Abstract
Lovastatin is a secondary metabolite produced by Aspergillus terreus. A chemically defined medium was developed in order to investigate the influence of carbon and nitrogen sources on lovastatin biosynthesis. Among several organic and inorganic defined nitrogen sources metabolized by A. terreus, glutamate and histidine gave the highest lovastatin biosynthesis level. For cultures on glucose and glutamate, lovastatin synthesis initiated when glucose consumption levelled off. When A. terreus was grown on lactose, lovastatin production initiated in the presence of residual lactose. Experimental results showed that carbon source starvation is required in addition to relief of glucose repression, while glutamate did not repress biosynthesis. A threefold-higher specific productivity was found with the defined medium on glucose and glutamate, compared to growth on complex medium with glucose, peptonized milk, and yeast extract.
In filamentous fungi many secondary metabolites with complex chemical structure are synthesized via the polyketide pathway (15, 33, 37). Lovastatin, monacolin J, monacolin L, and mevastatin can be produced by Monascus ruber (7), Penicillium brevicompactum, and Aspergillus terreus (1, 36). Lovastatin is an inhibitor of the enzyme hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase (mevalonate:NADP+ oxidoreductase [EC 1.1.1.34]) that catalyzes the reduction of HMG-CoA to mevalonate during synthesis of cholesterol (14, 23, 36). The biosynthetic pathway of lovastatin in A. terreus has been investigated by nuclear magnetic resonance and mass spectroscopy (5, 26, 38). These studies concluded that lovastatin is composed of two distinct polyketide chains joined through an ester linkage. Proof that these two polyketides are assembled by two discrete polyketide synthases came from the cloning and partial characterization of the lovastatin biosynthetic gene cluster from A. terreus (16, 18).
Despite the knowledge of the genes and the enzymes involved in the biosynthetic pathway, little is known about the regulation and the physiology of lovastatin biosynthesis. Reported growth and production conditions for lovastatin are from batch fermentations performed on media with glucose and a complex nitrogen source (1, 4, 13, 22, 28). Both carbon and nitrogen sources are thought to exert complex regulation on gene expression and enzyme activities for polyketide synthesis, possibly at the level of catabolite repression or signaling due to limitation in growth rate or substrate limitation.
The objective of this work was to investigate the influence of glucose and nitrogen source on the physiology of A. terreus and lovastatin production. Several carbon and nitrogen sources were tested in order to develop a chemically defined medium. The aim was to monitor biomass formation and lovastatin production in relation to the consumption pattern of carbon and nitrogen sources. We show here that although growth occurred on a large variety of substrates, the choice of nitrogen source had a major impact on lovastatin production. In addition, lovastatin biosynthesis was initiated only when assimilation of the carbon source was limited.
MATERIALS AND METHODS
Microorganism and growth conditions.
Stock culture of A. terreus Thom ATCC 74135 was maintained on potato dextrose agar (Oxoid). The slants were stored at 4°C after growth on potato dextrose agar for 8 days at 28°C. Conidiospores were harvested with sterile solution (0.9% NaCl, 0.05% Tween 80), washed twice with sterile buffer (20 mM KH2PO4, adjusted to pH 2.0 with HCl), and enumerated.
Culture on complex medium.
A 250-ml Erlenmeyer flask containing 40 ml of medium A [10 g of glucose, 5 g of corn steep liquor (Sigma catalog no. C-4648), 40 g of tomato paste, 10 g of oatmeal, and 10 ml of trace elements—1 g of FeSO4 · 7H2O, 1 g of MnSO4 · 4H2O, 200 mg of ZnSO4 · 7H2O, 100 mg of CaCl2 · 2H2O, 25 mg of CuCl2 · 2H2O, 56 mg of H3BO3, and 19 mg of (NH4)6Mo7O24 · 4H2O—per liter of solution (1)] was inoculated with 2 · 106 conidiospores. The flasks were shaken at 220 rpm for 1 day at 28°C. A second culture was prepared by inoculating 200 ml of medium B (containing [per liter] 45 g of glucose or lactose, 24 g of peptonized milk [Oxoid catalog no. LP032J], 2.5 g of yeast extract [Difco catalog no. 0127-17], 2.5 g of polyethylene glycol P2000 [Fluka Chemika catalog no. 81221] [1]) with 6 ml of the previous culture in a 1-liter Erlenmeyer flask. The flasks were shaken at 200 rpm and incubated at 28°C for 12 days. For cultivations in fermentors, a 1-liter Erlenmeyer flask containing 200 ml of medium A was inoculated with 4 ml (107) of conidiospore suspension. The flask was shaken at 220 rpm for 1 day and then transferred to a fermentor. Batch cultivations were performed in duplicate at 28°C in a 7- or 15-liter bioreactor (New MBR, Zurich, Switzerland) with a culture volume of 4 or 6 liters of medium B, respectively. The reactor was inoculated with 3% inoculum. An overpressure of 0.3 · 105 Pa was applied to the reactor. Dissolved oxygen was continuously monitored with an oxygen probe (Mettler Toledo, Greifensee, Switzerland). The stirrer speed was kept at 400 rpm, and the airflow rate was controlled at 0.4 or 1 vol/vol/min (vvm) use of a mass flow meter (Bronkhorst, Ruurlo, The Netherlands).
