Abstract
Background
Adults who have been infected with SARS-CoV-2 can develop a multisystem inflammatory syndrome (MIS-A), including fulminant myocarditis. Yet, several patients fail to meet MIS-A criteria, suggesting the existence of distinct phenotypes in fulminant COVID-19–related myocarditis.
Objectives
This study sought to compare the characteristics and clinical outcome between patients with fulminant COVID-19–related myocarditis fulfilling MIS-A criteria (MIS-A+) or not (MIS-A−).
Methods
A monocentric retrospective analysis of consecutive fulminant COVID-19–related myocarditis in a 26-bed intensive care unit (ICU).
Results
Between March 2020 and June 2021, 38 patients required ICU admission (male 66%; mean age 32 ± 15 years) for suspected fulminant COVID-19–related myocarditis. In-ICU treatment for organ failure included dobutamine 79%, norepinephrine 60%, mechanical ventilation 50%, venoarterial extracorporeal membrane oxygenation 42%, and renal replacement therapy 29%. In-hospital mortality was 13%. Twenty-five patients (66%) met the MIS-A criteria. MIS-A− patients compared with MIS-A+ patients were characterized by a shorter delay between COVID-19 symptoms onset and myocarditis, a lower left ventricular ejection fraction, and a higher rate of in-ICU organ failure, and were more likely to require mechanical circulatory support with venoarterial extracorporeal membrane oxygenation (92% vs 16%; P < 0.0001). In-hospital mortality was higher in MIS-A− patients (31% vs 4%). MIS-A+ had higher circulating levels of interleukin (IL)-22, IL-17, and tumor necrosis factor-α (TNF-α), whereas MIS-A− had higher interferon-α2 (IFN-α2) and IL-8 levels. RNA polymerase III autoantibodies were present in 7 of 13 MIS-A− patients (54%) but in none of the MIS-A+ patients.
Conclusion
MIS-A+ and MIS-A− fulminant COVID-19–related myocarditis patients have 2 distinct phenotypes with different clinical presentations, prognosis, and immunological profiles. Differentiating these 2 phenotypes is relevant for patients’ management and further understanding of their pathophysiology.
Key Words: COVID-19, cytokines, fulminant myocarditis, multisystem inflammatory syndrome, RNA polymerase III autoantibodies, SARS-CoV-2, VA-ECMO
Central Illustration
COVID-19–related myocarditis has been reported since the beginning of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) outbreak.1, 2, 3, 4, 5, 6 Fulminant myocarditis is a rare, but life-threatening, form of myocarditis leading to significant morbidity and mortality especially in young patients.7 First described in children8 and subsequently in adults,9 the multisystem inflammatory syndrome (MIS-C and MIS-A, respectively) accounts for a large proportion of COVID-19–related myocarditis. The U.S. Centers for Disease Control and Prevention has developed case definition criteria to standardize its diagnosis.10 Yet, some patients do not meet these criteria, suggesting the existence of distinct phenotypes in COVID-19–related myocarditis. We conducted a study to compare the clinical, biological, and immunological characteristics of patients with fulminant COVID-19–related myocarditis meeting or not meeting MIS-A criteria.
Methods
Patients and controls
We retrospectively reviewed the database of our 26-bed intensive care unit (ICU) between March 2020 and June 2021, and included all patients admitted for clinically suspected myocarditis with proven SARS-CoV-2 infection. Clinically suspected myocarditis was then adjudicated as definite or probable myocarditis according to the definition by Bonaca et al11 (Supplemental Appendix) following clinical investigations. Proven SARS-CoV-2 infection was confirmed by positive reverse transcription polymerase chain reaction (RT-PCR) in either nasopharyngeal aspirate, lower airway respiratory samples, or serum and/or positive serology showing the presence of circulating anti-nucleocapsid protein (anti-N) or anti-spike protein (anti-S) receptor binding domain antibodies in patients not vaccinated against COVID-19. All laboratory analyses were performed as the standard of care in the myocarditis workup of our institution. In addition, healthy SARS-CoV-2–negative individuals (n = 10) were included as control subjects for cytokine measurements.
