.
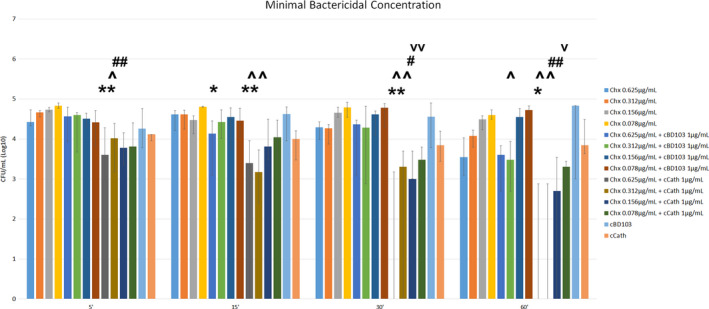
Median of the colony forming units (cfu) recovered after 5, 15, 30 and 60 min incubation with chlorhexidine digluconate (Chx) and host defence peptides (HDPs) alone or in combination.
Groups were compared using Friedman’s test with Dunn's multiple comparison test. *: comparison with Chx 0.625 μg/mL (*, P ≤ 0.05; **,P ≤ 0.01); ^: comparison with Chx 0.312 μg/mL (^, P ≤ 0.05; ^^, ≤ 0.01; ^^^, P ≤ 0.001); #: comparison with Chx 0.156 μg/mL (#, P ≤ 0.05; ##, P ≤ 0.01); v: comparison with Chx 0.0.078 μg/mL (v, P ≤ 0.05; vv, P ≤ 0.01). HDPs: cBD103, canine β‐defensin 103; cCath, canine cathelicidin. Lines on bars indicate upper and lower quartiles.
Time–kill method
A reduction of ≥1‐log10 was seen for the combination of Chx and cCath at most of the concentrations and time points tested (Figure 2). A 2‐log10 reduction was achieved for the combination of Chx and cCath used at the highest concentrations of Chx after 30 min (0.625 µg/mL) and 60 min (0.625 µg/mL and 0.312 µg/mL) of incubation. A lack of effect was observed with the combination of Chx and cBD103.
Figure 2.
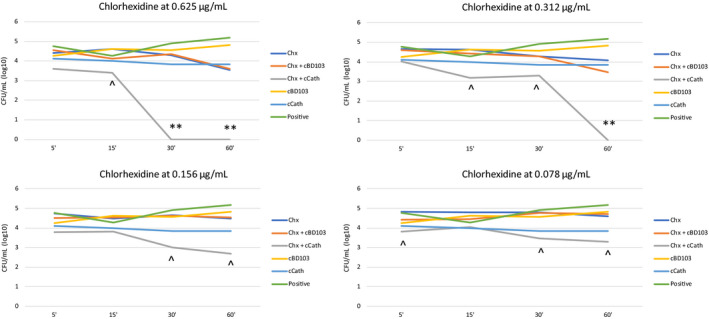
Time–kill curves of chlorhexidine digluconate (Chx) and host defence peptide (HDP) alone or in combination.
**, synergy; ^, additivity/neutrality. HDPs: cBD103, canine β‐defensin 103; cCath, canine cathelicidin. Positive indicates bacteria incubated in absence of antimicrobials (positive control).
Discussion
This is the first study in veterinary medicine demonstrating a synergistic/additive effect between Chx and HDPs. The MoA of such combinations is not clear. However, one potential MoA could involve the membrane‐disruptive action of one antimicrobial (e.g. HDPs) on Staphylococci making the other (e.g. Chx) more effective. In fact, both HDPs tested here and Chx are cationic antimicrobials whose major MoAs are the binding to and disruption of bacterial membranes. 3 , 4 , 5
The exact amounts of readily available (released) HDPs on the cutaneous surface currently are unknown rendering it difficult to decide the correct amount of HDP to be tested. The choice of using a concentration of 1 µg/mL HDP was based on previous in vitro and in vivo studies. 15 , 16 , 18 , 20 , 21 , 22 Such studies showed an amount of ≤0.4 µg/mL HDPs secreted in the skin wash of healthy and atopic dogs. 22 However, because of the increased adhesion of HDPs to the stratum corneum demonstrated in canine skin, 21 the amount of HDPs is likely to be greater than the one secreted in skin washes. Finally, the MIC/MBC of cBD103 and cCath for methicillin‐resistant SP has been found to be ˜25 µg/mL, 20 a much greater concentration than those recovered in skin washes. 22 Thus, based on these studies, a sub‐MIC/MBC concentration of 1 µg/mL HDPs was selected as this was reasonably present on canine skin. However, it is noteworthy to mention that in vivo a multitude of antimicrobial molecules work together against multiple pathogens.