Culture on synthetic medium.
The chemically defined fermentation medium developed in this study contained (per liter of distilled water) the following: 45 g of glucose (Fluka Chemika catalog no. 49159), 12.5 g of mono-hydrate sodium glutamate (Merck catalog no. 6445), 5 g of KH2PO4 (Merck catalog no. 104873), 5 g of K2HPO4 (Merck catalog no. 105101), 0.2 g of FeSO4 · 7H2O (Merck catalog no. 3965), 0.1 g of MnSO4 · 4H2O (Merck catalog no. 102786), 0.2 g of ZnSO4 · 7H2O (Merck catalog no. 8883), 0.1 g of MgSO4 · 7H2O (Fluka Chemika catalog no. 63138), 20 mg of CaCl2 · 2H2O (Fluka Chemika catalog no. 21097), 5 mg of CuCl2 · 2H2O (Merck catalog no. 2791), 11 mg of H3BO3 (Merck catalog no. 100165), and 5 mg of (NH4)6Mo7O24 · 4H2O (Merck catalog no. 101182). The pH of the medium was adjusted to 6.5 with HCl (2 N) or KOH (2 N). A suspension of 107 conidiospores was used to inoculate a 1-liter Erlenmeyer flask containing 200 ml of medium with 10 g of glucose liter−1. The inoculum was agitated at 200 rpm and incubated at 28°C for 1 day and then transferred to a fermentor containing 4.5 liters of synthetic medium. The cultivations were performed at a temperature of 28°C, an agitation rate of 400 rpm, and a volumetric aeration rate of 1 vvm.
Analytical methods.
Biomass was determined by gravimetric analysis after filtration of cell samples through preweighed nylon filters (45-mm diameter; pore size, 0.8 μm) and dried at 95°C to a constant weight. Glucose, lactose, ethanol, and organic acid concentrations in the filtrate were determined by high-performance liquid chromatography (HPLC) (HPX-87H+ column; temperature, 35°C; mobile phase, 5 mM H2SO4; flow rate, 0.6 ml min−1; detection at 210 nm with a diode-array detector). The concentration of amino acids in the medium was determined by HPLC (Hewlett-Packard) on an AccQ Tag column (3.9 by 150 mm; Waters catalog no. WATO 52885), after derivatization of samples by 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (Waters AccQ-Fluor method). The two eluents were 10% AccQ Tag (Waters catalog no. WATO 52890) (eluent A) and 60% acetonitrile (eluent B), and the flow rate was 1 ml min−1. The following separation gradient was applied to the first solvent: 0% eluent A to 100% eluent B within 49 min and 100% eluent A to 0% eluent B within 11 min. The detector used was a HP 1046A fluorimetric detector (λEX = 250 nm and λEM = 395 nm).
Lovastatin was determined by HPLC. A Nucleosil 100-5 C18 column (250 by 4 mm; Macherey & Nagel) was used with a precolumn (Lichrospher 100 RP-18; Merck). Solvent A was 0.05% H3PO4 in water, and solvent B was acetonitrile. The separation gradient was linear, starting with 95% solvent A and 5% solvent B, reaching 50% solvent A and 50% solvent B in 45 min, 30% solvent A and 70% solvent B in 46 min, 10% solvent A and 90% solvent B in 48 min, and 0% solvent A and 100% solvent B in 50 min and finally continued with an isocratic run for 4 min. Initial conditions were maintained for 6 min to reequilibrate the column. The flow rate was 1 ml min−1. The absorption was measured at a wavelength of 254 nm (Hewlett Packard G 1315 A, series 1100 detector). The detection level in sample broth was 1 mg liter−1, and we have confirmed the presence of lovastatin in the filtrate by mass spectroscopy. For all HPLC methods, identities of metabolites were confirmed by comparison of retention times with standards.
RESULTS
Batch fermentation of A. terreus in complex medium.
