Abstract
BACKGROUND
Taro (Colocasia esculenta cv. Daikichi) is believed to be one of the earliest cultivated tuber crops and it is a staple food in many parts of the world. The mother corm and side cormels (daughter and granddaughter tubers) form the major consumed parts; however, the former is rarely preferred. Taro is mainly cultivated using either unflooded or flooding cultivation, under dryland‐rainfed and wetland‐irrigated conditions, respectively. Although flooding cultivation has several advantages, such as lower risk of diseases, weeds, and insect pests, contributing to increased tuber yield, its effects on the quality characteristics of the tubers are largely unknown. In this study, the effects of controlled flooding cultivation on the quality of mother corm and side cormels were investigated. Their taste, color, physical properties, antioxidant activity, and starch, oxalic acid, nitrate ion, arabinogalactan (AG)/AG protein (AGP), γ‐aminobutyric acid (GABA), and total polyphenol content was compared with those under unflooded cultivation.
RESULTS
Flooding cultivation increased polyphenol levels and antioxidant activity and decreased oxalate, nitrate ion, GABA, and AG/AGP levels. Flooding cultivation also reduced the harshness and increased the hardness and stickiness of steamed mother corm paste, generally discarded under unflooded cultivation, thus rendering it suitable for consumption.
CONCLUSION
Controlled flooding cultivation has economic advantages and the potential to improve the quality of cultivated taro. © 2021 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Colocasia esculenta, flooding cultivation, oxalate, polyphenol, γ‐aminobutyric acid, antioxidant activity
INTRODUCTION
Alarming threats of global food security, resulting from several factors including the growing population, climate change, and low food‐crop productivity, have warranted the identification of potential alternative sources like ‘orphan crops’ or ‘underutilized crops’ to improve the availability of food. 1 Taro (Colocasia esculenta (L.) Schott), the most widely cultivated species of the genus Colocasia, is a tuber crop consumed in many parts of the world. 2 , 3 , 4 , 5 It is believed to have been one of the earliest cultivated plants, originated in the Bay of Bengal region of southeast Asia, and is thought to have been introduced into Japan more than 2500 years ago. 2 , 3 , 4 , 5 The primary edible parts of taro differ based on their cultivars. In Japan, the daughter and granddaughter cormels are consumed from the main varieties, such as Daikichi, Ishikawa‐wase, and Dotare. 6
The cultivation methods of taro vary in different parts of the world. However, the unflooded and flooding methods are the two widely used cultivation methods, used under dryland‐rainfed and wetland‐irrigated conditions, respectively. 2 , 3 In Japan, taro is cultivated mostly using the unflooded cultivation method (hereafter called unflooded cultivation), whereas in the Ryukyu and the Nansei Islands it is grown using the flooding cultivation method, like that used for paddy cultivation. 7 It has been reported that the yield and shapes of cultivated taro are adversely affected by drought. 2 Furthermore, increased infestation of insects and pests such as nematodes, and epidemics also affect the quality of the seed cormel. 8 These bottlenecks highlight the need for technology to stabilize the production of disease‐free and of superior quality taro.
Recently, controlled flooding cultivation (hereafter referred to as flooding cultivation), a new method of taro cultivation, has been developed. 9 In this method, water is supplied continuously for only 3 months during the cultivation period, and it maintains productivity without the injuries caused by continuous cropping. Previous studies have shown that the roots of taro grow normally, without moisture damage, and maintain a high rate of photosynthesis under flooding conditions due to the development of aeration tissue in the roots, similar to that in rice, which results in higher yields. 10 Flooding cultivation also suppresses the damage from nematodes or insects (data not published); therefore, it produces high‐quality and healthy seed cormels. Despite the reported advantages of flooding cultivation, its effects on taro corm and cormel composition and quality have not been explored. There are only a few studies available on the effects of land environment on the composition of taro. 11 Enhanced photosynthesis may lead to a difference in the starch content. Moreover, the levels of osmotic control factors such as soluble and insoluble oxalate, 12 , 13 arabinogalactan (AG)/AG protein (AGP), 14 , 15 and γ‐amino butyric acid (GABA) 16 , 17 could also be altered. As flooding converts the soil from aerobic to anaerobic conditions, changes in nitrogen utilization are also to be expected. 18 Furthermore, changes in palatability associated with the alteration of these factors are also anticipated. It has been shown that different cultivation environments alter the composition of crops and add new bioregulatory functions. 19 , 20 , 21
The purpose of this study was to evaluate the taste of the mother corms; quantify the levels of oxalates, nitrate ions (NO3 −), AG/AGP, GABA, and total polyphenol; evaluate their antioxidant activity, and compare these factors for taro corms and cormels cultivated with the flooding and the unflooded methods.
MATERIALS AND METHODS
Cultivation conditions
Taro (C. esculenta (L) schott cv. Daikichi) was cultivated in the open experimental paddy fields of Kagoshima Prefectural Institute for Agricultural Development (31° 48′ 20.71″ N, 130° 34′ 13.63″ E) located in Minami‐Satsuma city, Kagoshima, Japan, in 2016. The same amount of fertilizer was applied to both the flooding and unflooded cultivation plots before planting as follows: cow compost (40 t ha−1), magnesian lime (1 t ha−1), and chemical fertilizer (N:P2O5:K2O = 150:150:150 kg ha−1). Seed corms were planted at a planting distance of 40 cm and a furrow distance of 1 m on April 20, 2016. Fifty corms were planted in one plot, and two replications were made in each plot in a randomized design. In the flooding plot, water was continuously supplied between ridges, and drained to maintain water depth of 10 cm from June 6 to September 9. In contrast, the unflooded plot was not irrigated. The plants were harvested on October 25, 2016. The harvesting time was determined such that the growth period would be similar to that used in a previous study. 9 After harvesting, taro was washed and separated into mother corm, daughter, and granddaughter cormels.
