Neurodegenerative disorders, most commonly stroke, Alzheimer’s disease, and Parkinson’s disease, affect millions of people worldwide (1). Despite their different etiologies, a frequent feature of neurodegenerative disorders is the persistent activation of the innate immune system (2). Controlling this inflammatory response in a way that positively affects clinical outcomes for cognition and behavior is a current major challenge and requires deeper understanding of the cellular and molecular mechanisms that govern neuroimmune interactions. On page 528 of this issue, Cserép et al. (3) identify a pathway through which microglia, the innate immune sensors of the central nervous system (CNS), monitor and influence neuronal health.
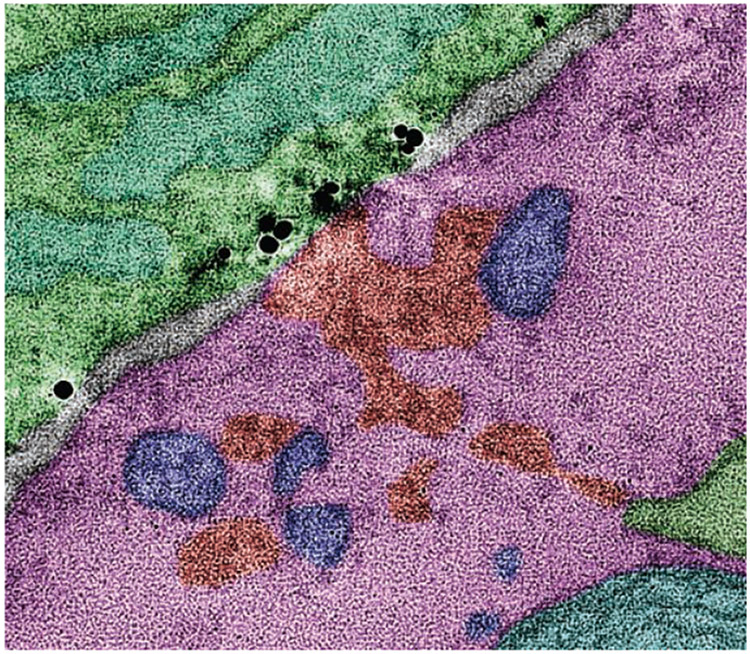
Microglia are damage sensors for the CNS. They continually surveil the parenchyma with highly motile processes and rapidly respond to tissue disturbances. However, the cellular and molecular mechanisms that control this structural and functional plasticity remain incompletely understood (4, 5). Much attention has focused on the interaction between microglial processes and neuronal synapses; this work has uncovered their important roles in activity-dependent synaptic plasticity and synapse elimination in health and neurodegenerative disorders (6). However, much less attention has focused on microglial interactions with other cell compartments, including the soma (cell body), where most of a neuron’s function is controlled. Cserép et al. show that targeted contact of microglial processes with neuronal somas (see the photo) is a prevalent feature of the mouse and human brain, rooted in specialized ultrastructure, and provides neuroprotection after injury. Inhibiting this protective response results in increased cell loss.
The death of a sufficient number of neurons can have devastating consequences for quality of life, as is evident in patients living with the effects of stroke, Parkinson’s disease, or spinal cord injury. Furthermore, given the limited regenerative capacity of the CNS, neurons and their thousands of synaptic connections are typically not replaced when these cells die prematurely (7). A key role of immune sentinels such as microglia is to protect neurons against intrinsic and extrinsic threats, such as disruption of the blood-brain barrier (which restricts extravasation of blood-borne substances into the brain) or viral infection. If the threat cannot be eliminated or contained and a neuron becomes critically injured or dysfunctional, microglia face a difficult decision: Should resources be directed toward rescuing this cell, or should the cell be removed to prevent further damage to neighboring and connected neurons? To help guide this cell fate decision, microglia rely on a number of soluble and membrane-bound signals (8).