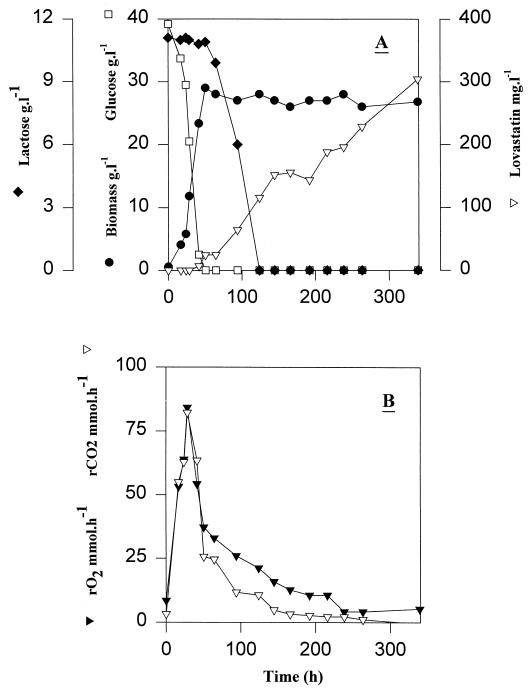
A batch fermentation of A. terreus grown on complex medium containing (per liter) 45 g of glucose, 24 g of peptonized milk, and 2.5 g of yeast extract, is depicted in Fig. 1. Lactose (11 g liter−1) detected at the beginning of the cultivation was derived from peptonized milk. Glucose was exhausted after 45 h of cultivation, while 11 g of lactose liter−1 remained in the medium (Fig. 1A). pH decreased from 6.5 to 4.9 during the phase of glucose consumption; thereafter pH increased slowly and reached 6.9 by the end of the culture. At the time of glucose exhaustion the biomass concentration was 24 g liter−1, corresponding to a biomass yield on glucose of 0.55 g g−1. During the glucose consumption phase, the specific growth rate, μ, was 0.07 h−1, and the specific glucose consumption rate, qglucose, was 0.13 g g−1 h−1. Lactose was consumed upon glucose exhaustion, and the biomass concentration reached 28 g liter−1. Ethanol and CO2 were the main fermentation products, while acetate was produced at a very low level only. Ethanol accumulated in the medium during the period of rapid glucose consumption. The maximal ethanol concentration was 1.3 g liter−1 after 42 h of cultivation. Ethanol was consumed after glucose and was exhausted after 80 h of cultivation. The gas exchange rates (Fig. 1B) increased during biomass formation and reached a maximum value at the time of glucose exhaustion.
FIG. 1.
Fermentation of A. terreus in complex medium with glucose (45 g liter−1), peptonized milk (24 g liter−1), and yeast extract (2.5 g liter−1) at an aeration rate of 1 vvm and agitation speed of 400 rpm. (A) Symbols: □, glucose; ●, biomass; ⧫, lactose; ▿, lovastatin. (B) Symbols: ▾, oxygen consumption rate (rO2); ▿, carbon dioxide production rate (rCO2).
Lovastatin production started after approximately 45 h of cultivation (Fig. 1A) when the residual glucose concentration was 2.5 g liter−1 and reached a concentration of 304 mg liter−1 by the end of the fermentation (350 h). The specific productivity, qlovastatin, was 0.034 g g−1 h−1. Neither monacolin J, monacolin L, nor mevastatin was detected in the fermentation broth.
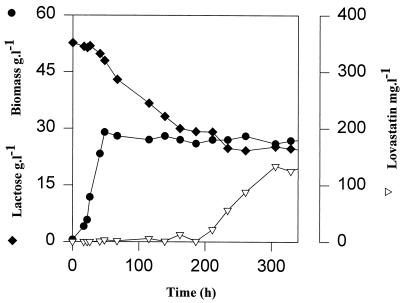
In a second experiment, lactose was tested as the sole nonrepressing carbon source (Fig. 2), with an initial concentration of 53 g liter−1 (no glucose was initially present in medium). Biomass was built up within 50 h, and from 50 to 200 h lactose was slowly consumed. After 200 h no lactose consumption was observed. The residual lactose concentration was about 25 g liter−1, and lovastatin started to be produced at a constant rate of 1 mg liter−1 h−1 until the end of the fermentation (250 h). Yeast extract and peptonized milk were the main substrates required for biomass formation. In shake flask experiments, biomass could be formed without addition of glucose or lactose to the complex medium (results not shown).
FIG. 2.
Fermentation of A. terreus in complex medium with lactose (53 g liter−1), peptonized milk (24 g liter−1), and yeast extract (2.5 g liter−1) at an aeration rate of 1 vvm and agitation speed of 400 rpm. ⧫, lactose; ●, biomass; ▿, lovastatin.
Influence of nitrogen source in chemically defined medium.
To investigate the effect of the substrates on lovastatin production, a defined medium with a sole carbon source and a sole nitrogen source was developed. Several inorganic and organic nitrogen sources were tested in shake flask cultures using a defined medium with 45 g of glucose liter−1 as the carbon source (Table 1). Ammonia, which plays a central role in nitrogen metabolism in filamentous fungi (2), was tested beside urea and nitrate, which can be consumed by some fungi (11). A. terreus grew on all nitrogen sources listed in Table 1, indicating their consumption. Glucose was exhausted after 140 h in all experiments except for that with ammonium acetate (Table 1). Although inorganic nitrogen sources like ammonium tartrate, ammonium nitrate, ammonium acetate, sodium nitrate, or urea were consumed for biomass formation, lovastatin production was very poor after 140 h of cultivation (Table 1 [first five rows of data]).
TABLE 1.
