Abstract
Introduction
While obesity is associated with adverse cancer outcomes in general, most retrospective clinical studies suggest a beneficial effect of obesity in non-small cell lung cancer (NSCLC).
Methods
Hypothesizing that this “obesity paradox” arises partly from the limitations of using body mass index (BMI) to measure obesity, we quantified adiposity using pre-operative CT images. This allowed the specific determination of central obesity as abdominal visceral fat area normalized to total fat area (visceral fat index or VFI). In addition, due to the previously reported salutary effect of metformin on high BMI patients with lung cancer, metformin-users were excluded. We then explored associations between visceral obesìty and outcomes after surgical resection of stage I/II non-small cell lung cancer. We also explored potential immunologic underpinnings of such as association using complimentary analyses of tumor gene expression data from NSCLC cancers and the tumor transcriptome and immune microenvironment in an immunocompetent model of lung cancer with diet induced obesity.
Results
We found that in 513 stage I/II NSCLC patients undergoing lobectomy, a high VFI is associated with decreased recurrence-free and overall survival. VFI was also inversely related to an inflammatory transcriptomic signature in NSCLC tumors, consistent with observations made in immunocompetent murine models where diet-induced obesity promoted cancer progression while exacerbating elements of immune suppression in the tumor niche.
Conclusion
In all, this study uses multiple lines to evidence to demonstrate the adverse effects of visceral obesity in NSCLC patients that align with those seen in animal models. Thus, the obesity paradox may, at least in part, be secondary to the use of BMI as a measure of obesity and the confounding effects of metformin use.
Keywords: Lung cancer, immunity, obesity, visceral adiposity
Introduction
An association between obesity and cancer at numerous sites has been recognized for almost two decades1. To date, thirteen human cancers have been associated with excess body weight2 – a fact made all the more pressing by the ongoing obesity epidemic and the rising prevalence of obesity among the U.S. population, which have increased to 38.3% among women and 34.3% among men3. Over the last four decades, the proportion of overweight (typically defined by a Body Mass Index or BMI > 25) individuals in the general population has increased to 66%1, with approximately half being classified as obese (BMI > 30). Correspondingly, the incidence of obesity associated cancers have also increased over the past ten years4, and obesity is now widely considered to be a carcinogen.
While the association between measures of obesity and both cancer incidence and outcome are clear in some solid tumor types such as breast, esophageal, and colon cancer, the relationship of obesity and lung cancer is more nuanced. Among cancers not traditionally thought to be obesity-related, the most prominent in terms of frequency and patient mortality is lung cancer. Contrary to many other cancers, obesity has been related to decreased incidence of lung cancer. For example, Smith et al. analyzed the NIH-AARP database and concluded that high BMI is inversely associated with lung cancer risk5. Notably, in this study, controlling for smoking increased the association between BMI and lung cancer incidence. This association is so consistent that, using data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, Tammemagi et al. developed a lung cancer risk prediction tool that incorporates BMI as a negative predictor of lung cancer risk6. In addition, several studies show that BMI is associated with improved long-term outcomes in patients with lung cancer, and this appears to be true in both early- and late-stage lung cancers7–9. Interestingly, several recent studies have also found that obese patients may respond better to anti-PD-1 immunotherapy than normal BMI patients6.
These and other clinical findings in lung cancer patients are strikingly at odds with the results of many animal experiments, which overwhelmingly demonstrate a deleterious effect of obesity in cancer. Several mechanisms have been proposed, from alteration of cancer cell metabolism to leptin mediated immune modulation10 It is widely recognized that obesity induces a state of chronic “meta-inflammation” typified by chronic cytokine production, widespread dysfunction of both innate and adaptive immune cells, and premature “immune aging” that yields an abundance of activated, yet exhausted and dysfunctional T cells11, 12The prolonged inflammatory elements of obesity have also been associated with increased frequencies of inhibitory myeloid derived suppressor cells (MDSCs) in mice and humans13 Notably, obesity has also been linked to the upregulation of immune checkpoint molecules that are well-characterized as obstacles preventing effective anti-tumor immune responses. In line with this, obese mice support more robust tumor growth while harboring CD8+ T cells with surface markers like PD-1, LAG3, and TIM3, and gene expression profiles that are associated with exhaustion14 This disconnect between the usual negative health related outcomes associated with obesity with its apparent beneficial effects in lung cancer has been termed the “Obesity Paradox” in lung cancer.
A closer examination of this apparent paradox reveals several concerns in drawing biological inferences from the clinical data. An important confounder of obesity’s contribution to lung cancer outcomes is the anti-diabetic agent, metformin. Though its molecular mechanism is poorly understood, metformin (N,N-dimethylbiguanide) is widely used to treat type II diabetes, a condition prevalent in the obese. The drug is also known to have anti-cancer activity15, for which myriad molecular and cellular mechanisms have been proposed16, 17. In lung cancer, metformin reduces tobacco carcinogen-induced lung carcinogenesis in animal and epidemiologic studies18–20. Additionally, we and others have shown that metformin use is associated with improved survival in patients with lung cancer21, 22. Of particular relevance to this study, we have recently demonstrated that the anti-cancer effect of metformin seems to disproportionately benefit overweight or obese individuals. Additional concerns stem from the potentially different smoking behaviors between obese and non-obese patients. However, the most important concern arises from the use of BMI to define excess body weight. While BMI is easy to measure, its use has been criticized due to its inability to discriminate between fat and lean body mass. BMI also fails to account for body fat distribution. It is becoming increasingly recognized that “visceral” or “central obesity” is the primary driver behind the health outcomes linked to high body fat23, 24.
Several methods of measuring central obesity exist. Anthropometric measures such as waist circumference and waist hip ratio have been used in large epidemiologic studies, are easy to measure, and are readily validated25. The primary limitation of these measures is that they need to be acquired prospectively. As patients may not be available or alive for remeasurement post-treatment, and since these measures may change over time, they do not lend themselves to retrospective studies. Image-based measures, on the other hand, such as those using computerized tomography (CT) and magnetic resonance imaging (MRI) are objective and can be studied retrospectively in human cancer provided the imaging modality is used during the clinical evaluation of the malignancy. The primary limitation of imaging modalities is the lack of validated cut-offs for classification of patients, particularly in cancer studies. CT is particularly feasible for retrospective measurements in lung cancer as virtually every patient undergoing treatment for lung cancer in the United States receives a combined positron emission tomography (PET)-CT scan before treatment initiation, and the CT images obtained as part of this evaluation can be used to measure visceral adiposity either using a single image at a predetermined level or a summation of the various components of adipose tissue as a volumetric analysis.
An early study by Jensen et al. demonstrated a close correlation between visceral adiposity as determined by analysis of a single slice of abdominal CT and a volumetric analysis of visceral fat26. This finding demonstrates the feasibility of using such image-based measurements in a large cohort of patients. While some studies have suggested that the specific vertebral level of CT imaging (L2/L3 vs. L4/L5) can lead to different adiposity measures, the ability of this approach to identify patients with complications of diabetes or metabolic syndrome seems comparable27, 28. In contrast, at least one study suggests that single slice measurement at L3/L4 vertebral levels most strongly correlates with volumetric determinations of visceral and subcutaneous adipose tissue29.
For any absolute measure of visceral fat area to reliably determine central obesity and be applicable across patients of different height, race or gender, normalization to body size is essential. A number of investigators have used height, subcutaneous fat area, or total fat area to achieve this normalization30–32. Thus, additional work is needed to develop and validate a method for determining obesity that is reliable and useful in retrospective applications. In our present study, we deployed a novel approach to measure visceral fat area at the L3 level relative to the total fat area at the same level to create a normalized metric useful in retrospective applications – the visceral fat index (VFI).
Based on the established knowledge described above, we sought to measure central obesity in a cohort of stage I NSCLC patients undergoing lobectomy at our institution and examine its association with oncologic outcomes. As early-stage patients are typically treated with surgery alone, without other treatments that may complicate analysis, they provide a context where the relationship between central obesity and tumor progression can be studied without additional therapy-associated confounders. For this reason, we focused our study on this subset of the NSCLC patient pool.
In the present study, we found that high visceral adiposity, as defined by a relatively high VFI, was indeed associated with poor overall and recurrence-free survival in early-stage NSCLC patients. A high VFI was also inversely related to an inflammatory gene expression signature in the tumor microenvironment of advanced NSCLC patients – observations suggestive of robust suppression of anti-tumor immunity in patients with excess visceral adipose tissue. While prior retrospective studies of obesity and lung cancer patient outcomes have generated paradoxical conclusions, our present findings suggest an effect of obesity on lung cancer that is aligned with both studies of other human cancers and comparisons of obese and normal mice in numerous preclinical tumor studies, including those we herein report that were generated using two widely used murine lung cancer models. These findings clarify the truly negative relationship that exists between central obesity and lung cancer outcomes, and they present a viable alternative to the use of BMI in retrospective studies of obesity rooted in biology with clear relevance to cancer outcomes.