Preparation of taro powder and paste
The outer skins of eight or nine corm/cormels from different plants were peeled off thinly. The mother corms and daughter/granddaughter cormels were halved lengthwise; one half was used as the raw sample and the other half was steamed for 50 min. The halved raw and steamed samples were divided into upper and lower portions, then again cut into 10 mm from the outside (marginal sample) and the central portion (central sample). The samples from each group were put together and frozen (−80 °C), dried in a vacuum dryer (VOS‐451SD, Tokyo Rika Kikai Co., Ltd, Tokyo, Japan), and powdered using a mill (OML‐1, Osaka Chemical Co., Ltd, Osaka, Japan). To prepare a paste, steamed samples from each group were put together and pressed out with a 5 mm diameter die using a mincing machine (WMG‐22, Watanabe Foodmach Co., Ltd., Nagoya, Japan).
Sensory evaluation of steamed tubers
The steamed mother corms, and daughter and granddaughter cormels were evaluated for the following sensory attributes: sweetness, harshness, hardness, stickiness, and overall evaluation. Each evaluated item was rated on a five‐point scale with scores between +2 to −2, using mother corms obtained from unflooded cultivation as a reference. The taste of unflooded steamed mother corms was given 0 point in each evaluate. The higher the score, the sweeter, harsher, harder, stickier, and better the overall rate. The evaluation was performed by 11 trained panelists of the Kagoshima Prefectural Osumi Food Technology Development Center, Kagoshima city, Japan.
Measurement of taste
Steamed powder and distilled water were mixed (20 g L−1) and stirred using a magnetic stirrer (SR 100, Advantec, Tokyo, Japan) for 30 min. It was then sonicated (ASU‐6M, As One Corporation, Osaka, Japan) for 15 min, followed by centrifugation at 4 °C and 1600×g for 10 min (RX‐200, Tomy, Tokyo, Japan), and the supernatant was collected. The supernatant was centrifuged again, and the resulting aliquot was taken as the sample using a taste‐recognition device (TS‐5000Z, Intelligent Sensor Technology, Inc., Kanagawa, Japan). The sensors used in the taste‐recognition device were preconditioned in the internal solution (3.33 mol L–1 potassium chloride and saturated silver chloride) and the reference solution for 24 h. The sensors and reference electrodes were attached to the taste‐recognition device and calibrated. Next, the sample solution was taken in a special cup, and the initial taste (sourness, saltiness, umami, acidic bitterness, astringency, and sweetness) and aftertaste (aftertaste from acidic bitterness, astringency, richness, basic bitterness, and hydrochloride salts) were measured.
Determination of starch content
The starch content was measured using the dinitrosalicylic acid (DNS) method. 22 Eighty percent ethanol (20 mL) (Wako Pure Chemical Industries, Ltd, Osaka, Japan) was mixed with 10 g of taro sample, and ground in a polytron homogenizer (PT 10–35, Kinematica AG, Lucerne, Switzerland). The crushed material was washed with 80% ethanol and filtered through a no. 6 filter paper (Advantec). The sugars in the residue were then washed with 80% ethanol, transferred to a beaker containing 50 mL of distilled water, and further ground in a homogenizer with 50 mL of 2 mol L−1 sodium hydroxide solution. Next, 0.1 mL isoamyl alcohol was added to the homogenate, and the volume was made up to 250 mL with distilled water and heated in a stainless‐steel bowl containing boiling water for 30 min. After cooling, 20 mL of this solution was fractionated, neutralized with 1.5 mol L−1 acetic acid to pH 4.5, filled to 50 mL with distilled water, and filtered through a no. 2 filter paper (Advantec). Then 0.25 mL of glucoamylase solution (15 U mL−1, Sigma‐Aldrich Co., Ltd, St. Louis, MO, USA) was added to 0.25 mL of the sample solution. After decomposition at 40 °C for 1 h, which is sufficient for the complete digestion of starch in the samples, the glucose content in the sample solution was determined using the DNS method. The starch content was obtained by multiplying the obtained glucose content with a conversion factor of 0.9.
Physical property and colorimetric analysis of taro paste
The texture of the pastes was measured using a rheometer (RE2‐33005, Yamaden Co., Ltd, Tokyo, Japan). A cylindrical plunger of 12 mm diameter was used to measure the texture at a speed of 10 mm s−1 and a strain factor of 30%. The sample was compressed twice, and the maximum load of the first compression was defined as hardness, and the load when the plunger was pulled up after the first compression was defined as stickiness. The color of the paste that was obtained was evaluated using a colorimeter (SQ2000, Nippon Denshoku Co., Ltd, Tokyo, Japan) with CIE L*a*b* coordinates.