Influence of nitrogen source on lovastatin productiona
| N source | Initial concn (g liter−1) | Residual concn (g liter−1) | Residual glucose concn (g liter−1) | Lovastatin production (mg liter−1) |
|---|---|---|---|---|
| Di-ammonium tartrate | 13.7 | —b | 0 | <1 |
| Ammonium nitrate | 4 | — | 0 | <1 |
| Ammonium acetate | 11 | — | 2.5 | <1 |
| Sodium nitrate | 4 | — | 0 | <1 |
| Urea | 4.5 | — | 0 | <1 |
| Sodium glutamate | 7 | 0.1 | 0 | 25 |
| Sodium glutamate | 12.5 | 0.4 | 0 | 47 |
| Sodium glutamate | 18 | 6.5 | 0 | 39 |
| Histidine | 12.5 | 6.0 | 0 | 46 |
| Glycine | 12.5 | 0.0 | 0 | 17 |
| Arginine | 12.5 | 7.0 | 0 | 1.5 |
| Isoleucine | 12.5 | 2.0 | 0 | <1 |
After cultivation for 140 h in shake flask in chemically defined medium with glucose (45 g liter−1).
—, not measured.
Amino acids can act both as a nitrogen source and a carbon source in filamentous fungi. Since no free ammonium could be detected in the supernatants (data not shown) during growth on amino acids, we assume that they were primarily used as a nitrogen source. Best lovastatin production was obtained with cultures grown on sodium glutamate (12.5 g liter−1) or histidine (12.5 g liter−1) (Table 1). A glutamate concentration of 12.5 g liter−1 was more favorable than 7 or 18 g liter−1 (Table 1). Glycine, arginine, and isoleucine (Table 1) were assimilated but gave poor lovastatin production. Because glutamate was consumed twice as fast as histidine during batch experiments (data not shown), glutamate was chosen as the nitrogen source for the chemically defined medium in order to allow rapid biomass formation.
Influence of carbon source in chemically defined medium.
In defined medium, the carbon and nitrogen sources play a critical role as a source of precursors and cofactors for synthesis of biomass building blocks and lovastatin production. In addition, the carbon source may exert complex regulation on gene expression and enzyme activities for polyketide synthesis. Lactose, glycerol, and ethanol as the sole C source or combined with glucose were tested in shake flasks cultures with sodium glutamate (12.5 g liter−1) as the nitrogen source. Lovastatin concentration, residual carbon source, and residual glutamate concentrations were measured 160 h after inoculation.
All single and combinations of carbon sources could be assimilated by A. terreus (Table 2). However, when used as the unique carbon source, lactose and ethanol were not completely exhausted after 140 h of cultivation in shake flasks (Table 2). The highest lovastatin production was obtained in the experiment with 20 or 45 g of glucose liter−1 and for growth on 45 g of lactose liter−1 (Table 2). Varying the initial glucose concentration from 20 to 45 g liter−1 had little effect on lovastatin concentration. By contrast, increasing the initial glucose concentration to 70 g liter−1 led to a significant decrease in lovastatin production (Table 2). After 160 h of culture, only traces of lovastatin were detected and 31 mg liter−1 were produced after 340 h. As observed during a batch experiment on complex medium with lactose (Fig. 2), lovastatin production was detected on defined medium at high residual lactose concentrations (Table 2).
TABLE 2.
Influence of carbon source on lovastatin productiona
| C sourcesb | Initial C source concn (g liter−1)b | Residual C source concn (g liter−1)b | Residual glutamic acid concn (g liter−1) | Lovastatin production (mg liter−1) |
|---|---|---|---|---|
| Glucose | 20 | 0 | 0 | 37 |
| Glucose | 45 | 0 | 0.4 | 35 |
| Glucose | 70 | 0 | 2.5 | <2 |
| Lactose | 45 | 6.3 | 0 | 25 |
| Glycerol | 20 | 0.3 | 0.2 | 6 |
| Ethanol | 20 | 8.1 | 4.4 | 4 |
| Lactose plus glucose | 20 and 20 | 11.3 and 0 | 0.4 | 54 |
| Glycerol plus glucose | 20 and 20 | 0.3 and 0 | 1.4 | 33 |
| Ethanol plus glucose | 20 and 20 | 12.2 and 0 | 0.3 | 14 |
After cultivation for 160 h in shake flask in chemically defined medium with glutamic acid (9.8 g liter−1) as N source (supplied as mono-hydrate sodium glutamate).
Respective concentrations are indicated when two substrates were used.
Ethanol and glycerol are reduced substrates that generate NADH, which is required for the biosynthesis of polyketides (27, 37). However, these two substrates proved to be very poor substrates for lovastatin production (Table 2). Furthermore, consumption of ethanol was very slow. Also a combination of glucose with ethanol resulted in poor lovastatin production (Table 2). Combination of glycerol with glucose (20 g liter−1 each) gave results similar to those of glucose alone (37 and 33 mg liter−1, respectively) (Table 2). By contrast, a combination of lactose with glucose (20 g liter−1 each) (Table 2) gave almost as much as the sum of glucose and lactose alone (54 compared to 37 and 25 mg liter−1, respectively). This indicates that a combination of both a rapidly and a slowly metabolized sugar may be beneficial to lovastatin production.