Taken together, our clinical and preclinical findings suggest a common potential mechanism for the accelerating effect of obesity on the development of tumor burden in the obese – namely, an apparently multifaceted immune dysfunction prevalent in the tumors of obese mice and patients. Thus, our study provides much needed clarity to the relationship between adiposity and NSCLC patient survival, providing firm scientific justification for the targeting of obesity’s pro-tumor effects which include decidedly adverse effects on the anti-tumor immune response. Our findings also reaffirm the utility and relevance of available mouse models to study further the mechanisms of obesity’s lung cancer promoting effects. Importantly, they also validate a refined approached for studying obesity in retrospective cancer patient data sets.
Materials and Methods
Clinical data
All data were acquired under Institutional Review Board approved protocols. For survival analyses, all consecutive patients with pathologic stage I and II undergoing pulmonary lobectomy at the Roswell Park Comprehensive Cancer Center from October 2008 to December 2015 were included. Relevant clinical data was extracted from the institutional thoracic surgery database as well as the medical record. Tumor stage, tumor grade, histology, overall survival (OS) and recurrence free survival (RFS) were extracted from the institutional tumor registry data. All confounders except for age were treated as categorical variables. For analysis, variables were categorized further as described in supplementary material (text S1, that has additional methodological details) Overall survival (OS) time started from the day of surgery and concluded at last contact or date of death. Recurrence free survival (RFS) time started from the day of surgery and ended with either the date of first recurrence (if exists) or the date of last contact or date of death (if no recurrence). For correlation with immune gene expression, advanced stage NSCLC patients undergoing molecular testing to guide therapy that had an immune report card generated as previously described 33 were analyzed. Of patients with these data available, those on metformin (both past and present metformin users) were excluded.
Image analysis
CT scans obtained as part of a pre-operative PET-CT were analyzed. A single image at L3 level was identified and exported as a DICOM image. In the unusual cases where a pre-operative PET-CT scan was unavailable in the clinical record, images at L2 or L1 obtained from chest CT scans were used for analysis. Images at L3, L2 and L1 levels were obtained for a cohort of 46 patients to assess the validity of using images at these levels in lieu of L3. Image acquisition was performed by CR, EK, XW, SS, RG and SY, and measurements were performed using NIH ImageJ (v1.52 Java 1.8.0_112), as described in supplementary data (text S1). The entire cross-section of the patient was selected and total fat area (TFA) was calculated. The visceral fat area (VFA) was then selected and measured. Central adiposity was estimated as the ratio between the visceral fat area (VFA) to the total fat area (TFA) and was labelled the viscera fat index (VFI).
Survival analyses
As established categories of VFI as a measure of central obesity do not exist, VFI was first analyzed as a continuous variable. Age, sex, grade, race, histology, diffusion capacity for carbon monoxide (DLCO), American Society of Anesthesiology Score (ASA) and smoking status were used as covariates in model generation. Univariate analyses (Kaplan Meier) were performed to evaluate the association of individual variables with OS and RFS. Only variables associated with OS and RFS on univariate analysis were included in model generation by multivariable analyses (Cox proportional hazards). Given the association of VFI with age and sex, interaction variables were included in model generation. Variables were excluded at a significance > 0.1 at each step of model generation. In a separate analysis, patients were classified into tertiles (‘Top’, ‘Middle’ and ‘Bottom’) based on VFI. Univariate (Kaplan-Meier) and multivariable (Cox proportional hazards) survival analyses were performed to compare the OS and RFS of the ‘Top’ and ‘Bottom’ tertile of patients. Similar analyses were performed with disease-specific survival (DSS) as well. All analyses were performed in SPSS ver. 25.
FFPE tumor gene expression
RNA was extracted from each FFPE sample and processed for targeted RNA-seq, as previously described34. Gene expression was evaluated by amplicon sequencing of 394 immune transcripts on samples that met validated quality control (QC) thresholds (text S1).
Murine lung cancer models
Age-matched cohorts of obese and non-obese male C57BL/6 mice were purchased from the Jackson Laboratory, Bar Harbor, ME (stock numbers 380050 and 380056, respectively). These mice were generated by feeding them a high-fat diet (5.2 kcal/gram, 60% fat calories) beginning at 6 weeks of age and continuing for approximately 14 weeks to induce obesity. Mice fed a conventional chow diet (3.8 kcal/gram, 5–10% fat calories) were used as normal-weight controls. Mice were weighed before and periodically after initiation of experiments to confirm obese/normal status. All mice were housed in a specific pathogen free facility and all procedures were approved by the Institutional Animal Care and Use Committee. The Lewis Lung Carcinoma (LLC) cell line and its EF1 promoter-driven firefly luciferase-expressing variant (LLC-luc) were purchased from ATCC, Manassas, VA (product identifiers CRL-1642 and CRL-1642-LUC2, respectively). Both cell lines were maintained as adherent cultures in DMEM medium (Gibco) supplemented with 10% v/v fetal bovine serum (FBS; Gemini Bioproducts). For tumor challenge experiments, cells were grown to near confluency, detached with trypsin/EDTA, washed and resuspended in sterile PBS. For subcutaneous (s.c.) tumor challenge, 1×105 tumor cells were implanted into the shaved flanks of obese and normal weight mice (n = 5–7/group). Tumor volumes were measured every 2–3 days using a digital caliper. About 21 days post-implantation mice were euthanized. Tumor sections were harvested and either snap frozen for RNA sequencing analysis or mechanical dissociation to generate single-cell suspensions. Tumor-infiltrating leukocytes (TILs) were recovered from the latter after washing and Percoll gradient centrifugation. These as well as the cells of both tumor-draining and tumor-distal lymph nodes and spleen were analyzed by flow cytometry. For studies of pulmonary metastases, 0.25×106 LLC-luc cells were injected intravenously (i.v.) by the tail vein into obese and non-obese mice (n = 5/group), and the development of secondary tumors primarily in the lung was quantified by in vivo bioluminescence imagery (BLI) using Xenogen IVIS Spectrum technology (Perkin-Elmer, Waltham, MA). Just prior to imaging session, mice were injected intraperitoneally (i.p.) with D-luciferin (150 mg/kg body weight; Gold Biotechnology) and anesthetized by isoflurane inhalation. Photonic flux (photon per second) in representative images was quantified with Living Image software (version 4.3.1.0.15880; Perkin-Elmer).
Mouse tumor gene expression
Gene expression of tumors of three each of control and obese mice from each of two independent experiments was obtained by mRNA sequencing (text S1). Raw sequencing data was deposited in the European Nucleotide Archive under accession number PRJEB34297. Tumors of control and obese mice were compared for differential gene expression and gene set enrichment using DESeq2 and gsva Bioconductor software, with false discovery rate (FDR) cut-offs of 0.05 and 0.25 respectively used for significance testing (text S1).
Flow cytometry analysis
TILs and cells from tumor associated lymphoid tissues were recovered from the mice described above. Single cell suspensions were generated and washed with PBS containing 2% v/v FBS before staining with fluorochrome-conjugated antibodies recognizing surface markers defining key immune cell subsets. Intracellular markers (e.g., FOXP3) were stained after fixation and permeabilized using specialized kits. For intracellular cytokine staining, cell suspensions were re-stimulated with phorbol myristate acetate (PMA) and Ionomycin (Millipore-Sigma) in the presence of Brefeldin-A (Thermofisher Scientific) for 4 hours at 37°C followed by washing, surface staining and fixation/permeabilization (eBioscience) prior to incubation with conjugated anti-IFNγ antibodies. Multi-color flow cytometry data was collected using a BD LSR II analyzer and analyzed using FlowJo software (v10.7, BD Biosciences). For specific antibodies used in this study, see Supplementary Table S1.
Results
Based on the described inclusion and exclusion criteria, 554 patients with stage I and II NSCLC undergoing lobectomy were selected for analysis. Of these, reliable fat area measurements were obtained in 513 patients – 499 (97.3 %) at L3, 5 (0.9%) at L2 and 9 (1.8%) at L1. Similarly, 159 patients with advanced stage NSCLC with molecular testing had reliable fat area measurements – 146 (91.8%) at L3, 10 at L2 (6.3%) and 3 at L1(1.9%). Inter-observer correlation of VFI measurements were good (n=53; R2=0.95; supplementary figure 1A). Correlation of measurements between L2 and L3 levels (n=29; R2= 0.92; Slope = 1.05) and between L1 and L3 levels (n=28; R2= 0.85; Slope = 1.05) were also good (Supplementary Figure S1). Therefore, in the few cases where measurements at L3 were not available, measurements at L2 or L1 were used. Importantly, CT scans of these patients could be used to clearly identify individuals with distinctly predominant visceral or subcutaneous adipose tissue distributions (Supplementary Figure S2A,B).
