Abstract
Introduction
The National Institute on Aging (NIA) provides funding to academic researchers and small businesses working in the Alzheimer's Disease (AD) and AD‐related dementia (ADRD) fields to help commercialize their products. The NIA uses Small Business Innovation Research (SBIR) awards to bridge the funding gap in the diagnostic, therapeutic, and care interventions areas, enabling startups to reach key value inflection points to achieve scientific milestones.
Methods
Only publicly available information is reported. The National Institutes of Health Report Portfolio Online Reporting Tool database and the commercial database Global Data, were used to track the progress of companies that received SBIR or Small Business Technology Transfer (STTR) funding from the NIA.
Results
Since 2008, the NIA has awarded $280 million—including $207 million from fiscal year (FY) 2015 to FY 2019—in new small business program awards for AD/ADRD research.
Discussion
NIA seed capital and mentoring programs are critical resources to help small businesses reach key value inflection points and advance their research from concept to commercialization.
Keywords: National Institute on Aging (NIA), National Institutes of Health (NIH), NIH Report Portfolio Online Reporting Tool (NIH RePORT), Small Business Education and Entrepreneurial Development (SEED), Small Business Innovation Research (SBIR), Small Business Technology Transfer (STTR)
Abbreviations
- AAP
Applicant Assistance Program
- ACTC
Alzheimer's Clinical Trials Consortium
- AD
Alzheimer's Disease
- ADDF
Alzheimer's Drug Discovery Foundation
- ADRD
Alzheimer's Disease and Related Dementias
- Aβ
Amyloid beta
- C3i
Concept to Clinic Commercializing Innovation Program
- CLIA
Clinical Laboratory Improvement Amendments
- CRP
Commercialization Readiness Pilot
- FDA
Food and Drug Administration
- FY
Fiscal Year
- GHR
Gerald and Henrietta Foundation
- HHS
Health and Human Services
- IP
Intellectual Property
- MCI
Mild Cognitive Impairment
- NAPA
National Alzheimer's Program Act
- NIA
National Institute on Aging
- NIH
National Institutes of Health
- NINDS
National Institute of Neurological Disorders and Stroke
- OSBR
Office of Small Business Research
- PET
Positron Emission Tomography
- PI
Principal Investigator
- R&D
Research and Development
- RePORT
Report Portfolio Online Reporting Tool
- SBIR
Small Business Innovation Research
- SEED
Small Business Education and Entrepreneurial Development
- STTR
Small Business Technology Transfer
1. INTRODUCTION
According to Lerner and Gompers, 1 90% of new ventures that cannot reach the key value inflection points needed to secure investments fail within their first 3 years. Value inflection points occur when a company achieves specific milestones in one phase, enabling it to enter the next phase to accelerate product commercialization. However, an inflection point is more than the small progress a company makes on a regular basis and can be identified as a key event or a milestone that can result in a significant change in the progress of the company, enabling it to enter the next phase. Depending upon the nature of the business, reaching an inflection point for a company could mean expanding into a new market, launching a new product, seeking capital for proving proof of concept, or helping with product commercialization. Therefore, small businesses need to raise capital to complete several milestones to reach an initial inflection point.
In the drug discovery process, a project reaches a major inflection point when it transitions from preclinical research to clinical trials. Significant funding is required not only for conducting the clinical trials but also to fulfill additional regulatory requirements, such as demonstrating a product's safety and efficacy, developing intellectual property (IP), and securing licensing. Completion of a set of such milestones corresponds to reaching a value inflection point. Often, a series of value inflection points are required to secure a private investment. The need to meet several value inflection points increases the risk of failure. 2
To prevent failures and encourage innovation, the U.S. Congress created the Small Business Innovation Research (SBIR) program in 1982 and the Small Business Technology Transfer (STTR) program in 1992. Both programs are congressionally mandated with set‐aside funds for U.S. small businesses. They are a resource for early‐stage capital to stimulate technological innovation and to help small businesses in research and development (R&D) with strong potential for commercialization. 3 , 4 While the terms SBIR and STTR represent two separate programs, this report will refer to these programs collectively as the small business programs.
According to a fiscal year (FY) 2015 National Science Board report, the Department of Health and Human Services (HHS) is the main federal funding source for health‐related R&D (Figure 1A). 5 Most of the HHS funds are obligated for the R&D activities of the National Institutes of Health (NIH), making NIH the largest federal funder of biomedical research (Figure 1B). 6 , 7 The NIH has been instrumental in supporting the discovery of novel therapeutic and diagnostic targets as well as initial technology development. 8 , 9
FIGURE 1.
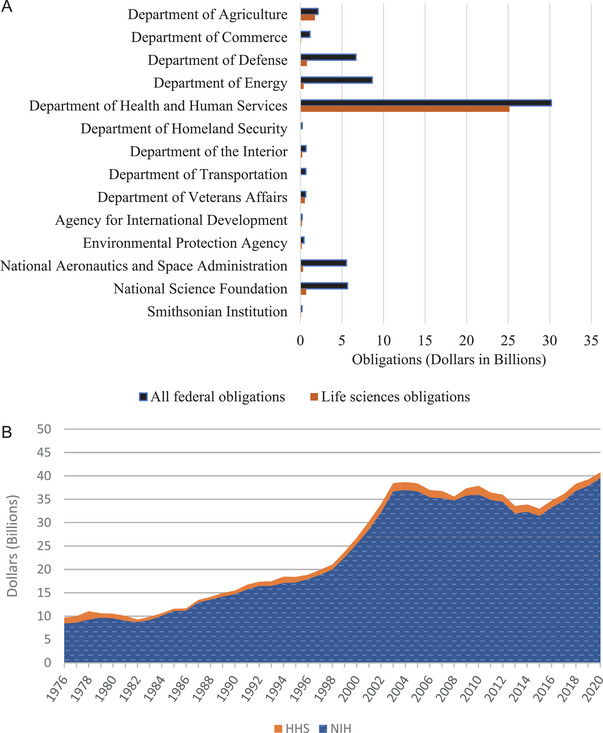
(A) Allocation of federal research funds for life sciences across agencies, based on a 2018 National Science Board report for life science research and development (R&D) obligations across federal departments and agencies. The Department of Health and Human Services (HHS) and the Department of Agriculture spent a major portion of their federal funding on life sciences research. (B) The National Institutes of Health (NIH) is the primary funder of biomedical R&D across the federal government. According to an American Association for the Advancement of Science R&D budget and policy report, since 1976, most of the HHS R&D funds are distributed through NIH
NIH‐supported inventions in university laboratories across the United States are spun out into startup companies to develop and commercialize products that can improve human health. 10 A major portion of the NIH's R&D budget funds basic research (new targets, interventions, and initial animal studies) by supporting investigators working in academic institutions across the United States, but translating basic research to innovations ready for further development and commercialization requires several additional steps. For example, target or lead optimization, initially in animals to estimate efficacy in humans, can be a costly but crucial aspect of drug development, and dedicated funding is needed to enable scientists developing promising innovations to reach critical value inflection points that can attract investors. 11 Because small businesses and startup companies play a key role in advancing such innovations toward commercialization, federal support for these businesses in early stages, before they can generate enough investor interest, is critical. By statute, any federal agency with an annual extramural R&D budget of at least $100 million is required to set aside 3.2% of that budget for the SBIR program. Those agencies whose extramural R&D budgets exceed $1 billion are required to set aside an additional 0.45% for the STTR program. During FY 2020, the NIH invested an estimated $1.187 billion in promoting innovations from small businesses across the United States.