Batch cultures of A. terreus in chemically defined medium.
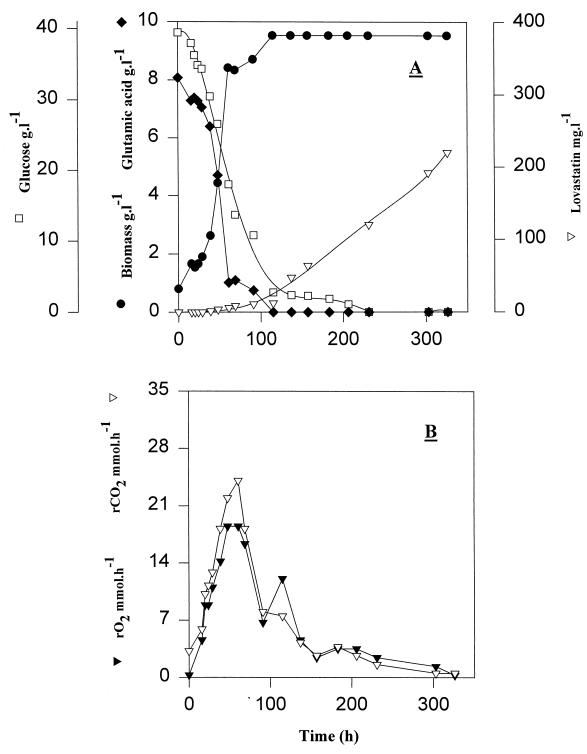
A batch cultivation experiment was performed on synthetic medium containing 45 g of glucose liter−1 and 12.5 g of sodium glutamate liter−1 as unique carbon and nitrogen sources, respectively. Fig. 3A shows that glutamate was exhausted after about 60 h of cultivation, while glucose was exhausted after about 130 h. No ammonium was detected in the culture supernatant throughout the experiment. The biomass concentration reached 9.5 g liter−1 at the time of glutamate exhaustion. On synthetic medium the specific growth rate was lower than on complex medium (0.052 and 0.07 h−1, respectively), and biomass yield on glucose was lower (0.25 and 0.55 g g−1, respectively), resulting in a period of glucose consumption three times longer than that on complex medium. The glucose specific consumption rate, qglucose, was 0.196 g g−1 h−1, and the glutamate specific consumption rate, qglutamate, was 0.045 g g−1 h−1. Metabolism remained purely oxidative. Both the oxygen uptake rate and the carbon dioxide production rate increased during the fermentation (Fig. 3C). Maximal values were observed after 61 h of cultivation when glutamate became limiting; the oxygen uptake rate reached 18 mmol h−1, and the carbon dioxide production rate reached 24 mmol h−1. Gas exchange rates were lower than those during growth on complex medium because glucose was metabolized significantly slower.
FIG. 3.
Fermentation of A. terreus in chemically defined medium with glucose (40 g liter−1) and glutamic acid (9.8 g liter−1) at an aeration rate of 1 vvm and agitation speed of 400 rpm. (A) Symbols: □, glucose; ●, biomass; ⧫, glutamic acid; ▿, lovastatin. (B) Symbols: ▾, oxygen consumption rate rO2; ▿, carbon dioxide production rate rCO2.
Production of lovastatin in synthetic medium initiated when glucose concentration dropped below 10 g liter−1 (Fig. 3A). The specific production rate of lovastatin reached 0.093 mg g−1 h−1 after glucose exhaustion, i.e., about three times higher than that on complex medium. A concentration of 220 mg liter−1 was measured after 13 days of cultivation. No monacolin J, monacolin L, or mevastatin was detected by mass spectroscopy (results not shown).
In order to determine whether lovastatin could be produced in the presence of residual glutamate, the initial glucose concentration was set to 20 g liter−1, while the initial glutamate concentration was kept to 12.5 g liter−1. Lovastatin started to accumulate slowly when the residual glucose concentration was about 10 g liter−1. More than 5 g of glutamate liter−1 remained in the culture supernatant when glucose exhaustion occurred. At that time point the rate of lovastatin biosynthesis increased while glutamate was still present in culture supernatant (data not shown).
DISCUSSION
In filamentous fungi, biosynthesis of secondary metabolites is subject to complex regulations. This study focused on the influence of N and C sources on the regulation of lovastatin biosynthesis in A. terreus. Under all cultivation conditions, in complex and defined media, A. terreus produced neither monacolin J nor monacolin L nor mevastatin.