Visceral obesity is associated with poor overall and recurrence free survival in early NSCLC patients undergoing surgical resection
We initially sought to establish the relationship between visceral obesity, as measured by VFI, and several clinically relevant metrics. We found that VFI was not associated with BMI (Pearson corr coeff = −0.06; P = 0.9; Supplementary Figure S2C). However, VFI is associated with sex as well as age. Specifically, the mean VFI of males is higher than females (0.56 vs. 0.37; P<0.01). VFI also tends to be elevated with increasing age (Pearson corr coeff 0.32; P<0.01).
Univariate analyses demonstrated a statistically significant association of sex, ASA score, tumor grade, histology, age, DLCO and VFI with OS. Similarly, sex, tumor grade, tumor stage, ASA score and VFI were associated with RFS. Multivariable analysis resulted in a final model of overall survival that retained age, sex, DLCO, ASA score, VFI, tumor grade and the interaction term between VFI and age. Similar analysis resulted in a final model of recurrence free survival that retained only tumor grade, tumor stage and VFI as predictive variables. These results are summarized in Table 1.
Table 1. Univariate associations and multivariable models of overall and recurrence free survival in patients with stage I and II NSCLC undergoing lobectomy.
Variables associated with survival on univariate analysis (P<0.1; in bold) were included in multivariable analyses. Only variables included in the final multivariable models are shown.
| Variable | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| Overall survival HR (95% CI) | Recurrence free survival | Overall survival HR (95% CI) | Recurrence free survival | ||
| Age | 67 ± 10.4 | 1.05 (1.03 – 1.06) | 1.01 (0.99 – 1.03) | 1.04(1.02–1.06) | |
| Sex (Female vs. male) | 305(59.5%) Female | 0.57 (0.41 – 0.80) | 0.65 (0.43 – 1.00) | 0.64(0.44 – 0.92) | |
| Race (White vs. others) | 462 (90.1%) White | 0.70 (0.36 – 1.31) | 0.40 (0.15 – 1.10) | ||
| DLCO | 77.3 ± 21.2 | 0.99 (0.98 – 1.00) | 0.99 (0.98 – 1.00) | 0.99(0.98–1.00) | |
| ASA score (High vs. Low) | 248 (48.3%) HIgh | 2.07 (1.47 – 2.90) | 1.49 (0.97 – 2.28) | 1.55(1.07 – 2.24) | |
| BMI | 27.2 ± 5.6 | 1.01 (0.98 – 1.04) | 1.01 (0.97 – 1.05) | ||
| VFI | 0.45 ± 0.14 | 7.30 (3.18 – 24.08) | 5.19 (1.14 – 23.69) | 4.51(0.93 – 21.76) | |
| Tumor grade (High vs. Low) | 181 (35.3%) High | 1.62 (1.16 – 2.26) | 1.95 (1.28 – 2.98) | 1.64(1.16–2.32) | 1.88(1.22 – 2.88) |
| Tumor stage (II vs. I) | 140 (27.3%) Stage II | 1.21 (0.84 – 1.74) | 2.19 (1.43 – 3.36) | 1.97(1.28 – 3.04) | |
| Histology Adeno vs. other SqCC vs. other | Adeno 317 (61.8%) SqCC 139 (27.1%) Other 57 (11.1%) | 0.81 (0.64 – 1.03) 1.33 (1.02 – 1.72) | 1.04 (0.76 – 1.42) 1.02 (0.71 – 1.47) | ||
| Smoking status Current vs. Never Former vs. Never | Never - 52 (10.1%) Former - 175 (34.1%) Current - 286 (55.8%) | 0.79 (0.53 – 1.17) 1.03 (0.78 – 1.37) | 0.94 (0.59 – 1.49) 1.01 (0.72 – 1.42) | ||
BMI = Body Mass Index; VFI = Visceral Fat Index; DLCO = Diffusion capacity for carbon monoxide; ASA – American Society of Anesthesiology; Adeno = Adenocarcinoma; SqCC – Squamous cell carcinoma.
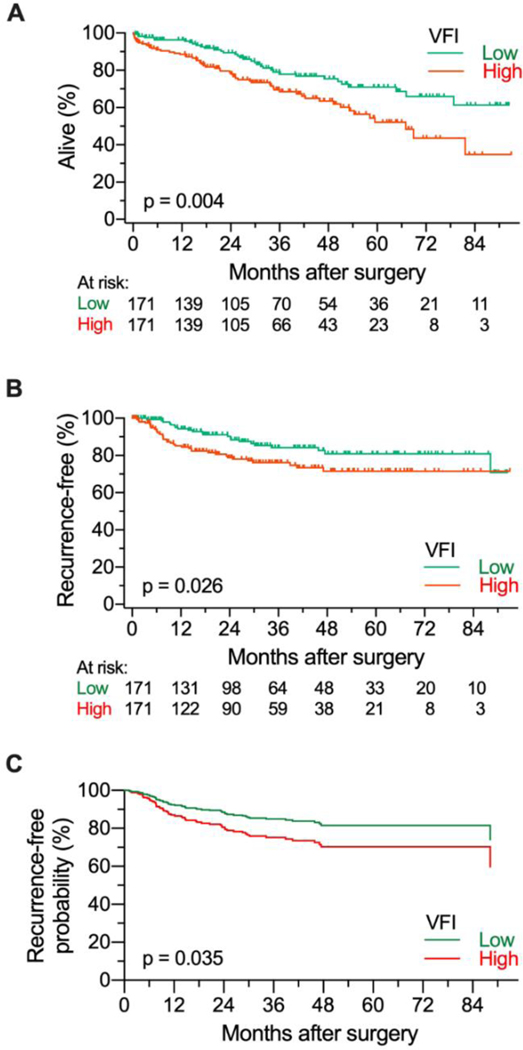
In order to observe the potential relationship between VFI and lung cancer survival outcomes, patients were stratified by VFI for Kaplan-Meier and Cox proportional hazards analyses. Comparison of the top and bottom VFI tertiles (VFItert) showed an association between high VFI and worse overall survival (OS) and recurrence free survival (RFS) (Figure 1A, B). Multivariable modeling confirmed these results with high VFI group associated with RFS (HR = 1.79; 95% CI= 1.04 – 3.08; Figure 1C). The model of overall survival retained VFI tertile as well as the interaction terms between VFI and age as well as VFI and sex (Supplementary table S2). These results suggest that in contrast to high-BMI, visceral obesity as defined by elevated VFI is negatively associated with survival in early-stage lung cancer patients.
Figure 1. Overall and recurrence free survival of patients with High and Low VFI.
A) Overall survival curves generated for 513 patients separated categorized as having High (top tertile; N=171) and Low (bottom tertile; N=171) visceral adiposity as defined by VFI score demonstrate decreased survival with high visceral adiposity (HR=1.84; 95% CI = 1.21 – 2.81). B) Recurrence free survival curves demonstrate decreased survival with high visceral adiposity (HR = 1.82; 95%CI = 1.06 – 3.11). C) Expected recurrence free survival curves using the Cox proportional hazards model for demonstrate decreased survival with high visceral adiposity (HR = 1.79; 95% CI = 1.04 – 3.08).
Of the 513 patients, cause of death was unknown in 41 patients. Therefore, DSS was analyzed in 472 patients. Similar to OS and RFS, VFI tertile (Top tertile vs. bottom tertile) was associated with DSS on Kaplan Meier analysis (HR = 2.1; P=0.025). VFI as a continuous variable did not reach statistical significance however (P=0.1). On multivariate analysis, VFI tertile (Top tertile vs. bottom tertile) was associated with worse DSS (HR = 2.25; 95% 95% CI= 1.16 – 4.37; P=0.016; Supplementary figure S3) and was the only variable retained in the model. When VFI was analyzed as a continuous variable, age (0.006), VFI (P=0.001), tumor grade (P=0.003) and the interaction variable between VFI and age (P<0.001) were associated with DSS.
