Abstract
Adult stem cells are fundamental to maintain tissue homeostasis, growth, and regeneration. They reside in specialized environments called niches. Following activating signals, they proliferate and differentiate into functional cells that are able to preserve tissue physiology, either to guarantee normal turnover or to counteract tissue damage caused by injury or disease. Multiple interactions occur within the niche between stem cell‐intrinsic factors, supporting cells, the extracellular matrix, and signaling pathways. Altogether, these interactions govern cell fate, preserving the stem cell pool, and regulating stem cell proliferation and differentiation. Based on their response to body needs, tissues can be largely classified into three main categories: tissues that even in normal conditions are characterized by an impressive turnover to replace rapidly exhausting cells (blood, epidermis, or intestinal epithelium); tissues that normally require only a basal cell replacement, though able to efficiently respond to increased tissue needs, injury, or disease (skeletal muscle); tissues that are equipped with less powerful stem cell niches, whose repairing ability is not able to overcome severe damage (heart or nervous tissue). The purpose of this review is to describe the main characteristics of stem cell niches in these different tissues, highlighting the various components influencing stem cell activity. Although much has been done, more work is needed to further increase our knowledge of niche interactions. This would be important not only to shed light on this fundamental chapter of human physiology but also to help the development of cell‐based strategies for clinical therapeutic applications, especially when other approaches fail.
Keywords: bone marrow, central nervous system, heart, skeletal muscle, skin, stem cell niches, tissue homeostasis
Adult stem cells reside in specialized environments called niches, where they may be kept quiescent or differentiate into functional cells preserving the tissue homeostasis. This review describes the main characteristics of stem cell niches in different tissues, highlighting the various components that influence stem cell activity. Improving our knowledge of niche interactions is important in the field of human physiology, which would also help to develop cell‐based strategies for clinical therapeutic applications.
1. INTRODUCTION
In postnatal life, tissue homeostasis mostly depends on the presence, self‐renewal, and differentiation potential of resident adult stem cells. Although they may remain quiescent indefinitely, under appropriate conditions they can evolve into specialized cells, to replace exhausted or dead cells (Fuchs & Blau, 2020; Montagnani et al., 2016). In mammals, adult stem cells have been identified and characterized in virtually all tissues, although some tissues are equipped with more efficient stem cell niches than others. Based on their response to body needs, tissues can be largely classified into three main categories: tissues that even in normal conditions are characterized by an impressive turnover, to replace rapidly exhausting cells (blood, skin epidermis, or intestinal epithelium); tissues that normally require only a basal cell replacement, though able to efficiently respond to increased needs, injury, or disease (skeletal muscle); tissues that are equipped with less powerful stem cell niches, whose repairing ability is unable to overcome severe damage (heart or nervous tissue). It is not a coincidence that heart and brain damage are the two most common causes of death worldwide.
In the first steps of embryogenesis, epiblast pluripotent stem cells generate the germ lineage and the three embryonic germ layers (ecto‐, meso‐, and endo‐derm). In each germ layer, tissue differentiation then evolves following a combination of transcription factors (Slack, 2008). However, a number of cells in each tissue would evade the instructions of early inducing signals, maintaining some features of their embryonic origin. Depending on their location, in the adult organism, these stem cells would normally produce elements of the tissue‐specific lineage, such as blood cells, epidermal cells, or neural cells.
Adult stem cells reside in specific anatomical locations called “niches” in which they are protected from external injury and damaging stimuli. First postulated by Schoefield in 1978 (Schofield, 1978), stem cell niches have been extensively explored and characterized. The niche is a specialized microenvironment where cell‐intrinsic regulatory mechanisms interact with extrinsic signals to determine stem cell fate. These interactions include cell‐to‐cell communication and cell–matrix interactions, which are influenced by external signals (Ferraro et al., 2010). As a result, stem cells can be kept in a quiescent state, induced to proliferate, or prompted to differentiate. Stem cell divisions may occur by symmetric or asymmetric division, mainly depending on the mitotic spindle orientation and microenvironment cues (Betschinger & Knoblich, 2004; Yamashita & Fuller, 2005). By symmetric divisions, both daughter cells encounter the same niche environment, and two identical stem cells are normally produced; this would increase the pool size, for example, during tissue expansion or in response to injury. By asymmetric divisions, when the mitotic spindle is perpendicular to the niche edges, one daughter cell normally stays inside the niche and inherits stem cell features, whereas the other cell moves away starting to differentiate into a tissue‐committed progenitor.
The main niche components are (A) Stromal support cells, which are normally located in close proximity to stem cells (i.e., osteoblasts in bone marrow [BM] or fibroblast in the skin), normally act through short‐range signals, which include secretion of soluble factors or membrane adhesion molecules; (B) Blood vessels, which are regularly in close contact with the niches, provide nutritional support, convey long‐range signals from other sources, and allow cell trafficking from and toward the niche; (C) Neural inputs, which integrate signals from different sources and often contribute to stem cell mobilization; and (D) Extracellular matrix (ECM) proteins, which provide mechanical support and biochemical signals (Assis‐Ribas et al., 2018). The ECM acts as a scaffolding system in which stem cells and stromal cells are closely embedded, interacting with each other and with external/internal molecular signals. The ECM can be structured in a two‐dimensional configuration (in the gut or in the skeletal muscle), or in a three‐dimensional arrangement, as in BM or in neural niches. It plays multiple roles, such as organizing stem cell positioning, governing chemical signals, and creating gradients that guide stem cell fate. Stem cells interact with ECM components through different cell surface receptors, such as integrins and cadherins, which, in addition to the adhesive function, are involved in external signal transduction to regulate stem cell processes.
Multiple signaling pathways are balanced inside niches. Although playing different roles, some of them are crucially involved in various tissues. Besides its involvement in embryonic development, Notch signaling is important in adult tissues to define stem cell features (Azizidoost et al., 2015). Other regulatory pathways include Wnt/β‐catenin (Clevers et al., 2014), bone morphogenetic protein (BMP), angiopoietin‐1, and several growth factors, such as fibroblast growth factor (FGF), insulin growth factor (IGF), vascular endothelial growth factor (VEGF), transforming growth factor (TGF)‐α, and platelet‐derived growth factor (PDGF) (Ferraro et al., 2010).
2. BONE MARROW
Studies on BM stem cells began more than 50 years ago when the first population of adult stem cells was discovered (Friedenstein et al., 1968). These cells were named hematopoietic stem cells (HSCs) because of their ability to form all blood cells. A second population called BM stromal cells (BMSCs) was identified a few years later (Friedenstein et al., 1970). BMSCs consist of a mixed population of cells, which, besides supporting HSC activity (Sacchetti et al., 2007), also feature self‐renewal ability, high proliferative potential, and the capacity to differentiate into mesodermal‐derived elements, such as chondrocytes, osteoblasts, and adipocytes (Kumar et al., 2018). For this reason, BMSCs were considered mesenchymal stem cells (MSCs) (Prockop, 1997).
HSCs are fundamental for blood homeostasis (Eaves, 2015; Seita & Weissman, 2010), being able to give rise to all functional blood cells. Moreover, by their self‐renewal ability, they also produce other undifferentiated HSCs, thus maintaining a constant pool. Originally generated from hemogenic endothelium in the ventral wall of the embryonic dorsal aorta (Drevon & Jaffredo, 2014; Ivanovs et al., 2014; Tavian et al., 2005), in adult mammals HSCs are predominantly located in BM niches (Figure 1).
Figure 1.
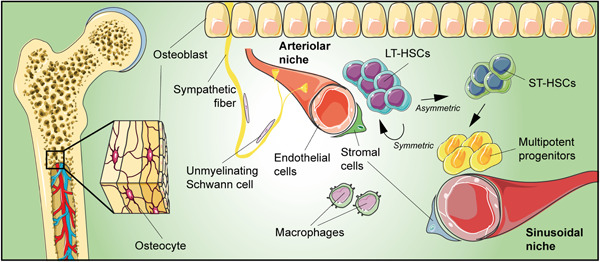
Schematic drawing of bone marrow niche summarizing its main components. Upon activation, long‐term hematopoietic stem cells (LT‐HSCs) divide asymmetrically, producing short‐term HSCs (ST‐HSCs) that give rise to multipotent progenitors that are able to ensure normal hematopoiesis for up to 3–4 months.
Two kinds of HSCs have been identified: long‐term HSCs (LT‐HSCs) and short‐term HSCs (ST‐HSCs) (Kosan & Godmann, 2016; Liu et al., 2012; Suda et al., 2011). Most LT‐HSCs are slow‐cycling or in a quiescent G0 phase, whereas ST‐HSCs are characterized by rapid cell‐cycle entry following mitogenic stimulation (Laurenti et al., 2015; Miftakhova et al., 2015). Upon activation, LT‐HSCs produce new LT‐HSCs and ST‐HSCs through asymmetric cell division. ST‐HSCs, in turn, give rise to multipotent progenitors that are able to ensure normal hematopoiesis for up to 3–4 months (Eaves, 2015; Kosan & Godmann, 2016; Liu et al., 2012; Seita & Weissman, 2010; Suda et al., 2011). ST‐HSCs and multipotent progenitor cells are still able to differentiate into all hematopoietic lineages, but they lose their self‐renewal capacity. HSCs can return to dormancy when homeostasis is restored, thus indicating that they can reversibly switch from dormancy to self‐renewal, according to hematopoietic needs (Wilson et al., 2008).
BM niches are the most extensively studied (Lutolf & Blau, 2009; Yu & Scadden, 2016). BM niche cells include osteoblasts, BMSCs, endothelial cells, macrophages, megakaryocytes, pericytes, and adipocytes (Kunisaki, 2019; Sanchez‐Aguilera & Mendez‐Ferrer, 2017). Niche cells are in close contact with the ECM, which consists of collagens, proteoglycans, and glycoproteins. ECM not only provides mechanical support for cell adhesion but also plays a crucial role in the interplay of growth factors and cytokines (D. Verma et al., 2020; Zanetti & Krause, 2020).
The heterogeneous population of BMSCs is fundamental, most of them secrete HSC‐supporting factors, such as C‐X‐C motif chemokine ligand 12 (CXCL12), angiopoietin, and stem cell factor (SCF) (Asada et al., 2017). Chemokine CXCL12 induces HSC quiescence and retention (Sugiyama et al., 2006), while its depletion or an impaired coupling of CXCL12 with its receptor CXCR4 produces significant reductions in HSC number and enhances their mobilization to the peripheral circulation. Activation of angiopoietin 1/Tie2 signaling inhibits HSC proliferation, promoting long‐term activity (Seita & Weissman, 2010). The interaction between Tie2‐expressing HSCs and angiopoietin 1–expressing stromal cells enhances the adhesion of HSCs to osteoblasts, favoring HSC quiescence (Mendelson & Frenette, 2014; Méndez‐Ferrer et al., 2010; Putnam, 2014). Similarly, the importance of CXCL12‐abundant reticular (CAR) cells, nestin‐expressing cells, and leptin receptor‐positive cells is demonstrated by the significant HSC reduction upon their deletion (Kosan & Godmann, 2016; Mendelson & Frenette, 2014; Méndez‐Ferrer et al., 2010; Sacchetti et al., 2007). According to their MSC nature, these cells can differentiate into osteoblasts and adipocytes (Sugiyama & Nagasawa, 2012). Macrophages also support HSC retention by inducing CXCL12 secretion; their depletion was found responsible for the marked reduction of endosteal osteoblasts, thus promoting HSC mobilization in the bloodstream. Endothelial cells secrete growth factors, cytokines, and adhesion molecules; sinusoidal endothelial cells express Notch ligands to promote HSC expansion (Butler et al., 2010). By activating Notch receptors, Jagged and Delta‐like Notch ligands increase stem cell numbers and self‐renewal. Especially after injury, the Notch signaling pathway promotes proliferation of short‐ and long‐term progenitors until homeostasis is reached (Azizidoost et al., 2015; Gomez‐Gaviro et al., 2012).
TGF‐β, secreted by nonmyelinating Schwann cells surrounding sympathetic nerves, induces the quiescence and contributes to HSC maintenance and self‐renewal. Neutralization of the TGF‐β/Smad signaling pathway induces the exit of hematopoietic progenitor cells from quiescence.
On the contrary, the mobilization of HSCs into the bloodstream has been associated with the circadian release of norepinephrine from sympathetic nerve terminals (Mendez‐Ferrer et al., 2010). Activation of β2‐ and β3‐adrenergic receptors produces a rhythmic downregulation of HSC maintenance genes, namely CXCL12, causing rhythmic HSC release. Granulocyte colony‐stimulating factor (G‐CSF) promotes HSC proliferation and mobilization in the bloodstream, likely increasing norepinephrine release from sympathetic fibers (Kunisaki et al., 2013). Parathormone administration increases HSCs and osteoblast numbers by stimulating osteoblastic differentiation from nestin‐positive MSCs. In fact, the proliferative fraction of these cells was found significantly greater after both chemical sympathectomy and parathormone administration.
Numerous investigations have been aimed at identifying the exact localization of BM niches. As in other tissues, the proximity to the vasculature appears of crucial importance (Putnam, 2014). Blood enters BM through arterioles in the endosteal region and reaches typical large‐diameter sinusoids in the marrow cavity. On this anatomical basis, two niche locations have been proposed, the endosteal and the central BM niche, each characterized by different blood vessels and cell types (Miftakhova et al., 2015; Sugiyama & Nagasawa, 2012). The endosteal niche, characterized by arterioles and osteoblasts, has been suggested as a preferential place for quiescent HSCs; the central BM niche, mainly associated with sinusoidal endothelial cells, pericytes, and stromal cells, would instead promote ST‐HSC differentiation and proliferation. This hypothesis was supported by data obtained using imaging techniques, exploring the localization of quiescent and nonquiescent HSCs in relation to arterioles, sinusoids, and osteoblasts (Kunisaki & Frenette, 2014; Kunisaki et al., 2013). These results would indicate that two distinct perivascular niches, quiescent or proliferative, are associated with arterioles and sinusoids, respectively. Because of their fenestrations, sinusoids are better suited for cell migration into the bloodstream. Partially discordant data, however, emerge from other studies, still carried out by using imaging techniques (Acar et al., 2015). According to these authors, most HSCs (both dividing and nondividing) would reside in perisinusoidal niches, diffusely distributed throughout BM. The hypoxic microenvironment around sinusoids is suitable for HSC metabolism (Spencer et al., 2014), mainly relying on cytoplasmic glycolysis rather than mitochondrial oxidative phosphorylation (Kocabas et al., 2015). Finally, it is important to point out that the close spatial relationship between BMSCs and HSCs is indicative of a unique BM niche in which the balance of these two types of stem cells can be governed by signaling pathways, as well as hormonal and nervous influences.