Data collection
The following information was collected on standardized forms: epidemiologic parameters; severity of underlying condition according to the McCabe–Jackson criteria; medical history; COVID-19 infection history, manifestations, and complications; MIS-A criteria (Supplemental Appendix); day 0 Sequential Organ Failure Assessment Score and Simplified Acute Physiology Score II; day 0 and in-ICU clinical and biological parameters; day 0 and in-ICU organ-failure support treatment; day 0, in-ICU, and last-follow-up echocardiography parameters; in-ICU cytokine profiling; in-ICU SARS-CoV-2 and myocarditis-specific treatment; in-ICU and follow-up computed tomography scan and cardiac magnetic resonance imaging (CMR); complications; and vital status at ICU and hospital discharge, as well as at last follow-up.
SARS-CoV-2 RT-PCR and serological analyses
Detection of SARS-CoV-2 was carried out by RT-PCR in clinical specimens, using the Cobas6800 SARS-CoV-2 Test (Roche Diagnostics) and serological detection of immunoglobulin G (IgG) anti-N and IgG anti-S SARS-CoV-2, was performed by enzyme-linked immunosorbent assay (ELISA) on the Abbott platform (Abbott Diagnostics) in accordance with the manufacturer’s specifications.
Cytokine measurement
Whole blood was collected in anticoagulant-free tubes, and serum was separated by centrifugation and stored at −80°C. Serum concentrations of interleukin (IL)-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-22, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α were measured on a Quanterix SP-X imaging and analysis platform using the Human CorPlex Cytokine Panel Array kit (Quanterix). Single-plex bead-based ultrasensitive immunodetection of IL-17A and IFN-α was performed by digital ELISA using the Simoa (single molecule array) HD-1 analyzer (Quanterix), according to the manufacturer’s instructions. Serum IFN-β levels were quantified using a highly sensitive ELISA kit (PBL Assay Science), according to the manufacturer’s instructions. Serum cytokine concentrations were interpolated from the correspondent calibration curve taking into account the dilution factor. All cytokine concentrations were expressed in pg/mL. Samples with nondetectable values or those above the detection range were replaced by the limit of detection value and the upper limit of quantification, respectively.
Anti–IFN-α and RNA polymerase III autoantibodies
Autoantibodies against IFN-α were quantified using the anti–IFN-α Antibody Human ELISA Kit (Thermo Fisher, Invitrogen), according to the manufacturer's instructions. Calibrators were run in duplicate and fit with a 4-parameter logistic regression. The concentration of anti–IFN-α antibodies in samples was interpolated from the calibration curve by multiplying the obtained values with the dilution factor. The positivity threshold was 15 ng/mL. For RNA polymerase III autoantibodies screening, an indirect immunofluorescence assay was run on HEp-2000 cells (Immuno Concepts). When positive (≥1/80) and when the immunofluorescence labeling pattern was evocative of RNA polymerase III autoantibodies (fine-speckled nuclear-labeling pattern with small dots), a confirmatory immunodot assay (Euroline Systemic Sclerosis Test, Bio Advance) was carried out.
Statistical analyses
Continuous variables are expressed as median (IQR: 25-75) and compared with Wilcoxon’s signed rank tests. Categorical variables are expressed as n (%) and compared with chi-square tests or Fisher exact tests. Cumulative probabilities of survival were calculated using the Kaplan-Meier method and compared with log-rank tests. A 2-tailed P value < 0.05 was considered statistically significant. Analyses were computed with StatView software v5.0 (SAS Institute) and IBM SPSS Statistics v22.0 software (IBM Corp). Unsupervised principal component analysis (PCA) was performed using R software v3.6.2 with the FactoExtra and FactoMineR functions, on z-scaled log10-transformed cytokine concentrations. Samples with missing data were excluded from the PCA analysis for 1 MIS-A+ patient and 2 MIS-A− patients.
Ethical considerations
This study was conducted in accordance with the declaration of Helsinki using the database registered at the Commission Nationale de l’Informatique et des Libertés (CNIL, registration no. 1950673). In agreement with the ethical standards of our hospital’s Institutional Review Board, the Committee for the Protection of Human Subjects, and French law, written informed consent was not needed for demographic, physiological, and hospital-outcome data analysis, because this observational study does not modify existing diagnostic or therapeutic strategies; however, patients and/or their relatives were informed of their anonymous inclusion in the study.