Visceral obesity is associated with alterations in the tumor immune microenvironment (TME) in late-stage NSCLC patients
Despite the reported survival advantage seen in high-BMI lung cancer patients7–9 a preponderance of data, including that obtained from a number of preclinical mouse tumor models, have demonstrated a profoundly negative effect of obesity on the cells of the anti-tumor immune response14, 35–37. To examine the potential association of VFI with tumor immune gene expression, we analyzed existing data from patients with advanced stage NSCLC that underwent molecular testing to guide their therapy. In these patients, targeted transcriptome analysis (an “immune report card”) was thus generated, as described in Methods. Thus, the expression of 395 immune genes was assessed for 159 patient lung tumors (see Supplementary Table S3 for a summary of patient characteristics).
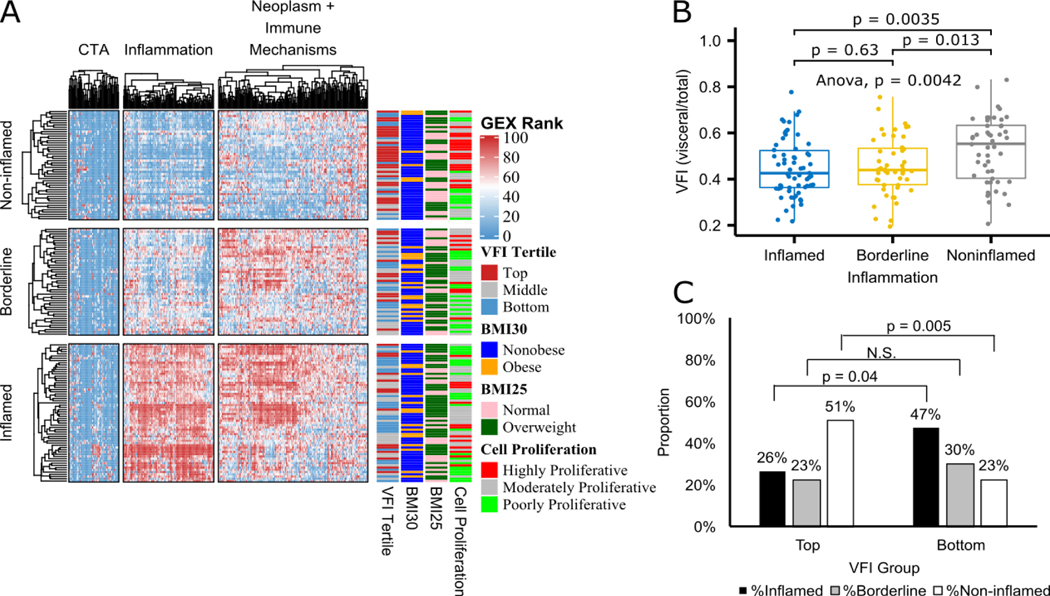
Unsupervised clustering analysis led to the discovery of three major inflammation clusters, namely, inflamed, borderline and non-inflamed tumors (Figure 2A). The inflammatory status of the tumors was significantly associated with both VFI as a continuous variable (Figure 2B) and VFI tertile as well (Figure 2C). Specifically, cases in the Top VFI tertile were significantly over-represented in the non-inflamed tumor cluster (p=0.04), whereas those in the bottom VFI tertile were over-represented among the inflamed tumors to a considerably significant degree (p= 0.0085) (Figure 2C). Within non-inflamed tumors (n=38), a significantly higher proportion of moderately proliferative tumor microenvironments were found in bottom tertile patients (p=0.015) compared to top tertile, where a higher proportion of highly proliferative tumor microenvironments were found, indicating that low visceral obesity may confer improved overall survival in NSCLC (Supplementary Figure S4)38. Further gene-wise differential gene expression analysis confirmed the significant down regulation of inflammation-associated genes in the top VFI tertile. Notably, tumors from High-VFI patients displayed enhanced expression of CDKN3 (a driver of cell proliferation linked to poor prognosis when overexpressed in lung cancer39), as well as BCL6, a promoter of tumor growth and survival in NSCLC40 and CD44, a marker of cancer stemness and poor prognoses in multiple cancers. Among the transcripts significantly under-represented in the tumors of High-VFI patients were those encoding chemokines or their receptors (including those associated with Thelper 1 (Th1)-type anti-tumor immune responses), the Th1 transcription factor Tbet, T cell lineage markers (e.g., CD4 and CD8), components of the T cell receptor and co-stimulatory signaling cascades (e.g., CD3, CD86), and the machinery of antigen presentation (Supplementary Table S4). These results strongly link central obesity (defined by high VFI) to a diminished immune activity in the lung tumor microenvironment (TME) that may stem from impaired T cell recruitment or expansion in this niche - conditions likely permissive to the growth and progression of lung cancers.
Figure 2. Changes in the tumor immune microenvironment with visceral obesity in advanced stage NSCLC.
A) Heatmap of unsupervised clustering of immune response genes expression ranks (GEX Rank: columns) and 159 samples (rows) annotated by visceral fat index (VFI) tertiles, body mass index (BMI) thresholds and cell proliferation groups. B) Boxplot of inflammation cluster groups for VFI as a continuous variable with Wilcoxon test p values shown for all pairwise comparisons. C) Bar chart showing distribution of Inflammation clusters within top and bottom VFI tertiles with chi-square proportion test p values shown.
We further explored the potential association of VFI with co-variates such as BMI, race, gender, primary histology and staging. As expected, BMI category was inversely associated with VFI (p=0.00652), and pathology stage was significantly associated with VFI tertile (p=0.0024). Additionally, male gender was highly associated with high VFI status (p=1.54E-11) (Supplementary Table S5). These findings are in line with the generally accepted notion that males are more prone to visceral adiposity than females41. They also merit further investigation into the role of gender in visceral versus subcutaneous fat distribution with the two genders and the potential implications for long-term lung cancer patient outcomes and the anti-tumor immune response.
Interestingly, examining the correlation between traditional obesity status (as defined by a BMI of 30 or greater) with patient VFI tertile revealed that a significantly higher proportion of obese cases were seen in bottom VFI tertile than in the top tertile (p=0.009) (Supplementary Figure S5B). This surprising result suggests that only 15% of the VFI high cases would be classified as obese using the prevailing method of identifying obese patients in retrospective studies. In addition, no association was found between standard BMI categories and tumor inflammation state (Supplementary Figure S5A). These results link visceral adiposity in lung cancer patients to potential immune dysfunction expected in the obese host, and they further highlight the markedly different relationships that exist between VFI and BMI and lung cancer biology.
Obesity exacerbates lung cancer progression in mice while altering TME gene expression
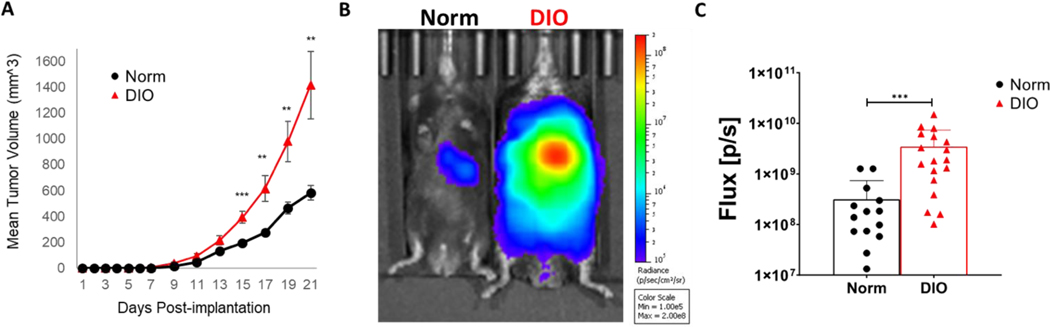
In parallel, we observed the effects of obesity on tumor progression and anti-tumor immunity in widely used pre-clinical models of lung cancer. For this, the in vivo conditions present in overweight and obese lung cancer patients were recreated using a well-characterized approach for diet-induced obesity (DIO) in mice. Prolonged administration of high fat diet (e.g., one containing 60% calories from fat) to mice of an obesity-susceptible genetic background, such as C57BL/6, results in progressive weight gain and an accumulation of visceral fat42 compared to normal diet-fed control mice. Cohorts of obese and normal weight mice were injected subcutaneously (s.c.) with Lewis lung carcinoma (LLC) cells, and subsequent tumor development was monitored. As generally seen in implantable mouse tumor models14, 37, obese mice supported more robust tumor growth compared to non-obese controls in this model (Figure 3A). In a complementary model of pulmonary metastatic disease, obese mice challenged intravenously (i.v.) with a modest number of luciferase-expressing LLC (LLC-Luc) cells developed markedly enhanced lung tumor burden compared to normal weight controls within 26 days post-injection (Figure 3B,C). These results demonstrate the decidedly pro-tumor effects associated with obesity in mouse models that align closely with the relationship between lung cancer outcomes and central obesity brought to light by the use of VFI in our clinical studies.