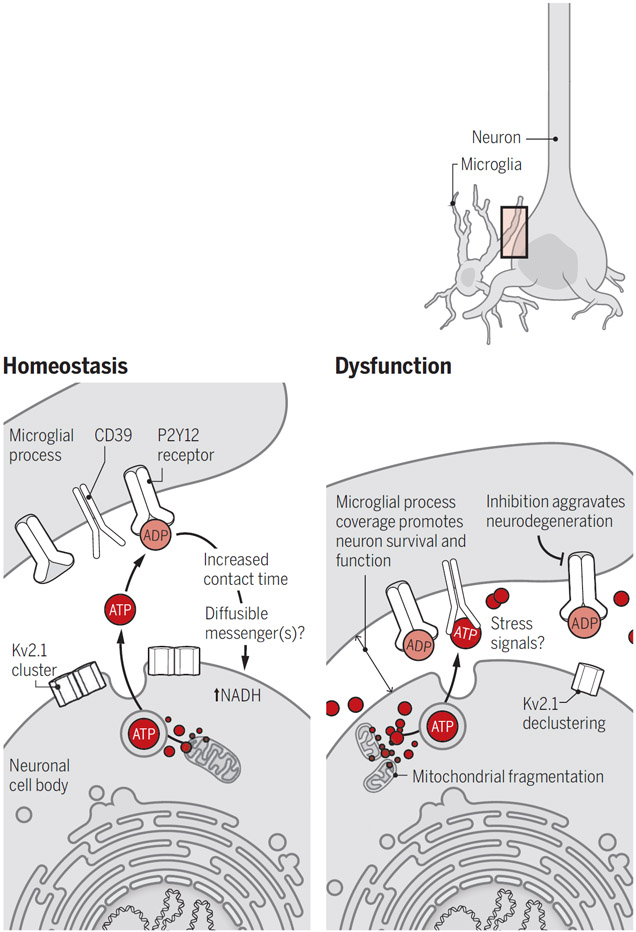
One important messenger in this context is adenosine triphosphate (ATP) and its metabolites, including adenosine diphosphate (ADP). High extracellular concentrations of ATP and ADP generated by injured and stressed cells are detected by purinergic receptors, including P2Y purinoceptor 12 (P2Y12 receptor), that are expressed on microglia. Thus, high extracellular ATP and ADP concentrations constitute a “find me” signal that guides microglia to the site of tissue damage. Lower concentrations of ATP are generally released under physiological conditions—for example, as a co-neurotransmitter at synapses (4, 5). The study of Cserép et al. shows that the activity-dependent release of ATP from anatomical specializations on neuronal somas is a ubiquitous feature of cortical and deep brain regions and serves to continually communicate with nearby microglia.
These “somatic purinergic junctions” comprise a distinct combination of proteins and organelles, including mitochondria, reticular membrane structures, intracellular tethers, vesicle-like membrane structures, and clusters of the voltage-gated potassium channel Kv2.1 (see the figure). Kv2.1 clusters localize to the soma and proximal dendrites of excitatory and inhibitory neurons, and functionally tether the plasma membrane to the endoplasmic reticulum, thereby providing sites of endocytosis and exocytosis (9). The authors show that ATP released through this machinery, and likely converted to ADP in the extracellular space, attracts microglial processes and controls their contact duration in a P2Y12 receptor–dependent manner. Junctional contact by microglial processes correlated with the amount of nicotinamide adenine dinucleotide (NADH) in nearby neuronal mitochondria, which suggests active communication, albeit through an unknown mechanism. Because P2Y12 receptor inhibition reduced contact lifetime but did not prevent cellular interactions, additional factors may regulate microglial sampling.
Microglia protect neurons.
During homeostasis, low amounts of ATP released from active neurons are converted to ADP and detected by microglial processes through P2Y12 receptors, leading to increased contact time with cell bodies and NADH in neurons. After neuronal injury, high concentrations of extracellular ATP increase coverage by microglial processes that protect viable neurons from cell death, although the precise mechanisms remain unknown.