Determination of oxalic acid content
Oxalic acid was quantified using high‐performance liquid chromatography (HPLC). 23 To extract soluble and total oxalates, taro powder was mixed with distilled water and 0.5 mol L–1 hydrochloride solution (20 mg mL−1). Samples were vortexed (Se‐08, Taitec, Saitama, Japan) and sonicated for 30 min each and centrifuged at 1600×g for 10 min (Model 3500, Kubota, Tokyo, Japan). The supernatant was collected and filtered through a 0.45 μm filter (DISMIC®‐13HP045AN, Advantec) and used as the sample for analysis. The HPLC unit comprised a pump (PU‐4180, Jasco, Japan), a detector (UV‐4075, Jasco, Tokyo, Japan), an autosampler (AS‐4050, Jasco, Tokyo, Japan), and a column oven (SLC‐25A, MEE, Japan). The HPLC analysis was performed using a C18 reversed‐phase column (Cosmosil 5C18‐PAQ, 4.6 mm I.D. × 250 mm, Nacalai Tesque, Kyoto, Japan) maintained at 40 °C. The eluent used was 20 mM phosphate buffer (pH 2.5) with a flow rate of 1.0 mL min−1, a detection wavelength at 254 nm, and an injection volume of 10 μL. The calibration curve was obtained using oxalic acid anhydride (Wako, Japan) as standards (0, 31.3, 62.5, 125, 250, 500, and 1000 μg mL−1, n = 3). Insoluble oxalate was calculated as the difference between the total and soluble oxalates.
Nitrate ion analysis
Sample solution was prepared using the same method that was used for water‐soluble oxalates and measured using the nitrate ion meter (Laqua twin B‐74, Horiba, Ltd., Kyoto, Japan). Briefly, the equipment was adjusted with two‐point calibration, using 68 and 1100 ppm NO3 −‐N standard solution (Model Y042 and Y041, Horiba, Ltd., Kyoto, Japan). Next, 300 μL of the sample solution was applied onto the ion meter to determine the concentration of nitrate ions.
Arabinogalactan (AG)/AG protein (AGP) assay
The AG/AGP was extracted as follows: taro powder and distilled water were mixed (200 mg mL−1), vortexed, and sonicated for 30 min each and left at 25 °C for 24 h. Next, it was centrifuged at 1600×g for 10 min at 4 °C, and the supernatant was collected. The AG/AGP assay was carried out according to a previously reported method. 24 Briefly, 1 mm thick gel was prepared by mixing and heating 1% (w/v) agarose, 0.15 mol L–1 NaCl, and 10 μg mL−1 β‐glucosyl Yariv reagent. The gel was drilled with a Pasteur pipette, followed by the addition of 0.8 μL of the sample solution. The gel was placed in a Tupperware container with water to prevent it from drying for 24 h. The area of the halos formed on the gel was measured using Fiji image analysis software 25 and calculated as gum arabic equivalent.
Analysis of γ‐aminobutyric acid (GABA) content
γ‐Aminobutyric acid was measured using HPLC (Jasco). 26 The taro powder and 75% (v/v) ethanol were mixed (25 mg mL−1), vortexed, and sonicated for 15 min each. The mixture was heated at 80 °C for 20 min (Thermomixer C, Eppendorf, Germany), centrifuged at 4 °C, 1600×g for 10 min, and the supernatant was collected. The supernatant that was obtained was concentrated and dried using a centrifuge concentrator (VEC‐260, Iwaki, Japan), and amino acid extracts were obtained. The dry matter that was obtained was dissolved in 0.1 mol L–1 sodium carbonate buffer (pH 8.9). The sample solution (200 μL) and 800 μL of dabsyl chloride solution (TCI, Tokyo, Japan) dissolved in acetonitrile (1.3 mg mL−1) was mixed and vortexed in a Thermomixer C (Eppendorf, Hamburg, Germany) at 70 °C, 2000 rpm for 30 min. After cooling, the sample was filtered through a 0.45 μm filter and used for HPLC. The mobile phase comprised 20 mM sodium acetate buffer (pH 6.5; solution A) and acetonitrile (solution B). The HPLC system used was the same that quantified oxalates. We used a linear gradient of 15% solution B at 0 min, with a direct increase in solution B up to 45% from 0 to 28 min. The flow rate was 1.0 mL min−1, and a detection wavelength was monitored at 465 nm.
Total polyphenol content
Total polyphenol analysis was performed using the Folin–Ciocalteu method. 27 Taro powder and 80% acetone were mixed (25 mg mL−1), followed by vortexing and sonication for 15 min each. The mixture was then centrifuged at 4 °C and 1600×g for 10 min, and the supernatant was collected (extracted twice). The supernatant that was obtained was concentrated and dried in a centrifugal concentrator (VEC‐260, Iwaki). The dry matter was dissolved in 50% methanol solution containing 0.1% acetic acid to prepare the sample for measurement. Then, 75 μL of 10% phenol reagent solution (Nacalai Tesque, Japan) was added to 10 μL of the sample solution and allowed to stand for 5 min, after which 75 μL of 2% sodium carbonate solution was added and allowed to stand for 15 min. The absorbance was measured using a plate reader (Infinite F200, Tecan, Meilen, Switzerland) at 750 nm and calculated as a gallic acid equivalent.