Results
General patient characteristics
Between March 2020 and June 2021, 38 patients requiring ICU admission for clinically suspected fulminant COVID-19–related myocarditis were included in this study. They were mostly men (66%) of young age (median age 27.5 years [IQR: 19-37 years]) with few comorbidities. Their baseline characteristics are reported in Table 1 and Supplemental Table 1. All had positive SARS-CoV-2 RT-PCR (37%) or serology (68%) with a median delay of 5 days between COVID-19 symptom onset and the first manifestation of myocarditis. None had previously received any COVID-19 vaccine. Most frequent symptoms were fever (95%), abdominal pain or nausea (60%), chest pain (47%), and dyspnea (42%).
Table 1.
COVID-19–Related MIS-A Criteria and SARS-CoV-2 Tests Results
| All Patients (N = 38) | MIS-A− (n = 13) | MIS-A+ (n = 25) | P Value | |
|---|---|---|---|---|
| Age, y | 27.5 (19.0-37.0) | 33.0 (21.5-38.5) | 25.0 (18.5-35.5) | 0.3 |
| Women | 13 (34) | 6 (46) | 7 (28) | 0.3 |
| BMI, kg/m2 | 24.8 (22.4-28.6) | 25.9 (23.3-28.9) | 24.7 (21.7-28.2) | 0.7 |
| Time from first COVID-19 symptoms to myocarditis, days | 5 (0-26) | 3 (0-5) | 8 (2-38) | 0.04 |
| Time from myocarditis symptoms onset to ICU, days | 3 (0-5) | 1 (0-3) | 4 (1-6) | 0.009 |
| ICU admission SOFA score | 8 (5-11) | 11 (8-13) | 6 (4-10) | 0.002 |
| ICU admission SAPS-II score | 30 (20-40) | 33 (25-49) | 25 (15-35) | 0.04 |
| MIS-A criteriaa | ||||
| Fever | 36 (95) | 9 (69) | 25 (100) | 0.01 |
| Primary clinical criteria | ||||
| Myocardial involvement | 38 (100) | 13 (100) | 25 (100) | na |
| Skin involvement | 14 (37) | 1 (8) | 13 (52) | 0.01 |
| Secondary clinical criteria | ||||
| Neurological involvement | 14 (37) | 4 (31) | 10 (40) | 0.5 |
| Shock or hypotension | 36 (95) | 13 (100) | 23 (92) | 0.1 |
| Abdominal involvement | 22 (58) | 7 (54) | 15 (60) | 0.7 |
| Platelets <150 × 109/L | 10 (26) | 5 (38) | 5 (20) | 0.09 |
| Laboratory evidence | ||||
| Inflammationb | 29 (76) | 2 (15) | 25 (100) | <0.0001 |
| Positive SARS-CoV-2 testc | 38 (100) | 13 (100) | 25 (100) | na |
| SARS-CoV-2 tests | ||||
| Positive nasopharyngeal RT-PCR | 14 (37) | 11 (85) | 4 (16) | <0.0001 |
| CT value | 26 (18-32) | 24 (18-28) | 32 (21-34) | 0.2 |
| Positive serology | 26 (68) | 2 (15) | 24 (96) | <0.0001 |
| IgG anti-S | 25 (67) | 2 (15) | 23/24 (96) | <0.0001 |
| Titer, UA/mL | 660 (156-1,440) | 2.3 (0.1-59) | 854 (528-2,575) | <0.0001 |
| IgG anti-N | 26 (68) | 2 (15) | 24 (96) | <0.0001 |
| Index, value | 2.2 (0.1-5.3) | 0.4 (0.03-0.8) | 4.8 (2.2-5.5) | <0.0001 |
Values are median (IQR) or n (%). Continuous variables are compared with Wilcoxon’s rank test; categorical variables are compared with Fisher exact test.
BMI = body mass index; CT = cycle threshold; ICU = intensive care unit; IgG = immunoglobulin G; MIS-A = multisystem inflammatory syndrome in adults; N = nucleocapsid protein; RT-PCR = reverse transcription polymerase-chain reaction; S = spike protein; SAPS-II = Simplified Acute Physiology Score-II; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2; SOFA = Sequential Organ Failure Assessment.
According the Centers for Disease Control and Prevention.
Elevated levels of at least 2 of the following biomarkers including: C-reactive protein >10 mg/L, procalcitonin >1 ng/mL, fibrinogen >5 g/L.
Positive RT-PCR, serology, or antigen for SARS-CoV-2.