Since growth requirements of A. terreus are limited, a chemically defined medium could be developed. The buffer capacity of this medium allowed us to keep the pH between 6.2 and 7.0. In the defined medium, all nitrogen sources tested (Table 2) were consumed, which was confirmed by biomass formation. However, lovastatin biosynthesis proved dependent on the nitrogen source, as no lovastatin was detected during cultivation in presence of ammonium, nitrate, or urea (Table 1). Only glutamate and histidine, and to a lesser extent glycine, supported lovastatin biosynthesis. Study of NAD- and NADP-glutamate dehydrogenase showed that glutamate and histidine play a key role in generation of idiophase conditions by the formation of α-ketoglutarate, which stimulates aflatoxin formation by inhibition of the tricarboxylic acid cycle (3). The influence of nitrogen sources on secondary metabolism has been illustrated for fumonisin B1 biosynthesis in Gibberella fujikuroi (32), for sterigmatocystin (10) and aflatoxin (6, 20) biosynthesis in Aspergillus nidulans and Aspergillus parasiticus, and for penicillin synthesis in Penicillium chrysogenum (9).
During growth on complex medium (glucose, peptonized milk, and yeast extract), glucose was rapidly metabolized under high oxygen consumption and biomass was formed (Fig. 1). Ethanol was also formed, probably as result of a glycolytic overflow. Lactose and ethanol were consumed only after glucose exhaustion. The onset of lovastatin biosynthesis after glucose consumption can be attributed either to relief from carbon catabolite repression or to carbon source limitation. Carbon catabolite repression in Aspergillus is mediated by the negative-acting creA gene product (30, 35). The binding of protein CreAp to specific promoter sites of structural or regulatory genes involved in the utilization of alternative carbon sources, prevents their expression in the presence of glucose or another repressing carbon source (e.g., for alc genes see reference 29 and for endoglucanase encoded by egl1 see reference 12). The utilization of ethanol in A. nidulans is repressed by double lock control by CreAp of activator alcR and structural gene alcA, encoding alcohol dehydrogenase I (19). The lovastatin biosynthetic gene cluster consists of 18 putative open reading frames (ORFs) (18), among which 2 were annotated to encode regulatory proteins, lovE and ORF 13. Analysis of the lovastatin biosynthetic gene cluster revealed that closely spaced SYGGRG consensus sequences, the motif of functional CreAp binding sites in vivo (25), are present in the 5′-proximal region of ORF 13 [SYGGRG(N)15SYGGRG] and in the putative promoter of the divergently transcribed ORF 8 and lovE [SYGGRG(N)7CYCCRW]. The presence of putative functional CreAp binding sites in two putative regulatory genes suggests that repression of lovastatin biosynthesis by glucose could be mediated by CreAp. Other carbon catabolite repression mechanisms might also be involved. Glucose represses penicillin synthesis in P. chrysogenum (9) by repressing pcbAB, pcbC, and penD, whereas in A. nidulans only pcbC is strongly repressed by glucose. In both species glucose repression of penicillin biosynthesis is not exclusively mediated by creA (8) but is mediated by another putative DNA-binding protein (24).
Batch experiments on lactose as the unique carbon source showed that lactose was consumed by A. terreus (Fig. 2) but consumption stopped when the residual concentration was 25 g liter−1. Lovastatin biosynthesis was low during ethanol and glycerol consumption in shake flasks (Table 2). As nonrepressing carbon sources, lactose, ethanol, and glycerol cannot activate CreAp. Interestingly, lovastatin biosynthesis started only when lactose consumption had stopped. This suggests that lovastatin synthesis was elicited at the cessation of substrate consumption or growth limitation, i.e., under starvation conditions. The role of starvation in secondary metabolism was illustrated in Trichoderma atroviride, in which chitinase gene ech42 is only expressed after glucose or glycerol starvation (21). An implication of starvation as an eliciting factor has been demonstrated for the induction of sterigmatocystin in A. nidulans (17, 34). The transcriptional regulator aflR is regulated by flbA, fluG, and aflR, showing that both asexual sporulation and sterigmatocystin require inactivation of proliferative growth through inhibition of fadA-dependent signaling (17). In A. nidulans, brlA plays a central role in the switch from vegetative growth to sporulation, and glucose starvation induces high expression levels of brlA (34).
A connection between secondary metabolism and sporulation was demonstrated in Streptomyces griseus, where a γ-butyrolactone-containing compound called A factor binds the ArpA DNA-binding protein, which in turn is no longer able to function as the negative regulator of genes required for sporulation and antibiotic production (31). These authors showed that addition of butyrolactone I (a γ-butyrolactone-containing compound inhibiting cyclin-dependent kinases which control cell cycle progression) to cultures of A. terreus resulted in submerged sporulation and increased production of lovastatin.
A threefold-higher specific productivity was found with the defined medium on glucose and glutamate as compared to complex medium with glucose, yeast extract, and peptonized milk. An explanation might be that stringent starvation conditions are required: in defined medium the only organic substrates are glucose and glutamate or histidine. Once they have been exhausted no alternative organic compound is available to the cells. By comparison, in a complex medium strict starvation conditions may not apply after glucose exhaustion.
ACKNOWLEDGMENTS
We thank M. Richard and P. A. Richon for technical assistance and P. van den Broek for stimulating discussions.