3. SKIN
The skin is the largest organ of the human body and covers multiple functions. It provides resistance to mechanical insults, prevents water loss, and is crucial for the regulation of body temperature. It also contributes to immune surveillance, acting as a barrier to microbial infection (Basler & Brandner, 2017; Hsu et al., 2014; Xie & Zhou, 2017).
The skin consists of three main layers (Figure 2). The epidermis is the outermost layer, further divisible into four strata (basal, spinosum, granulosum, and corneum). It also produces multiple appendages, such as hair follicles (HFs), sebaceous glands, sweat glands, and nails (Blanpain & Fuchs, 2006; Veltri et al., 2018). The epidermis and its appendages are continuously subjected to insults from the external environment. In fact, superficial cells incessantly flake off and are continuously replaced by new ones moving outward from inner layers. Indeed, only stem cells in the basal layer are proliferative. They unceasingly replenish the basal layer and, after detachment from the basal lamina, stop proliferating and, moving upward, progressively differentiate into cells of the spinous, granular, and corneum layers. In the last step, they undergo a programmed cell death known as cornification, losing their organelles and even their nucleus, and become rich in insoluble bundles of keratin filaments. For this reason, epidermal cells are also called keratinocytes. At the end of this process, they build up a formidable physical barrier, resistant but flexible, indispensable for protecting the internal environment (Hardman et al., 1998; Veniaminova et al., 2019).
Figure 2.
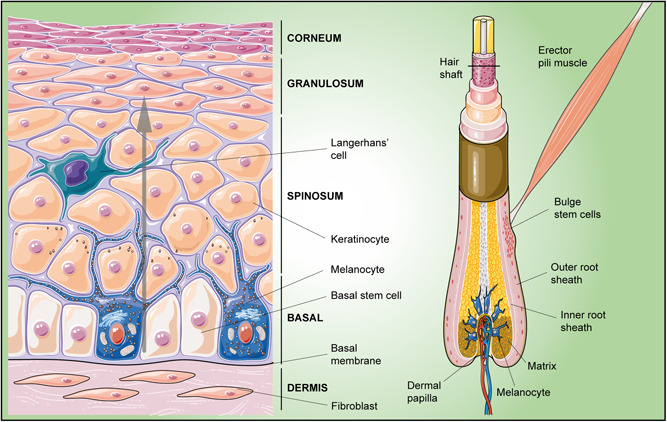
Schematic drawing of skin epidermal niches. Left: Interfollicular epidermis, the epidermal region located between hair follicles; basal stem cells continuously produce daughter cells, which moving upward (gray arrow), progressively differentiate into suprabasal layer cells. Right: During the hair cycle, primed bulge stem cells migrate along the outer root sheath of the hair shaft toward the dermal papilla within a cluster of cells called the matrix; here they proliferate and differentiate to produce the hair shaft and the inner root sheath
In the adult, the epithelium is composed of several building blocks, each consisting of a pilosebaceous unit (HF and sebaceous gland) and the interfollicular epidermis (IFE), the epidermal region located between HFs. Stem cells are present both in the IFE and in specific niches within HFs (Schepeler et al., 2014). In the IFE, they are mostly organized as proliferative units at the base of rete ridges, the deepest and most protected region of the epidermis, just above the basement membrane, rich in ECM and growth factors. The basement membrane is likely secreted by both basal keratinocytes and underlying fibroblasts. Adhesion of basal progenitors to the basement membrane occurs through integrins, mainly α3β1 and α6β4. Basal integrins and apical‐lateral adherens junctions with surrounding cells define the progenitor cell polarity (Muroyama & Lechler, 2012). By asymmetric divisions, basal stem cells generate a basal progenitor cell and a daughter cell that will differentiate into suprabasal cells while migrating upward. In this way, the number of progenitor cells is kept constant as well as the number of the other suprabasal epidermal cells: lost cells of the superficial layer are exactly replaced by newly generated ones (Lechler & Fuchs, 2005).
Whereas basal cells in the IFE are characterized by continuous proliferation, the HFs go through cycles of degeneration and regeneration. Stem cells are located in a specific region at the insertion site of the erector pili muscle, called the bulge. Unlike IFE, where unipotent progenitors are responsible for tissue homeostasis (Ito et al., 2005), bulge stem cells feature the multipotent ability to generate any of the three lineages of the skin epithelium (IFE, HFs, and sebaceous glands). In fact, the bulge also represents a reservoir of multipotent stem cells that can be mobilized in response to injury (Ji et al., 2017).
Located in the outmost layer at the base of the bulge niche, stem cells supply the sequential stages of the hair cycle: active hair growth (anagen), quiescence (telogen), and destruction (catagen). Two populations of stem cells were identified: a quiescent slow‐cycling population and a population of primed stem cells, with a higher propensity to proliferate (Chen et al., 2020). Upon activation, primed stem cells migrate along the outer root sheath of the hair shaft (Fuchs, 2018) toward the dermal papilla, within a cluster of cells below the bulge called the matrix. Here they evolve into TA cells that proliferate and differentiate to produce the hair shaft and its inner root sheath. In the progression from early to late telogen, dermal papilla levels of activating factors (FGF‐7, FGF‐10, TGF‐β2, and the BMP inhibitor noggin) increase, whereas BMP4 levels in dermal fibroblasts and BMP2 in mature adipocytes decrease, leading to HF stem cell activation (Yan et al., 2019).
The balance between the two main counteracting signaling pathways, BMP and Wnt/β‐catenin, are mainly involved in HF dynamic events. Whereas BMP induces a quiescent condition, Wnt signaling promotes cell proliferation and differentiation during hair growth (Chen et al., 2020; Ji et al., 2017; Myung et al., 2013). During anagen onset, the follicular epithelium is responsible for the secretion of Wnt ligands, whereas both HF epithelial and mesenchymal components contribute at later stages. BMPs are mainly secreted by dermal fibroblasts and subcutaneous adipocytes, as well as by cells within the bulge.
Other pathways are, however, involved. For example, dermal papilla‐derived TGF‐β2 exerts a positive effect on HF stem cell activation (Oshimori & Fuchs, 2012). Nerve‐derived hedgehog signaling would be relevant for hair bulge maintenance. In HFs, the Notch signaling pathway is required for an appropriate hair tissue differentiation by suppressing epidermal fate. In the IFE, Notch signaling promotes the main processes of epidermal stratification: basal cell proliferation, their detachment from the basal membrane, and their differentiation into spinous layer cells as they move upwards. A disrupted Notch signaling results in reduced basal cell proliferation and suppression of spinous layer formation.
In cases of a skin wound, stem cells rapidly respond to repair tissue damage and restore the disrupted barrier (Gonzales & Fuchs, 2017; Hsu et al., 2014). The process begins with the initial dermal contraction and platelet aggregation to form a temporary barrier. Various leukocyte lineages participate in this phase, providing immune protection against infections. Moreover, by releasing growth factors and cytokines, they promote new blood vessel formation and the local migration/proliferation of epidermal keratinocytes, which start to re‐epithelialize the wound area. Eventually, the underlying dermis and ECM are restored. Although stem cells from the HFs may contribute, the IFE plays the most important role. In fact, skin repair normally occurs also in hairless skin regions.
4. GUT
Intestinal stem cell (ISC) niches probably carry out the heaviest renewal work in the entire organism, being able to replace most of the intestinal epithelial cells in about 5 days. When activated, ISCs can provide a rapid and efficient turnover (Jasper, 2020) to ensure that a single thin layer of epithelial cells may efficiently fulfill its absorption and protective roles (Pentinmikko & Katajisto, 2020).
In the small intestine, the epithelium is organized in luminal protrusions (villi) and deep invaginations (crypts of Lieberkühn), which constitute an autonomous unit, the crypt‐villus (Figure 3). Here, different cell types carry out either absorption mechanisms (enterocytes) or secretory activities (goblet cells, Paneth cells, enteroendocrine cells, and tuft cells). Numerous investigations indicate that ISCs are positioned at the base of the crypts where they interact with the ECM and neighboring cells, such as fibroblasts, myofibroblasts, endothelial cells, pericytes, and immune cells. In the niche, ISCs may divide symmetrically, producing daughter stem cells (Snippert et al., 2010), or asymmetrically, giving rise to transit‐amplifying (TA) cells (Schellenberg, 1996) that account for about two‐thirds of the cells at the base of the crypt. TA cells proliferate very rapidly, differentiating into the various absorptive and secretory cell types as they migrate toward the apex of the villus (Meran et al., 2017). Eventually, they lose their anchorage to the ECM and are released into the intestinal lumen. Alternatively, some TA cells move back to the bottom of the crypt, differentiating into Paneth cells, the closest cells to ISCs (Grossmann et al., 2001).
Figure 3.
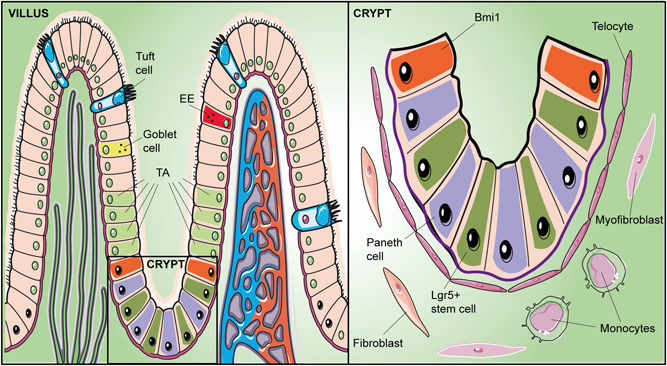
Schematic drawing of the crypt‐villus axis in the small intestine summarizing its main components. Left: The main types of epithelial cells are indicated in two adjacent villi. Right: Enlargement of the crypt bottom, containing intestinal stem cells (slow‐cycling B‐cell‐specific Moloney murine leukemia virus insertion site 1, Bmi1, and fast‐cycling leucine‐rich repeat‐containing G protein–coupled receptor 5 positive, Lgr5+). Lgr5+ divide producing transit‐amplifying (TA) cells that differentiate into the various absorptive and secretory cell types as they migrate toward the apex of the villus. Moving to the bottom of the crypt, some TA cells differentiate into Paneth cells. EE, enteroendocrine cell; TA, transit‐amplifying
Two types of ISCs have been identified: fast‐cycling and quiescent ISCs. Fast‐cycling ISCs are closely adjacent to Paneth cells and are characterized by the expression of leucine‐rich repeat‐containing G protein‐coupled receptor 5 (LGR5) (Barker et al., 2007). Quiescent ISCs express B‐cell‐specific Moloney murine leukemia virus insertion site 1 (Bmi1) and, because of their position, they are named +4 cells: 4‐cell diameters from the base of the crypt (Sangiorgi & Capecchi, 2008). Bmi1 cells support stem cell self‐renewal and crypt maintenance; their deletion results in complete crypt disruption. Instead, LGR5 cell loss may be counterbalanced by the activity of Bmi1 cells, which enables to restore cell population at the base of the crypt (Jasper, 2020).
Paneth cells support ISCs by expressing multiple signaling factors (epidermal growth factor, EGF, TGF‐α, Wnt3, and the Notch ligand Dll4) (Porter et al., 2002; Sato et al., 2011). However, ablation of Paneth cells does not abolish ISC proliferation and differentiation, likely because of compensatory mechanisms exerted by other niche cells. Even though enteroendocrine cells represent a very small number of intestinal epithelial cells, they are crucial for regulating intestinal physiology and contribute to the niche microenvironment (Cani et al., 2013). They are able to detect chemical stimuli from the intestinal lumen (nutrients, bile acids, microbes, etc.) and secrete regulatory hormones, such as cholecystokinin, gastrointestinal inhibitory peptide, and ghrelin (Sykaras et al., 2014). Stromal cells are heavily involved by releasing various regulatory factors. Subepithelial MSCs produce BMPs, antagonizing Wnt signaling along the crypt‐villus axis.
The Wnt/β‐catenin signaling pathway plays a crucial role in crypt physiology (Clevers, 2013). Binding to Frizzled and Lrp receptors, Wnt ligands activate downstream signaling, leading to cytoplasmic β‐catenin accumulation. After entering the nucleus, β‐catenin binds to TCF and activates transcription of Wnt/Tcf target genes. According to Derksen et al., the β‐catenin/TCF complex represents the ‘‘master switch’' that controls proliferation versus differentiation (Derksen et al., 2004). In the absence of Wnt, cytoplasmic β‐catenin is degraded by a complex of proteins, including Axin and Adenomatous Polyposis Coli (Parker & Neufeld, 2020). Aberrations of Wnt/β‐catenin signaling pathway components may be at the basis of colorectal cancers, such as the hereditary syndrome termed “familiar adenomatous polyposis” (Nusse & Clevers, 2017).
BMP activity improves epithelial cell differentiation and inhibits ISC expansion. Vice versa, BMP inhibitors secreted by myofibroblasts induce an improved Wnt‐mediated ISC self‐renewal (Le Guen et al., 2015). Similar to subepithelial myofibroblasts, pericytes release Wnt ligands and BMP antagonists. Subepithelial telocytes, expressing the winged‐helix transcription factor (Foxl1) and the canonic hedgehog signaling mediator (Gli1), are part of MSCs residing in the submucosa. They arrange a continuous plexus underneath the intestinal epithelium and produce numerous factors, including those of the Wnt family and BMP antagonists, such as Gremlin 1 and Gremlin 2 (Kaestner, 2019); their ablation severely compromises crypt proliferation. Macrophages are essential elements for protective immunity. Located in the lamina propria, they are closely associated with the crypt epithelium and preserve intestinal homeostasis by counteracting microbiota and food antigens (Bain & Mowat, 2014). Macrophage depletion negatively affects intestinal epithelial differentiation (Sehgal et al., 2018).
Signaling regulatory pathways differently act along the crypt‐villus axis. Notch activation is particularly evident at the base of the crypt where it improves ISC maintenance and proliferation (Santos et al., 2018). A gradient of Wnt activity improves ISC self‐renewal, mostly at the base. The activity of Wnt ligands is strongly improved by R‐spondins, likely released by myofibroblasts, exerting synergic effects on the increase of the Lgr5 population (de Lau et al., 2012); Wnt loss leads to ISC depletion and complete disruption of intestinal crypts. An opposite gradient of activity can be revealed for TGF‐β/BMP signaling, which inhibits stem cell proliferation and stimulates epithelial differentiation (Qi et al., 2017). As a result, differentiation processes are mainly activated in the upper part of the crypt, toward the villus apex, whereas they are inhibited at the base of the crypt, by BMP antagonists. Ephrin ligand‐Eph receptor coupling exerts dynamic actions. At the bottom of the crypt, it supports the proliferative cells activating the Wnt pathway; in the upper part, it prevents cell migration maintaining epithelial cell positioning along the axis (Y. Wang et al., 2018). Hedgehog ligands are expressed by epithelial cells in the crypt (Sonic Hedgehog) and in villi (Indian Hedgehog). Overall, interactions between epithelial and mesenchymal cells through the Hedgehog signaling pathway promote both epithelial proliferation and differentiation (Kosinski et al., 2010).