At admission, patients had severely impaired left ventricular function (left ventricular ejection fraction [LVEF] 20% [IQR: 14%-37%], LVOT-VTI 11 cm [IQR: 6-15 cm]), an increased high-sensitivity T-troponin (median 1,300 ng/mL [IQR: 486-4,750 ng/mL]), and 79% presented with cardiogenic shock. When performed (n = 10), coronary angiography was normal. COVID-19 pneumonia was noted on computed tomography scan examination in 29% of cases. In the 26 patients who had CMR evaluation, myocardial edema, and late gadolinium enhancement were reported in 73% and 54%, respectively.
Three patients without recovery of cardiac function underwent myocardial biopsy. None were CMR-guided, as all were taken under mechanical circulatory support. Two surgical biopsies were taken in patients during venoarterial extracorporeal membrane oxygenation (VA-ECMO) centralization. The first one was an apical surgical biopsy in a patient cannulated under cardiopulmonary resuscitation, which was inconclusive. The second surgical biopsy highlighted myocarditis with an inflammatory infiltrate associated with myocyte dystrophy and edema. SARS-CoV-2 RT-PCR was negative, and electron microscopy analysis failed to identify viral particles in cardiomyocytes despite active myocarditis lesions on the evaluated sample. The last biopsy was endomyocardial and disclosed a mild lymphohistiocytic myocarditis with no edema, but with severe necrosis. SARS-CoV-2 RT-PCR was negative. Twenty-nine patients (76%) met the Bonaca classification criteria for definite myocarditis, whereas the others had probable myocarditis (Table 2 ).
Table 2.
Fulminant COVID-19–Related Myocarditis Findings
| na | All Patients (N = 38) | MIS-A− (n = 13) | MIS-A+ (n = 25) | P Value | |
|---|---|---|---|---|---|
| Clinical symptoms | |||||
| Chest pain | 18 (47) | 9 (69) | 9 (36) | 0.09 | |
| Faintness | 4 (10) | 4 (31) | 0 (0) | 0.01 | |
| Syncope | 2 (5) | 1 (8) | 1 (4) | 1.0 | |
| Sudden death | 1 (3) | 1 (8) | 0 (0) | 0.3 | |
| Laboratory findings | |||||
| Troponin, ng/mL | 526 (224-1,227) | 441 (177-1,089) | 712 (217-2,025) | 0.2 | |
| Highest value in ICU | 1,300 (486-4,750) | 2,836 (450-9,634) | 1,000 (471-3,036) | 0.2 | |
| NT-proBNP, ng/L | 1 | 9,931 (2,367-23,934) | 2,755 (1,044-8,271) | 12,525 (7,000-32,500) | 0.007 |
| Creatine phosphokinase, UI/L | 312 (131-1,150) | 586 (388-1,802) | 190 (115-435) | 0.003 | |
| Electrocardiogram findings | |||||
| Normal electrocardiogram | 14 (37) | 5 (38) | 9 (36) | 1.0 | |
| Sinus rhythm | 36 (95) | 12 (92) | 24 (96) | 1.0 | |
| Atrial fibrillation | 2 (5) | 1 (8) | 1 (4) | 1.0 | |
| ST-segment elevation | 10 (26) | 6 (46) | 4 (16) | 0.06 | |
| ST-segment depression | 6 (16) | 2 (15) | 4 (16) | 1.0 | |
| Negative T wave | 10 (26) | 1 (8) | 9 (36) | 0.1 | |
| Complete heart block | 1 (3) | 0 (0) | 1 (4) | 1.0 | |
| Bundle branch block | 5 (13) | 1 (8) | 4 (16) | 0.6 | |
| Ventricular rhythm disorders | 3 (8) | 3 (23) | 0 (0) | 0.03 | |
| Echocardiography findings | |||||
| LVEF, % | |||||
| First evaluation | 30 (20-45) | 30 (15-45) | 30 (25-42) | 0.5 | |
| On ICU admission | 20 (14-37) | 10 (5-30) | 30 (15-45) | 0.01 | |
| Lowest value in ICU | 20 (10-30) | 10 (5-25) | 20 (15-30) | 0.02 | |
| ICU discharge | 42 (30-54) | 35 (17-57) | 45 (35-52) | 0.1 | |
| Last follow-up | 10 | 60 (50-64) | 59 (44-60) | 60 (50-65) | 0.5 |
| LVOT VTI, cm | |||||
| First evaluation | 12 (8-16) | 8 (7-17) | 12 (9-15) | 0.2 | |
| On ICU admission | 11 (6-15) | 5 (2-9) | 13 (10-17) | <0.0001 | |
| ICU discharge | 17 (12-18) | 12 (7-18) | 17 (15-19) | 0.08 | |