ADP, adenosine diphosphate; ATP, adenosine triphosphate; Kv2.1, voltage-gated potassium channel 2.1; NADH, nicotinamide adenine dinucleotide; P2Y12 receptor, P2Y purinoceptor 12.
Cserép et al. investigated how the microglia-neuron interaction is altered in disease. After inducing middle cerebral artery occlusion (MCAo) in mice, which causes ischemia as well as neuron stress and injury (as occurs in stroke), they found that microglia in penumbral regions markedly increased the process coverage of stressed but morphologically intact neurons hours after reperfusion. This was inhibited by administration of the P2Y12 receptor antagonist PSB0739 before or immediately after MCAo, resulting in a larger region of neuron damage. Additionally, they show that ischemia led to degradation of the purinergic junctions in neurons, including mitochondrial fragmentation and Kv2.1 declustering. Increased microglial process coverage was also found in post-mortem stroke patient tissue.
Although P2Y12 receptor–mediated signaling in the microglial response to injury is important, the findings of Cserép et al. raise the question of what role the purinergic junctions play at different time points after stroke. Initially, neuronal hyperexcitability likely causes massive ATP release that attracts microglia to stressed neurons. However, as purinergic junctions disintegrate and ATP concentration drops, the mechanisms that sustain microglial contact remain unknown. Changes in extracellular ATP and ADP concentration are likely insufficient, and they may not allow microglia to discriminate neurons that can be rescued. Instead, such discrimination likely involves the integration of a variety of soluble and membrane-bound factors, including those derived from mitochondrial stress. Microglial ensheathment likely provides neuroprotection through various mechanisms, such as limiting the exposure of neurons to excitotoxic substances (e.g., high glutamate concentrations or leaked blood plasma components), controlling the ion flux of neurons, or providing them with trophic factors.
The identification by Cserép et al. of somatic purinergic junctions broadens our knowledge of the mechanistic basis underlying neuroimmune communication in the CNS. Alterations in this pathway may have implications for other disorders. For example, Kv2.1 declustering occurs in response to neuronal hyperactivity (9) and may therefore modulate neuron-microglia communication in epilepsy or neuropathic pain. Additionally, the neuroprotective abilities of microglia are likely impaired by dysregulation of their function during aging or neurodegenerative disease. Recent genetic analyses have revealed that many of the genes associated with the risk of neurodegenerative diseases are expressed in the CNS predominantly or exclusively by microglia (e.g., in Alzheimer’s disease, which is associated with prominent down-regulation of P2Y12 receptor expression) (6).
Perhaps the most important question is how this new knowledge can be translated into better treatments. In stroke, the timing and route of drug administration can make the difference between protective and detrimental treatment outcomes (10). Better understanding of this spatiotemporal interaction will facilitate the design of treatments that either augment the beneficial effects or reduce the pathogenic effects of immune responses, ultimately improving the lives of patients.
A microglial process (green) with P2Y purinoceptor 12 (black) contacts a neuron cell body (pink) in mouse.
ACKNOWLEDGMENTS
Thanks to G. S. Shadel and G. Lemke for discussions. Supported by NIH grants NS108034, NS103522, and NS112959 (to A.N.) and CA014195 (to the Salk Institute).
REFERENCES AND NOTES
- 1.GBD 2016 Neurology Collaborators, Lancet Neurol. 18, 459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson J et al. , Immunology 154, 204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cserép C et al. , Science 367, 528 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo P, Attwell D, Madry C, Trends Neurosci. 42, 278 (2019). [DOI] [PubMed] [Google Scholar]
- 5.York EM, Bernier L-P, MacVicar BA, Dev. Neurobiol. 78, 593 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Salter MW, Stevens B, Nat. Med. 23, 1018 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Grade S, Götz M, NPJ Regen. Med. 2, 29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemke G, Nat. Rev. Immunol. 19, 539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson B, Leek AN, Tamkun MM, Channels 13, 88 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoval KE, Witt KA, Neurobiol. Dis. 32, 200 (2008). [DOI] [PubMed] [Google Scholar]