Besides the federal government, sources of funding for late‐stage and commercialization‐oriented development efforts also include state funding, biotechnology companies, pharmaceutical companies, private investors, and nonprofit foundations and advocacy groups, such as the Alzheimer's Drug Discovery Foundation and the Alzheimer's Association. 10 Funding from each of these sources may have advantages and disadvantages for the startup. Advantages of NIH small business funding include the validation that often results from obtaining an award through the NIH peer review and award selection process. In addition, the funding is non‐dilutive in nature; there is no repayment or royalties, and the awardee retains their intellectual property. One limitation of the funding is that the diligent, multi‐step review and selection process takes time—both the time required to develop a competitive application and the time from application to award, which at the National Institute on Aging (NIA) is typically between 5 and 12 months.
RESEARCH IN CONTEXT
Systematic review: The authors performed a literature search using the publicly available National Institutes of Health Report Portfolio Online Reporting Tool database, the commercial database Global Data, and various credible sources. Data were analyzed and tabulated in Microsoft Excel.
Interpretation: Our findings demonstrate that National Institute on Aging federal seed funding is an effective funding resource to enable Alzheimer's disease (AD) startups to bridge financial gaps, enabling them to reach key value inflection points to translate their ideas from bench to bedside. This is consistent with public literature.
Future directions: This article focuses on one method of funding to address the overall fight against AD and AD‐related dementias. We propose additional studies to analyze how collaborative efforts by innovators of all backgrounds could help companies reach key value inflection points, and ultimately, commercial success.
Federal funding is important for small businesses because private funders usually invest in established entrepreneurs or in businesses that are at a mature stage, often after a product's viability has been demonstrated. The government (NIH/NIA), on the other hand, uses small business seed funding programs to invest in small businesses that may or may not have a proven record but are pursuing innovative ideas that could help the community in the future. Federal funding decisions are not solely dependent on commercial track record or a high financial return on investment. For example, small businesses with novel device, diagnostic, or software technologies that address significant unmet needs but lack the data to secure private funding may receive federal funding to demonstrate proof of concept data and reach a key value inflection point.
Additionally, NIH/NIA small business program funds are non‐dilutive, which means they do not affect the small company's stock or shares. Because the funding is not a loan, no repayment is required. Non‐dilutive funding reduces the innovators’ and potential partners’ risk of giving up any equity or ownership but supports data generation for the initial proof of concept. With funding from NIH/NIA, small businesses retain their IP rights. They also can access additional federal resources, such as early market intelligence, entrepreneurial training, business development resources, and—more importantly—a seal of approval for their product from a panel of expert peer reviewers, all of which can facilitate further fundraising efforts. 12 Besides providing funding and other resources, NIA also showcases small business awardees at various industry partnering and investment conferences to help them attract additional funding.
Although small business program funding is needed in a variety of disease areas, it may be most critical in areas that private investors consider to be higher risk. One such area is Alzheimer's disease (AD) and AD‐related dementias (ADRD), including frontotemporal dementia, vascular dementia, and Lewy body dementia. AD and ADRD are progressive brain disorders that result in impaired memory, behavior, thinking, and judgment. In 2011, the National Alzheimer's Project Act (NAPA) was signed into law, calling for an aggressive and coordinated national plan to accelerate research on AD/ADRD, and to provide better clinical care and services for people living with dementia and their families. The national plan, developed in 2012, includes the ambitious goal to prevent and effectively treat AD/ADRD by 2025. 13
The cost of care for AD symptom management is exorbitant: from $215 billion in 2010, it is estimated to have cost society $255 billion in 2020 14 , 15 —and the number of AD/ADRD cases is expected to increase. 16
AD drug development is slow and costly. It takes approximately 13 years and costs an average of $5.6 billion for an AD drug candidate to receive final U.S. Food and Drug Administration (FDA) approval. 17 Because of the high failure rate and associated cost of AD drug development, pharmaceutical companies and private investors typically avoid investing in unproven ideas. Therefore, federal funding is critical to encourage innovative ideas in this field.
In 2013, a G8 summit titled “Tackling gaps in developing life‐changing treatments for dementia” aimed to identify research and innovation gaps that can be strategically targeted to prevent and treat AD/ADRD. The summit identified six innovation gaps 18 :
Understanding the biological processes affected by newly identified genetic risk factors
Understanding neuronal resilience for novel drug development
Drug‐target validation
Selection of appropriate subjects for proof‐of‐concept clinical trials
Drug‐target engagement in humans
Innovative approaches for clinical trials for early detection of AD/ADRD
Based on these recommendations, the NIA has recognized and developed funding opportunities for these innovation gaps that include the development of novel cell and gene therapies, bioinformatics and data science technologies and methods, prevention and therapeutics, and assistive technologies, devices, and mobile applications for the treatment of AD patients.
Following the passing of NAPA, NIH/NIA has seen large increases in congressionally directed appropriations to boost AD/ADRD research, starting in 2015. Funding for AD/ADRD research has surpassed the overall NIA base budget for non‐AD related aging research (Figure 2). With an overall increase in the NIA budget, by statute, the NIA small business program funding budget has also increased. The small business program not only funds small businesses, but also academic investigators who partner with small businesses to translate innovative research ideas into viable commercial products that address aging and aging‐related dementias.
FIGURE 2.