Likewise, the range of concentrations tested for Chx was based on previous studies on SA and SP. 27 , 28 , 29 , 30 In particular, a starting working concentration of 0.625 µg/mL was chosen based on the high variability on the antimicrobial action of Chx shown against SA (0.625–250 µg/mL) and SP (7 µg/mL). 27 , 28 , 29 , 30 To assess a potential synergy between Chx and HDPs, several sublethal dilutions of Chx were selected. It was found that higher concentrations of Chx resulted in a bactericidal effect of Chx, not allowing further comparison between Chx alone and the combinations of Chx and HDP.
In order to assess the potential synergy between Chx and HDPs, a time–kill method was selected. This method was chosen because it is very flexible and better suited to the assessment of MBCs over time. 31 Because of the short contact time achieved by Chx formulations in practice, an incubation time of ≤60 min was selected, yet a potentially extended time–kill curve, encompassing 10 days of exposure, would have accounted for the residual effect of Chx demonstrated in some studies. 32 , 33 , 34 Based on this method, the results of this study show that a synergic effect is present between Chx and cCath, and not CHx and cBD103. However, an additive/neutral effect was seen between Chx and both HDPs tested for most of the concentrations and times analysed. These results are in line with a previous study showing an enhanced antimicrobial effect of human BD3 (human orthologue of cBD103) when associated with Chx. 13 This seemingly positive association between Chx and HDPs, within the narrow concentration range as used in this manuscript, should now be assessed for a wider range of bacterial lineages.
One limitation of this study is the lack of epidemiological characterisation of the bacterial isolates. However, although a multilocus sequence typing (MLST) characterisation was not performed, based on the current knowledge on MRSP epidemiology, it is likely that all of the isolates belonged to the same type.
In conclusion, these preliminary data show the potentiation of Chx’s antimicrobial effects against MDR‐SP when associated with cCath and cBD103. The degree of the potentiation depends on the concentration of Chx, the incubation time and the HDP tested. How this phenomenon works in nature is hard to determine, yet it is possible to speculate that Chx activity when in contact with the skin and the naturally secreted HDPs may increase its efficacy and kill speed. This would explain the positive clinical benefit of Chx against MDR bacterial pyoderma in dogs. Beyond the purpose of this study is the potential direct effect of Chx on HDPs. It would be interesting to test whether or not Chx (with or without an antifungal agent) increases the presence and antimicrobial effect of natural canine HDPs, or if the combination of Chx and HDPs would increase the susceptibility of Chx‐resistant organisms.
Author contributions
Domenico Santoro: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing‐original draft, Writing‐review & editing. Lopamudra Kher: Data curation, Investigation, Writing‐review & editing. Vanessa Chala: Conceptualization, Writing‐review & editing. Christelle Navarro: Conceptualization, Writing‐review & editing.
Supporting information
Table S1. Susceptibility of Staphylococcus pseudintermedius clinical isolates, n = 10. MDR is defined by methicillin resistance and resistance to at least one agent (bold) in three or more antimicrobial categories. 1 , 2
Sources of Funding: This study was funded by Virbac Corporation
Conflicts of Interest: DS received reimbursements, fees, funding or salary from Virbac. VC and CN are employees of Virbac.