Figure 3. Obesity-mediated effects on progression of Lewis lung carcinoma tumors in mice.
A. C57BL/6 mice were fed a high-fat (to induce obesity) or normal diet for ~14–16 weeks before s.c. implantation of 10×105 cells by s.c. injection into the shaved flanks. Mean tumor volumes were calculated using the formula: volume=0.5×lengthxwidth2. (B,C) Obese and normal mice were challenged with intravenous tail vein injection of 0.25×106 LLC-Luc cells. 26 days after injection, tumor burden (pulmonary metastases) were visualized by BLI after i.p. injection of d-luciferin (150mg/kg) using IVIS technology. P < 0.05 (*), < 0.02 (**), <0.001 (***) in standard t test. Error bars depict the SEM. Shown are a representative image (B) and mean tumor burden measurements (A and C) from 3–5 independent experiments.
To gain insight into the potential mechanisms underlying the effects of obesity on tumor progression, we set out to document the transcriptomic changes in the TME associated with obesity. RNA was harvested from s.c. tumor sections (n=6 each) generated in the experiments described in Figure 3A, and RNASeq analysis was carried out. Expression of 187 and 217 genes was respectively up- and down-regulated by > 1.2x in tumors of obese compared to normal with adjusted Wald test p < 0.05 in analysis with DESeq243. Single-sample geneset enrichment analysis (GSEA) using the geneset variation analysis (GSVA) method44 revealed significant divergences of multiple genesets related to metabolism and cancer biology (Bayes-moderated t test p < 0.05 with false discovery rate [FDR] < 0.20).
Of the 18,025 genes identified as expressed in the tumors, expression of only 33 differed by ≥ 1.5-fold between obese and control mice at FDR < 0.05 (Supplementary Table S6). The five genes that were most up-regulated in obese tumors compared to controls were all found to either encode proteins involved in fat metabolism (i.e., pyruvate dehydrogenase kinase 4, lipase K, fatty acid binding protein 4) and lipid oxidation in particular45 or to have been previously associated with adiposity (i.e., angiotension II receptor 246, bone morphogenetic protein 547). Examination of gene expression at the level of biological processes revealed a 1.2–1.3-fold enrichment of the gene sets involved in adipogenesis and oxidative phosphorylation in the tumors of obese mice. As might be expected given the robust tumor growth in the obese mice, genes involved in angiogenesis, epithelial-mesenchymal transition, hypoxia, and glycolysis were also upregulated in obese tumors. On the other hand, expression of genes associated with the immunologically relevant IL6-JAK-STAT3 signaling pathway were significantly reduced in the tumors of obese compared to control mice. Meanwhile expression of Stat4, a central driver of Th1 immunity and Areg, a gene known to be upregulated by Tregs in peripheral tissues (including lungs) were nominally down- and up-modulated in obese tumors, respectively (Supplementary Figure S5, Supplementary Table S6).
These findings suggest that obesity’s still poorly understood impact on the TME and its potential fueling of tumor progression may be multi-faceted in nature, involving processes capable of aiding tumors directly and by opposing the activity of immune cells in the TME. Indications of reduced IL-6/STAT3 signaling were surprising, however, since elevated serum levels of IL-6 have been reported in the obese48 and this cascade is generally thought to have multiple-pro-tumor effects in cancer cells themselves including expression of genes that promote cell proliferation, survival, angiogenesis, invasiveness, metastasis, and stemness49. Interestingly though, an inverse relationship has been reported between STAT3 and commitment to oxidative metabolism/TCA cycle activity in other cancers (i.e., prostate cancer)50. Also, given the known involvement of STAT3 signaling in glycolytic metabolism51 a reduced engagement of this cascade could also reflect a favoring of oxidative metabolism in the TME. Indeed, metabolic pathways that consume the available oxygen and enforce hypoxia in the TME were recently shown to be an obstacle for the mounting of effective immune cell activity52, and altered lipid transport and metabolism that can alter the availability of free fatty acids can also modify immune function in the TME37. A relative upregulation of genes involved in TGF-beta-signaling suggests that along with these more recently described mechanisms obesity may enhance the notoriously anti-inflammatory cytokine contributing to a staunchly immune-suppressive TME under obese conditions.
Obesity attenuates anti-lung cancer immune responses in mice
It is well appreciated that metabolic factors play a non-trivial part in regulating the phenotypic differentiation, fitness, and activity of immune cells53, 54. Obesity is also known to have a profound effect on metabolism at an organismal or systemic level that is accompanied by immune dysfunction and smoldering inflammation55. Yet, the precise effects of obesity on critical participants in the cellular response to lung cancer in mice and patients with lung cancer are just beginning to be understood. We therefore set out to dissect the impact of the obese state on the immune cell constituents of the TME in the mice described in Figure 3A using a multi-color flow cytometry-based approach. Our findings revealed multiple indications that obesity had a deleterious effect on the potency of the anti-tumor response.
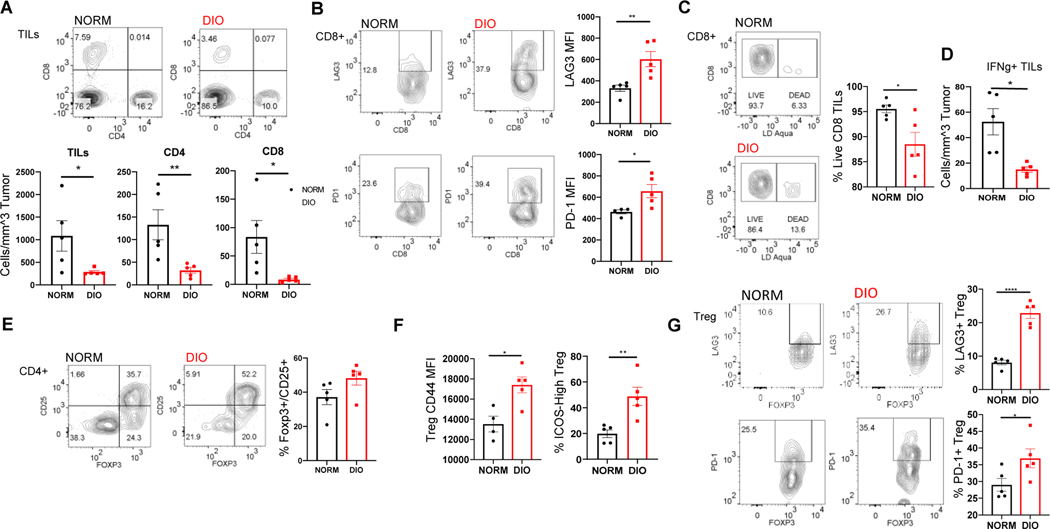
Leukocyte infiltration of s.c. LLC tumors was markedly suppressed in obese (DIO) mice relative to normal weight (NORM) control tumors. This reduced intratumoral cellularity reflected a relative dearth of CD4+ and CD8+ T cells in the obese TME (Figure 4A). The potential tumoricidal CD8+ T cell compartment of obese mice were further observed to express considerably elevated levels of the immune checkpoint/exhaustion markers LAG3 and PD-1 on their surface relative to their normal weight counterparts (Figure 4B) in line with previous assessments of T cell phenotypes in obese tumor-bearing mice14, 56. Similarly enhanced checkpoint expression levels were seen on conventional Thelper CD4+ T (Tconv) cells from DIO tumors (data not shown), and the CD8+ T cells recovered from obese mouse tumors stained more prominently with the dead cell-identifying dye LD Aqua (Figure 4C), indicative of an obesity-related defect in the fitness as well as the anti-tumor potential of intra-tumoral cells. Further suggesting an obesity-associated suppression of anti-tumor responses, the numbers of TILs capable of producing the tumoricidal Th1 cytokine IFNγ were much lower in the TILs recovered from DIO mice (Figure 4D).
Figure 4. Obesity-mediated effects on the anti-tumor immune response to s.c. LLC tumors.