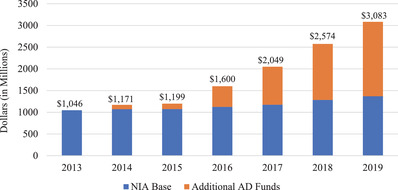
National Institute on Aging (NIA) Appropriations, FY2013‐FY2019. Congressionally appropriated AD/ADRD funds increased NIA's base budget starting in FY2014 and approximately tripled by the end of FY2019, thereby increasing the funding for small business and technology awards via the small business funding mechanisms
At NIA, SBIR and STTR awards for AD/ADRD (through an AD/ADRD‐specific funding opportunity) are funded in phases based on the stage of the proposed research (Table 1).
TABLE 1.
Alzheimer's Disease (AD) and AD‐related diseases small business grant funding phases and maximum award sizes as of fiscal year 2020
| Phase | Duration | Stage | Funding |
|---|---|---|---|
| Phase I | 1 year | Discovery and feasibility | Up to $500,000 |
| Phase II | 2 years | Research and development | Up to $2.5 million |
| Phase IIB | 3 years | Subsequent development (to accelerate commercialization) | Up to $3.0 million |
Phase I supports the demonstration of the proof of concept, while Phase II supports further research and development. Phase IIB awards provide strong support to help businesses generate data to attract private investors for further commercialization, a key value inflection point. The NIA website provides detailed information on specific funding.
Additional institutes at the NIH, such as the National Institute of Neurological Disorders and Stroke (NINDS), also use their small business program to achieve the mission of the NIA by supporting innovative ideas at different stages of development, including applied bench research, translational research, and early‐stage clinical trials. The NIA and NINDS have shared interests in AD/ADRD, with the NINDS as the lead for vascular contributions to cognitive impairment and dementia, Lewy body dementia, and frontotemporal lobar degeneration, and the NIA as the lead for applications focused on Alzheimer's Disease.
In addition to funding innovative research, the NIH/NIA provides non‐funding resources, initiatives, and mentoring programs that help address gaps to increase the reach and impact of the small business programs. These additional programs enhance innovators’ business development knowledge and facilitate access to investors and strategic partners. As a result, in the last decade, small business program‐funded innovators from academia have produced several commercially viable products, including a non‐invasive radioactive tracer for the detection of amyloid plaques, compounds to normalize protein trafficking in AD/ADRD patients, and technologies to improve care of AD patients.
Addressing AD will require approaching the problem from multiple angles, including prevention, treatment, diagnostics, and care management. It will require testing the most innovative approaches across a wide variety of technologies, modalities, and biological targets. It will also require funding efforts from innovators of all backgrounds around the world. In the United States, SBIR and STTR funding can support a broad group of innovators and innovations.
This report evaluates the impact of small business program funding in addressing gaps in the diagnostic, therapeutics, and care intervention areas within the AD/ADRD R&D continuum, and how this funding provides opportunities for small businesses to fill these gaps. It also presents product development case studies in which SBIR funding enabled small businesses to reach key value inflection points that allowed them to transition from initial ideas to commercialization.
2. METHODS
2.1. Data sources
We used the publicly available NIH Research Portfolio Online Reporting Tools (NIH RePORT) to identify all new small business program grants awarded from FY 2008 through FY 2019.
We then filtered the list to include only companies that received Phase II or Commercial Readiness Program (CRP) funding. Receiving Phase II or CRP funding indicates that these companies have met their initial value inflection points. To guide the selection of portfolio companies included in this report, we evaluated success and achievement of value inflection points. We defined success as using small business program funding and/or resources to reach key value inflection points that lead to further investments to advance a technology toward commercialization. Achievement of value inflection points could be demonstrated via regulatory approvals, licensing, investments, sales, and generation of IP. We used the paid commercial databases GlobalData Medical Devices and GlobalData Pharmaceuticals to ascertain the post‐award success of these funded companies. All data were tabulated and analyzed in Microsoft Excel.
We obtained information on NIA's Office of Small Business Research (OSBR) non‐funding and mentoring resources from the OSBR and the NIH Office of Extramural Research's Small Business Education and Entrepreneurial Development (SEED) websites and informational interviews with the coordinators of these programs. To obtain Applicant Assistance Program (AAP) success rate statistics, we acquired a list of companies that were admitted to and completed the program from the OSBR AAP coordinator.
2.2. Research areas
To capture the broad range of the NIA OSBR portfolio, we selected three research areas: care interventions, diagnostics, and therapeutics. We also selected two or three NIA small business program awardees as representatives of the funded research in each area. All information obtained and reported here is publicly available and does not infringe the IP of the investigators and companies.
3. RESULTS
From FY 2008 through FY 2019, the NIA awarded $280 million for AD/ADRD research (Figures 3A and 3B), funding 233 small businesses. Specifically, the NIA funded 613 AD/ADRD‐related small business grants to companies located in 37 states. Since the 2015 increase in the appropriation of funds, the NIA has invested $50 to $70 million per year in funding small businesses’ AD and ADRD research, focusing on innovation, intervention solutions, tools, and technology. Not surprisingly, a major portion of the $280 million investment in the last 11 years—approximately $207 million—was spent between FY 2015 and FY 2019 (Figure 3A), with awards made to companies located in 32 states (Figure 4). From FY 2016 through FY 2019, the NIA funded 182 small businesses that submitted Phase I and Phase II applications in response to 48 different funding opportunity announcements related to AD/ADRD research (Figure 3B). The NIA emphasized supporting companies that had not previously received small business program funding from the Institute. This emphasis is reflected in the proportion of awarded grants: More than 50% of companies awarded between FY 2017 and FY 2019 were receiving their first small business grant from the NIA. In FY 2019 alone, 63% of the small business program recipients were new NIA awardees (Figure 5). This approach enabled the NIA to fund a wider variety of targets and technological solutions. Overall, the NIH small business programs are competitive funding programs. Between 2008 and 2019, the funding rate of Phase I applicants has remained fairly stable at the NIH (Figure 6). The NIH does not release application data at the NIH Institute and Center level, but the additional AD/ADRD funding allowed the NIA to fund additional awards and to fund larger budgets, which were often critical because of the level of risk in AD research and development.
FIGURE 3.
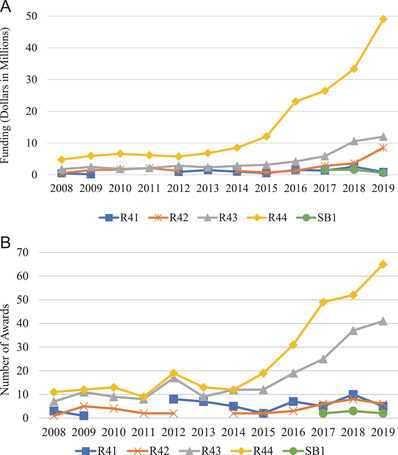
(A) Small business funding, fiscal year (FY) 2008 to FY 2019. Small Business Technology Transfer (STTR) funding for Phase I is under the R41 mechanism, and STTR Phase II is funded under the R42 mechanism. Small Business Innovation Research funding is represented as R43 (Phase I) and R44 (Phase II), and the SB1 mechanism represents grants funded under the Commercial Readiness Program (CRP). (B) The number of small business awards, FY 2008 to FY 2019. STTR funds are shown as R41 (Phase I) and R42 (Phase II); SBIR funds are shown as R43 (Phase I) and R44 (Phase II); CRP funds are shown as SB1
FIGURE 4.