REFERENCES
- 1.Alberts A W, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrianopoulos A, Kourambas S, Sharp J A, Davis M A, Hynes M J. Characterization of the Aspergillus nidulans nmrA gene involved in nitrogen metabolite repression. J Bacteriol. 1998;180:1973–1977. doi: 10.1128/jb.180.7.1973-1977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnagar R K, Ahmad S, Mukerji K G, Venkitasubramanian T A. Nitrogen metabolism in Aspergillus parasiticus NRRL 3240 and A. flavus NRRL 3537 in relation to aflatoxin production. J Appl Bacteriol. 1986;60:203–211. doi: 10.1111/j.1365-2672.1986.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 4.Buckland B, Gbewonyo K, Hallada T, Kaplan L, Masurekar P. Production of lovastatin, an inhibitor of cholesterol accumulation in humans. In: Demain A L, Somkuti G A, Hunter-Cevera J C, Rossmoore H W, editors. Novel microbial products for medicine and agriculture. Society for Industrial Microbiology. Amsterdam, The Netherlands: Elsevier Science Ltd.; 1989. pp. 161–169. [Google Scholar]
- 5.Chan J K, Moore R N, Nakashima T T, Vederas J C. Biosynthesis of mevinolin. Spectral assignment by double-quantum coherence NMR after high carbon-13 incorporation. J Am Chem Soc. 1983;105:3334–3336. [Google Scholar]
- 6.Chang P K, Ehrlich K C, Yu J, Bhatnagar D, Cleveland T E. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl Environ Microbiol. 1995;61:2372–2377. doi: 10.1128/aem.61.6.2372-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot. 1979;32:852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- 8.Espeso E A, Tilburn J, Arst H N, Jr, Penalva M A. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 1993;12:3947–3956. doi: 10.1002/j.1460-2075.1993.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, Friedlin E, Marzluf G A. A reporter gene analysis of penicillin biosynthesis gene expression in Penicillium chrysogenum and its regulation by nitrogen and glucose catabolite repression. Appl Environ Microbiol. 1994;60:4432–4439. doi: 10.1128/aem.60.12.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng G H, Leonard T J. Culture conditions control expression of the genes for aflatoxin and sterigmatocystin biosynthesis in Aspergillus parasiticus and A. nidulans. Appl Environ Microbiol. 1998;64:2275–2277. doi: 10.1128/aem.64.6.2275-2277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett R H, Amy N K. Nitrate assimilation in fungi. Adv Microb Physiol. 1978;18:1–65. doi: 10.1016/s0065-2911(08)60414-2. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez R, Perez-Gonzalez J A, Gonzalez-Candelas L, Ramon D. Transcriptional regulation of the Trichoderma longibrachiatum egl1 gene. FEMS Microbiol Lett. 1994;122:303–307. doi: 10.1111/j.1574-6968.1994.tb07184.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan M D, Yudkovitz J B. Mevinolinic acid biosynthesis by Aspergillus terreus and its relationship to fatty acid biosynthesis. J Bacteriol. 1985;162:704–707. doi: 10.1128/jb.162.2.704-707.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunde-Cimerman N, Plemenitas A, Cimerman A. Pleurotus fungi produce mevinolin, an inhibitor of HGM CoA reductase. FEMS Microbiol Lett. 1993;133:333–338. doi: 10.1111/j.1574-6968.1993.tb06536.x. [DOI] [PubMed] [Google Scholar]
- 15.Hajjaj H, Klaebe A, Loret M O, Tzedakis T, Goma G, Blanc P J. Production and identification of N-glucosylrubropunctamine and N-glucosylmonascorubramine from Monascus ruber and the occurrence of electron donor-acceptor complexes in these red pigments. Appl Environ Microbiol. 1997;63:2671–2678. doi: 10.1128/aem.63.7.2671-2678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrickson L, Davis C R, Roach C, Nguyen D K, Aldrich T, McAda P C, Reeves C D. Lovastatin biosynthesis in Aspergillus terreus: characterization of blocked mutants, enzyme activities and a multifunctional polyketide synthase gene. Chem Biol. 1999;6:429–439. doi: 10.1016/s1074-5521(99)80061-1. [DOI] [PubMed] [Google Scholar]
- 17.Hicks J K, Yu J, Keller N P, Adams T. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 1997;16:4916–4923. doi: 10.1093/emboj/16.16.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy J, Auclair K, Kendrew S G, Park C, Vederas J C, Hutchinson C R. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science. 1999;284:1368–1372. doi: 10.1126/science.284.5418.1368. [DOI] [PubMed] [Google Scholar]