The basement membrane that separates epithelial cells from the mesenchymal compartment represents a specialized ECM component. It consists of two layers: the basal lamina (positioned beneath epithelial cells) and a reticular network that links epithelial cells to the lamina propria (Laurie et al., 1982). The basal lamina is produced by both epithelial and stromal cells and includes collagen, laminin, and fibronectin. Collagen is the main structural protein; laminin is crucial for defining epithelial cell polarity (Teller & Beaulieu, 2001); fibronectin is particularly important for cell adhesion, migration, and differentiation. Moreover, glycosaminoglycan molecules, because of their high viscosity and low compressibility, provide lubrication, structural integrity, and facilitate cell migration (Hodde et al., 1996). A continuous ECM remodeling occurs, mainly related to angiogenesis, cell migration, and differentiation processes (Bonnans et al., 2014).
A different organization exists in colon stem cell niches, whose cell population has not yet been precisely defined. For example, it remains to be identified which cells produce Wnt since, unlike the small intestine, Paneth cells are not present in colonic crypts. Probably, deep crypt secretory cells, called Paneth/goblet‐like cells, intermingled with the Lgr5 + stem cells, produce Wnt and Notch factors to support colonic niche growth and maintenance (Hageman et al., 2020; Sasaki et al., 2016); the ablation of these cells in mouse experiments resulted in the loss of stem cell function. Some evidence indicates that Wnt ligands can be secreted by GLI1‐expressing subepithelial mesenchymal cells, located at the base of the crypt (Degirmenci et al., 2018). In particular, it has been reported in mice that blocking their Wnt secretion impairs stem cell renewal, leading to a loss of stem cells and to the disruption of the colonic epithelium.
5. SKELETAL MUSCLE
Skeletal muscle tissue mainly consists of multinucleated contractile muscle cells, also named myofibers, which originate during embryonic development from the fusion of mesodermal progenitors, called myoblasts. In normal conditions, skeletal muscle shows only a limited turnover, mainly addressed to manage tissue growth and the daily wear and tear due to its physiological activity. In response to injury, however, it features a considerable regenerative potential by which new myofibers, adequately vascularized and innervated, are generated (Gulino et al., 2019). Repair mechanisms and tissue growth ability mainly rely on the presence of resident stem cells that, due to their position, are commonly called satellite cells (Musumeci et al., 2015). Described by Alexander Mauro in 1961 (Mauro, 1961), they are small mononucleated cells characterized by a high ratio of nuclear to cytoplasmic volume, located between the myofiber membrane (sarcolemma) and the basement membrane (Figure 4), with which they establish intimate contacts (Rayagiri et al., 2018; Yin et al., 2013). Their distribution may be considerably variable. In the same myofiber, they are more densely present at the extremities, where myofiber longitudinal growth occurs; a preferential location has been found in proximity to neuromuscular junctions and capillaries. The number of satellite cells associated with slow muscle fibers is generally higher than those associated with fast muscle fibers.
Figure 4.
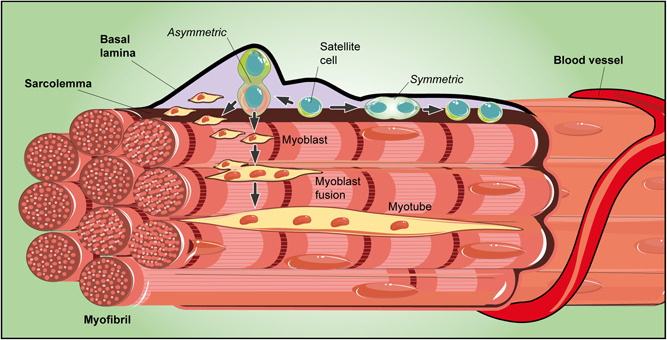
Schematic drawing of a skeletal muscle niche. Satellite cells are small mononucleated cells located between the sarcolemma and the basal lamina. Satellite cells may undergo symmetric or asymmetric division. By symmetric division, two identical progenitor cells are produced, both equally in contact with the sarcolemma and the basal lamina. Otherwise, by asymmetric division, the daughter cell in contact with the basal lamina inherits the role of an uncommitted progenitor, whereas the other, adjacent to the sarcolemma, may undergo myogenic differentiation. Proliferating myoblasts are then produced and, after their fusion, new syncytial contractile muscle cells are assembled
The sublaminal region that harbors satellite cells is acknowledged as a stem cell niche (Dinulovic et al., 2017). Myofibers and the surrounding ECM, including several related diffusible molecules (Wnt, IGF, and FGF) released by both components, can be considered the main niche components. Satellite cells can also interact with each other, by means of cell‐to‐cell communication and autocrine/paracrine signals.
By symmetric divisions (Kuang et al., 2007), two identical progenitor cells are produced, equally in contact with the sarcolemma and the basal lamina. Instead, by asymmetric, apical‐basal‐oriented divisions, the daughter cell in contact with the basal lamina inherits the role of an uncommitted progenitor, whereas the other, adjacent to the sarcolemma (apical), may undergo myogenic differentiation.
When activated, satellite cells start to proliferate, giving origin to myogenic precursor cells (MPCs). In this way, an expansion of existing muscle fibers can be achieved during muscle growth or in other physiological conditions (i.e., following intense physical exercise). Instead, the formation of new myofibers occurs during tissue regeneration following injury or disease. In fact, muscle regeneration is completely abolished after the ablation of satellite cells (von Maltzahn et al., 2013).
In homeostatic conditions, myofibers mostly keep satellite cells in a quiescent state (G0 phase), either by direct contact or by releasing chemical signals (Bischoff, 1990). Further influences are exerted by multiple stimuli from the surrounding environment, which comprises interstitial cells, the microvascular network, nerve fibers, and their associated factors. The basal lamina, also in direct contact with satellite cells, separates the niche from the muscle interstitium and consists of a complex molecular network, including type IV collagen, laminin, fibronectin, and glycoproteins (Csapo et al., 2020).
After muscle injury there is tissue regeneration; the process begins with the necrosis of damaged muscle fibers, which is associated with sarcolemma dissolution and increased membrane permeability. After the consequent inflammatory response, activated satellite cells start to proliferate giving origin to myoblasts that will produce new myofibers. The entire process is governed by the interplay between satellite cell‐intrinsic factors and signaling factors from the external microenvironment.
Quiescent satellite cells express Pax7 and Myf5 but not MyoD or Myogenin. Following activation and proliferation, myoblasts begin their differentiation path by downregulating Pax7 and expressing the myogenic transcription factors MyoD and Myf5. Terminal differentiation and fusion are characterized by the expression of Myogenin and MyoD. These molecular signatures allow us to recognize a hierarchical classification within the quiescent satellite cell population. About 10% of quiescent Pax7‐expressing satellite cells are considered true stem cells, having never expressed Myf5, the earliest marker of myogenic commitment. In response to injury, Pax7 + /Myf5‐ cells become activated and begin proliferation, generating basal Pax7 + /Myf5‐ and apical Pax7 + /Myf5 + cells. Whereas Myf5‐ cells preserve the satellite stem cell pool, the majority of Myf5 + cells undergo myogenic differentiation (Kuang et al., 2007). Terminal differentiation and fusion will be characterized by the expression of MyoD and Myogenin. At the end of the regeneration progression, some myoblasts reacquire high Pax7 levels and return to quiescence for future tissue needs. Returning to homeostatic conditions, the heterogeneous quiescent satellite cell population is composed of a small population of true stem cells (satellite stem cells, having never expressed Myf5), and a much larger population (about 90%) of Myf5 + committed MPCs (Beauchamp et al., 2000). After in vivo transplantation, only satellite stem cells are able to repopulate host muscle niches, ensuring long‐term muscle regeneration, whereas satellite myogenic cells preferentially proceed to their differentiation fate.
Regulatory factors mainly involved in the modulation of satellite cell activity include Notch and Wnt/β‐catenin signaling pathways, with opposite effects (Girardi & Le Grand, 2018; M. Verma et al., 2018; Yartseva et al., 2020). Notch activation and low activity of Wnt signaling promote the expansion of proliferating myoblasts for their successive differentiation. Notch inactivation is, however, required for terminal differentiation and myoblast fusion. Wnt signaling promotes myogenic commitment and terminal differentiation. Activation of Wnt/β‐catenin signaling is able to increase Desmin expression in myogenic cells and the size of newly formed myofibers.
In a recent paper, a transient niche for stem cell proliferation was proposed for muscle regeneration following injury. In this niche, proliferative signals would be provided by a subset of macrophages through the secretion of the cytokine nicotinamide phosphoribosyl transferase, acting on the C‐C motif chemokine receptor type 5, which is expressed on muscle stem cells. The authors suggest that this macrophage‐derived pathway could be usefully explored as a new therapeutic approach for skeletal muscle repair.
6. HEART
For many decades, it was assumed that the heart was a terminally differentiated organ, without an effective regeneration capability following injury. However, experiments in rodents have shown that DNA synthesis, very high during development and the early postnatal period, remains at lower levels in adult tissue (Soonpaa & Field, 1998). These findings suggest the existence of a limited proliferative capacity of adult cardiomyocytes. Quantitative estimates in rodents suggest a proliferation rate of <0.01% per year, which may increase after injury (Eschenhagen et al., 2017). Therefore, it can be concluded that in the adult the mechanism to increase cardiac mass mainly relies on cell enlargement, whereas the low cell turnover would essentially contribute to heart structural maintenance (Aguilar‐Sanchez et al., 2018).
It has long been debated if cardiac regeneration could be due to dedifferentiation and proliferation of cardiomyocytes (Bloomekatz et al., 2016) or to differentiation of resident cardiac stem cells (Liang & Phillips, 2013; van Berlo & Molkentin, 2014). Indeed, the existence of putative cardiac stem/progenitor cells in the heart has been suggested by several studies (Le & Chong, 2016; Lyngbaek et al., 2007). The term cardiac progenitor cells was proposed since, unlike stem cells, they would not be able to divide indefinitely and feature a limited multipotent differentiation ability (Mauretti et al., 2017). To accomplish their role, they should be able to differentiate into at least three of the four cardiac cell types (cardiomyocytes, endothelial cells, smooth muscle cells, and fibroblasts) (Mauretti et al., 2017).
Experimental data indicate the existence of cardiac stem/progenitor cells (CSCs) in myocardial tissue (Gomez‐Gaviro et al., 2012; Le & Chong, 2016; Urbanek et al., 2006), although our knowledge about CSC niches is still limited after almost two decades from their discovery (Beltrami et al., 2003). Localizing CSC niches has been hampered by the lack of the typical basal‐apical orientation in myocardial tissue. Subepicardial regions have been indicated as preferential locations for CSC niches in the adult heart (Di Meglio et al., 2010; Limana et al., 2010). However, they can also be found scattered throughout the myocardium, particularly in the atria and apex, cardiac regions characterized by low hemodynamic stress (Vukusic et al., 2019). Experiments on CSC localization have been mostly carried out in rodents (Barreto et al., 2019). CSC distribution in cardiac tissue was explored by cell labeling with nucleotide analogs, such as BrdU or 3[H]‐thymidine, which are incorporated into the nuclei during the S‐phase (Braun et al., 2003). A considerable dilution of the label occurs in rapidly dividing cells, whereas it is preserved in slow‐cycling cells. Using this method, it was also possible to follow the growth and phenotypic changes of CSCs and their progeny. While the number of bright BrdU‐CSCs (slow cycling) decreased rapidly, the number of fleble BrdU‐CSCs (fast cycling) increased over time. It was concluded that bright BrdU represents true CSCs, whose number remained constant over the observation period (Urbanek et al., 2006). They were mostly present in the atrial and apical myocardium, compared with the base‐mid‐region of the heart (Sanada et al., 2014).
CSC localization was also investigated in the presence of specific surface markers, such as c‐KIT, whose expression is considered necessary to define stem cells (Marino et al., 2019; Vicinanza et al., 2017). c‐KIT expressing CSCs was first isolated in female rat hearts, particularly in the atria and the ventricular apex (Beltrami et al., 2003). However, they were able to generate cardiomyocytes only at earlier stages of embryonic development and in perinatal age, whereas they virtually lost this ability in the adult heart (Jesty et al., 2012). The same localization was largely confirmed by analyzing SCA1 expression, commonly associated with stem/progenitor cells. First isolated in mouse hearts, SCA1‐expressing CSCs were predominantly found in the atrium, the intra‐atrial septum, the atrium‐ventricular boundary, and scattered along the epicardial region (van Vliet et al., 2008); they were able to differentiate into three cardiac lineages (Oh et al., 2003). In vivo experiments showed that, after injury, SCA1 CSCs have the homing ability and contribute to neoangiogenesis (X. Wang et al., 2006). However, it was also shown that beneficial effects were mainly due to their paracrine activity (Huang et al., 2011). In addition, SCA1‐expressing CSCs were only demonstrated in mice, whereas no human homolog has yet been identified (Holmes & Stanford, 2007). According to Vukusic et al. (Vukusic et al., 2019), the atrio‐ventricular junction at the insertion point of the mitral valve may represent a crucial niche region, from which CSCs would promote regeneration processes for both the valve, atria, and ventricles.
Cardiomyocytes and fibroblasts represent key cellular components of the niche. Both types of cells are intimately connected to CSCs through gap and adherens junctions, for reciprocal information transfer: cardiomyocytes are critical to promote CSC differentiation, and CSCs express growth factors and cytokines for cardiomyocyte survival (Mauretti et al., 2017). Fibroblasts maintain the supporting matrix and induce CSC differentiation through the Wnt signaling pathway; they also release pro‐ and antiangiogenic factors to modulate postinjury angiogenesis (Deb & Ubil, 2014). Fibroblasts originate not only from the epicardium but also from a fraction of endothelial cells undergoing endothelial–mesenchymal transition (Moore‐Morris et al., 2014).