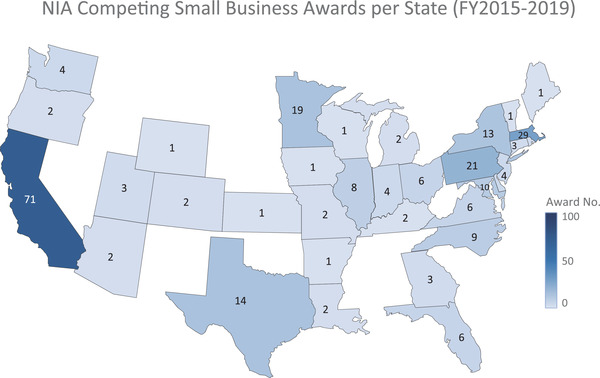
FIGU National Institute on Aging competing small business awards per state from fiscal year (FY) 2015 to FY 2019
FIGURE 5.
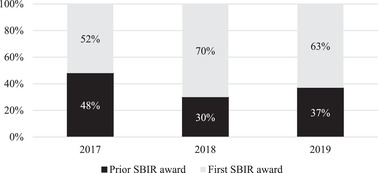
Companies awarded for Alzheimer's disease (AD) and AD‐related diseases research, showing the percentages of companies who received their first versus subsequent Small Business Innovation Research awards during fiscal years 2017 to 2019
FIGURE 6.
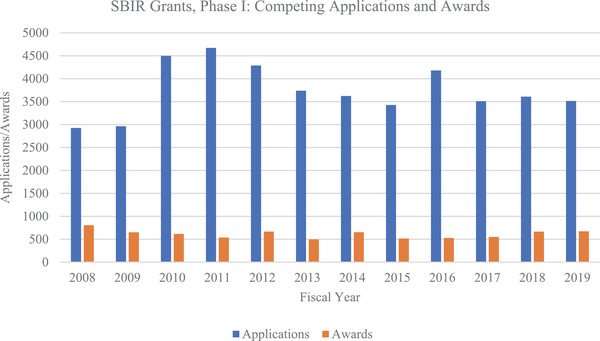
The number of National Institutes of Health Small Business Innovation Research Phase I applications and awards between fiscal year 2008 and 2019
Several small companies that received small business program funding from the NIA for AD/ADRD‐related projects have commercialized their innovations in care interventions, diagnostics, and therapeutics. Many of these companies were spun out of academia, including Avid Radiopharmaceuticals, founded by an investigator from the University of Pennsylvania. Avid developed a radioactive tracer to detect beta‐amyloid (Aβ) plaques via positron emission tomography (PET) scans. Another company, Cognition Therapeutics, was started by an academic investigator from the University of Irvine, California. The company developed a small‐molecule therapeutic targeting AD and other neurocognitive disorders.
In this report, we will showcase a few representative examples to demonstrate how federal funding has enabled innovators from academia and small businesses to reach key value inflection points and develop their ideas from bench to bedside.
3.1. Care interventions
Care interventions are technologies that improve the ability to provide, manage, and enhance care for patients and caregivers.
3.1.1. BioSensics
BioSensics is a biomedical engineering company that develops wearable sensors and digital technologies for clinical trials and health care applications. BioSensics developed IADLSys, an integrated technology platform that enables objective assessment of instrumental activities of daily living, digital social interactions, and life space. With a total of $7.2 million in SBIR funding, BioSensics conducted preliminary studies, developed a prototype, and partnered with Baylor College of Medicine to further assess the platform's efficacy and advance the IADLSys prototype via a Phase I/II clinical trial.
BioSensics has also used small business program funding to develop fall detection technologies. The company used a Phase I/II STTR award from the NIA to refine and validate its fall detection technologies, which resulted in the commercialization of ActivePERS, an advanced medical alert pendant with automatic fall detection, activity monitoring, and non‐compliance alerts. With an additional $3.4 million in NIA small business funding, BioSensics further developed the fall risk feature of ActivePERS. ActivePERS was licensed by and integrated into medical alert devices sold by GreatCall (now part of Best Buy Co., Inc.), Mytrex, and Essence Group, resulting in the technology's widespread use. In August 2019, Best Buy Co., Inc., acquired a portion of BioSensics’ assets. The NIA small business funding was critical in strengthening the credibility of the company's data.
“NIA funding shows that the scientific rationale behind your technology and efforts has been validated,” said Ashkan Vaziri, PhD, the principal investigator (PI) on the small business awards.
Currently, small business program funding is enabling BioSensics to focus on applying its underlying technology to the continuous monitoring of cognitive decline and developing a robust care coordination and management platform for older adults with dementia.
3.1.2. People Power Company
People Power Company produces software and mobile apps to enable caregivers to better care for their families. Its leading product, People Power Caregiver, uses in‐home sensors, artificial intelligence, and cloud services to track the daily activities of ADRD patients and alert their caregivers to any potential hazards. The company conducted significant research in collaboration with the Berkeley Psychophysiology Laboratory at the University of California, Berkeley, with funding from a $4.5 million NIA SBIR Fast Track grant. These funds enabled People Power Company to deliver four improved versions of the product, test its efficacy in a small‐scale clinical trial, and expand into a larger‐scale effectiveness trial to confirm the product's validity. According to Gene Wang, CEO of People Power,
"The NIA SBIR grant enabled People Power to focus on providing important solutions to reduce caregivers’ anxiety and depression while increasing their well‐being."
The success of People Power Caregiver 3.0 led to a collaboration with the hardware company Develco Products, which will further advance the research funded by the NIA small business program, 19 and the acquisition of Tri‐Cura, which provides software to homecare agencies. 20 People Power is now preparing for pilot releases with more than 100 homecare or chronic care management agencies.
3.2. Diagnostics
Innovative diagnostic tools play a crucial role in enabling health care providers to identify and diagnose cognitive decline, which can pay dividends in both prevention of late‐stage disease and clinical development of novel therapeutics.