| Ventricular hypertrophy | 16 (42) | 8 (62) | 8 (32) | 0.1 | |
| Ventricular dilation | 5 (13) | 2 (15) | 3 (12) | 1.0 | |
| LVEDD, mm | 50 (47-56) | 48 (46-55) | 50 (47-56) | 0.7 | |
| Right ventricular involvement | 15 (39) | 7 (54) | 8 (32) | 0.3 | |
| TAPSE, mm | 20 | 14 (12-17) | 12 (8-16) | 14 (12-17) | 0.2 |
| S wave, cm/s | 21 | 9 (7-11) | 6 (1-11) | 10 (8-11) | 0.1 |
| Mitral valve regurgitation | 9 (24) | 3 (23) | 6 (24) | 1.0 | |
| Aortic valve regurgitation | 3 (8) | 0 (0) | 1 (4) | 1.0 | |
| Tricuspid valve regurgitation | 3 (8) | 0 (0) | 3 (12) | 0.5 | |
| Pericardial effusion | 15 (39) | 8 (62) | 7 (28) | 0.08 | |
| Pericardiocentesis | 4 (10) | 4 (31) | 0 (0) | 0.01 | |
| CMR findings | |||||
| Number performed in ICU/hospital | 26 (68) | 5 (38) | 21 (84) | ||
| Time from symptoms to CMR, days | 7 (4-18) | 16 (9-33) | 5 (4-10) | ||
| Myocardial edema | 19/26 (73) | 3/5 (60) | 16/21 (76) | 0.6 | |
| Late gadolinium enhancement | 14/26 (54) | 4/5 (80) | 10/21 (48) | 0.3 | |
| Myocarditis classificationb | |||||
| Definite myocarditis | 29 (76) | 9 (69) | 20 (80) | ||
| Probable myocarditis | 9 (24) | 4 (31) | 5 (20) | ||
| Pathology | 2 (5) | 2 (15) | 1 (4) | ||
| Imaging | |||||
| Cardiac magnetic resonance | 22 (58) | 4 (31) | 18 (72) | ||
| Echocardiography WMA | 32 (84) | 13 (100) | 24 (96) | ||
| Coronary angiography performed and normal | 10 (26) | 6 (46) | 4 (16) | ||
| Electrocardiogram | 23 (60) | 8 (61) | 15 (60) | ||
| Syndrome | 38 (100) | 13 (100) | 25 (100) | ||
| Biomarkers | 38 (100) | 13 (100) | 25 (100) |
Values are median (IQR), n (%), or n/N (%), unless otherwise indicated. Continuous variables are compared with Wilcoxon’s rank test; categorical variables are compared with Fisher exact test.
CMR = cardiac magnetic resonance; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; LVOT VTI = left ventricle outflow tract velocity-time integral; NT-proBNP = N-terminal pro–B-type natriuretic peptide; TAPSE = tricuspid annular plane systolic excursion; WMA = wall motion abnormality; other abbreviations as in Table 1.
Number of missing values.
According to the myocarditis classification proposed by Bonaca et al.11
In-ICU evolution and outcomes
Median length of stay in ICU was 6 days. Seventy-nine percent of the patients received dobutamine, 60% norepinephrine, 50% mechanical ventilation, and 29% renal replacement therapy (Table 3 ). Four patients had a large pericardial effusion requiring drainage. Sixteen patients (42%) required mechanical circulatory support with VA-ECMO 1 day (IQR: 0-1 day) following ICU admission, for a median duration of 7 days. Twenty-eight (74%) were treated with corticosteroids, and 27 (74%), with intravenous immunoglobulins. In-hospital mortality was 13%. None of the survivors required cardiac transplantation or long-term ventricular assist device implantation. Among the 5 deceased patients, all had multiorgan failure before VA-ECMO implantation, including 3 cannulations during cardiopulmonary resuscitation. None could be weaned from VA-ECMO because of severe cardiac dysfunction. Median LVEF at ICU and hospital discharge was 42% (IQR: 30%-54%) and 60% (IQR: 50%-64%), respectively. Twenty-one survivors (64%) received beta-blockers at discharge, and 25 (76%) were treated with angiotensin-converting enzyme inhibitors, until distant evaluation with a cardiologist. At the last follow-up (median: 235 days [IQR: 155-359 days]), 32 patients were alive, and all but 1 had a normal LVEF. One patient was lost to follow-up.