C57BL/6 mice were fed a high-fat (to induce obesity) or normal diet for 16 weeks before implantation of 1×105 cells by injection in shaved flanks (n=5–7/group). The density of tumor infiltrating leukocytes (TILs) were found, and flow cytometry analysis of tumor cell suspensions obtained in the experiment presented in Fig. 3 revealed the frequencies of CD4+ and CD8+ T cells amongst the tumor infiltrating leukocytes (TILs; A). The proportions of CD8+ T cells expressing the checkpoint molecules LAG3 and PD-1 and the viable fraction of CD8+ T cells were found and quantified (B, C). The levels of IFNγ producing TILs in obese and non-obese mice were also found (D). The relative proportions of Tregs among the TILs were found (E) as well as the levels of activated Treg markers. (F) and the frequencies of Foxp3+ Tregs expressing the checkpoint molecules LAG3 and PD-1 (F). P < 0.05 (*), < 0.02 (**), (****)<0.001 in standard t test. Error bars depict the SEM. Shown are representative flow plots and the mean quantification of replicates from one of 2–3 independent experiments.
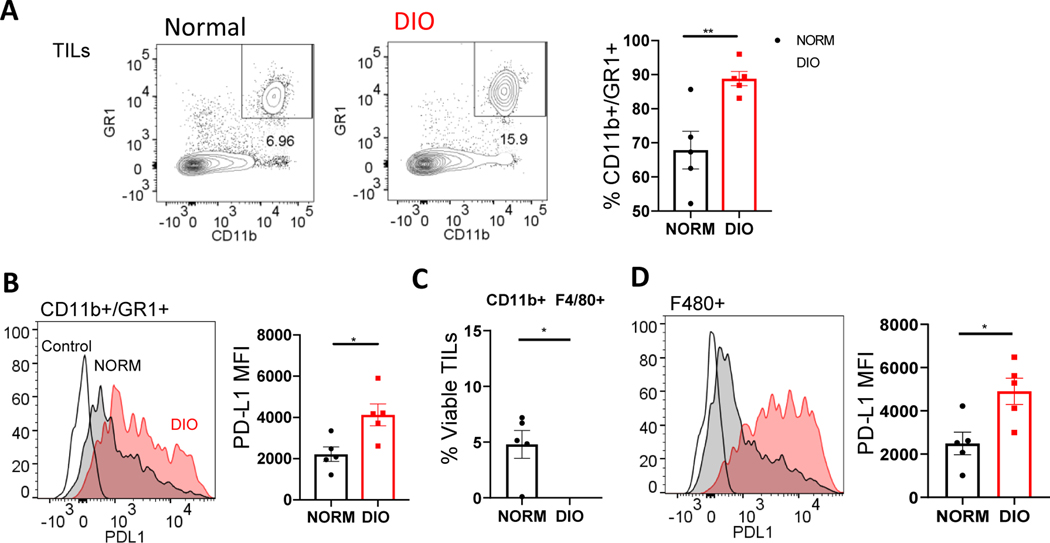
In addition to effector cell deficits, enhanced suppressive cell phenotypes were also seen in the tumors of obese mice. Foxp3+ regulatory T (Treg) cells were modestly yet consistently enriched in the tumors of obese mice (Figure 4E). Moreover, these obese tumor-infiltrating Tregs displayed considerable up-regulated markers of an activated phenotype (CD44, ICOS) (Figure 4F). Since the activated or effector-like Treg phenotype is associated with both robust suppressive potency and a tendency to accumulate in murine and human tumors57, 58, this observation suggests that obesity may bolster this subpopulation of suppressor cell known to be responsible for pathological immune suppression in the cancer setting. Concordantly, the levels of immune checkpoint molecules LAG3 and PD-1 expected to be expressed on the surface of intra-tumoral Tregs57 were enhanced in DIO Tregs compared to those recovered from controls (Figure 4G). We also observed a marked elevation in the abundance of tumor CD11b+GR1(Ly6C/G)+ myeloid derived suppressor cells (MDSCs) in obese mice-an observation in agreement with previous studies13 and these cells displayed higher levels of PD-L1 on their surface in the obese setting compared to normal weight controls (Figure 5A, B). Obesity also appeared to bolster proportions of potentially suppressive tumor-associated macrophages (CD11b+/F480+) as well as expression of the immune checkpoint molecule PDL-1 on these cells (Figure 5C, D). In general, these differences in T and myeloid cell phenotypes were muted in other tissues surveyed including tumor-draining and distal lymph nodes and the spleens compared to the tumors of obese and control mice (data not shown) suggesting the immune cells of the TME may be particularly susceptible to obesity-related modulation. Interestingly, our findings suggest that, in contrast to the known ability of obesity to down-regulate Treg abundance in visceral fat59 and blood, obesity can actually enhance Treg presence and activation in the TME and tumor-associated tissues. This previously unappreciated effect, potentially in concert with the potentiation of several other notoriously suppressive elements of the immune response (i.e., MDSCs, PD-L1:PD-1 signaling, exhausted CD8 T cells) may contribute to the enhanced tumor progression seen in obese mice.
Figure 5. Obesity-mediated effects on myeloid derived suppressor cells in s.c. LLC tumors.
C57BL/6 mice were fed a high-fat (to induce obesity) or normal diet before implantation of 1×105 cells by injection in shaved flanks (n=5–7/group). Flow cytometry analysis of tumor cell suspensions obtained from Fig. 3. The frequencies of MDSC (GR1+/CD11b+) among the TILs were found by flow cytometry (A) as were levels of PD-L1 expression on these suppressor cells (B). Similarly, we determined the frequencies of tumor associated macrophages (CD11b+/F480+) and the surface levels of PDL1 on these cells (C,D). P < 0.05 (*), < 0.02 (**), in standard t test. Error bars depict the SEM. Shown are representative flow plots and the mean quantification of replicates from one of 2 independent experiments.
Perturbance by obesity of immune pathways in mouse tumors can be discerned in tumor transcriptome
To obtain insights into the molecular mechanisms by which obesity may influence tumor biology, we compared mRNA sequencing-based transcriptomes of LLC tumors of obese and normal mice (n=6 each). Expression of 187 and 217 genes was respectively up- and down-regulated by > 1.2x in tumors of obese compared to normal with adjusted Wald test p < 0.05 in analysis with DESeq243. Single-sample geneset enrichment analysis (GSEA) using the geneset variation analysis (GSVA) method44 revealed significantly increased expression of multiple genesets related to metabolism in tumors of obese mice (Bayes-moderated t test p < 0.05 with false discovery rate [FDR] < 0.20) (Supplementary Figure S6) as well as cancer biology (such as angiogenesis and epithelial mesenchymal transition; data not shown). On the other hand, tumors of obese mice had significantly diminished expression of many immune-related genesets (Supplementary Figure S5). These results suggest that obesity triggers marked alterations in the tumor microenvironment.
In all, these results illustrate the multi-faceted nature of the immune dysfunction associated with obesity across varied mouse tumor models. These effects, which include enhanced suppressor cells presence and function as well as effector cell deficiencies resonate with our transcriptomic characterization of high-VFI patient tumors as harboring a low degree of immunologic/inflammatory activity. When taken together, these preclinical and clinical findings strongly suggest that the poor outcomes seen in both centrally obese mice and patients are rooted in common biological underpinning, i.e., obesity-mediated effects on the anti-tumor immune response. While further work will be necessary to pin-point the precise mechanisms at play in the subversion of anti-tumor immunity in the obese, and indeed several potential mechanisms have been proposed to date, our results suggest that at least some of the key elements at play (e.g., obesity-altered tumor metabolism and immune-suppression) may be modeled in mouse tumor models for in-depth, mechanistic studies.
Discussion
Obesity is an established risk factor in the development of multiple cancer types and a negative prognostic factor for many as well2, 60, 61. However, the relationship between obesity and lung cancer outcomes is less clear. While obese mice generally display accelerated tumor development and growth compared to normal weight controls in preclinical models of lung cancer and other malignancies, analysis of clinical data have suggested a survival benefit and better responses to therapies among obese patients (particularly when obesity is defined by BMI) - giving rise to a so-called “obesity paradox”. However, a number of potential confounding factors may account for this surprising association.
One potential confounder in clinical studies of obesity and lung cancer outcomes is the relationship between smoking and obesity. It is possible that obese patients smoke less, and this may be misinterpreted as a beneficial effect of obesity. For example, Lam et al. showed that obesity is not associated with increased lung cancer in never smokers62 However, a large study of ~450,000 patients examined this in detail, and after adjusting for smoking, the relationship of obesity (measured by BMI) to lung cancer incidence still held5 suggesting that the possible confounding issue of smoking is not causatively important, but nevertheless essential to consider in order to avoid erroneous interpretations. Another pitfall in the interpretation of clinical results concerning obesity’s effects on lung cancer survival seems to involve the widespread use of metformin to treat type II diabetes – a common comorbidity in overweight and obese patients. This drug has long been studied for its potential anti-cancer effects in preclinical models and clinical data sets with varying degrees of potency reported in the literature, and it has been demonstrated to have immunomodulatory effects52, 63, 64. We recently linked metformin use to significantly better survival outcomes specifically in obese early-stage lung cancer patients65. It is possible that the context-specific benefit of this drug may account at least partially for the better lung cancer outcomes seen in high-BMI patients.