3.2.1. Avid Radiopharmaceuticals
Avid Radiopharmaceuticals, a spin‐off out of the University of Pennsylvania, developed a diagnostic tool called Amyvid. Amyvid is a radioactive tracer that detects Aβ plaque density via PET scans in patients with cognitive impairment. In the early stages of development—a time when feasibility and proof‐of‐concept studies are critical—the NIA small business program awarded Avid $1.5 million. The funding, awarded between 2007 and 2009, laid the foundation for the research and development of Amyvid, which eventually resulted in it becoming the first FDA‐approved method of directly detecting the pathology of AD to reach the market. Amyvid has been widely used in clinical trials, including a trial for Parkinson's disease.
Avid strategically used the small business program to fund early innovation and development. The funding directly enabled key steps in discovery of molecules and demonstrating key features of the tracers in animal and human research studies. As a small business program awardee, the company had scientific credibility that helped attract $100 million in venture capital funding to further develop and test its technology, as well as a partnership with PETNET Solutions, Inc., that enabled the manufacture of Amyvid. Avid was acquired by Eli Lilly in 2010.
Avid founder Daniel M. Skovronsky, MD, PhD, credits the NIA small business program for the entrepreneurial shift in his career path.
“Initial SBIR support was the most important part, because it's always hard to get a company started,” he said.
Skovronsky believed that small business funds provided him the “confidence and time” to start his company and transition into translation science.
3.2.2. DiamiR, LLC
DiamiR is a molecular diagnostics company that develops minimally invasive, accurate solutions for early detection and monitoring of brain health conditions to enable early intervention. The company's lead product, which is in the late stages of development, is CogniMIR, a test for early detection and prediction of progression of AD and mild cognitive impairment (MCI) based on quantitative analysis of brain‐enriched and inflammation‐associated microRNA biomarkers in plasma. Securing $4.8 million in SBIR awards allowed the company to establish feasibility, conduct larger cross‐sectional and longitudinal studies for validation, and define pre‐analytical and analytical workflows. Successful research outcomes led to a clinical research collaboration with Janssen Pharmaceuticals, additional funding from the Alzheimer's Drug Discovery Foundation, and a CRP award from the NIA small business program.
President and chief scientific officer Samuil Umansky, MD, PhD, DrSci, highlighted the benefits of NIA small business funding:
"This funding strengthens our ability to collaborate with key opinion leaders in the field of neurodegenerative diseases, to conduct larger studies, to further validate our targeted platform technology, and to advance the development of our first test, CogniMIR™, for early, even pre‐symptomatic, detection of MCI and AD with the initial application in clinical trials support." 21
The CRP funding allowed DiamiR to complete the development of CogniMIR and validate it as a Clinical Laboratory Improvement Amendments (CLIA)–compliant lab‐developed test.
3.2.3. C2N Diagnostics
C2N Diagnostics is a diagnostic company that focuses on neurodegeneration. C2N Diagnostics collaborated with the BrightFocus Foundation to develop a blood‐based assay, APTUS‐Aβ, to detect early cases of AD. APTUS‐Aβ measures the levels of two forms of Aβ (42 and 40) to predict levels of plaque formation in the brain. 22 With an initial $225,000 from the NIA small business program, C2N validated its data and moved to Phase II trials to further develop the assay. An additional $1.7 million of NIA small business awards funded trials that demonstrated high diagnostic power. The FDA granted the assay a Breakthrough Device Designation, and the company secured a $20 million investment from the Gerald and Henrietta Foundation to position the assay to enter the clinic. C2N Diagnostics recently received CLIA certification and commercially launched the test as PrecivityAD, a tool to help clinicians diagnose AD. Although it cannot diagnose AD by itself, it has proven effective in aiding the evaluation process. In 686 patients over age 60, the PrecivityAD test correctly identified brain amyloid plaque status in 86% of the patients. 23 PrecivityAD is non‐invasive and radiation free, making it much more accessible to physicians than other diagnostic methods. 23
3.3. Therapeutics
Although diagnostic tools give us effective means for prevention and accurate diagnoses and can aid therapeutics development, therapeutic drug interventions are crucial in the fight against AD.
3.3.1. Cognition Therapeutics
Cognition Therapeutics is a drug discovery and development company that aims to develop a pipeline of disease‐modifying small‐molecule drug therapeutics targeting AD and other neurocognitive disorders. Its lead candidate, CT1812, is a novel first‐in‐class small molecule that has shown potential in initial clinical studies to normalize the protein trafficking and lipid metabolism pathways that are disrupted in AD and to enable the protection and restoration of synapses from Aβ oligomer‐induced neurotoxicity.
Early Investigational New Drug studies, funded by the NIA R01 grant mechanism, provided data to confirm the safety of CT1812, which laid the groundwork for later SBIR‐funded clinical trials. Clinical trial data from these SBIR grants resulted in Fast Track designation from the FDA in 2017 for treatment of AD patients. With a $1 million Fast Track SBIR award, Cognition Therapeutics generated data that demonstrated proof of concept, supported three ongoing Phase II clinical trials, and made the company attractive for outside partnerships. Susan Catalano, PhD, co‐founder, and chief science officer, noted the following:
"NIA support has been critical for CT1812's discovery and development. The commitment of NIA to innovative and diverse approaches to treating this disease is unequalled in the private sector and a critical linchpin in the fight against this devastating disease."
In June 2020, the NIA awarded Cognition Therapeutics $75.8 million over a 5‐year period to support a Phase II study in collaboration with the Alzheimer's Clinical Trials Consortium (ACTC).
3.3.2. Tetra Therapeutics
Tetra Therapeutics is a drug development company that focuses on restoring clarity of thought in patients affected by AD and other conditions that impair cognitive function. Its leading drug candidate, BPN14770, is a novel, small‐molecule drug that is designed to target and modulate PDE4D, a phosphodiesterase enzyme. Mutations in PDE4D result in impaired cognitive function due to an alteration in cAMP signaling. By modulating instead of inhibiting PDE4D, the company hopes to prolong cAMP activity while maintaining safe levels of the enzyme. 24
The NIA small business program kick‐started clinical development by providing Tetra Therapeutics with $1.9 million to fund its Phase I multiple ascending dose study, which led to a cascade of further investments and alliances. 25 After completing its Phase I trials, the company began Phase II clinical trials for BPN14770's applications in AD in 2019.