Although lacking tight junctions, endothelial cells and smooth muscle cells are also considered niche cells since CSCs are often found in the perivascular area. The Notch signaling pathway is mainly involved in CSC‐endothelial cell interactions, promoting cardiomyogenic differentiation and vessel formation (Gude et al., 2015). VEGF‐mediated interactions with endothelial cells promote CSC migration and regulate their differentiation into endothelial or smooth muscle cells (Yoon et al., 2007). Immune cells such as macrophages, natural killer cells, and mast cells influence CSC behavior. Macrophages promote CSC proliferation and differentiation into cardiomyocytes and endothelial cells, releasing growth factors, including IGF‐1, VEGF, and TGF‐β. The crosstalk between CSCs with natural killer cells improves cardiac regeneration, downregulating their toxicity by switching cytokine secretion toward an anti‐inflammatory state (Boukouaci et al., 2014). Interactions between CSCs and mast cells likely occur through paracrine effects since direct cell contact has not been described; mast cells produce several cytokines, growth factors, and angiogenic factors that are involved in cardiac repair.
Through paracrine actions, epicardium‐derived cells (Lie‐Venema et al., 2007) stimulate CSC migration and proliferation, improving cardiac function. Indeed, it has been suggested that the epicardium itself is a source of progenitor cells (Bollini et al., 2011), able to differentiate into different cell types such as coronary smooth muscle cells, cardiomyocytes, endothelial cells, and interstitial fibroblasts (Laugwitz et al., 2008). However, they mainly contribute to cardiomyocyte formation during early development, rather than in the adult heart. Subepicardial telocytes facilitate cardiac repair, interacting with CSCs through stromal synapses and adherens junctions (Bani, 2016). Telocyte influences are exerted via growth factors (VEGF) and macromolecular signals, such as microRNAs (Albulescu et al., 2015).
Cardiac cells are embedded in a complex ECM, composed of different proteins, proteoglycans, and glycosaminoglycans (Jourdan‐Lesaux et al., 2010). Structural components include collagen types I, III, and V, and elastin. CSC adhesion to the ECM is assured by transmembrane protein complexes, such as integrins, which also allow the external microenvironment to influence CSC intracellular activity. Within the ECM framework, CSC behavior is governed by soluble macromolecules (VEGF, TGF‐β, and stromal cell‐derived factor 1‐α), released by the different cell types (Corda et al., 2000). Cardiac fibroblasts are predominantly responsible for ECM formation and remodeling. Available data indicate that also a low oxygen tension, which occurs after myocardial infarction, may increase CSC proliferation and motility (van Oorschot et al., 2011). Overall, although some repair mechanisms occur, the myocardial regenerative potential is clearly lower than in other tissues, unable to counteract efficiently severe injuries or diseases. Despite continuous advances in the clinical treatment of heart failure, cardiovascular diseases still represent a major health priority, and heart transplantation is often the only choice to avoid death. However, transplantation‐related problems and the limited organ availability encourage exploration for alternative therapeutic approaches. Cell‐based approaches to replace damaged heart cells have been extensively explored in preclinical studies, but only moderate beneficial effects have been induced in animal models of cardiac ischemic injury (Muller et al., 2018; Vagnozzi et al., 2020). Even more modest improvements are reported in humans (Bardelli & Moccetti, 2016). The microenvironment of the stem cell niche in the adult myocardium might then be considered a potential therapeutic target to improve the regenerative potential of the heart after injury. For example, modulation of the Notch signaling pathway may ameliorate the performance of the postinfarcted heart (Gude et al., 2015).
7. CENTRAL NERVOUS SYSTEM
In the adult central nervous system (CNS), it was assumed for a long time that only glial cells could proliferate, whereas neurons were supposed to be unable to divide. Indeed, the presence of neurogenic areas was identified in at least two brain regions, which can be considered as neural niches (Figure 5): the subventricular zone of the lateral ventricle (SVZ) and the subgranular zone (SGZ) of the dentate gyrus within the hippocampus (Andreotti et al., 2019; Kempermann et al., 2015; Riquelme et al., 2008). In both the SVZ and the SGZ, neural stem cells (NSCs) are characterized by long‐term self‐renewal, proliferative potential, and the ability to differentiate into the three main CNS cell types: neurons, astrocytes, and oligodendrocytes. Expressing the astrocyte marker glial fibrillary acidic protein (GFAP), NSCs are also called GFAP‐positive astrocytes.
Figure 5.
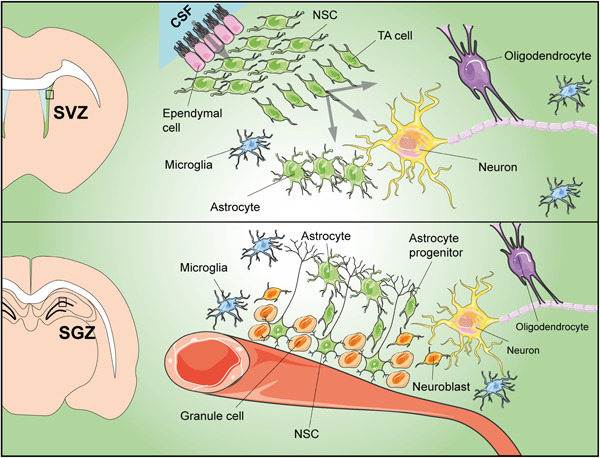
Schematic drawing of neural niches and their main components. Top: Neural stem cell (NSC) niche in the subventricular zone of the lateral ventricle (SVZ); located underneath ependymal cells, NSCs produce transit‐amplifying (TA) progenitors that are able to differentiate into the three main nervous cell types: neurons, astrocytes, and oligodendrocytes. Bottom: Neural stem cells in the subgranular zone of the hippocampus (SGZ) reside at the interface between the hilus and the granule cell layer of the dentate gyrus; in this niche, NSCs give rise to neurons through intermediate precursors that migrate toward the granule cell layer and become mature neurons. CSF, cerebrospinal fluid
The SVZ niche is located along the lateral wall of the lateral ventricles where NSCs are organized in a thin layer underneath the ependymal cells that are in contact with the cerebral spinal fluid (CSF); the CSF acts as a vehicle for trophic factors and neuroendocrine peptides affecting NSC maintenance and proliferation. In this subependymal region, NSCs and their progeny include three different cell types: GFAP expressing slowly proliferating cells, rapidly dividing TA progenitors, and migratory neuroblasts (Doetsch, 2003). After migrating toward the olfactory bulb, neuroblast‐derived periglomerular and granule mature neurons are involved in olfactory learning, memory, and discrimination (Rochefort et al., 2002). Ependymal cells in the wall of the lateral ventricle are linked to NSCs through gap adherens junctions, which allow reciprocal interchanges (Riquelme et al., 2008; Ruddy & Morshead, 2018). By producing noggin, ependymal cells promote neurogenesis, antagonizing BMP, which favors glial differentiation. Other niche components comprise the endothelial cells of blood vessels and their basal lamina, as well as perivascular cells and axon terminals (Lim et al., 2000).
In the hippocampal SGZ, NSCs reside at the interface between the hilus and the granule cell layer of the dentate gyrus. Their apical processes extend up to the granule layer and through basal processes, they contact blood vessels below the dentate gyrus (Walton, 2012). Unlike the SVZ, hippocampal NSCs are not in contact with CSF (Clarke & van der Kooy, 2011). In this niche, NSCs give rise to neurons through intermediate precursors that migrate toward the granule cell layer where they become mature neurons. Newly formed neurons likely contribute to typical hippocampal functions, such as learning and memory (Andreotti et al., 2019).
In both neurogenic areas, microglia are important cellular components. In the SVZ, they promote the survival and migration of neuroblasts. Microglial processes are closely associated with blood vessels as well as with astrocytes, stem cells, TA cells, and migrating neuroblasts (Solano Fonseca et al., 2016). In the SGZ, they are responsible for the removal of apoptotic new neurons since only some of them are incorporated into the hippocampal network, whereas many others die within the initial days (Sierra et al., 2010).
Blood vessels are essential components of neural niches (Tavazoie et al., 2008), which are more vascularized than other brain areas. In the perivascular microenvironment, a variety of cell types, such as perivascular astrocytes, macrophages, fibroblasts, and adventitial cells contribute to the niche dynamics. In the hippocampus, neurogenesis is closely associated with active angiogenesis (Palmer et al., 2000). In the SVZ, where blood vessels constitute a relatively stable vascular bed, signaling cues from vessels are provided through gaps in astrocyte end‐feet and incomplete pericyte coverage.
The ECM structure in neural niches consists of various components, such as laminin, collagen, and heparin sulfate proteoglycan (Ruddy & Morshead, 2018). Through galectin family proteins, the ECM also controls cell adhesion and migration of neural precursors (Comte et al., 2011). In the SVZ niche, a key component is represented by a basal lamina organized in “fractones”, so‐called because of their fractal organization. The base of each fractone is in contact with perivascular macrophages and, after crossing the SVZ with the stem, it eventually forms a bulb beneath the ependymal cells. This particular arrangement allows fractones to interact with blood vessels, astrocytes, ependymal and microglial cells, and precursor cells.
Multiple regulatory factors come from the blood, the CSF, or are released by surrounding cells. In both neural niches, EGF and FGF are required for NSC proliferation. By acting on TA cells or precursor cells, EGF promotes an increased glial rather than neuronal production. Limited to the SVZ, FGF mainly promotes an increase in new neurons (Kuhn et al., 1997). Produced by the choroid plexus and endothelial cells, IGF‐1 supports cell proliferation and survival, inhibiting apoptosis (Nieto‐Estevez et al., 2016). Nonetheless, about 50% of the newly formed cells of the neurogenic lineage die within the first 7 days (Ryu et al., 2016). The Wnt/β‐catenin signaling pathway stimulates proliferation, migration, and differentiation of newly formed neurons. Combined IGF‐1 and Wnt signaling improves neurogenesis and the NSC pool size in the early postnatal brain (Zhang et al., 2007). Secreted by Cajal–Retzius neurons, reelin is important for the initial organization of the hippocampal dentate gyrus. In adult life, it creates a signaling gradient that organizes the migration of new neurons for the formation of the molecular layer (Frotscher, 2010). After the initial reduction of precursors and new neurons in the first two postnatal months, Notch pathways help to stabilize the pool size (Sibbe et al., 2009). In the SVZ, brain‐derived neurotrophic factor (BDNF) stimulates TA cell proliferation and neuroblast migration. BDNF and serotonin are associated with exercise‐induced neurogenesis in the SGZ of running rats (Ieraci et al., 2016).
To date, our knowledge about neural niches in the human brain is still quite limited and most experimental findings have been gathered from studies in rodents. Although many similarities undoubtedly exist, some differences have been outlined. For example, both in humans and in rodents, hippocampal NSCs become dentate granule neurons (Spalding et al., 2013). Instead, SVZ neural progenitors are predestined to become medium striatal spiny neurons in humans, and olfactory interneurons in rodents (Ernst et al., 2014).
Divergent hypotheses have been made about neurogenesis and aging. According to some authors (Boldrini et al., 2018), neurogenesis in the hippocampus of healthy individuals is preserved with aging. Instead, other authors (Sorrells et al., 2018) suggest that the number of hippocampal stem cells/young neurons rapidly decreases during the first decade of life. Overall, it is widely accepted that age‐related reduced neurogenesis negatively affects neuron replacement and repair mechanisms: the aged brain would have both a restricted rescue potential and a higher predisposition to neurodegenerative diseases (Conover & Shook, 2011). Age‐related decreased production of new neurons in the hippocampus dentate gyrus has been associated with learning and memory impairments, whereas a reduced SVZ neurogenesis may be implicated in Huntington's disease (Seib & Martin‐Villalba, 2015).
Besides these two “classical” neural niches, two other neurogenic regions have been identified, in the hypothalamus and in the olfactory mucosa. At the hypothalamic level, they are located along the lateral wall of the third ventricle and in the median eminence region at the bottom (Rojczyk‐Golebiewska et al., 2014). In these regions, neural precursors, also called tanycytes, express typical neural precursor markers, such as nestin, vimentin, and doublecortin‐like protein. Tanycytes adjacent to the median eminence are influenced by molecules present in the CSF, as well as by hormones and nutritional signals conveyed by blood vessels; this is not surprising since the hypothalamus is involved in the regulation of energy homeostasis.
In the olfactory mucosa, lifelong neurogenesis results from the continuous proliferation and differentiation of progenitor cells within the olfactory epithelium. This is indispensable for replacing olfactory neurons that, exposed to the external environment, are subject to lesions or traumatic injury (Mackay‐Sim, 2010; Pellitteri et al., 2010). NSCs in the olfactory epithelium, remain quiescent and maintaining long‐term regenerative ability (Carter et al., 2004). Supporting glial cells, known as olfactory ensheathing cells, contribute to NSC differentiation releasing ECM proteases, and secreting neurotrophic/axonal growth factors (Russo et al., 2020; Simón et al., 2011; Vicario et al., 2017).
In conclusion, it can be hypothesized that increased neurogenesis can generate new neural cells to replace neurons or glial cells in damaged areas (Kokaia & Lindvall, 2003). However, these self‐repair mechanisms do not seem to be able to efficiently counteract diffuse neuronal death as it occurs in severe injury or neurodegenerative diseases.
8. CONCLUSIONS
Stem cell niches have been focused on in this review to highlight the importance of these particular internal microenvironments in tissue homeostasis. The vital importance of niches for stem cell features is demonstrated by the following critical observations: (i) once stem cells lose contact with the niche, they rapidly differentiate into specialized cells, losing their long‐lasting physiological role (Lutolf & Blau, 2009). On the contrary, (ii) niches are able to preserve their functional properties even in the absence of stem cells; for example, following radiation‐induced stem cell depletion, BM niches can still functionally harbor implanted HSCs (Dominici et al., 2009; Ferraro et al., 2010). This fundamental aspect was already demonstrated about 70 years ago when the intravenous injection of BM cells was able to restore hematopoiesis in irradiated mice (Jacobson et al., 1951). A number of studies in animals show that (iii) tissue aging is largely related to niche aging: stem cells from young animals acquire an aged phenotype when transplanted into old mice, whereas those from old animals seem rejuvenated when transplanted into young animals (Ferraro et al., 2010).