Our present findings and those of others suggest that a major contributor to the obesity paradox arises from the manner in which obesity is typically measured. Most retrospective studies examining the association between obesity and lung cancer use BMI as an anthropomorphic measure of obesity. There is growing evidence that the use of BMI has serious limitations in the study of obesity and its effects on human health and disease outcome. While different anatomical distribution patterns of adipose tissue accumulation (i.e., different body compositions) have major implications for human health, BMI measurements are not capable of differentiating between these patterns66. As such, BMI is a poor measure of visceral obesity, and this is particularly limiting for the effects of obesity in diverse patient demographics. Specifically, central obesity is known to occur in Asian Americans and East Asians, at a lower BMI than Caucasians67. Also, germane to the study of the obesity-lung cancer connection, recent studies suggest that an ability to discern between visceral and s.c. obesity is critically important for defining obesity in a meaningful way and for exploring the interaction of obesity and processes determining lung cancer outcomes.
It is important to note that others have used waist circumference (WC), waist-hip ratio (WHR), or imaging to explore the relationship between lung cancer outcomes and central obesity, specifically, with findings in agreement with our own. Leitzmann et al., analyzed the NIH-AARP study dataset with 225,712 individuals and found that patients with a higher WC had a higher lung cancer-specific mortality68. This was again demonstrated in a pooled analysis of 1.6 million patients with 23,732 incident lung cancer in which WC was associated with a higher risk of lung cancer69. These findings are in agreement with those of Ardesch et al., that implicated a positive association between lung cancer risk and an obese body shape (as determined by Waist circumference (WC), Waist-to-Hip Ratio (WHR), Body Shape Index) 70. Similarly, measurement of visceral adipose tissue was also associated with poor lung cancer prognosis in patients undergoing chemotherapy71. The findings of these studies and those of our own, clearly illustrate the vital need for accurate measures of central obesity in studies of lung cancer.
Our clinical findings are not only in line with the effects of obesity and tumor progression seen in many murine lung cancer models, but they also reflect the logical outcome of the roundly pro-tumor alterations that obesity triggers in human and murine gene expression (Figure 2 and Supplementary Tables S4, S6) and its effects on the immune cell composition of the TME reported by us65 (Figure 3) and others.
Deploying a novel CT-based approach for the retrospective quantification of visceral and s.c. adipose tissue, we here show definitely that central or visceral adiposity (i.e., high VFI status) is negatively associated with both OS and RFS in early-stage NSCLC patients. This finding contrasts starkly with previous observations made using BMI that have, in part, given rise to the obesity paradox. Also, unlike the disease outcomes seen in high-BMI patients, high VFI status corresponded, at least in a general sense, with the course of disease seen in obese tumor bearing mice. Indeed, in both mice and human central obesity appears linked to worse outcomes, namely accelerated tumor growth (Figure 3), and shorter survival times (Figure 1), respectively.
Our parallel observations made in human lung TME gene expression and the cellular analysis of murine tumors clearly suggest that obesity undercuts key elements of the T cell- and inflammatory response in the tumor niche while enhancing known-mediators of immune suppression that include environmental stresses, immune-dampening signaling pathways, and pro-tumor phenotypes in notorious cellular antagonists of anti-tumor immunity. In particular, side-by-side assessment of human and murine lung tumors reveal common indications of a suppressed immune presence in the TME of obese hosts, albeit through distinct approaches in some cases. Perhaps most notably, in both human and mouse tumors transcripts encoding elements of Th1 immunity were reduced by central obesity. In our comparison of immune-relevant transcripts among top- and bottom-VFI tertile patient tumors, Th1 chemokines CXCL10, CXCL11, and CXCL9; and Th1 transcription factors STAT1 and Txb21/Tbet were found to be underrepresented in the high-VFI samples as were IFIT1 and IFIT3, which are reported to be induced by IFNgamma signaling72 (comparison p values <0.05; Supplemental Table S4). Meanwhile, in our analysis of mouse tumor gene expression, STAT4, which plays an important role in the generation of Th1 immunity was among the genes down-regulated by obesity (Supplementary table S6), and both these observations were very much in line with our cellular analysis of the mouse tumors in the s.c. LLC model showing a lower density of IFNgamma-producing TILs in obese tumors (Figure 4D). It is interesting to note that while our flow cytometric characterization of mouse tumors revealed enhancements in Tregs and PD-L1 expression by myeloid cells, while general levels of CD274 (PD-L1) and Foxp3 transcript were found to be underrepresented in high-VFI tumors. This apparent incongruity could reflect, in the case of PD-L1, a low-level of IFNgamma signaling73 implicated by our other observations to be active in the obese TME or the expression of these factors by a number of tumor residents74 including tumor cells themselves.
Across species we observed indications of a stymied T cell presence in the tumors of high-VFI patients and DIO mice indicated by reduced pan-T cell and lineage defining transcripts (CD2, CD3, CD4, CD8, CD247) and the scarcity of CD4+ and CD8+ TILs in our flow cytometry analysis, respectively (Supplemental Table S4, Fig.4). Also, among the transcripts relatively down-regulated in high-VFI tumors were several involved in the process of antigen presentation (CIITA and several HLA transcripts; Supplemental Table S4), an observation is compatible with a more immunologically quiet TME in the obese and potentially explanative as defective stimulation of effector T cell immunity by antigen presenting cells in the obese TME may in part contribute to the aforementioned defects in the tumor T cell presence due to poor expansion. The compromised viability of CD8 T cells suggested by our mouse model experiments (Figure 4C) presents another possible explanation for both a lack of cells with anti-tumor potential and the enhancement of tumor growth seen in the obese context – namely that the already inhospitable TME is made even less conducive to T cell infiltration by factors stemming from an obesity state. Additionally, the obesity-enhanced suppressor cell phenotypes among the myeloid compartment of the murine TILs (i.e., elevated MDSCs and Tumor-associated macrophages marked by high PD-L1 expression; Figure 5) are also in line with defective immune priming (in favor of immune suppression). However, since a number of chemokine genes are relatively under-expressed in high-VFI patient tumors (Supplemental Table S4), deficient recruitment of effector T cells is another potential mechanism at play. As the previously unappreciated obesity-associated enhancement of the TIL Treg phenotype that we observe in our mouse studies (Figure 4E–G) remains to explored in depth, further study is needed to define the relative importance of this and the other potential mechanisms of obeserelated immune dysfunction to overall disease outcome. Nevertheless, our present findings implicate the soundly predicted, likely multi-faceted, but incompletely delineated suppressive effects of obesity on the immune TME of lung tumors as probable contributors to obesity’s pro-tumor effects in lung cancer.
This study marks an important step in fostering a better understanding of the so-called obesity paradox, and its findings clarify the impact of distinct body-fat distribution patterns on lung cancer outcomes. Its findings may inform continued efforts to better predict lung cancer outcomes, management and apply treatments, and develop novel therapeutic approaches to prevent or control early-stage lung cancers across a patient pool that is increasingly overweight and obese.
Supplementary Material
Acknowledgements
We thank the Genomics Shared Resource facility of RPCCC for performing the RNA sequencing experiments.
Funding source
This work was supported by intramural research support from Roswell Park Comprehensive Cancer Center (RPCCC) to SY and JB, American Lung Association Lung Cancer Discovery Award to JB, and National Cancer Institute, USA grant P30-CA016056 to RPCCC.
Abbreviations
- LLC
Lewis lung carcinoma
- NSCLC
non-small cell lung cancer
- OS
overall survival
- RFS
recurrencefree survival
- VFA
visceral fat area
- VFI
visceral fat index
Footnotes
Conflicts of interest
Drs. Pabla, and Seagar are employees of OmniSeq Inc. of Buffalo, NY, and Dr. Pabla holds restricted stock in the molecular diagnostics company. Roswell Park Comprehensive Cancer Center is a shareholder of OmniSeq. These and other authors declare no other conflict of interest.