Mark Gurney, PhD, MBA, chairman and CEO, recognizes the impact small business funds have had on early‐stage research. In March 2020, Tetra formed a strategic alliance with Shionogi & Co., Ltd., to develop and commercialize BPN14770 for the treatment of AD. Shionogi & Co. subsequently acquired Tetra for up to $500 million in upfront fees, regulatory, and commercialization milestones. 26
4. DISCUSSION
The NIA small business funding program enables startups to accelerate translation of their research ideas from bench to bedside by providing the initial non‐dilutive funding to test their hypotheses. With a remarkably diverse portfolio (Figure 7), NIA funds research in various fields, including research tools, therapeutics, sensors, and monitoring technologies. The companies highlighted in this report demonstrate that SBIR funding can help small businesses succeed, allowing them to provide patients and their caregivers products resulting from extensive NIH‐funded AD/ADRD research. SBIR funding has also enabled several academic investigators to spin out startup companies from U.S. universities.
FIGURE 7.
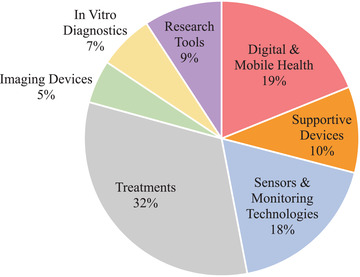
National Institute on Aging overall fiscal year 2019 small business programs’ active portfolio classifications by research area
Limitations of this analysis include the inability to accurately calculate the number of funded companies that originated from U.S. universities or to track the rate of commercialization among NIA SBIR/STTR awardees. These limitations are due to the difficulty of connecting funding for basic and translational research from other NIH funding mechanisms to a small business grant. The underlying research does not always directly result in product development, and personnel changes, mergers, and licensing events often occur between early development and commercialization. Additionally, past evaluation efforts have demonstrated that because SBIR/STTR awards often support early‐stage development, a technology may not be commercialized until several years after SBIR/STTR funding. Since the bulk of NIA's AD/ADRD small business funding has occurred in the years since 2015, it may be premature to quantitatively capture the rate of commercialization. Future efforts at NIA are expected to include evaluation of small business program initiatives and the impact of small business funding. The NIA OSBR ensures that real‐time data collected at the time of award and upon award completion will enable future evaluation efforts.
In 2015, the National Academies of Sciences, Engineering, and Medicine (NASEM) published the results of a comprehensive study of the SBIR program at the NIH. The study, conducted by the Committee on Capitalizing on Science, Technology, and Innovation, investigated how the program has stimulated technological innovation and used small businesses to meet federal research and development needs. 27 The committee's findings and recommendations are based on a complement of quantitative and qualitative tools, including a survey, case studies of award recipients, agency data, public workshops, and agency interviews. The survey, administered in 2014, captured results from 726 NIH Phase II SBIR/STTR awardees. The survey was sent to all PIs in companies that received a Phase II award from NIH between FY 2001 and FY 2010 whose contact information was available. The study demonstrated that SBIR/STTR projects at NIH between 2001 and 2010 were commercialized at a substantial rate. Forty‐nine percent of SBIR and STTR respondents reported some sales or licensing revenues at the time of the survey, and a further 25% expected sales in the future. However, the amount of sales was often small. Of those with some sales, 39% had sales less than $100,000; only 6% had sales over $10 million. The study also demonstrated that SBIR/STTR funding makes a substantial difference in determining project initiation, scope, and timing: 74% of respondents reported that the project probably or definitely would not have proceeded without SBIR/STTR funding. NASEM is currently conducting an updated study of the economic and health care benefits of the SBIR and STTR programs at the NIH and the effectiveness of the strategies the NIH has employed to enhance the programs.
Although small business program funding has played a role in advancing innovations toward commercialization, tremendous unmet needs remain within the AD/ADRD space. A recent review highlighted several pharmacological agents that are currently in clinical trials: disease‐modifying agents, cognitive enhancing agents, and treatments for neuropsychiatric and behavioral symptoms. However, the high rate of failure in AD drug development means that the field needs more R&D to keep moving forward. 28 With the congressionally directed increase in NIA AD/ADRD funding, the small business funding program can have a greater impact in advancing technologies across the space.
Innovators at small companies and academic institutions are working on various translational ideas that could grow into commercialized products. However, some investigators may need help and mentoring to prevent initial failures that keep these innovative ideas from reaching key value inflection points. Because of the endless challenges in the space, the NIA must proactively manage its small business funding program to ensure programmatic success. Thus, in 2018, the NIA launched the OSBR, which identifies gaps that can limit programmatic impact and develops initiatives to fill them.
The NIA OSBR ensures that the funding programs are optimally designed to meet the needs of the innovator community. Recognizing that AD and AD/ADRD research and development is complex and costly, the NIA increased the budget limits on SBIR Phase I and Phase II awards to $500,000 and to $2.5 million, respectively. These limits are significantly higher than the limits for typical NIH SBIR awards. The NIA also recognizes that it is often most efficient for small businesses to outsource certain research activities to Contract Research Organizations and other organizations that possess the specialized equipment and skill sets to conduct certain assays and techniques. The NIA allows small business program applicants to include fee‐for‐service activities as part of the small business’ effort if the small business is conducting specialized experimental design and data analysis activities.
The NIA has also launched or joined several initiatives that address gaps and aim to increase the small business program's reach and impact within the AD space. Because these programs are new and have limited data, future efforts will focus on analyzing their merits.
Applicant Assistance Program (AAP): AAP is an NIH‐wide 10‐week coaching program that helps small businesses prepare Phase I applications for the NIA small business programs at no cost to the applicant. Through one‐on‐one coaching from subject matter experts, the program demystifies the federal grant application process and offers step‐by‐step assistance in completing all components of a Phase I grant application. Subject matter experts also assist with application reviews, preparation, and required registrations. Although all small businesses may apply, the program encourages participation from underrepresented small businesses, especially women‐owned, socially and economically disadvantaged companies, and companies from under‐represented geographic areas, such as the states shown in white in Figure 4.
Innovation‐Corps (I‐Corps) at NIH: The I‐Corps at NIH program, launched in 2014, provides funding, mentoring, and networking opportunities to help investigators develop skills and strategies that will reduce risk during commercialization. Using a systematic, hands‐on approach, teams focus on customer discovery to improve their odds of commercializing. Teams conduct more than 100 customer interviews during the 8‐week program. Between 2017 and 2019, four NIA small business–funded companies completed the program. Longitudinal metrics for the I‐Corps at NIH program demonstrate the substantial impact thus far. A survey of 13 NIH awardees, 3 years after completion of the pilot program, revealed that these companies had secured a total of $78 million in follow‐up funding, eight strategic partnerships, 18 patents, and two spin‐off companies. 29
Concept to Clinic Commercializing Innovation (C3i) Program: The C3i Program is a 24‐week entrepreneurial commercialization assistance training experience. It uses a curriculum established by the Coulter Foundation, which develops distinctive educational programs to accelerate academic innovations to the marketplace. The C3i Program provides investigators with the specialized business frameworks and essential tools that drive translation of medical devices from lab to market. The goal is to engage investigators who want to better understand their innovations’ value and assess their commercial viability and potential business opportunities. Recognizing the importance of this resource for small business program awardees, the NIA joined this effort in 2019 and is now one of six Institutes offering the C3i Program. Four NIA‐supported companies have completed the program.