The importance of the environment on stem cell fate has been widely demonstrated by an impressive number of experiments in vitro, showing that differentiation processes can be significantly manipulated by adding a variety of chemical agents or growth factors to the culture medium. These strategies have been widely used on multipotent MSCs derived from BM (Chu et al., 2020) and adipose tissue (Si et al., 2019), to obtain cellular elements not only of mesodermal origin (Mannino et al., 2020; Szychlinska et al., 2020) but also neural (Lo Furno et al., 2018) and epithelial cells (Sierra‐Sanchez et al., 2018). It is worth noting that these pre‐differentiated MSCs represent a useful tool in cell‐based medicine, even for allogeneic administrations, since like other cells, they do not evoke a significant immune response (Gao et al., 2016; Vancheri et al., 2005). Therefore, investigating the mechanisms occurring within stem cell niches is important not only to shed light on this fundamental chapter of human physiology but also to help develop therapeutic applications, especially when other approaches fail. To date, only a limited number of stem cell‐based therapies are approved for wide clinical use. BM transplants for blood diseases and skin transplants in cases of severe burns are probably the best examples. Much has been done in the last decades but much more work is needed for a better understanding of the complex interplay between the various niche components (Bardelli & Moccetti, 2017; Lutolf & Blau, 2009). In this respect, a remarkable number of experiments on artificial niches have been designed to improve the translation of experimental outcomes from bench to bedside. For example, the development of xeno‐free/serum‐free culture media that mimic, as much as possible, in vivo conditions. In addition, a careful selection of growth factors and chemical additives surely helps to meet the requests of regulatory authorities for wider and safer clinical applications. The most ambitious goal will be achieved when it is possible to develop therapeutic interventions targeting individual niche components of selected tissues/organs to improve in loco the regenerative potential of already existing stem cells, to minimize the adverse effects of aging, injury, or disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to thank Dr Antony Bridgewood of the Scientific Bureau of the University of Catania for language support. This study was supported by the University of Catania, Italy, “Piano Triennale per la Ricerca 2020‐2022—Grant PIACERI.” The figures were produced using Servier Medical Art. Open access funding provided by Universita degli Studi di Catania within the CRUI‐CARE Agreement.
Mannino, G. , Russo, C. , Maugeri, G. , Musumeci, G. , Vicario, N. , Tibullo, D. , Giuffrida, R. , Parenti, R. , & Lo Furno, D. (2022). Adult stem cell niches for tissue homeostasis. J Cell Physiol, 237, 239–257. 10.1002/jcp.30562
Giuliana Mannino and Cristina Russo should be considered joint first author.
Rosalba Parenti and Debora Lo Furno should be considered joint senior author.
REFERENCES
- Acar, M. , Kocherlakota, K. S. , Murphy, M. M. , Peyer, J. G. , Oguro, H. , Inra, C. N. , Jaiyeola, C. , Zhao, Z. , Luby‐Phelps, K. , & Morrison, S. J. (2015). Deep imaging of bone marrow shows non‐dividing stem cells are mainly perisinusoidal. Nature, 526(7571), 126–130. 10.1038/nature15250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar‐Sanchez, C. , Michael, M. , & Pennings, S. (2018). Cardiac stem cells in the postnatal heart: Lessons from development. Stem Cells International, 2018, 1247857. 10.1155/2018/1247857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu, R. , Tanase, C. , Codrici, E. , Popescu, D. I. , Cretoiu, S. M. , & Popescu, L. M. (2015). The secretome of myocardial telocytes modulates the activity of cardiac stem cells. Journal of Cellular and Molecular Medicine, 19(8), 1783–1794. 10.1111/jcmm.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti, J. P. , Silva, W. N. , Costa, A. C. , Picoli, C. C. , Bitencourt, F. , Coimbra‐Campos, L. , Resende, R. R. , Magno, L. , Romano‐Silva, M. A. , Mintz, A. , & Birbrair, A. (2019). Neural stem cell niche heterogeneity. Seminars in Cell and Developmental Biology, 95, 42–53. 10.1016/j.semcdb.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada, N. , Kunisaki, Y. , Pierce, H. , Wang, Z. , Fernandez, N. F. , Birbrair, A. , Ma'ayan, A. , & Frenette, P. S. (2017). Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nature Cell Biology, 19(3), 214–223. 10.1038/ncb3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis‐Ribas, T. , Forni, M. F. , Winnischofer, S. M. B. , Sogayar, M. C. , & Trombetta‐Lima, M. (2018). Extracellular matrix dynamics during mesenchymal stem cells differentiation. Developmental Biology, 437(2), 63–74. 10.1016/j.ydbio.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Azizidoost, S. , Bavarsad, M. S. , Bavarsad, M. S. , Shahrabi, S. , Jaseb, K. , Rahim, F. , Shahjahani, M. , Saba, F. , Ghorbani, M. , & Saki, N. (2015). The role of notch signaling in bone marrow niche. Hematology, 20(2), 93–103. 10.1179/1607845414Y.0000000167 [DOI] [PubMed] [Google Scholar]
- Bain, C. C. , & Mowat, A. M. (2014). Macrophages in intestinal homeostasis and inflammation. Immunological Reviews, 260(1), 102–117. 10.1111/imr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani, D. (2016). Telocytes in cardiac tissue architecture and development. Advances in Experimental Medicine and Biology, 913, 127–137. 10.1007/978-981-10-1061-3_8 [DOI] [PubMed] [Google Scholar]
- Bardelli, S. , & Moccetti, M. (2016). Stem cell banking and its impact on cardiac regenerative medicine. Advances in Experimental Medicine and Biology, 951, 163–178. 10.1007/978-3-319-45457-3_14 [DOI] [PubMed] [Google Scholar]
- Bardelli, S. , & Moccetti, M. (2017). Remodeling the human adult stem cell niche for regenerative medicine applications. Stem Cells International, 2017, 6406025. 10.1155/2017/6406025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, N. , van Es, J. H. , Kuipers, J. , Kujala, P. , van den Born, M. , Cozijnsen, M. , Haegebarth, A. , Korving, J. , Begthel, H. , Peters, P. J. , & Clevers, H. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449(7165), 1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barreto, S. , Hamel, L. , Schiatti, T. , Yang, Y. , & George, V. (2019). Cardiac progenitor cells from stem cells: Learning from genetics and biomaterials. Cells, 8(12), 1536. 10.3390/cells8121536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler, K. , & Brandner, J. M. (2017). Tight junctions in skin inflammation. Pflugers Archiv European Journal of Physiology, 469(1), 3–14. 10.1007/s00424-016-1903-9 [DOI] [PubMed] [Google Scholar]
- Beauchamp, J. R. , Heslop, L. , Yu, D. S. , Tajbakhsh, S. , Kelly, R. G. , Wernig, A. , Buckingham, M. E. , Partridge, T. A. , & Zammit, P. S. (2000). Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. Journal of Cell Biology, 151(6), 1221–1234. 10.1083/jcb.151.6.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami, A. P. , Barlucchi, L. , Torella, D. , Baker, M. , Limana, F. , Chimenti, S. , Kasahara, H. , Rota, M. , Musso, E. , Urbanek, K. , Leri, A. , Kajstura, J. , Nadal‐Ginard, B. , & Anversa, P. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell, 114(6), 763–776. 10.1016/s0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- Betschinger, J. , & Knoblich, J. A. (2004). Dare to be different: Asymmetric cell division in Drosophila, C. elegans and vertebrates. Current Biology, 14(16), R674–R685. 10.1016/j.cub.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Bischoff, R. (1990). Interaction between satellite cells and skeletal muscle fibers. Development, 109(4), 943–952. [DOI] [PubMed] [Google Scholar]
- Blanpain, C. , & Fuchs, E. (2006). Epidermal stem cells of the skin. Annual Review of Cell and Developmental Biology, 22, 339–373. 10.1146/annurev.cellbio.22.010305.104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomekatz, J. , Galvez‐Santisteban, M. , & Chi, N. C. (2016). Myocardial plasticity: Cardiac development, regeneration and disease. Current Opinion in Genetics & Development, 40, 120–130. 10.1016/j.gde.2016.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini, M. , Fulmore, C. A. , Tartt, A. N. , Simeon, L. R. , Pavlova, I. , Poposka, V. , Rosoklija, G. B. , Stankov, A. , Arango, V. , Dwork, A. J. , Hen, R. , & Mann, J. J. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell, 22(4), 589–599. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollini, S. , Smart, N. , & Riley, P. R. (2011). Resident cardiac progenitor cells: At the heart of regeneration. Journal of Molecular and Cellular Cardiology, 50(2), 296–303. 10.1016/j.yjmcc.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Bonnans, C. , Chou, J. , & Werb, Z. (2014). Remodelling the extracellular matrix in development and disease. Nature Reviews Molecular Cell Biology, 15(12), 786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukouaci, W. , Lauden, L. , Siewiera, J. , Dam, N. , Hocine, H. R. , Khaznadar, Z. , Tamouza, R. , Borlado, L. R. , Charron, D. , Jabrane‐Ferrat, N. , & Al‐Daccak, R. (2014). Natural killer cell crosstalk with allogeneic human cardiac‐derived stem/progenitor cells controls persistence. Cardiovascular Research, 104(2), 290–302. 10.1093/cvr/cvu208 [DOI] [PubMed] [Google Scholar]
- Braun, K. M. , Niemann, C. , Jensen, U. B. , Sundberg, J. P. , Silva‐Vargas, V. , & Watt, F. M. (2003). Manipulation of stem cell proliferation and lineage commitment: visualisation of label‐retaining cells in wholemounts of mouse epidermis. Development, 130(21), 5241–5255. 10.1242/dev.00703 [DOI] [PubMed] [Google Scholar]
- Butler, J. M. , Nolan, D. J. , Vertes, E. L. , Varnum‐Finney, B. , Kobayashi, H. , Hooper, A. T. , Seandel, M. , Shido, K. , White, I. A. , Kobayashi, M. , Witte, L. , May, C. , Shawber, C. , Kimura, Y. , Kitajewski, J. , Rosenwaks, Z. , Bernstein, I. D. , & Rafii, S. (2010). Endothelial cells are essential for the self‐renewal and repopulation of Notch‐dependent hematopoietic stem cells. Cell Stem Cell, 6(3), 251–264. 10.1016/j.stem.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D. , Everard, A. , & Duparc, T. (2013). Gut microbiota, enteroendocrine functions and metabolism. Current Opinion in Pharmacology, 13(6), 935–940. 10.1016/j.coph.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Carter, L. A. , MacDonald, J. L. , & Roskams, A. J. (2004). Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. Journal of Neuroscience, 24(25), 5670–5683. 10.1523/JNEUROSCI.0330-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. L. , Huang, W. Y. , Wang, E. H. C. , Tai, K. Y. , & Lin, S. J. (2020). Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. Journal of Biomedical Science, 27(1), 43. 10.1186/s12929-020-0624-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D. T. , Phuong, T. , Tien, N. , Tran, D. K. , Thanh, V. V. , Quang, T. L. , Truong, D. T. , Pham, V. H. , Ngoc, V. , Chu‐Dinh, T. , & Kushekhar, K. (2020). An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. International Journal of Molecular Sciences, 21(3), 708. 10.3390/ijms21030708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, L. , & van der Kooy, D. (2011). The adult mouse dentate gyrus contains populations of committed progenitor cells that are distinct from subependymal zone neural stem cells. Stem Cells, 29(9), 1448–1458. 10.1002/stem.692 [DOI] [PubMed] [Google Scholar]
- Clevers, H. (2013). The intestinal crypt, a prototype stem cell compartment. Cell, 154(2), 274–284. 10.1016/j.cell.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Clevers, H. , Loh, K. M. , & Nusse, R. (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 346(6205), 1248012. 10.1126/science.1248012 [DOI] [PubMed] [Google Scholar]
- Comte, I. , Kim, Y. , Young, C. C. , van der Harg, J. M. , Hockberger, P. , Bolam, P. J. , Poirier, F. , & Szele, F. G. (2011). Galectin‐3 maintains cell motility from the subventricular zone to the olfactory bulb. Journal of Cell Science, 124(Pt 14), 2438–2447. 10.1242/jcs.079954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover, J. C. , & Shook, B. A. (2011). Aging of the subventricular zone neural stem cell niche. Aging and Disease, 2(1), 49–63. [PMC free article] [PubMed] [Google Scholar]
- Corda, S. , Samuel, J. L. , & Rappaport, L. (2000). Extracellular matrix and growth factors during heart growth. Heart Failure Reviews, 5(2), 119–130. 10.1023/A:1009806403194 [DOI] [PubMed] [Google Scholar]
- Csapo, R. , Gumpenberger, M. , & Wessner, B. (2020). Skeletal muscle extracellular matrix ‐ what do we know about its composition, regulation, and physiological roles? A narrative review. Frontiers in Physiology, 11, 253. 10.3389/fphys.2020.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau, W. B. , Snel, B. , & Clevers, H. C. (2012). The R‐spondin protein family. Genome Biology, 13(3), 242. 10.1186/gb-2012-13-3-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb, A. , & Ubil, E. (2014). Cardiac fibroblast in development and wound healing. Journal of Molecular and Cellular Cardiology, 70, 47–55. 10.1016/j.yjmcc.2014.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirmenci, B. , Valenta, T. , Dimitrieva, S. , Hausmann, G. , & Basler, K. (2018). GLI1‐expressing mesenchymal cells form the essential Wnt‐secreting niche for colon stem cells. Nature, 558(7710), 449–453. 10.1038/s41586-018-0190-3 [DOI] [PubMed] [Google Scholar]
- Derksen, P. W. , Tjin, E. , Meijer, H. P. , Klok, M. D. , MacGillavry, H. D. , van Oers, M. H. , Lokhorst, H. M. , Bloem, A. C. , Clevers, H. , Nusse, R. , van der Neut, R. , Spaargaren, M. , & Pals, S. T. (2004). Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proceedings of the National Academy of Sciences of the United States of America, 101(16), 6122–6127. 10.1073/pnas.0305855101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio, F. , Castaldo, C. , Nurzynska, D. , Miraglia, R. , Romano, V. , Russolillo, V. , Giuseppina, L. , Vosa, C. , & Montagnani, S. (2010). Localization and origin of cardiac CD117‐positive cells: identification of a population of epicardially‐derived cells in adult human heart. Italian Journal of Anatomy and Embryology, 115(1‐2), 71–78. [PubMed] [Google Scholar]