References
- 1. Detwiler A, Bloom P, Petersen A et al. Multi‐drug and methicillin resistance of staphylococci from canine patients at a veterinary teaching hospital (2006–2011). Vet Q 2013; 33: 60–67. [DOI] [PubMed] [Google Scholar]
- 2. Morris DO, Loeffler A, Davis MF et al. Recommendations for approaches to meticillin‐resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2017; 28: 304–e69. [DOI] [PubMed] [Google Scholar]
- 3. Karpiński TM, Szkaradkiewicz AK. Chlorhexidine–pharmaco‐biological activity and application. Eur Rev Med Pharmacol Sci 2015; 19: 1,321–1,326. [PubMed] [Google Scholar]
- 4. Boothe HW. Antiseptics and disinfectants. Vet Clin North Am Small Anim Pract 1998; 28: 233–248. [DOI] [PubMed] [Google Scholar]
- 5. Carlotti DN, Majart P. La chlorhexidine, revue bibliographique. Prat Méd Chirurg Anim Compagnie 1996; 31: 553–563. [Google Scholar]
- 6. Mueller RS, Bergvall K, Bensignor E et al. A review of topical therapy for skin infections with bacteria and yeast. Vet Dermatol 2012; 23: 330–341, e62. [DOI] [PubMed] [Google Scholar]
- 7. Johnson RC, Schlett CD, Crawford K et al. Recurrent methicillin‐resistant Staphylococcus aureus cutaneous abscesses and selection of reduced chlorhexidine susceptibility during chlorhexidine use. J Clin Microbiol 2015; 53: 3,677–3,682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Q, Zhao H, Han L et al. Frequency of biocide‐resistant genes and susceptibility to chlorhexidine in high‐level mupirocin‐resistant, methicillin‐resistant Staphylococcus aureus (MuH MRSA). Diagn Microbiol Infect Dis 2015; 82: 278–283. [DOI] [PubMed] [Google Scholar]
- 9. Clark SM, Loeffler A, Bond R. Susceptibility in vitro of canine methicillin‐resistant and ‐susceptible staphylococcal isolates to fusidic acid, chlorhexidine and miconazole: opportunities for topical therapy of canine superficial pyoderma. J Antimicrob Chemother 2015; 70: 2,048–2,052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brochmann RP, Helmfrid A, Jana B et al. Antimicrobial synergy between carprofen and doxycycline against methicillin‐resistant Staphylococcus pseudintermedius ST71. BMC Vet Res 2016; 12: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santoro D, Ahrens K, Vesny R et al. Evaluation of the in vitro effect of Boldo and Meadowsweet plant extracts on the expression of antimicrobial peptides and inflammatory markers in canine keratinocytes. Res Vet Sci 2017; 115: 255–262. [DOI] [PubMed] [Google Scholar]
- 12. Santoro D, Bohannon M, Ahrens K et al. Evaluation on the effects of 0.1% Peumus boldus leaf and Spiraea ulmaria plant extract combination on bacterial colonization in canine atopic dermatitis: a preliminary randomized, placebo controlled, double‐blinded study. Res Vet Sci 2018; 118: 164–170. [DOI] [PubMed] [Google Scholar]
- 13. Maisetta G, Batoni G, Esin S et al. Activity of human β‐Defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob Agents Chemother 2003; 47: 3,349–3,351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolska KI, Grześ K, Kurek A. Synergy between novel antimicrobials and conventional antibiotics or bacteriocins. Pol J Microbiol 2012; 61: 95–104. [PubMed] [Google Scholar]
- 15. Sang Y, Ortega MT, Blecha F et al. Molecular cloning and characterization of three β‐defensins from canine testes. Infect Immun 2005; 73: 2,611–2,620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sang Y, Ortega MT, Rune K et al. Canine cathelicidin (K9CATH): gene cloning, expression, and biochemical activity of a novel pro‐myeloid antimicrobial peptide. Dev Comp Immunol 2007; 31: 1,278–1,296. [DOI] [PubMed] [Google Scholar]
- 17. Santoro D, Bunick D, Graves TK et al. Expression and distribution of antimicrobial peptides in the skin of healthy beagles. Vet Dermatol 2011; 22: 61–67. [DOI] [PubMed] [Google Scholar]
- 18. Erles K, Brownlie J. Expression of β‐defensins in the canine respiratory tract and antimicrobial activity against Bordetella bronchiseptica . Vet Immunol Immunopathol 2010; 135: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leonard BC, Marks SL, Outerbridge CA et al. Activity, expression and genetic variation of canine β‐defensin 103: a multifunctional antimicrobial peptide in the skin of domestic dogs. J Innate Immun 2012; 4: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santoro D, Maddox CW. Canine antimicrobial peptides are effective against resistant bacteria and yeasts. Vet Dermatol 2014; 25: 35–e12. [DOI] [PubMed] [Google Scholar]
- 21. Santoro D, Archer L, Kelley K. A defective release of host defense peptides is present in canine atopic skin. Comp Immunol Microbiol Infect Dis 2019; 65: 65–69. [DOI] [PubMed] [Google Scholar]
- 22. Santoro D. Evaluation of the secretion of antimicrobial peptides and antimicrobial effect of skin wash in atopic and healthy dogs: a preliminary study. Vet Dermatol 2018; 29: 402–e132. [DOI] [PubMed] [Google Scholar]
- 23. CLSI . Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals – 3rd ed. CLSI supplement VET01S. Wayne, PA: Clinical and Laboratory Standards Institute, 2015.