Table 3.
Organ Failure Support, Myocarditis Treatment, Complications, and Outcome in ICU
| All Patients (N = 38) | MIS-A− (n = 13) | MIS-A+ (n = 25) | P Value | |
|---|---|---|---|---|
| Time in ICU, days | 6 (4-16) | 12 (7-30) | 5 (2-6) | <0.0001 |
| Cardiac arrest before ICU | 1 (3) | 1 (8) | 0 (0) | 0.3 |
| Organ failure support in ICU | ||||
| Dobutamine | 30 (79) | 12 (92) | 18 (72) | 0.2 |
| Norepinephrine | 23 (60) | 12 (92) | 11 (44) | 0.005 |
| Mechanical ventilation | 19 (50) | 11 (85) | 8 (32) | 0.005 |
| Time on mechanical ventilation, days | 15 (6-28) | 15 (8-35) | 11 (4-25) | 0.3 |
| Renal replacement therapy | 11 (29) | 8 (61) | 3 (12) | 0.003 |
| VA-ECMO | 16 (42) | 12 (92) | 4 (16) | <0.0001 |
| VA-ECMO under CPR | 4 (11) | 4 (31) | 0 (0) | 0.02 |
| Time on VA-ECMO, days | 7 (5-12) | 8 (6-12) | 5 (4-12) | 0.1 |
| Time from admission to VA-ECMO, days | 1 (0-1) | 0 (0-1) | 1 (0-5) | 0.4 |
| VV-ECMO | 4 (10) | 2 (15) | 2 (8) | 0.6 |
| Time on VV-ECMO, days | 18 (14-29) | 24 (16-24) | 17 (14-17) | 0.4 |
| Myocarditis treatment in ICU | ||||
| Corticosteroids | 28 (74) | 7 (54) | 21 (84) | 0.06 |
| Intravenous immunoglobulins | 27 (71) | 8 (61) | 19 (76) | 0.5 |
| Tocilizumab | 0 (0) | 0 (0) | 0 (0) | Na |
| Outcome | ||||
| In-ICU mortality | 5 (13) | 4 (31) | 1 (4) | 0.04 |
| In-hospital mortality | 5 (13) | 4 (31) | 1 (4) | 0.04 |
| 3-month probability of survival, %a | 86 ± 6 | 68 ± 13 | 96 ± 4 | 0.01 |
Values are median (IQR), n (%), or mean ± SD. Continuous variables are compared with Wilcoxon’s rank test; categorical variables are compared with Fisher exact test.
CPR = cardiopulmonary resuscitation; VA-ECMO = venoarterial extracorporeal membrane oxygenation; VV-ECMO = venovenous extracorporeal membrane oxygenation; other abbreviations as in Table 1.
Probability of survival were calculated using Kaplan-Meier method and compared with log-tank tests.
Comparison between MIS-A+ and MIS-A− patients
Twenty-five patients (66%) met the MIS-A criteria (Table 1). By definition, MIS-A+ patients had more frequent fever, skin rash, enanthema, pharyngitis, and conjunctivitis, as compared with MIS-A− patients (Table 1). In addition, they had, as expected by the MIS-A definition, higher levels of systemic inflammation markers, including circulating leukocytes, procalcitonin, C-reactive protein, and fibrinogen (Table 4 ).
Table 4.