- Dinulovic, I. , Furrer, R. , & Handschin, C. (2017). Plasticity of the muscle stem cell microenvironment. Advances in Experimental Medicine and Biology, 1041, 141–169. 10.1007/978-3-319-69194-7_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch, F. (2003). A niche for adult neural stem cells. Current Opinion in Genetics & Development, 13(5), 543–550. 10.1016/j.gde.2003.08.012 [DOI] [PubMed] [Google Scholar]
- Dominici, M. , Rasini, V. , Bussolari, R. , Chen, X. , Hofmann, T. J. , Spano, C. , Bernabei, D. , Veronesi, E. , Bertoni, F. , Paolucci, P. , Conte, P. , & Horwitz, E. M. (2009). Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood, 114(11), 2333–2343. 10.1182/blood-2008-10-183459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon, C. , & Jaffredo, T. (2014). Cell interactions and cell signaling during hematopoietic development. Experimental Cell Research, 329(2), 200–206. 10.1016/j.yexcr.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Eaves, C. J. (2015). Hematopoietic stem cells: Concepts, definitions, and the new reality. Blood, 125(17), 2605–2613. 10.1182/blood-2014-12-570200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, A. , Alkass, K. , Bernard, S. , Salehpour, M. , Perl, S. , Tisdale, J. , Possnert, G. , Druid, H. , & Frisén, J. (2014). Neurogenesis in the striatum of the adult human brain. Cell, 156(5), 1072–1083. 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- Eschenhagen, T. , Bolli, R. , Braun, T. , Field, L. J. , Fleischmann, B. K. , Frisén, J. , Giacca, M. , Hare, J. M. , Houser, S. , Lee, R. T. , Marbán, E. , Martin, J. F. , Molkentin, J. D. , Murry, C. E. , Riley, P. R. , Ruiz‐Lozano, P. , Sadek, H. A. , Sussman, M. A. , & Hill, J. A. (2017). Cardiomyocyte regeneration: A consensus statement. Circulation, 136(7), 680–686. 10.1161/CIRCULATIONAHA.117.029343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, F. , Celso, C. L. , & Scadden, D. (2010). Adult stem cels and their niches. Advances in Experimental Medicine and Biology, 695, 155–168. 10.1007/978-1-4419-7037-4_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein, A. J. , Chailakhjan, R. K. , & Lalykina, K. S. (1970). The development of fibroblast colonies in monolayer cultures of guinea‐pig bone marrow and spleen cells. Cell and Tissue Kinetics, 3(4), 393–403. 10.1111/j.1365-2184.1970.tb00347.x [DOI] [PubMed] [Google Scholar]
- Friedenstein, A. J. , Petrakova, K. V. , Kurolesova, A. I. , & Frolova, G. P. (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation, 6(2), 230–247. [PubMed] [Google Scholar]
- Frotscher, M. (2010). Role for Reelin in stabilizing cortical architecture. Trends in Neurosciences, 33(9), 407–414. 10.1016/j.tins.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Fuchs, E. (2018). Skin stem cells in silence, action, and cancer. Stem Cell Reports, 10(5), 1432–1438. 10.1016/j.stemcr.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, E. , & Blau, H. M. (2020). Tissue stem cells: Architects of their niches. Cell Stem Cell, 27(4), 532–556. 10.1016/j.stem.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, F. , Chiu, S. M. , Motan, D. A. , Zhang, Z. , Chen, L. , Ji, H. L. , Tse, H. F. , Fu, Q. L. , & Lian, Q. (2016). Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death & Disease, 7, e2062. 10.1038/cddis.2015.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi, F. , & Le Grand, F. (2018). Wnt signaling in skeletal muscle development and regeneration. Progress in Molecular Biology and Translation Science, 153, 157–179. 10.1016/bs.pmbts.2017.11.026 [DOI] [PubMed] [Google Scholar]
- Gomez‐Gaviro, M. V. , Lovell‐Badge, R. , Fernandez‐Aviles, F. , & Lara‐Pezzi, E. (2012). The vascular stem cell niche. Journal of Cardiovascular Translational Research, 5(5), 618–630. 10.1007/s12265-012-9371-x [DOI] [PubMed] [Google Scholar]
- Gonzales, K. A. U. , & Fuchs, E. (2017). Skin and its regenerative powers: An alliance between stem cells and their niche. Developmental Cell, 43(4), 387–401. 10.1016/j.devcel.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann, J. , Walther, K. , Artinger, M. , Kiessling, S. , & Scholmerich, J. (2001). Apoptotic signaling during initiation of detachment‐induced apoptosis ("anoikis") of primary human intestinal epithelial cells. Cell Growth and Differentiation, 12(3), 147–155. [PubMed] [Google Scholar]
- Gude, N. , Joyo, E. , Toko, H. , Quijada, P. , Villanueva, M. , Hariharan, N. , Sacchi, V. , Truffa, S. , Joyo, A. , Voelkers, M. , Alvarez, R. , & Sussman, M. A. (2015). Notch activation enhances lineage commitment and protective signaling in cardiac progenitor cells. Basic Research in Cardiology, 110(3), 29. 10.1007/s00395-015-0488-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino, R. , Vicario, N. , Giunta, M. , Spoto, G. , Calabrese, G. , Vecchio, M. , Gulisano, M. , Leanza, G. , & Parenti, R. (2019). Neuromuscular plasticity in a mouse neurotoxic model of spinal motoneuronal loss. International Journal of Molecular Sciences, 20(6). 10.3390/ijms20061500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman, J. H. , Heinz, M. C. , Kretzschmar, K. , van der Vaart, J. , Clevers, H. , & Snippert, H. J. G. (2020). Intestinal regeneration: Regulation by the microenvironment. Developmental Cell, 54(4), 435–446. 10.1016/j.devcel.2020.07.009 [DOI] [PubMed] [Google Scholar]
- Hardman, M. J. , Sisi, P. , Banbury, D. N. , & Byrne, C. (1998). Patterned acquisition of skin barrier function during development. Development, 125(8), 1541–1552. [DOI] [PubMed] [Google Scholar]
- Hodde, J. P. , Badylak, S. F. , Brightman, A. O. , & Voytik‐Harbin, S. L. (1996). Glycosaminoglycan content of small intestinal submucosa: A bioscaffold for tissue replacement. Tissue Engineering, 2(3), 209–217. 10.1089/ten.1996.2.209 [DOI] [PubMed] [Google Scholar]
- Holmes, C. , & Stanford, W. L. (2007). Concise review: Stem cell antigen‐1: Expression, function, and enigma. Stem Cells, 25(6), 1339–1347. 10.1634/stemcells.2006-0644 [DOI] [PubMed] [Google Scholar]
- Hsu, Y. C. , Li, L. , & Fuchs, E. (2014). Emerging interactions between skin stem cells and their niches. Nature Medicine, 20(8), 847–856. 10.1038/nm.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Gu, H. , Yu, Q. , Manukyan, M. C. , Poynter, J. A. , & Wang, M. (2011). Sca‐1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF‐1 following myocardial ischemia/reperfusion. PLOS One, 6(12), e29246. 10.1371/journal.pone.0029246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci, A. , Madaio, A. I. , Mallei, A. , Lee, F. S. , & Popoli, M. (2016). Brain‐derived neurotrophic factor Val66Met human polymorphism impairs the beneficial exercise‐induced neurobiologicalchanges in mice. Neuropsychopharmacology, 41(13), 3070–3079. 10.1038/npp.2016.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, M. , Liu, Y. , Yang, Z. , Nguyen, J. , Liang, F. , Morris, R. J. , & Cotsarelis, G. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Medicine, 11(12), 1351–1354. 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- Ivanovs, A. , Rybtsov, S. , Anderson, R. A. , Turner, M. L. , & Medvinsky, A. (2014). Identification of the niche and phenotype of the first human hematopoietic stem cells. Stem Cell Reports, 2(4), 449–456. 10.1016/j.stemcr.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, L. O. , Simmons, E. L. , Marks, E. K. , & Eldredge, J. H. (1951). Recovery from radiation injury. Science, 113(2940), 510–511. 10.1126/science.113.2940.510 [DOI] [PubMed] [Google Scholar]
- Jasper, H. (2020). Intestinal stem cell aging: Origins and interventions. Annual Review of Physiology, 82, 203–226. 10.1146/annurev-physiol-021119-034359 [DOI] [PubMed] [Google Scholar]
- Jesty, S. A. , Steffey, M. A. , Lee, F. K. , Breitbach, M. , Hesse, M. , Reining, S. , Lee, J. C. , Doran, R. M. , Nikitin, A. Y. , Fleischmann, B. K. , & Kotlikoff, M. I. (2012). c‐kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proceedings of the National Academy of Sciences of the United States of America, 109(33), 13380–13385. 10.1073/pnas.1208114109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, J. , Ho, B. S. , Qian, G. , Xie, X. M. , Bigliardi, P. L. , & Bigliardi‐Qi, M. (2017). Aging in hair follicle stem cells and niche microenvironment. Journal of Dermatology, 44(10), 1097–1104. 10.1111/1346-8138.13897 [DOI] [PubMed] [Google Scholar]
- Jourdan‐Lesaux, C. , Zhang, J. , & Lindsey, M. L. (2010). Extracellular matrix roles during cardiac repair. Life Sciences, 87(13‐14), 391–400. 10.1016/j.lfs.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner, K. H. (2019). Theintestinal stem cell niche: A central role for Foxl1‐expressing subepithelial telocytes. Cellular and Molecular Gastroenterology and Hepatology, 8(1), 111–117. 10.1016/j.jcmgh.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann, G. , Song, H. , & Gage, F. H. (2015). Neurogenesis in the adult hippocampus. Cold Spring Harbor Perspectives in Biology, 7(9), a018812. 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabas, F. , Xie, L. , Xie, J. , Yu, Z. , DeBerardinis, R. J. , Kimura, W. , Thet, S. , Elshamy, A. F. , Abouellail, H. , Muralidhar, S. , Liu, X. , Chen, C. , Sadek, H. A. , Zhang, C. C. , & Zheng, J. (2015). Hypoxic metabolism in human hematopoietic stem cells. Cell & Bioscience, 5, 39. 10.1186/s13578-015-0020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia, Z. , & Lindvall, O. (2003). Neurogenesis after ischaemic brain insults. Current Opinion in Neurobiology, 13(1), 127–132. 10.1016/s0959-4388(03)00017-5 [DOI] [PubMed] [Google Scholar]
- Kosan, C. , & Godmann, M. (2016). Genetic and epigenetic mechanisms that maintain hematopoietic stem cell function. Stem Cells International, 2016, 5178965. 10.1155/2016/5178965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski, C. , Stange, D. E. , Xu, C. , Chan, A. S. , Ho, C. , Yuen, S. T. , Mifflin, R. C. , Powell, D. W. , Clevers, H. , Leung, S. Y. , & Chen, X. (2010). Indian hedgehog regulates intestinal stem cell fate through epithelial‐mesenchymal interactions during development. Gastroenterology, 139(3), 893–903. 10.1053/j.gastro.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, S. , Kuroda, K. , Le Grand, F. , & Rudnicki, M. A. (2007). Asymmetric self‐renewal and commitment of satellite stem cells in muscle. Cell, 129(5), 999–1010. 10.1016/j.cell.2007.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, H. G. , Winkler, J. , Kempermann, G. , Thal, L. J. , & Gage, F. H. (1997). Epidermal growth factor and fibroblast growth factor‐2 have different effects on neural progenitors in the adult rat brain. Journal of Neuroscience, 17(15), 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Godavarthy, P. S. , & Krause, D. S. (2018). The bone marrow microenvironment in health and disease at a glance. Journal of Cell Science, 131(4). 10.1242/jcs.201707 [DOI] [PubMed] [Google Scholar]
- Kunisaki, Y. (2019). Pericytes in bone marrow. Advances in Experimental Medicine and Biology, 1122, 101–114. 10.1007/978-3-030-11093-2_6 [DOI] [PubMed] [Google Scholar]
- Kunisaki, Y. , Bruns, I. , Scheiermann, C. , Ahmed, J. , Pinho, S. , Zhang, D. , Mizoguchi, T. , Wei, Q. , Lucas, D. , Ito, K. , Mar, J. C. , Bergman, A. , & Frenette, P. S. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 502(7473), 637–643. 10.1038/nature12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki, Y. , & Frenette, P. S. (2014). Influences of vascular niches on hematopoietic stem cell fate. International Journal of Hematology, 99(6), 699–705. 10.1007/s12185-014-1580-4 [DOI] [PubMed] [Google Scholar]
- Laugwitz, K. L. , Moretti, A. , Caron, L. , Nakano, A. , & Chien, K. R. (2008). Islet1 cardiovascular progenitors: A single source for heart lineages? Development, 135(2), 193–205. 10.1242/dev.001883 [DOI] [PubMed] [Google Scholar]
- Laurenti, E. , Frelin, C. , Xie, S. , Ferrari, R. , Dunant, C. F. , Zandi, S. , Neumann, A. , Plumb, I. , Doulatov, S. , Chen, J. , April, C. , Fan, J. B. , Iscove, N. , & Dick, J. E. (2015). CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell, 16(3), 302–313. 10.1016/j.stem.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie, G. W. , Leblond, C. P. , & Martin, G. R. (1982). Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. Journal of Cell Biology, 95(1), 340–344. 10.1083/jcb.95.1.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen, L. , Marchal, S. , Faure, S. , & de Santa Barbara, P. (2015). Mesenchymal‐epithelial interactions during digestive tract development and epithelial stem cell regeneration. Cellular and Molecular Life Science, 72(20), 3883–3896. 10.1007/s00018-015-1975-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, T. , & Chong, J. (2016). Cardiac progenitor cells for heart repair. Cell Death Discovery, 2, 16052. 10.1038/cddiscovery.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T. , & Fuchs, E. (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature, 437(7056), 275–280. 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S. X. , & Phillips, W. D. (2013). Migration of resident cardiac stem cells in myocardial infarction. Anatomical Record, 296(2), 184–191. 10.1002/ar.22633 [DOI] [PubMed] [Google Scholar]
- Lie‐Venema, H. , van den Akker, N. M. , Bax, N. A. , Winter, E. M. , Maas, S. , Kekarainen, T. , Hoeben, R. C. , de Ruiter, M. C. , Poelmann, R. E. , & Gittenberger‐de Groot, A. C. (2007). Origin, fate, and function of epicardium‐derived cells (EPDCs) in normal and abnormal cardiac development. The Scientific World Journal, 7, 1777–1798. 10.1100/tsw.2007.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, D. A. , Tramontin, A. D. , Trevejo, J. M. , Herrera, D. G. , Garcia‐Verdugo, J. M. , & Alvarez‐Buylla, A. (2000). Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron, 28(3), 713–726. 10.1016/s0896-6273(00)00148-3 [DOI] [PubMed] [Google Scholar]