-
Small Business Education and Entrepreneurial Development (SEED) Resources: Developing products across the biomedical spectrum requires NIH collaboration with universities and research institutions, small businesses, trade associations and societies, angel investors, venture capitalists, and strategic partners. SEED, established in 2019, supports the innovator community through programs that develop these relationships and build opportunities for NIH innovators to further their product development efforts. SEED leads initiatives in three areas: academic innovation, management and enhancement of NIH's small business programs, and innovator support for academic and small business innovators.
The academic innovation team develops and facilitates programs that move innovations from academic laboratories to the marketplace. This includes coordination of NIH's proof‐of‐concept center consortium, which enables academic innovators to validate the potential health impacts of promising scientific discoveries and advance them into health care products and services. The small business programs team works with the NIH Institutes and Centers to implement strategic approaches and policies to enhance the return on the NIH small business funding programs and serve as a central source of information on the NIH's small business programs and resources. The innovator support team delivers product development guidance from industry veterans to NIH's academic and small business innovators, provides entrepreneurial training to NIH awardees and program staff, and facilitates strategic alliances between federal and private‐sector stakeholders. SEED work in each area enhances the NIA's efforts to facilitate the commercialization of products to advance prevention, treatment, diagnosis, management, and caregiving for AD/ADRD.
-
Outreach and Stakeholder Engagement: Since the FY 2015 increase in small business funding for AD/ADRD‐focused projects, the NIA has dramatically expanded its outreach efforts to increase the quantity and quality of applications. The number of attendees at NIA SBIR‐focused outreach events increased more than eightfold between FY 2018 and FY 2019, from approximately 230 attendees in FY 2018 to approximately 2,000 in FY 2019. This increase has helped support a sustained increase in the number of AD/ADRD applications. The increase in outreach and guidance available to applicants may have contributed to a significantly improved median score in FY 2020 compared to previous years.
The NIA has conducted outreach to both the general applicant community and stakeholder organizations through in‐person and virtual workshops. One key example of the reach obtained through NIA's outreach efforts is the J.P. Morgan Healthcare Conference and relevant co‐located meetings in January 2020. NIA OSBR representatives presented at three different conferences during the conference week and met with more than 200 potential applicants. The NIA OSBR not only makes presentations at several domain‐relevant conferences, it also hosts sessions designed to meet the needs of the applicant and awardee community. In 2020, the NIA hosted virtual workshops on the goals, structure, and application specifics of later stage funding opportunities that can help awardees meet their value inflection points. For example, in July 2020, 529 participants from 49 states and territories attended a virtual workshop on preparing to submit CRP and Phase IIB applications, and 29% of the attendees were from underrepresented small businesses. The NIA's ability to reach such broad audiences creates an opportunity for future virtual workshops, held on a monthly or bimonthly basis, that can provide guidance to potential applicants and training to awardees on relevant business development topics.
In addition to direct outreach, stakeholder engagement has also been critical in expanding the reach of NIA's small business programs. Expanded presence at AD‐relevant innovator conferences, such as the Alzheimer's Association International Conference® and relevant pre‐conferences, Sachs Neuroscience Innovation Forum, Longevity Venture Summit, and the Alzheimer's Drug Discovery Foundation's (ADDF's) International Conference on Alzheimer's Drug Discovery, has raised awareness of the programs in the community and helped raise the number of new applicants and awardees each year. OSBR also teams up with nonprofit trade associations, such as Alzheimer's Association Business Consortium, ADDF, and the Rainwater Charitable Foundation's Tau Consortium, and several state Biotechnology Innovation Organization associations and regional innovation organizations to create a wider network and help our applicants gain access to additional capital, potential partners, and other business services. This comprehensive approach to outreach, stakeholder engagement, and innovator support is critical to the sustained efforts to enhance the impact of NIA's small business programs.
Commercialization Readiness Pilot (CRP) Program: The recently released CRP funding opportunities aim to facilitate the transition of previously funded small business Phase II and Phase IIB projects to the commercialization stage by providing support for technical assistance not typically supported through small business grants. The CRP program can support critical technical assistance and late‐stage R&D activities that are essential to bring innovations to the marketplace. Since 2016, the NIA has used the first NIH iteration of the CRP to award six companies a total of $11 million to cover these costs. A second iteration of the CRP, released in 2019, enables companies to secure both a CRP and a Phase IIB. This increased support is critical in research areas with high commercial risk, such as AD/ADRD.
5. CONCLUSIONS
The fight against AD/ADRD has been and remains a challenge. With the future projected increase in the AD/ADRD population, associated high cost of care, and high failure rate of AD/ADRD drug development, it is crucial to invest capital for the early‐stage and often high‐risk development of innovations that can substantially improve our ability to prevent, diagnose, treat, and manage AD/ADRD.
As the world's foremost medical experts seek more efficient prevention, treatment, and care management tools, they will inevitably encounter financial difficulties that will result in promising technologies remaining on a lab bench instead of entering the commercial market. Addressing AD/ADRD will require a collaborative effort by innovators of all backgrounds. The NIA small business funding program is just one of the valuable resources companies can use—and have successfully used—to bridge financial gaps to reach the value inflection points that are critical in securing downstream private investments, developing partnerships, and launching their innovations to market. Since the FY 2015 increase in the congressionally directed appropriation for AD/ADRD research, the small business program at the NIA has brought several innovations for early detection, therapeutics, and care interventions toward the market relatively quickly. The NIA strives to continue these efforts by optimizing our funding programs and enhancing our entrepreneurial training, networking, and outreach initiatives. Our goal is to ensure that no innovation that could lead to AD/ADRD prevention or treatment falls short because of a lack of early‐stage product development funding and support that the NIA small business program could provide.
DECLARATIONS OF INTEREST
None.
ACKNOWLEDGMENTS
Amy Schneider of Palladian Partners, Inc., for manuscript editorial support.