Measurement of antioxidant activity
Antioxidant activity was measured with a 1,1‐diphenyl‐2‐picrylhydrazyl (DPPH) radical scavenging assay. Taro powder and methanol/water/acetic acid (90/9.5/0.5, v/v/v) solution were mixed (25 mg mL−1), vortexed, and sonicated for 15 min each. The mixture was then centrifuged at 4 °C and 1600×g for 10 min, and the supernatant was collected (extracted twice). The supernatant that was obtained was concentrated and dried. The resulting dry matter was dissolved in 50% ethanol to prepare the sample for measurement. After adding 50 μL of the sample solution to a microplate, 100 μL of 50% ethanol was added and mixed. Next, 50 μL of 800 μM DPPH solution was added, and the plate was incubated in the dark for 20 min. The absorbance was measured at 540 nm using a plate reader and calculated as Trolox equivalent.
Statistical analysis
Quantitative analysis of all components was repeated three times independently. The data were analyzed for statistical significance using Welch's t‐test. Differences between the cultivation methods were assessed using a two‐sided test with an α level of 0.05 using Microsoft Excel 2013. Data are represented as the means ± standard deviations (SD). P < 0.05 was considered statistically significant. Correlation analysis was performed to investigate the effect of different cultivation methods using RStudio (ver. 1.3.959).
RESULTS
Improvement of the taste and physical properties of the mother corm
The sensory evaluation of the steamed taro indicated that the mother corms from flooding cultivation showed a weak inclination toward reducing harshness (Fig. 1(A)). The overall scoring of the mother corm was rated better under flooding cultivation. Taste measurement using the taste‐recognition device indicated reduced harsh taste (astringent taste immediately after placement in the mouth) with flooding cultivation (Fig. 1(B)). The hardness of the paste from the mother corm also increased significantly (P < 0.05) and tended to increase the stickiness according to the physical property analysis (Table 1). Starch content did not show significant differences between unflooded and flooding cultivation but tended to increase in flooding cultivation (Table 1).
Figure 1.

Effects of different cultivation methods on the taste and physical properties of mother corm. (A) Result of sensory evaluation. 0 denotes the result of unflooded cultivated taro. Data are expressed as mean ± SD (n = 11). (B) The relative value of harsh taste. □ Unflooded, ■ Flooding. Data are expressed as mean ± SD (n = 3). *P < 0.05 (flooding versus unflooded cultivation).
Table 1.
Effect of different cultivation methods on starch content and physical properties of mother corm
| Starch content | Physical property of paste | |||
|---|---|---|---|---|
| Raw (%) | Steamed (%) | Hardness (N) | Stickiness (kJ/m3) | |
| Unflooded | 20.1 | 12.8 | 3.57 ± 0.55 | 1.22 ± 0.62 |
| Flooding | 21.9 | 19.3 | 5.54 ± 0.19* | 1.50 ± 0.27 |
Data of physical properties are expressed as mean ± SD (n = 3).
P < 0.05 (flooding versus unflooded cultivation).
Soluble and insoluble oxalate content
As shown in Fig. 2, the water‐soluble oxalate was distributed over the entire mother corm (Fig. 2(A)). However, its content was higher in the daughter and granddaughter cormel than in the mother corm (Fig. 2(B)). The insoluble oxalate (Fig. 2(C) and (D)) was also located in the marginal part of the mother corm, and its content was lower in the daughter/granddaughter cormel. The soluble oxalate content enhanced under flooding cultivation in raw mother corm and granddaughter cormel, which can affect moisture content of cormels (Fig. 2(B)). As seen in Fig. 2(D), several samples did not show insoluble oxalates because there was no difference in the amount of total oxalate and water‐soluble oxalate.
Figure 2.
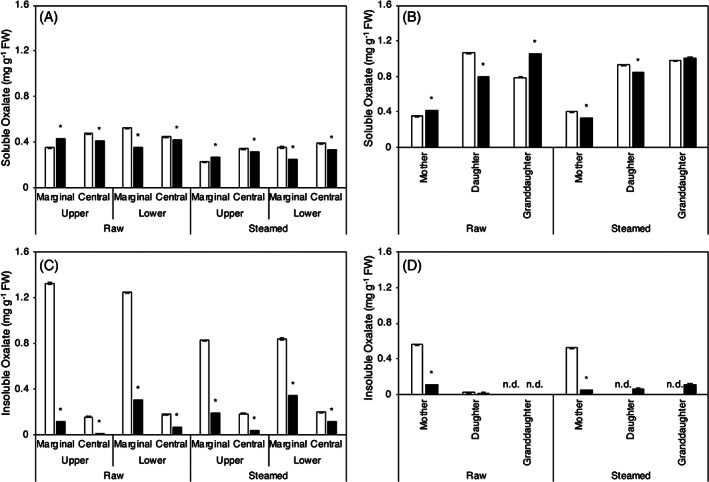
Effects of different cultivation methods on the oxalate content of taro. (A) Distribution of the soluble oxalate in different parts of mother corm. (B) Soluble oxalate content in mother, daughter, and granddaughter cormels. (C) The distribution of the insoluble oxalates in different parts of the mother corm. (D) The insoluble oxalate contents in mother, daughter, and granddaughter cormels. □ Unflooded, ■ Flooding. FW means the fresh weight of taro. Data are expressed as mean ± SD (n = 3). *P < 0.05, flooding versus unflooded cultivation.
Nitrate ion content
As shown in Fig. 3, the nitrate ions were distributed in the upper and lower periphery of the corm. However, after flooding cultivation, the nitrate ions were significantly reduced despite the area (Fig. 3(A)). Nitrate ions were also decreased in the daughter and granddaughter cormels and the mother corm (Fig. 3(B)) of the unflooded‐cultivated taro.