- Limana, F. , Bertolami, C. , Mangoni, A. , Di Carlo, A. , Avitabile, D. , Mocini, D. , Iannelli, P. , De Mori, R. , Marchetti, C. , Pozzoli, O. , Gentili, C. , Zacheo, A. , Germani, A. , & Capogrossi, M. C. (2010). Myocardial infarction induces embryonic reprogramming of epicardial c‐kit(+) cells: Role of the pericardial fluid. Journal of Molecular and Cellular Cardiology, 48(4), 609–618. 10.1016/j.yjmcc.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Papa, E. F. , Dooner, M. S. , Machan, J. T. , Johnson, K. W. , Goldberg, L. R. , Quesenberry, P. J. , & Colvin, G. A. (2012). Homing and long‐term engraftment of long‐ and short‐term renewal hematopoietic stem cells. PLoS One, 7(2), e31300. 10.1371/journal.pone.0031300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Furno, D. , Mannino, G. , Giuffrida, R. , Gili, E. , Vancheri, C. , Tarico, M. S. , Perrotta, R. E. , & Pellitteri, R. (2018). Neural differentiation of human adipose‐derived mesenchymal stem cells induced by glial cell conditioned media. Journal of Cellular Physiology, 233(10), 7091–7100. 10.1002/jcp.26632 [DOI] [PubMed] [Google Scholar]
- Lutolf, M. P. , & Blau, H. M. (2009). Artificial stem cell niches. Advanced Materials, 21(32‐33), 3255–3268. 10.1002/adma.200802582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngbaek, S. , Schneider, M. , Hansen, J. L. , & Sheikh, S. P. (2007). Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Research in Cardiology, 102(2), 101–114. 10.1007/s00395-007-0638-3 [DOI] [PubMed] [Google Scholar]
- Mackay‐Sim, A. (2010). Stem cells and their niche in the adult olfactory mucosa. Archives Italiennes de Biologie, 148(2), 47–58. [PubMed] [Google Scholar]
- Mannino, G. , Gennuso, F. , Giurdanella, G. , Conti, F. , Drago, F. , Salomone, S. , Furno, D. L. , Bucolo, C. , & Giuffrida, R. (2020). Pericyte‐like differentiation of human adipose‐derived mesenchymal stem cells: An in vitro study. World Journal of Stem Cells, 12(10), 1152–1170. 10.4252/wjsc.v12.i10.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, F. , Scalise, M. , Cianflone, E. , Mancuso, T. , Aquila, I. , Agosti, V. , Torella, M. , Paolino, D. , Mollace, V. , Nadal‐Ginard, B. , & Torella, D. (2019). Role of c‐Kit in myocardial regeneration and aging. Frontiers in Endocrinology, 10, 371. 10.3389/fendo.2019.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauretti, A. , Spaans, S. , Bax, N. A. M. , Sahlgren, C. , & Bouten, C. V. C. (2017). Cardiac progenitor cells and the interplay with their microenvironment. Stem Cells International, 2017, 7471582. 10.1155/2017/7471582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro, A. (1961). Satellite cell of skeletal muscle fibers. Journal of Biophysical and Biochemical Cytology, 9, 493–495. 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson, A. , & Frenette, P. S. (2014). Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature Medicine, 20(8), 833–846. 10.1038/nm.3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez‐Ferrer, S. , Battista, M. , & Frenette, P. S. (2010). Cooperation of beta(2)‐ and beta(3)‐adrenergic receptors in hematopoietic progenitor cell mobilization. Annals of the New York Academy of Sciences, 1192, 139–144. 10.1111/j.1749-6632.2010.05390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez‐Ferrer, S. , Michurina, T. V. , Ferraro, F. , Mazloom, A. R. , Macarthur, B. D. , Lira, S. A. , Scadden, D. T. , Ma'ayan, A. , Enikolopov, G. N. , & Frenette, P. S. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 466(7308), 829–834. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran, L. , Baulies, A. , & Li, V. S. W. (2017). Intestinal stem cell niche: The extracellular matrix and cellular components. Stem Cells International, 2017, 7970385. 10.1155/2017/7970385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miftakhova, R. , Hedblom, A. , Batkiewicz, L. , Anagnosaki, L. , Zhang, Y. , Sjölander, A. , Wingren, A. G. , Wolgemuth, D. J. , & Persson, J. L. (2015). Cyclin A1 regulates the interactions between mouse haematopoietic stem and progenitor cells and their niches. Cell Cycle, 14(12), 1948–1960. 10.1080/15384101.2015.1026513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani, S. , Rueger, M. A. , Hosoda, T. , & Nurzynska, D. (2016). Adult stem cells in tissue maintenance and regeneration. Stem Cells International, 2016, 7362879. 10.1155/2016/7362879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore‐Morris, T. , Guimarães‐Camboa, N. , Banerjee, I. , Zambon, A. C. , Kisseleva, T. , Velayoudon, A. , Stallcup, W. B. , Gu, Y. , Dalton, N. D. , Cedenilla, M. , Gomez‐Amaro, R. , Zhou, B. , Brenner, D. A. , Peterson, K. L. , Chen, J. , & Evans, S. M. (2014). Resident fibroblast lineages mediate pressure overload‐induced cardiac fibrosis. Journal of Clinical Investigation, 124(7), 2921–2934. 10.1172/JCI74783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, P. , Lemcke, H. , & David, R. (2018). Stem cell therapy in heart diseases–cell types, mechanisms and improvement strategies. Cellular Physiology and Biochemistry, 48(6), 2607–2655. 10.1159/000492704 [DOI] [PubMed] [Google Scholar]
- Muroyama, A. , & Lechler, T. (2012). Polarity and stratification of the epidermis. Seminars in Cell and Developmental Biology, 23(8), 890–896. 10.1016/j.semcdb.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci, G. , Castrogiovanni, P. , Coleman, R. , Szychlinska, M. A. , Salvatorelli, L. , Parenti, R. , Magro, G. , & Imbesi, R. (2015). Somitogenesis: From somite to skeletal muscle. Acta Histochemica, 117(4‐5), 313–328. 10.1016/j.acthis.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Myung, P. S. , Takeo, M. , Ito, M. , & Atit, R. P. (2013). Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. Journal of Investigative Dermatology, 133(1), 31–41. 10.1038/jid.2012.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto‐Estevez, V. , Defterali, C. , & Vicario‐Abejon, C. (2016). IGF‐I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Frontiers in Neuroscience, 10, 52. 10.3389/fnins.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse, R. , & Clevers, H. (2017). Wnt/beta‐catenin signaling, disease, and emerging therapeutic modalities. Cell, 169(6), 985–999. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Oh, H. , Bradfute, S. B. , Gallardo, T. D. , Nakamura, T. , Gaussin, V. , Mishina, Y. , Pocius, J. , Michael, L. H. , Behringer, R. R. , Garry, D. J. , Entman, M. L. , & Schneider, M. D. (2003). Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proceedings of the National Academy of Sciences of the United States of America, 100(21), 12313–12318. 10.1073/pnas.2132126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimori, N. , & Fuchs, E. (2012). Paracrine TGF‐beta signaling counterbalances BMP‐mediated repression in hair follicle stem cell activation. Cell Stem Cell, 10(1), 63–75. 10.1016/j.stem.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, T. D. , Willhoite, A. R. , & Gage, F. H. (2000). Vascular niche for adult hippocampal neurogenesis. Journal of Comparative Neurology, 425(4), 479–494. [DOI] [PubMed] [Google Scholar]
- Parker, T. W. , & Neufeld, K. L. (2020). APC controls Wnt‐induced beta‐catenin destruction complex recruitment in human colonocytes. Scientific Reports, 10(1), 2957. 10.1038/s41598-020-59899-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellitteri, R. , Spatuzza, M. , Stanzani, S. , & Zaccheo, D. (2010). Biomarkers expression in rat olfactory ensheathing cells. Frontiers in Bioscience, 2, 289–298. 10.2741/s64 [DOI] [PubMed] [Google Scholar]
- Pentinmikko, N. , & Katajisto, P. (2020). The role of stem cell niche in intestinal aging. Mechanisms of Ageing and Development, 191, 111330. 10.1016/j.mad.2020.111330 [DOI] [PubMed] [Google Scholar]
- Porter, E. M. , Bevins, C. L. , Ghosh, D. , & Ganz, T. (2002). The multifaceted Paneth cell. Cellular and Molecular Life Science, 59(1), 156–170. 10.1007/s00018-002-8412-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop, D. J. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science, 276(5309), 71–74. 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- Putnam, A. J. (2014). The instructive role of the vasculature in stem cell niches. Biomaterials Science, 2(11), 1562–1573. 10.1039/C4BM00200H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Z. , Li, Y. , Zhao, B. , Xu, C. , Liu, Y. , Li, H. , Zhang, B. , Wang, X. , Yang, X. , Xie, W. , Li, B. , Han, J. J. , & Chen, Y. G. (2017). BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nature Communications, 8, 13824. 10.1038/ncomms13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayagiri, S. S. , Ranaldi, D. , Raven, A. , Mohamad Azhar, N. I. F. , Lefebvre, O. , Zammit, P. S. , & Borycki, A. G. (2018). Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self‐renewal. Nature Communications, 9(1), 1075. 10.1038/s41467-018-03425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme, P. A. , Drapeau, E. , & Doetsch, F. (2008). Brain micro‐ecologies: Neural stem cell niches in the adult mammalian brain. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1489), 123–137. 10.1098/rstb.2006.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort, C. , Gheusi, G. , Vincent, J. D. , & Lledo, P. M. (2002). Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. Journal of Neuroscience, 22(7), 2679–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojczyk‐Golebiewska, E. , Palasz, A. , & Wiaderkiewicz, R. (2014). Hypothalamic subependymal niche: A novel site of the adult neurogenesis. Cellular and Molecular Neurobiology, 34(5), 631–642. 10.1007/s10571-014-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy, R. M. , & Morshead, C. M. (2018). Home sweet home: The neural stem cell niche throughout development and after injury. Cell and Tissue Research, 371(1), 125–141. 10.1007/s00441-017-2658-0 [DOI] [PubMed] [Google Scholar]
- Russo, C. , Patanè, M. , Vicario, N. , Di Bella, V. , Cosentini, I. , Barresi, V. , Gulino, R. , Pellitteri, R. , Russo, A. , & Stanzani, S. (2020). Olfactory ensheathing cells express both ghrelin and ghrelin receptor in vitro: A new hypothesis in favor of a neurotrophic effect. Neuropeptides, 79, 101997. 10.1016/j.npep.2019.101997 [DOI] [PubMed] [Google Scholar]
- Ryu, J. R. , Hong, C. J. , Kim, J. Y. , Kim, E. K. , Sun, W. , & Yu, S. W. (2016). Control of adult neurogenesis by programmed cell death in the mammalian brain. Molecular Brain, 9, 43. 10.1186/s13041-016-0224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti, B. , Funari, A. , Michienzi, S. , Di Cesare, S. , Piersanti, S. , Saggio, I. , Tagliafico, E. , Ferrari, S. , Robey, P. G. , Riminucci, M. , & Bianco, P. (2007). Self‐renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell, 131(2), 324–336. 10.1016/j.cell.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Sanada, F. , Kim, J. , Czarna, A. , Chan, N. Y. , Signore, S. , Ogórek, B. , Isobe, K. , Wybieralska, E. , Borghetti, G. , Pesapane, A. , Sorrentino, A. , Mangano, E. , Cappetta, D. , Mangiaracina, C. , Ricciardi, M. , Cimini, M. , Ifedigbo, E. , Perrella, M. A. , Goichberg, P. , … Leri, A. (2014). c‐Kit‐positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circulation Research, 114(1), 41–55. 10.1161/CIRCRESAHA.114.302500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Aguilera, A. , & Mendez‐Ferrer, S. (2017). The hematopoietic stem‐cell niche in health and leukemia. Cellular and Molecular Life Science, 74(4), 579–590. 10.1007/s00018-016-2306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi, E. , & Capecchi, M. R. (2008). Bmi1 is expressed in vivo in intestinal stem cells. Nature Genetics, 40(7), 915–920. 10.1038/ng.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, A. J. M. , Lo, Y. H. , Mah, A. T. , & Kuo, C. J. (2018). The intestinal stem cell niche: Homeostasis and adaptations. Trends in Cell Biology, 28(12), 1062–1078. 10.1016/j.tcb.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, N. , Sachs, N. , Wiebrands, K. , Ellenbroek, S. I. , Fumagalli, A. , Lyubimova, A. , Begthel, H. , van den Born, M. , van Es, J. H. , Karthaus, W. R. , Li, V. S. , López‐Iglesias, C. , Peters, P. J. , van Rheenen, J. , van Oudenaarden, A. , & Clevers, H. (2016). Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proceedings of the National Academy of Sciences of the United States of America, 113(37), E5399–E5407. 10.1073/pnas.1607327113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T. , van Es, J. H. , Snippert, H. J. , Stange, D. E. , Vries, R. G. , van den Born, M. , Barker, N. , Shroyer, N. F. , van de Wetering, M. , & Clevers, H. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature, 469(7330), 415–418. 10.1038/nature09637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg, E. G. (1996). Expectancy in melody: Tests of the implication‐realization model. Cognition, 58(1), 75–125. 10.1016/0010-0277(95)00665-6 [DOI] [PubMed] [Google Scholar]
- Schepeler, T. , Page, M. E. , & Jensen, K. B. (2014). Heterogeneity and plasticity of epidermal stem cells. Development, 141(13), 2559–2567. 10.1242/dev.104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, R. (1978). The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells, 4(1‐2), 7–25. [PubMed] [Google Scholar]
- Sehgal, A. , Donaldson, D. S. , Pridans, C. , Sauter, K. A. , Hume, D. A. , & Mabbott, N. A. (2018). The role of CSF1R‐dependent macrophages in control of the intestinal stem‐cell niche. Nature Communications, 9(1), 1272. 10.1038/s41467-018-03638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib, D. R. , & Martin‐Villalba, A. (2015). Neurogenesis in the normal ageing hippocampus: A mini‐review. Gerontology, 61(4), 327–335. 10.1159/000368575 [DOI] [PubMed] [Google Scholar]