CRediT statement not available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 2.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Fakhouri TH, Carroll MD, et al. Prevalence of Obesity Among Adults, by Household Income and Education - United States, 2011–2014. MMWR Morb Mortal Wkly Rep 2017;66:1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele CB, Thomas CC, Henley SJ, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2017;66:1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith L, Brinton LA, Spitz MR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst 2012;104:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ten Haaf K, Jeon J, Tammemagi MC, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med 2017;14:e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung CC, Lam TH, Yew WW, et al. Lower lung cancer mortality in obesity. Int J Epidemiol 2011;40:174–182. [DOI] [PubMed] [Google Scholar]
- 9.Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017;104:52–57. [DOI] [PubMed] [Google Scholar]
- 10.Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014;10:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canter RJ, Le CT, Beerthuijzen JMT, et al. Obesity as an immune-modifying factor in cancer immunotherapy. J Leukoc Biol 2018;104:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 13.Ostrand-Rosenberg S. Myeloid derived-suppressor cells: their role in cancer and obesity. Curr Opin Immunol 2018;51:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014;10:143–156. [DOI] [PubMed] [Google Scholar]
- 16.Vancura A, Bu P, Bhagwat M, et al. Metformin as an Anticancer Agent. Trends Pharmacol Sci 2018;39:867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurelac I, Umesh Ganesh N, Iorio M, et al. The multifaceted effects of metformin on tumor microenvironment. Semin Cell Dev Biol 2019. [DOI] [PubMed] [Google Scholar]
- 18.Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777. [DOI] [PubMed] [Google Scholar]
- 20.Thakkar B, Aronis KN, Vamvini MT, et al. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism 2013;62:922–934. [DOI] [PubMed] [Google Scholar]
- 21.Dhillon SS, Groman A, Meagher A, et al. Metformin and Not Diabetes Influences the Survival of Resected Early Stage NSCLC Patients. J Cancer Sci Ther 2014;6:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Rexrode KM, van Dam RM, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008;117:1658–1667. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 25.Litwin SE. Which measures of obesity best predict cardiovascular risk? J Am Coll Cardiol 2008;52:616–619. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MD, Kanaley JA, Reed JE, et al. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–278. [DOI] [PubMed] [Google Scholar]
- 27.Balentine CJ, Marshall C, Robinson C, et al. Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res 2010;164:18–22. [DOI] [PubMed] [Google Scholar]
- 28.Kuk JL, Church TS, Blair SN, et al. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care 2006;29:679–684. [DOI] [PubMed] [Google Scholar]
- 29.Irlbeck T, Massaro JM, Bamberg F, et al. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013;216:1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukiyama H, Nagai Y, Matsubara F, et al. Proposed cut-off values of the waist circumference for metabolic syndrome based on visceral fat volume in a Japanese population. J Diabetes Investig 2016;7:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi MH, Choi JI, Park MY, et al. Validation of intimate correlation between visceral fat and hepatic steatosis: Quantitative measurement techniques using CT for area of fat and MR for hepatic steatosis. Clin Nutr 2018;37:214–222. [DOI] [PubMed] [Google Scholar]
- 33.Paluch BE, Glenn ST, Conroy JM, et al. Robust detection of immune transcripts in FFPE samples using targeted RNA sequencing. Oncotarget 2017;8:3197–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conroy JM, Pabla S, Glenn ST, et al. Analytical Validation of a Next-Generation Sequencing Assay to Monitor Immune Responses in Solid Tumors. J Mol Diagn 2018;20:95–109. [DOI] [PubMed] [Google Scholar]
- 35.Algire C, Amrein L, Bazile M, et al. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 2011;30:1174–1182. [DOI] [PubMed] [Google Scholar]
- 36.Nimri L, Saadi J, Peri I, et al. Mechanisms linking obesity to altered metabolism in mice colon carcinogenesis. Oncotarget 2015;6:38195–38209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringel AE, Drijvers JM, Baker GJ, et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020;183:1848–1866 e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pabla S, Conroy JM, Nesline MK, et al. Proliferative potential and resistance to immune checkpoint blockade in lung cancer patients. J Immunother Cancer 2019;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan C, Chen L, Huang Q, et al. Overexpression of major CDKN3 transcripts is associated with poor survival in lung adenocarcinoma. Br J Cancer 2015;113:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deb D, Rajaram S, Larsen JE, et al. Combination Therapy Targeting BCL6 and Phospho-STAT3 Defeats Intratumor Heterogeneity in a Subset of Non-Small Cell Lung Cancers. Cancer Res 2017;77:3070–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauli AM, Matin S. Why Do Men Accumulate Abdominal Visceral Fat? Front Physiol 2019;10:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magnuson AM, Regan DP, Fouts JK, et al. Diet-induced obesity causes visceral, but not subcutaneous, lymph node hyperplasia via increases in specific immune cell populations. Cell Prolif 2017;50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettersen IKN, Tusubira D, Ashrafi H, et al. Upregulated PDK4 expression is a sensitive marker of increased fatty acid oxidation. Mitochondrion 2019;49:97–110. [DOI] [PubMed] [Google Scholar]
- 46.Xue Q, Chen P, Li X, et al. Maternal High-Fat Diet Causes a Sex-Dependent Increase in AGTR2 Expression and Cardiac Dysfunction in Adult Male Rat Offspring. Biol Reprod 2015;93:49. [DOI] [PubMed] [Google Scholar]
- 47.Shao GC, Luo LF, Jiang SW, et al. A C/T mutation in microRNA target sites in BMP5 gene is potentially associated with fatness in pigs. Meat Sci 2011;87:299–303. [DOI] [PubMed] [Google Scholar]
- 48.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74. [DOI] [PubMed] [Google Scholar]
- 50.Oberhuber M, Pecoraro M, Rusz M, et al. STAT3-dependent analysis reveals PDK4 as independent predictor of recurrence in prostate cancer. Mol Syst Biol 2020;16:e9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valle-Mendiola A, Soto-Cruz I. Energy Metabolism in Cancer: The Roles of STAT3 and STAT5 in the Regulation of Metabolism-Related Genes. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharping NE, Menk AV, Whetstone RD, et al. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol Res 2017;5:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beckermann KE, Dudzinski SO, Rathmell JC. Dysfunctional T cell metabolism in the tumor microenvironment. Cytokine Growth Factor Rev 2017;35:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Romero P. Metabolic Control of CD8(+) T Cell Fate Decisions and Antitumor Immunity. Trends Mol Med 2018;24:30–48. [DOI] [PubMed] [Google Scholar]
- 55.Deng T, Lyon CJ, Bergin S, et al. Obesity, Inflammation, and Cancer. Annu Rev Pathol 2016;11:421–449. [DOI] [PubMed] [Google Scholar]
- 56.Kado T, Nawaz A, Takikawa A, et al. Linkage of CD8(+) T cell exhaustion with high-fat diet-induced tumourigenesis. Sci Rep 2019;9:12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao JL, Savage PA. Unlocking the Complexities of Tumor-Associated Regulatory T Cells. J Immunol 2018;200:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plitas G, Konopacki C, Wu K, et al. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016;45:1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin 2019;69:88–112. [DOI] [PubMed] [Google Scholar]
- 61.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 62.Lam TK, Moore SC, Brinton LA, et al. Anthropometric measures and physical activity and the risk of lung cancer in never-smokers: a prospective cohort study. PLoS One 2013;8:e70672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A 2015;112:1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Li F, Tian Y, et al. Metformin Enhances the Antitumor Activity of CD8(+) T Lymphocytes via the AMPK-miR-107-Eomes-PD-1 Pathway. J Immunol 2020;204:2575–2588. [DOI] [PubMed] [Google Scholar]
- 65.Yendamuri S, Barbi J, Pabla S, et al. Body Mass Index Influences the Salutary Effects of Metformin on Survival After Lobectomy for Stage I NSCLC. J Thorac Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cespedes Feliciano EM, Kroenke CH, Caan BJ. The Obesity Paradox in Cancer: How Important Is Muscle? Annu Rev Nutr 2018;38:357–379. [DOI] [PubMed] [Google Scholar]
- 67.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 68.Leitzmann MF, Moore SC, Koster A, et al. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS One 2011;6:e18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu D, Zheng W, Johansson M, et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. J Natl Cancer Inst 2018;110:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ardesch FH, Ruiter R, Mulder M, et al. The Obesity Paradox in Lung Cancer: Associations With Body Size Versus Body Shape. Front Oncol 2020;10:591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nattenmuller J, Wochner R, Muley T, et al. Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. PLoS One 2017;12:e0169136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng W, Miyazato A, Chen G, et al. Interferon-gamma-induced gene expression in CD34 cells: identification of pathologic cytokine-specific signature profiles. Blood 2006;107:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cerezo M, Guemiri R, Druillennec S, et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat Med 2018;24:1877–1886. [DOI] [PubMed] [Google Scholar]
- 74.Dimitrakopoulos FI, Papadaki H, Antonacopoulou AG, et al. Association of FOXP3 expression with non-small cell lung cancer. Anticancer Res 2011;31:1677–1683. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.