Figure 3.
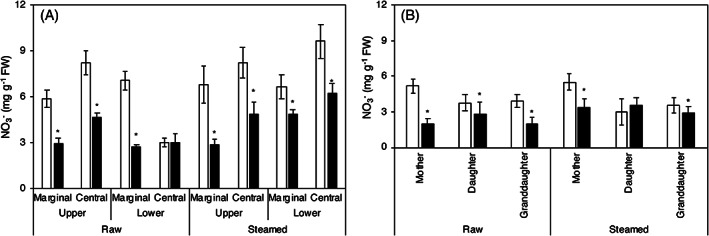
The effect of different cultivation methods on the nitrate content of taro. (A) The distribution of nitrate in different parts of the mother corm. (B) The nitrate ion content in mother, daughter, and granddaughter cormels. □ Unflooded, ■ Flooding. FW means the fresh weight of taro. Data are expressed as mean ± SD (n = 3). *P < 0.05; flooding versus unflooded cultivation.
AG/AGP content
In both raw and steamed taro, AG/AGP was decreased by flooding. This decrease was especially pronounced in mother corm (Fig. 4).
Figure 4.

Effect of different cultivation methods on the AG/AGP content of taro in mother, daughter, and granddaughter cormels. □ Unflooded, ■ Flooding. FW means the fresh weight of taro. Data are expressed as mean ± SD (n = 3). *P < 0.05; flooding versus unflooded cultivation.
GABA content
The distribution of GABA in the mother corm was not characterized (Fig. 5(A)). The GABA content of mother corm, daughter, and granddaughter cormels significantly decreased in flooding cultivation (Fig. 5(B)).
Figure 5.
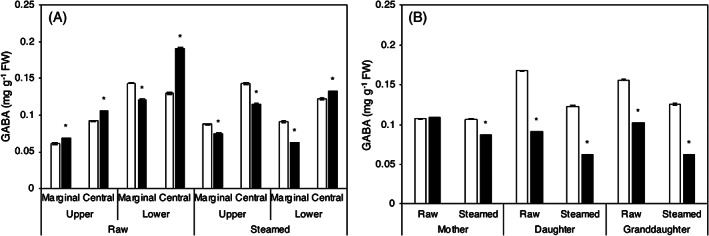
Effect of different cultivation methods on the GABA content of taro. (A) In different parts of the mother corm. (B) In mother, daughter, and granddaughter cormels. □ Unflooded, ■ Flooding. FW means the fresh weight of taro. Data are expressed as mean ± SD (n = 3). *P < 0.05; flooding versus unflooded cultivation.
Color of taro paste
Table 2 shows the color values of the unflooded and flooding‐cultivated mother corms. Taro produced under flooding cultivation had lower L* values for brightness and slightly higher a* values for redness than unflooded cultivation.
Table 2.
Effect of different cultivation methods on colorimetric parameters of mother corm paste
| Colorimetric parameters | |||
|---|---|---|---|
| L* | a* | b* | |
| Unflooded | 67.3 ± 0.4 | 1.9 ± 0.1 | 6.0 ± 0.1 |
| Flooding | 62.7 ± 0.4 † | 2.7 ± 0.1 † | 3.4 ± 0.1 † |
Data are expressed as mean ± SD (n = 3).
P < 0.05 (flooding vs unflooded cultivation).
The asterisks (*) after L*, a*, and b* are pronounced star and are part of the full name.
Total polyphenol content
As shown in Fig. 6, the total polyphenol content increased mainly on the marginal side of the mother corm (Fig. 6(A)) and in the daughter and granddaughter cormels (Fig. 6(B)) under flooding cultivation.
Figure 6.

Effect of different cultivation methods on the total polyphenol contents. (A) In different parts of the mother corm. (B) In mother, daughter, and granddaughter cormels. □ Unflooded, ■ Flooding. FW means the fresh weight of taro. Data are expressed as mean ± SD (n = 3). *P < 0.05; flooding versus unflooded cultivation.
Antioxidant activity
Regardless of flooding or unflooded cultivation, the antioxidant activity of the mother corm was higher in marginal than in central (Fig 7(A)). In mother corm, daughter, and granddaughter cormels, the antioxidant activity of flooding‐cultivated taro increased than those obtained from unflooded conditions (Fig. 7 (B)). In contrast, the steamed samples did not show significant differences in antioxidant activities of the flooding and unflooded cultivations (Fig. 7(A) and (B)). Furthermore, a positive correlation (r = 0.7547) was observed between the total polyphenol content and antioxidant activity (Fig. 7(C)).
Figure 7.

Antioxidant activity of taro. (A) In different parts of mother corm. (B) In mother, daughter, and granddaughter cormels. □ Unflooded, ■ Flooding. (C) Correlation between total polyphenol contents and antioxidant activity. FW: Fresh weight of taro. Data are expressed as mean ± SD (n = 3). *P < 0.05; flooding versus unflooded cultivations.
Correlation analysis
The effect of different cultivation methods on the quality of taro was evaluated using Spearman's correlation coefficients, and the results are shown in Table 3. While positive correlations were found for all factors in the mother corms, no correlations were observed for NO3 − and GABA in different generations, and correlations also tended to be weak for other factors.