- Seita, J. , & Weissman, I. L. (2010). Hematopoietic stem cell: Self‐renewal versus differentiation. Wiley Interdisciplinary Reviews: Systems Biologyand Medicine, 2(6), 640–653. 10.1002/wsbm.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si, Z. , Wang, X. , Sun, C. , Kang, Y. , Xu, J. , Wang, X. , & Hui, Y. (2019). Adipose‐derived stem cells: Sources, potency, and implications for regenerative therapies. Biomedicine & Pharmacotherapy = Biomédecine & Pharmacothérapie, 114, 108765. 10.1016/j.biopha.2019.108765 [DOI] [PubMed] [Google Scholar]
- Sibbe, M. , Forster, E. , Basak, O. , Taylor, V. , & Frotscher, M. (2009). Reelin and Notch1 cooperate in the development of the dentate gyrus. Journal of Neuroscience, 29(26), 8578–8585. 10.1523/JNEUROSCI.0958-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, A. , Encinas, J. M. , Deudero, J. J. , Chancey, J. H. , Enikolopov, G. , Overstreet‐Wadiche, L. S. , Tsirka, S. E. , & Maletic‐Savatic, M. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell, 7(4), 483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra‐Sanchez, A. , Ordonez‐Luque, A. , Espinosa‐Ibanez, O. , Ruiz‐Garcia, A. , & Arias‐Santiago, S. (2018). Epithelial in vitro differentiation of mesenchymal stem cells. Current Stem Cell Research & Therapy, 13(6), 409–422. 10.2174/1574888X13666180501120416 [DOI] [PubMed] [Google Scholar]
- Simón, D. , Martín‐Bermejo, M. J. , Gallego‐Hernández, M. T. , Pastrana, E. , García‐Escudero, V. , García‐Gómez, A. , Lim, F. , Díaz‐Nido, J. , Avila, J. , & Moreno‐Flores, M. T. (2011). Expression of plasminogen activator inhibitor‐1 by olfactory ensheathing glia promotes axonal regeneration. GLIA, 59(10), 1458–1471. 10.1002/glia.21189 [DOI] [PubMed] [Google Scholar]
- Slack, J. M. (2008). Origin of stem cells in organogenesis. Science, 322(5907), 1498–1501. 10.1126/science.1162782 [DOI] [PubMed] [Google Scholar]
- Snippert, H. J. , van der Flier, L. G. , Sato, T. , van Es, J. H. , van den Born, M. , Kroon‐Veenboer, C. , Barker, N. , Klein, A. M. , van Rheenen, J. , Simons, B. D. , & Clevers, H. (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell, 143(1), 134–144. 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Solano Fonseca, R. , Mahesula, S. , Apple, D. M. , Raghunathan, R. , Dugan, A. , Cardona, A. , O'connor, J. , & Kokovay, E. (2016). Neurogenic niche microglia undergo positional remodeling and progressive activation contributing to age‐associated reductions in neurogenesis. Stem Cells and Development, 25(7), 542–555. 10.1089/scd.2015.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonpaa, M. H. , & Field, L. J. (1998). Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circulation Research, 83(1), 15–26. 10.1161/01.res.83.1.15 [DOI] [PubMed] [Google Scholar]
- Sorrells, S. F. , Paredes, M. F. , Cebrian‐Silla, A. , Sandoval, K. , Qi, D. , Kelley, K. W. , James, D. , Mayer, S. , Chang, J. , Auguste, K. I. , Chang, E. F. , Gutierrez, A. J. , Kriegstein, A. R. , Mathern, G. W. , Oldham, M. C. , Huang, E. J. , Garcia‐Verdugo, J. M. , Yang, Z. , & Alvarez‐Buylla, A. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature, 555(7696), 377–381. 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding, K. L. , Bergmann, O. , Alkass, K. , Bernard, S. , Salehpour, M. , Huttner, H. B. , Boström, E. , Westerlund, I. , Vial, C. , Buchholz, B. A. , Possnert, G. , Mash, D. C. , Druid, H. , & Frisén, J. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153(6), 1219–1227. 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, J. A. , Ferraro, F. , Roussakis, E. , Klein, A. , Wu, J. , Runnels, J. M. , Zaher, W. , Mortensen, L. J. , Alt, C. , Turcotte, R. , Yusuf, R. , Côté, D. , Vinogradov, S. A. , Scadden, D. T. , & Lin, C. P. (2014). Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature, 508(7495), 269–273. 10.1038/nature13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda, T. , Takubo, K. , & Semenza, G. L. (2011). Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell, 9(4), 298–310. 10.1016/j.stem.2011.09.010 [DOI] [PubMed] [Google Scholar]
- Sugiyama, T. , Kohara, H. , Noda, M. , & Nagasawa, T. (2006). Maintenance of the hematopoietic stem cell pool by CXCL12‐CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity, 25(6), 977–988. 10.1016/j.immuni.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Sugiyama, T. , & Nagasawa, T. (2012). Bone marrow niches for hematopoietic stem cells and immune cells. Inflammation & Allergy Drug Targets, 11(3), 201–206. 10.2174/187152812800392689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykaras, A. G. , Demenis, C. , Cheng, L. , Pisitkun, T. , McLaughlin, J. T. , Fenton, R. A. , & Smith, C. P. (2014). Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology, 155(9), 3339–3351. 10.1210/en.2013-2165 [DOI] [PubMed] [Google Scholar]
- Szychlinska, M. A. , Calabrese, G. , Ravalli, S. , Parrinello, N. L. , Forte, S. , Castrogiovanni, P. , Pricoco, E. , Imbesi, R. , Castorina, S. , Leonardi, R. , Di Rosa, M. , & Musumeci, G. (2020). Cycloastragenol as an exogenous enhancer of chondrogenic differentiation of human adipose‐derived mesenchymal stem cells. A morphological study. Cells, 9(2). 10.3390/cells9020347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie, M. , Van der Veken, L. , Silva‐Vargas, V. , Louissaint, M. , Colonna, L. , Zaidi, B. , Garcia‐Verdugo, J. M. , & Doetsch, F. (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell, 3(3), 279–288. 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavian, M. , Zheng, B. , Oberlin, E. , Crisan, M. , Sun, B. , Huard, J. , & Peault, B. (2005). The vascular wall as a source of stem cells. Annals of the New York Academy of Sciences, 1044, 41–50. 10.1196/annals.1349.006 [DOI] [PubMed] [Google Scholar]
- Teller, I. C. , & Beaulieu, J. F. (2001). Interactions between laminin and epithelial cells in intestinal health and disease. Expert Reviews in Molecular Medicine, 3(24), 1–18. 10.1017/S1462399401003623 [DOI] [PubMed] [Google Scholar]
- Urbanek, K. , Cesselli, D. , Rota, M. , Nascimbene, A. , De Angelis, A. , Hosoda, T. , Bearzi, C. , Boni, A. , Bolli, R. , Kajstura, J. , Anversa, P. , & Leri, A. (2006). Stem cell niches in the adult mouse heart. Proceedings of the National Academy of Sciences of the United States of America, 103(24), 9226–9231. 10.1073/pnas.0600635103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi, R. J. , Maillet, M. , Sargent, M. A. , Khalil, H. , Johansen, A. , Schwanekamp, J. A. , York, A. J. , Huang, V. , Nahrendorf, M. , Sadayappan, S. , & Molkentin, J. D. (2020). An acute immune response underlies the benefit of cardiac stem cell therapy. Nature, 577(7790), 405–409. 10.1038/s41586-019-1802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo, J. H. , & Molkentin, J. D. (2014). An emerging consensus on cardiac regeneration. Nature Medicine, 20(12), 1386–1393. 10.1038/nm.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oorschot, A. A. , Smits, A. M. , Pardali, E. , Doevendans, P. A. , & Goumans, M. J. (2011). Low oxygen tension positively influences cardiomyocyte progenitor cell function. Journal of Cellular and Molecular Medicine, 15(12), 2723–2734. 10.1111/j.1582-4934.2011.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet, P. , Roccio, M. , Smits, A. M. , van Oorschot, A. A. , Metz, C. H. , van Veen, T. A. , Sluijter, J. P. , Doevendans, P. A. , & Goumans, M. J. (2008). Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 16(5), 163–169. 10.1007/BF03086138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancheri, C. , Mastruzzo, C. , Trovato‐Salinaro, E. , Gili, E. , Lo Furno, D. , Pistorio, M. P. , Caruso, M. , La Rosa, C. , Crimi, C. , Failla, M. , & Crimi, N. (2005). Interaction between human lung fibroblasts and T‐lymphocytes prevents activation of CD4+ cells. Respiratory Research, 6, 103. 10.1186/1465-9921-6-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltri, A. , Lang, C. , & Lien, W. H. (2018). Concise review: Wnt signaling pathways in skin development and epidermal. Stem Cells, 36(1), 22–35. 10.1002/stem.2723 [DOI] [PubMed] [Google Scholar]
- Veniaminova, N. A. , Grachtchouk, M. , Doane, O. J. , Peterson, J. K. , Quigley, D. A. , Lull, M. V. , Pyrozhenko, D. V. , Nair, R. R. , Patrick, M. T. , Balmain, A. , Dlugosz, A. A. , Tsoi, L. C. , & Wong, S. Y. (2019). Niche‐specific factors dynamically regulate sebaceous gland stem cells in the skin. Developmental Cell, 51(3), 326–340. 10.1016/j.devcel.2019.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, D. , Zanetti, C. , Godavarthy, P. S. , Kumar, R. , Minciacchi, V. R. , Pfeiffer, J. , Metzler, M. , Lefort, S. , Maguer‐Satta, V. , Nicolini, F. E. , Burroni, B. , Fontenay, M. , & Krause, D. S. (2020). Bone marrow niche‐derived extracellular matrix‐degrading enzymes influence the progression of B‐cell acute lymphoblastic leukemia. Leukemia, 34(6), 1540–1552. 10.1038/s41375-019-0674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, M. , Asakura, Y. , Murakonda, B. , Pengo, T. , Latroche, C. , Chazaud, B. , Mcloon, L. K. , & Asakura, A. (2018). Muscle satellite cell cross‐talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell, 23(4), 530–543. 10.1016/j.stem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario, N. , Calabrese, G. , Zappalà, A. , Parenti, C. , Forte, S. , Graziano, A. , Vanella, L. , Pellitteri, R. , Cardile, V. , & Parenti, R. (2017). Inhibition of Cx43 mediates protective effects on hypoxic/reoxygenated human neuroblastoma cells. Journal of Cellular and Molecular Medicine, 21(10), 2563–2572. 10.1111/jcmm.13177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicinanza, C. , Aquila, I. , Scalise, M. , Cristiano, F. , Marino, F. , Cianflone, E. , Mancuso, T. , Marotta, P. , Sacco, W. , Lewis, F. C. , Couch, L. , Shone, V. , Gritti, G. , Torella, A. , Smith, A. J. , Terracciano, C. M. , Britti, D. , Veltri, P. , Indolfi, C. , … Torella, D. (2017). Adult cardiac stem cells are multipotent and robustly myogenic: c‐kit expression is necessary but not sufficient for their identification. Cell Death and Differentiation, 24(12), 2101–2116. 10.1038/cdd.2017.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn, J. , Jones, A. E. , Parks, R. J. , & Rudnicki, M. A. (2013). Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America, 110(41), 16474–16479. 10.1073/pnas.1307680110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic, K. , Sandstedt, M. , Jonsson, M. , Jansson, M. , Oldfors, A. , Jeppsson, A. , Dellgren, G. , Lindahl, A. , & Sandstedt, J. (2019). The atrioventricular junction: A potential niche region for progenitor cells in the adult human heart. Stem Cells and Development, 28(16), 1078–1088. 10.1089/scd.2019.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, R. M. (2012). Postnatal neurogenesis: Of mice, men, and macaques. Veterinary Pathology, 49(1), 155–165. 10.1177/0300985811414035 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Hu, Q. , Nakamura, Y. , Lee, J. , Zhang, G. , From, A. H. , & Zhang, J. (2006). The role of the sca‐1+/CD31‐ cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells, 24(7), 1779–1788. 10.1634/stemcells.2005-0386 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Kim, R. , Hinman, S. S. , Zwarycz, B. , Magness, S. T. , & Allbritton, N. L. (2018). Bioengineered systems and designer matrices that recapitulate the intestinal stem cell niche. Cellular and Molecular Gastroenterology and Hepatology, 5(3), 440–453. 10.1016/j.jcmgh.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A. , Laurenti, E. , Oser, G. , van der Wath, R. C. , Blanco‐Bose, W. , Jaworski, M. , Offner, S. , Dunant, C. F. , Eshkind, L. , Bockamp, E. , Lió, P. , Macdonald, H. R. , & Trumpp, A. (2008). Hematopoietic stem cells reversibly switch from dormancy to self‐renewal during homeostasis and repair. Cell, 135(6), 1118–1129. 10.1016/j.cell.2008.10.048 [DOI] [PubMed] [Google Scholar]
- Xie, W. , & Zhou, J. (2017). Regulation of mitotic spindle orientation during epidermal stratification. Journal of Cellular Physiology, 232(7), 1634–1639. 10.1002/jcp.25750 [DOI] [PubMed] [Google Scholar]
- Yamashita, Y. M. , & Fuller, M. T. (2005). Asymmetric stem cell division and function of the niche in the Drosophila male germ line. International Journal of Hematology, 82(5), 377–380. 10.1532/IJH97.05097 [DOI] [PubMed] [Google Scholar]
- Yan, H. , Gao, Y. , Ding, Q. , Liu, J. , Li, Y. , Jin, M. , Xu, H. , Ma, S. , Wang, X. , Zeng, W. , & Chen, Y. (2019). Exosomal micro RNAs derived from dermal papilla cells mediate hair follicle stem cell proliferation and differentiation. International Journal of Biological Sciences, 15(7), 1368–1382. 10.7150/ijbs.33233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartseva, V. , Goldstein, L. D. , Rodman, J. , Kates, L. , Chen, M. Z. , Chen, Y. J. , Foreman, O. , Siebel, C. W. , Modrusan, Z. , Peterson, A. S. , & Jovičić, A. (2020). Heterogeneity of satellite cells implicates DELTA1/NOTCH2 signaling in self‐renewal. Cell Reports, 30(5), 1491–1503. 10.1016/j.celrep.2019.12.100 [DOI] [PubMed] [Google Scholar]
- Yin, H. , Price, F. , & Rudnicki, M. A. (2013). Satellite cells and the muscle stem cell niche. Physiological Reviews, 93(1), 23–67. 10.1152/physrev.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, J. , Choi, S. C. , Park, C. Y. , Shim, W. J. , & Lim, D. S. (2007). Cardiac side population cells exhibit endothelial differentiation potential. Experimental & Molecular Medicine, 39(5), 653–662. 10.1038/emm.2007.71 [DOI] [PubMed] [Google Scholar]
- Yu, V. W. , & Scadden, D. T. (2016). Hematopoietic stem cell and its bone marrow niche. Current Topics in Developmental Biology, 118, 21–44. 10.1016/bs.ctdb.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti, C. , & Krause, D. S. (2020). “Caught in the net”: The extracellular matrix of the bone marrow in normal hematopoiesis and leukemia. Experimental Hematology, 89, 13–25. 10.1016/j.exphem.2020.07.010 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Moats‐Staats, B. M. , Ye, P. , & D'Ercole, A. J. (2007). Expression of insulin‐like growth factor system genes during the early postnatal neurogenesis in the mouse hippocampus. Journal of Neuroscience Research, 85(8), 1618–1627. 10.1002/jnr.21289 [DOI] [PMC free article] [PubMed] [Google Scholar]