- 19.Kulmburg P, Mathieu M, Dowzer C, Kelly J, Felenbok B. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol Microbiol. 1993;7:847–857. doi: 10.1111/j.1365-2958.1993.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 20.Luchese R H, Harrigan W F. Biosynthesis of aflatoxin—the role of nutritional factors. J Appl Bacteriol. 1993;74:5–14. doi: 10.1111/j.1365-2672.1993.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 21.Mach R L, Peterbauer C K, Payer K, Jaksits S, Woo S L, Zeilinger S, Kullnig C M, Lorito M, Kubicek C P. Expression of two major chitinase genes of Trichoderma atroviride (T. harzianum P1) is triggered by different regulatory signals Appl. Environ Microbiol. 1999;65:1858–1863. doi: 10.1128/aem.65.5.1858-1863.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzoni M, Rollini M, Bergomi S, Cavazzoni V. Production and purification of statins from Aspergillus terreus strains. Biotechnol Techniques. 1998;12:529–532. [Google Scholar]
- 23.Manzoni M, Bergomi S, Rollini M, Cavazzoni V. Production of statins by filamentous fungi. Biotechnol Lett. 1999;21:253–257. [Google Scholar]
- 24.Martin J F, Casqueiro J, Kosalkova K, Marcos A T, Gutierrez S. Penicillin and cephalosporin biosynthesis: mechanism of carbon catabolite regulation of penicillin production. Antonie Leeuwenhoek. 1999;75:21–31. doi: 10.1023/a:1001820109140. [DOI] [PubMed] [Google Scholar]
- 25.Mathieu M, Fillinger S, Felenbock B. In vivo studies of upstream regulatory cis-acting elements of the alcR gene encoding the transactivator of the ethanol regulon in Aspergillus nidulans. Mol Microbiol. 2000;36:123–131. doi: 10.1046/j.1365-2958.2000.01833.x. [DOI] [PubMed] [Google Scholar]
- 26.Moore R N, Bigam G, Chan J K, Hogg A M, Nakashima T T, Vederas J C. Biosynthesis of the hypocholesterolemic agent mevinolin by Aspergillus terreus. Determination of the origin of carbon, hydrogen, and oxygen by 13C NMR and mass spectroscopy. J Am Chem Soc. 1985;107:3694–3701. [Google Scholar]
- 27.Niehaus W G, Dilts R P. Purification and characterization of mannitol dehydrogenase from Aspergillus parasiticus. J Bacteriol. 1982;151:243–250. doi: 10.1128/jb.151.1.243-250.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak N, Gerdin S, Berovic M. Increased lovastatin formation by Aspergillus terreus using repeated fed-batch process. Biotechnol Lett. 1997;19:947–948. [Google Scholar]
- 29.Panozzo C, Cornillot E, Felenbok B. The CreA repressor is the sole DNA-binding protein responsible for carbon catabolite repression of the alcA gene in Aspergillus nidulans via its binding to a couple of specific sites. J Biol Chem. 1998;273:6367–6372. doi: 10.1074/jbc.273.11.6367. [DOI] [PubMed] [Google Scholar]
- 30.Ruijter G J, Visser J. Carbon repression in Aspergilli. FEMS Microbiol Lett. 1997;151:103–114. doi: 10.1111/j.1574-6968.1997.tb12557.x. [DOI] [PubMed] [Google Scholar]
- 31.Schimmel T G, Coffman A D, Parsons S J. Effect of butyrolactone I on the producing fungus, Aspergillus terreus. Appl Environ Microbiol. 1998;64:3707–3712. doi: 10.1128/aem.64.10.3707-3712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim W B, Woloshuk C P. Nitrogen repression of fumonisin B1 biosynthesis in Gibberella fujikuroi. FEMS Microbiol Lett. 1999;177:109–116. doi: 10.1111/j.1574-6968.1999.tb13720.x. [DOI] [PubMed] [Google Scholar]
- 33.Simpson T J. Studies of polyketide chain-assembly processes. Amsterdam, The Netherlands: Elsevier; 1986. pp. 85–96. [Google Scholar]
- 34.Skromne L, Sanchez O, Aguirre J. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology. 1995;141:21–28. doi: 10.1099/00221287-141-1-21. [DOI] [PubMed] [Google Scholar]
- 35.Strauss J, Horvath H K, Abdallah B M, Kindermann J, Mach R L, Kubicek C P. The function of CreA, the carbon catabolite repressor of Aspergillus nidulans, is regulated at the transcriptional and post-transcriptional level. Mol Microbiol. 1999;32:169–178. doi: 10.1046/j.1365-2958.1999.01341.x. [DOI] [PubMed] [Google Scholar]
- 36.Treiber L R, Reamer R A. Origin of monacolin L from Aspergillus terreus cultures. J Antibiot. 1989;XLII:30–36. doi: 10.7164/antibiotics.42.30. [DOI] [PubMed] [Google Scholar]
- 37.Turner W B. Fungal metabolites: polyketides. London, United Kingdom: Academic Press; 1971. pp. 445–476. [Google Scholar]
- 38.Yoshizawa Y, Witter D J, Liu Y, Vederas J C. Revision of the biosynthetic origin of oxygens in mevinolin (lovastatin), a hypocholesterolemic drug from Aspergillus terreus MF 4845. J Am Chem Soc. 1994;116:2693–2694. [Google Scholar]