Table 3.
Spearman's correlation coefficients for quality of taro obtained using different cultivation methods
| Spearman's correlation coefficients | ||
|---|---|---|
| Parts of mother corm | Different generations | |
| Soluble oxalate | 0.5313* | 0.4097 |
| Insoluble oxalate | 0.7113* | 0.4407 |
| NO3 − | 0.6319* | −0.0124 |
| AG/AGP | 0.3044 | |
| GABA | 0.7043* | 0.0361 |
| Total polyphenol content | 0.9185* | 0.9635* |
| Antioxidant activity | 0.9509* | 0.7774* |
NO3 −, nitrate ion; AG, arabinogalactan; AGP, AG protein; GABA, γ‐aminobutyric acid.
P < 0.05 (flooding versus unflooded cultivation).
DISCUSSION
Colocasia esculenta (L.) Schott cv. Daikichi, one of the major cultivars of taro in Japan, is normally cultivated under unflooded conditions. 7 Flooding cultivation is a newly developed method of taro cultivation. 9 Although it has been found that the yield of corms/cormels increases, differences in taro quality with traditional and new methods remain unknown. As there are significant changes in cultivation conditions, a major change in taro quality is also expected. Daughter and granddaughter cormels of Daikichi are primarily consumed in Japan. However, in contrast, mother corm is not used as food because of its harsh taste and hardness. In this study, we evaluated the differences in edible quality of taro produced using the two different cultivation methods.
The mother corms obtained from flooding cultivation demonstrated an inclination toward reduced harshness. Using a taste‐recognition device, we confirmed the taste differences quantitatively, as the subject's sense and preference could influence the sensory evaluation test. The taste‐measurement results showed that the harsh taste experienced immediately after the taro was placed in the mouth was reduced in the mother corm. This was consistent with the results of the sensory evaluation. Physical property analysis showed the tendency of increased hardness and stickiness of the paste in the flooding cultivation samples. Despite the alteration in physical properties, the sensory evaluation did not show significant differences. The starch content showed a tendency to increase in both raw and steamed mother corm. Photosynthesis was enhanced in the flooding cultivation samples, 10 which, in turn, might have increased the starch content as an assimilation product and affected the physical properties of taro. Furthermore, the tendency of increased stickiness and hardness in mother corms may be responsible for activating starch synthesis during enhanced photosynthesis.
The soluble oxalates were distributed in all parts of mother corms, regardless of cultivation methods or processing, and were more abundant in the daughter and granddaughter cormels than mother corms. The raw mother corms and granddaughter cormels of flooding cultivation showed significantly high content of soluble oxalates; this may have affected moisture content (data not shown). Insoluble oxalate was distributed on the surface of the mother corms regardless of the cultivation method. This result agreed with the results in a previous report. 28 Moreover, insoluble oxalate was more abundant in mother corms than in the daughter and granddaughter cormels; insoluble oxalate content in both of which was reduced by flooding cultivation. The oxalate content of the unflooded taro reported in this study was not markedly different from that previously reported, 11 and the differences that occurred were thought to be due to differences in cultivation environment and variety. The significant reduction in insoluble oxalate in mother corm under flooding cultivation may be due to the sufficient water supply, which reduces the demand for calcium oxalate for osmotic adjustment. 12 , 13 From the viewpoint of palatability, a significant decrease in insoluble oxalate contributed to the decreasing trend in harshness of the mother corms.
In field cultivation, NO3 − is preferentially supplied to plants as a nitrogen source because nitrification of organic nitrogen is actively carried out owing to the aerobic soil conditions. 29 In contrast, under flooding cultivation, nitrification is suppressed because of anaerobic soil conditions. This is thought to increase the ratio of NH4 + as a nitrogen source. 30 Under flooding conditions, taro plants develop aerobic tissue in their roots to maintain the rhizosphere in an aerobic state. 18 Taro can also utilize NH4 + as well as NO3 − as nitrogen. 31 Thus, the decrease in NO3 − levels in the soil and the increase in the use of NH4 + as a substitute for nitrogen could have led to the decrease in NO3 − in flooding cultivation.
Although AG/AGP is a primary component of taro mucilage, 32 the effects of cultivation methods on the AG/AGP content were not discussed sufficiently. The role of mucilage in plants is to prevent osmotic stresses and use soil water effectively. 14 , 15 Under unflooded cultivation, certain drought stress occurs, and plants may protect themselves by upregulating the AG/AGP production to use the limited water efficiently. In contrast, under flooding cultivation, sufficient water is supplied so the drought stress is decreased, resulting in lower AG/AGP production. As for the physical properties, the stickiness of taro paste from the flooding cultivation tended to increase despite decreasing AG/AGP. The ratio of AG/AGP in flesh weight was approximately 0.1%, and it was thought to have negligible effect on stickiness.