- 24. Magiorakos A, Srinivasan A, Carey RB et al. Multidrug‐resistant, extensively drug resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–281. [DOI] [PubMed] [Google Scholar]
- 25. Fazakerley J, Crossley J, McEwan N et al. In vitro antimicrobial efficacy of β‐defensin 3 against Staphylococcus pseudintermedius isolates from healthy and atopic canine skin. Vet Dermatol 2010; 21: 463–468. [DOI] [PubMed] [Google Scholar]
- 26. Fritz P, Beck‐Jendroschek V, Brasch J. Inhibition of dermatophytes by the antimicrobial peptides human β‐defensin‐2, ribonuclease 7 and psoriasin. Med Mycol 2012; 50: 579–584. [DOI] [PubMed] [Google Scholar]
- 27. Gava Mazzola P, Jozala F, Novaes LCL et al. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz J Pharm Sci 2009; 45: 241–248. [Google Scholar]
- 28. Odore R, Colombatti Valle V, Re G. Efficacy of chlorhexidine against some strains of cultured and clinically isolated microorganisms. Vet Res Commun 2000; 24: 229–238. [DOI] [PubMed] [Google Scholar]
- 29. Delany CM, Yong S, Gajjar M et al. In vitro study of the efficacy of chlorhexidine in the management of infectious keratitis. Invest Ophthalmol Vis Sci 2005; 46: 4881. [Google Scholar]
- 30. Banovic F, Bozic F, Lemo N. In vitro comparison of the effectiveness of polihexanide and chlorhexidine against canine isolates of Staphylococcus pseudintermedius, Pseudomonas aeruginosa and Malassezia pachydermatis . Vet Dermatol 2013; 24: 409–413, e88–9. [DOI] [PubMed] [Google Scholar]
- 31. White RL, Burgess DS, Manduru M et al. Comparison of three different in vitro methods of detecting synergy: time‐kill, checkerboard, and E test. Antimicrob Agents Chemother 1996; 40: 1,914–1,918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramos SJ, Woodward M, Hoppers SM et al. Residual antibacterial activity of canine hair treated with five mousse products against Staphylococcus pseudintermedius in vitro . Vet Dermatol 2019; 30: 183–e57. [DOI] [PubMed] [Google Scholar]
- 33. Mesman ML, Kirby AL, Rosenkrantz WS et al. Residual antibacterial activity of canine hair treated with topical antimicrobial sprays against Staphylococcus pseudintermedius in vitro . Vet Dermatol 2016; 27: 261–e61. [DOI] [PubMed] [Google Scholar]
- 34. Kloos I, Straubinger RK, Werckenthin C et al. Residual antibacterial activity of dog hairs after therapy with antimicrobial shampoos. Vet Dermatol 2013; 24: 250–e54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Susceptibility of Staphylococcus pseudintermedius clinical isolates, n = 10. MDR is defined by methicillin resistance and resistance to at least one agent (bold) in three or more antimicrobial categories. 1 , 2