Ghazarian AL, Haim T, Sauma S, Katiyar P. National Institute on Aging seed funding enables Alzheimer's disease startups to reach key value inflection points. Alzheimer's Dement. 2022;18:348–359. 10.1002/alz.12392
REFERENCES
- 1. Lerner J, Gompers PA. The money of invention: how venture capital creates new wealth. Ubiquity. https://ubiquity.acm.org/article.cfm?id=763904. Accessed January, 2002. [Google Scholar]
- 2. Hall S, Wood AJJ. Financial Growing Pains of a Biotech. The Scientist. https://www.the‐scientist.com/uncategorized/financial‐growing‐pains‐of‐a‐biotech‐44936. Accessed August, 2008. [Google Scholar]
- 3. SBIR‐STTR: America's Seed Fund . The SBIR and STTR Programs. https://www.sbir.gov/about/about‐sbir#sbir‐mission.
- 4. U.S. Small Business Administration , Performance benchmark requirements for phase I. SBIR‐STTR: America's Seed Fund. https://www.sbir.gov/performance‐benchmarks.
- 5. Board NS. National Science Board: Science & Engineering Indicators 2018. Recent Trends in Federal support for U.S. R&D. https://www.nsf.gov/statistics/2018/nsb20181/report/sections/research‐and‐development‐u‐s‐trends‐and‐international‐comparisons/recent‐trends‐in‐federal‐support‐for‐u‐s‐r‐d#distribution‐of‐federal‐funding‐for‐research‐by‐s‐e‐fields.
- 6. American Association for the Advancement of Science. Historical Trends in Federal R&D. https://www.aaas.org/programs/r‐d‐budget‐and‐policy/historical‐trends‐federal‐rd.
- 7. Jefferson RS, How the largest public funder of biomedical research in the world spends your money. Forbes. https://www.forbes.com/sites/robinseatonjefferson/2018/12/21/how‐the‐largest‐public‐funder‐of‐biomedical‐research‐in‐the‐world‐spends‐your‐money/#50e5936327b9. Accessed December 21, 2018.
- 8. Onken J, Miklos AC, Dorsey TF, Aragon R, Calcagno AM. Using database linkages to measure innovation, commercialization, and survival of small businesses. Eval Program Plann. 2019;77:101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Packalen M, Bhattacharya J. NIH funding and the pursuit of edge science. Proc Natl Acad Sci U S A. 2020;117(22):12011‐12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummings J, Reiber C, Kumar P. The price of progress: funding and financing Alzheimer's disease drug development. Alzheimers Dement (N Y). 2018;4:330‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabbagh JJ, Kinney JW, Cummings JL. Animal systems in the development of treatments for Alzheimer's disease: challenges, methods, and implications. Neurobiol Aging. 2013;34(1):169‐183. [DOI] [PubMed] [Google Scholar]
- 12. Ben‐Menachem G, Ferguson SM, Balakrishnan K. Doing business with the NIH. Nat Biotechnol. 2006;24(1):17‐20. [PMC free article] [PubMed] [Google Scholar]
- 13. National Alzheimer's Project Act . National Plan to Address Alzheimer's Disease. https://aspe.hhs.gov/national‐plans‐address‐alzheimers‐disease. Accessed May 15, 2012.
- 14. Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing Alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):189‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010‐2050) estimated using the 2010 census. Neurology. 2013;80(19):1778‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott TJ, O'Connor AC, Link AN, Beaulieu TJ. Economic analysis of opportunities to accelerate Alzheimer's disease research and development. Ann N Y Acad Sci. 2014;1313:17‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mauricio R, Benn C, Davis J, et al. Tackling gaps in developing life‐changing treatments for dementia. Alzheimers Dement (N Y). 2019;5:241‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. PRWeb Cision . Breakthrough dementia homecare solution available today at no cost, funded by National Institutes of Health. http://www.prweb.com/releases/breakthrough_dementia_homecare_solution_available_today_at_no_cost_funded_by_national_institutes_of_health/prweb16955794.htm. Accessed March 9, 2020.
- 20. Mesa A. GlobalNewsWire. Iveda and People Power announce IoT collaboration for senior care. https://www.globenewswire.com/news‐release/2019/12/23/1964001/0/en/Iveda‐and‐People‐Power‐Announce‐IoT‐Collaboration‐for‐Senior‐Care.htmlhttps://www.globenewswire.com/news‐release/2019/12/23/1964001/0/en/Iveda‐and‐People‐Power‐Announce‐IoT‐Collaboration‐for‐Senior‐Care.html. Accessed December 23, 2019.
- 21. Sheinerman K. DiamiR announces $1.5M SBIR Phase II funding for further evaluation of the microRNA biomarkers for early detection of Alzheimer's disease. GlobalNewsWire. https://www.globenewswire.com/news‐release/2015/03/16/1078510/0/en/DiamiR‐Announces‐1‐5M‐SBIR‐Phase‐II‐Funding‐for‐Further‐Evaluation‐of‐the‐microRNA‐Biomarkers‐for‐Early‐Detection‐of‐Alzheimer‐s‐Disease.html. Accessed March 16, 2015.
- 22. C2N Diagnostics. APTUS™‐Aβ blood test for Alzheimer's disease. https://www.c2ndiagnostics.com/products/home.
- 23. Diagnostics CN. C2N Diagnostics. Alzheimer's breakthrough: C2N first to offer a widely accessible blood test. https://www.c2ndiagnostics.com/press/press/2020/10/28/alzheimers‐breakthrough‐cn‐first‐to‐offer‐a‐widely‐accessible‐blood‐test. Accessed October 29, 2020.
- 24. Ricciardi L. Cognition Therapeutics. Cognition Therapeutics receives $75.8 million NIA grant for 540‐patient Phase 2 study of CT1812 in collaboration with the Alzheimer's Clinical Trials Consortium. https://cogrx.com/cognition‐receives‐nia‐grant‐for‐actc‐study/. Accessed June 8, 2020.
- 25. Bloomberg . Tetra Therapeutics and Shionogi announce expanded alliance. https://www.bloomberg.com/press‐releases/2020‐03‐06/tetra‐therapeutics‐and‐shionogi‐announce‐expanded‐alliance. Accessed March 6, 2020.
- 26. Tetra Therapeutics . What is the science behind BPN14770? https://tetratherapeutics.com/science/our‐focus/.
- 27. National Academies of Sciences, Engineering, and Medicine . SBIR/STTR at the National Institutes of Health. Washington, DC: The National Academies Press; 2015. 10.17226/21811 [DOI] [Google Scholar]
- 28. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (N Y). 2019;5:272‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Canaria CA, Portilla L, Weingarten M. I‐Corps at NIH: entrepreneurial training program creating successful small businesses. Clin Transl Sci. 2019;12(4):324‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]