The amino acid profile of paddy and upland cultivated corm has been previously reported. 11 However, comparative analysis of GABA has not been performed. Further, the effect of differences in cultivation on GABA content is still unknown. γ‐Aminobutyric acid is produced by glutamate decarboxylase (GAD, glutamate decarboxylase; EC 4.1.1.15), which is known to GABA synthase, and functions as a stress signal. 16 , 17 , 33 The results of the present study showed that taro contains GABA regardless of the cultivation method and generation, whereas the GABA content was lower in taro under flooding cultivation than under unflooded condition, especially in the daughter and granddaughter cormels. Thus, drought stress and levels of GABA, the stress mediator, were decreased under flooding cultivation. The distribution of GABA in mother corms was also more significant in the marginal parts than in the central parts. Ca2+ influences GAD activity, and as Ca2+ is predominantly reported in the central part of the corm, 34 the distribution of Ca2+ may have changed under flooding cultivation. With regard to the difference between the GABA content of the raw and steamed samples, the decrease is thought to be the result of the leaching of GABA into vapor. 35
The total polyphenol content increased under flooding cultivation regardless of the generation. In the mother corm, the total polyphenol content in the upper epidermis was increased. The results also showed an increase in antioxidant activity in water‐soluble extracts of the flood‐cultivated taro, and a positive correlation was found between the antioxidant activity and total polyphenol content. These results indicated that the increase in antioxidant activity could be due to the increase in polyphenol content. It has been reported that the enhanced photosynthesis and metabolisms in flooding cultivation generate a high number of reactive oxygen species (ROS) and promote polyphenol synthesis. 36 Enhanced polyphenol synthesis is a defense mechanism of the plants against ROS‐induced oxidative stress. 37 , 38 With respect to the decreased total polyphenol and antioxidant activity in steamed samples, our results are consistent with those previously reported. 32 Longer steaming time may enhance the degradation of the total polyphenol contents and affect antioxidant activity.
CONCLUSIONS
The unflooded cultivation of taro has been shown to develop defects such as blistering due to water and calcium deficits and low yields because of injury caused by continuous cropping. However, the flooding cultivation method allows cultivars that have been considered for field use to grow without problems, and they have many advantages, such as increased yields due to the activation of photosynthesis and reduced application of herbicides and insecticides. This study revealed that the harvested corm and cormel from flooding cultivation had different characteristics from those grown in the unflooded condition. The edible quality of mother corms improved because of the decreased insoluble oxalates, thus enabling the farmers to use the mother corms, which were discarded in the past. This can help the farmers increase their income and even result in the development of new processed products. This cultivation method can also reduce the number of nitrate ions due to the changes in nitrogen usage, GABA, and AG/AGP, caused by relief from drought stress. An increase in the polyphenol content also augmented the antioxidant activity of taro, which could lead to its use as a source of high added‐value components and raises the possibility of using it as a raw material for functional foods.
Taken together, the results of this study, which proved the superiority of taro generated by the flooding cultivation method, could contribute to the promotion of flooding cultivation, an increase in taro consumption, and the revitalization of agriculture by increasing the income of farmers.
ACKNOWLEDGEMENTS
This research was supported by grants from JSPS KAKENHI (17K07795 and 20H02936) and the Project of the NARO Bio‐oriented Technology Research Advancement Institution (the special scheme project on regional developing strategy; No. 16789619). We would like to thank Dr Fumio Yagi for his technical advice. This research was part of the dissertation submitted by the first author in partial fulfillment of a PhD degree. All authors have provided consent.
REFERENCES
- 1. FAO, IFAD, UNICEF, WFP and WHO , The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets. FAO, Rome: (2020). [Google Scholar]
- 2. Onwueme I, Taro Cultivation in Asia and the Pacific. FAO RAP Publication 1999/16, Bangkok, Thailand (1999). [Google Scholar]
- 3. Linus UO, Edible Aroids: Post‐Harvest Operation. AGST/FAO, Rome, Italy (2003). [Google Scholar]
- 4. O'Hair SK and Maynard DN, Vegetables of tropical climates | Edible Aroids, in Encyclopedia of Food Sciences and Nutrition, 2nd edn, Academic Press, Amsterdam, Netherlands, pp. 5970–5973 (2003). [Google Scholar]
- 5. Matthews PJ, Genetic diversity in Taro, and the preservation of culinary knowledge. Ethnobot Res Appl 2:55–71 (2004). [Google Scholar]
- 6. Kumazawa S, Niuchi K and Honda F, Classification of the taro varieties in Japan. J Jpn Soc Hortic Sci 25:1–10 (1956). [Google Scholar]
- 7. Konishi T, A special importance on the “Imo” for the food, taro [in Japanese]. Int Crop Agric For 31:12–20 (2009). [Google Scholar]
- 8. Bridge J, Plant‐parasitic nematode problems in the Pacific Islands. J Nematol 20:173–183 (1988). [PMC free article] [PubMed] [Google Scholar]
- 9. Ikezawa K, Fukumoto S, Onjo M, Yoshida R and Iwai S, Effects of flooding on growth and yield of Taro (Colocasia esculenta Schott cv. ‘Daikichi’) in pot culture. Hortic Res 13:35–40 (2014). [Google Scholar]
- 10. Ikezawa K, Onjo M, Yoshida R, Yamamoto M and Iwai S, Effects of flooding on photosynthesis in Eddo. J Crop Sci 84:150–154 (2015). [Google Scholar]
- 11. Huang CC, Chen WC and Wang CCR, Comparison of Taiwan paddy‐ and upland‐cultivated taro (Colocasia esculenta L.) cultivars for nutritive values. Food Chem 102:250–256 (2007). [Google Scholar]
- 12. Gouveia CSS, Ganança JFT, Lebot V and MÂAP d C, Quantitation of oxalates in corms and shoots of Colocasia esculenta (L) Schott under drought conditions. Acta Physiol Plant 40:214 (2018). [Google Scholar]
- 13. Nakata PA, Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci 164:901–909 (2003). [Google Scholar]
- 14. Ahmed MA, Kroener E, Holz M, Zarebanadkouki M and Carminati A, Mucilage exudation facilitates root water uptake in dry soils. Funct Plant Biol 41:1129–1137 (2014). [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Dong M and Huang Z, Role of mucilage in the germination of Artemisia sphaerocephala (Asteraceae) achenes exposed to osmotic stress and salinity. Plant Physiol Biochem 48:131–135 (2010). [DOI] [PubMed] [Google Scholar]
- 16. Ramos‐Ruiz R, Martinez F and Knauf‐Beiter G, The effects of GABA in plants. Cogent Food Agric 5:1670553 (2019). [Google Scholar]
- 17. Kinnersley AM and Turano FJ, Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509 (2000). [Google Scholar]
- 18. Deenik JL, Penton CR and Bruland G, Nitrogen cycling in flooded Taro agriculture. Soil Crop Manage 31:1–8 (2013). [Google Scholar]
- 19. Kajiya K, Yamanouchi H, Tanaka Y, Hayashi H and Minami Y, Capsicum cultivated under adverse conditions produces high concentrations of antioxidants and capsaicinoids. J Agric Sci 12:1 (2020). 10.5539/jas.v12n2p1. [DOI] [Google Scholar]
- 20. Wakamatsu M, Yamanouchi H, Sahara H, Iwanaga T, Kuroda R, Yamamoto A et al., Catechin and caffeine contents in green tea at different harvest periods and their metabolism in miniature swine. Food Sci Nutr 7:2769–2778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuroda R, Kazumura K, Ushikata M, Minami Y and Kajiya K, Elucidating the improvement in vascular endothelial function from Sakurajima Daikon and its mechanism of action: a comparative study with Raphanus sativus . J Agric Food Chem 66:8714–8721 (2018). [DOI] [PubMed] [Google Scholar]
- 22. Miller GL, Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428 (1959). [Google Scholar]
- 23. NACALAI TESQUE, INC. COSMOSIL application data [homepage on the Internet]. Application No. AP‐1279. Available: https://www.nacalai.co.jp/cosmosil/data/csmosrchtop.cfm. [17 August 2021].
- 24. van Holst GJ and Clarke AE, Quantification of arabinogalactan‐protein in plant extracts by single radial gel diffusion. Anal Biochem 148:446–450 (1985). [DOI] [PubMed] [Google Scholar]
- 25. Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al., Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9:676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. TOSOH Corpotation TSKgel technical information [homepage on the Internet]. technical information No. 044. Available: https://www.separations.asia.tosohbioscience.com/litjp/techinfo. [17 August 2021].
- 27. Ministry of Education , Culture, Sports, Science and Technology, The Analytical Manual for the Standard Tables of Food Composition in Japan 2015, 7th Revised edn, MEXT, Tokyo, Japan, pp. 204–207 (2015). [Google Scholar]
- 28. Sunell LA and Healey PL, Distribution of calcium oxalate crystal idioblasts in corms of taro (Colocasia esculenta). Am J Bot 9:1029–1032 (1979). [Google Scholar]
- 29. Alexander M, Nitrification, in Soil Nitrogen, ed. by Bartholomew WV and Clark FE. The American Society of Agronomy, Madison, pp. 307–343 (1965). [Google Scholar]
- 30. Strock JS, Ammonification, in Encyclopedia of Ecology, ed. by Jørgensen SE and Fath BD. Academic Press, Oxford, pp. 162–165 (2008). [Google Scholar]
- 31. Osorio NW, Shuai X, Miyasaka S, Wang B, Shirey RL and Wigmore WJ, Nitrogen level and form affect Taro growth and nutrition. HortScience 38:36–40 (2003). [Google Scholar]
- 32. Jiang G and Ramsden L, Characterisation and yield of the arabinogalactan–protein mucilage of taro corms. J Sci Food Agric 79:671–674 (1999). [Google Scholar]
- 33. Akama K and Takaiwa F, C‐terminal extension of rice glutamate decarboxylase (OsGAD2) functions as an autoinhibitory domain and overexpression of a truncated mutant results in the accumulation of extremely high levels of GABA in plant cells. J Exp Bot 58:2699–2707 (2007). [DOI] [PubMed] [Google Scholar]
- 34. Mergedus A, Kristl J, Ivancic A, Sober A, Sustar V, Krizan T et al., Variation of mineral composition in different parts of taro (Colocasia esculenta) corms. Food Chem 170:37–46 (2015). [DOI] [PubMed] [Google Scholar]
- 35. Lee K, Lee H, Choi Y, Kim Y, Jeong HS and Lee J, Effect of different cooking methods on the true retention of vitamins, minerals, and bioactive compounds in Shiitake mushrooms (Lentinula edodes). Food Sci Technol Res 255:115–122 (2019). [Google Scholar]
- 36. Farooq MA, Niazi AK, Akhtar J, Saifullah Farooq M, Souri Z, Karimi N et al., Acquiring control: the evolution of ROS‐induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem 141:353–369 (2019). [DOI] [PubMed] [Google Scholar]
- 37. Xu Z and Rothstein SJ, ROS‐induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav 13:e1451708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakabayashi R, Yonekura‐Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T et al., Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