Laboratory Findings and Cytokine Profiling in the ICU
| Day 0 Laboratory Findings | na | All Patients (N = 38) | MIS-A− (n = 13) | MIS-A+ (n = 25) | P Value |
|---|---|---|---|---|---|
| Hemogram and hemostasis | |||||
| Leukocytes, 109/L | 12.6 (9.2-19.7) | 8.7 (5.7-11.4) | 18.5 (11.7-21.0) | <0.001 | |
| Lymphocytes, 109/L | 0.8 (0.5-1.5) | 1.2 (0.6-2.3) | 0.8 (0.5-1.2) | 0.08 | |
| Polymorphonuclear cells, 109/L | 10.7 (5.8-18.0) | 5.8 (3.4-8.1) | 15.6 (10.3-19.0) | <0.001 | |
| Hemoglobin, g/dL | 12,1 (11.1-13.5) | 12.5 (10.4-16.0) | 12 (11.6-13.3) | 0.8 | |
| Platelets 109/L | 192 (152-247) | 192 (92-258) | 206 (160-243) | 0.7 | |
| Prothrombin time, % | 72 (64-81) | 65 (56-90) | 72 (69-77) | 0.4 | |
| D-dimers, μg/L | 3 | 3,860 (1,290-6,700) | 2,500 (396-20,000) | 4,217 (1,602-6,035) | 0.6 |
| Inflammatory parameters | |||||
| C-reactive protein, mg/L | 5 | 257 (110-329) | 5 (4-72) | 277 (226-376) | <0.0001 |
| Procalcitonin, ng/mL | 7.4 (0.5-46) | 0.2 (0.1-1.1) | 12.8 (3.7-65) | <0.0001 | |
| Fibrinogen, g/L | 6.8 (4.2-8.5) | 3.2 (2.2-4.3) | 7.9 (6.8-9.2) | <0.0001 | |
| Biochemical findings | |||||
| Serum creatinine, μmol/L | 105 (69-156) | 85 (60-105) | 134 (71-265) | 0.038 | |
| LDH, IU/L | 2 | 419 (315-634) | 619 (320-973) | 385 (307-526) | 0.2 |
| AST, IU/L | 83 (46-139) | 70 (42-168) | 94 (46-129) | 0.9 | |
| ALT, IU/L | 50 (32-101) | 39 (26-110) | 60 (37-101) | 0.4 | |
| Serum total bilirubin, μmol/L | 11 (8-19) | 6 (4-14) | 12 (10-21) | 0.006 | |
| pH | 1 | 7.43 (7.30-7.46) | 7.31 (7.15-7.42) | 7.44 (7.41-7.47) | 0.004 |
| pO2, mm Hg | 1 | 90 (70-120) | 106 (80-235) | 81 (69-99) | 0.06 |
| pCO2, mm Hg | 1 | 30 (24-36) | 29 (20-46) | 30 (27-36) | 0.7 |
| Serum bicarbonates, mmol/L | 2 | 19 (15-23) | 16 (10.4-19.4) | 21 (17-24) | 0.005 |
| Arterial lactate, mmol/L | 2 | 2.5 (1.7-3.9) | 5.5 (1.8-8.2) | 2.1 (1.5-2.7) | 0.009 |
| Highest value in ICU, mmol/L | 2 | 3.1 (2.4-7.1) | 7.5 (5.2-15.5) | 2.7 (1.7-3.4) | <0.0001 |
| Serum protein, g/L | 61 (52-68) | 51 (40-57) | 65 (58-70) | <0.0001 | |
| Serum albumin, g/L | 25 (22-28) | 27 (23-33) | 25 (20-27) | 0.1 | |
| Triglycerides, mmol/L | 15 | 2 (1.7-3) | 2.0 (1.1-3.0) | 2.3 (1.8-3.2) | 0.4 |
| Immunological findings | |||||
| RNA polymerase 3 autoantibodies | 7 (18) | 7 (54) | 0 (0) | 0.001 | |
| Serum cytokine levels in ICU | |||||
| IL-12p70, pg/mL | 3 | 0.03 (0.01-0.4) | 0.03 (0.01-0.1) | 0.03 (0.01-0.4) | 0.3 |
| IL-1β, pg/mL | 3 | 0.2 (0.02-0.4) | 0.3 (0.01-0.9) | 0.2 (0.02-0.3) | 0.5 |
| IL-4, pg/mL | 3 | 0.4 (0.2-1.1) | 0.3 (0.3-0.5) | 0.6 (0.2-2.1) | 0.3 |
| IL-5, pg/mL | 3 | 0.1 (0.01-0.5) | 0.04 (0.01-0.6) | 0.3 (0.06-0.6) | 0.1 |
| IFN-γ, pg/mL | 3 | 0.4 (0.2-2.2) | 0.4 (0.09-2.0) | 1.2 (0.2-2.6) | 0.2 |
| IL-6, pg/mL | 3 | 55.2 (25.1-207.6) | 39.6 (16.6-225.4) | 57.8 (26.9-198.9) | 0.7 |
| IL-8, pg/mL | 3 | 82.7 (58.2-166.4) | 158.7 (74.9-784.2) | 65.7 (55.7-118.3) | 0.02 |
| IL-22, pg/mL | 3 | 6.4 (2.3-15.7) | 1.5 (0.7-2.9) | 9.93 (5.28-28.99) | <0.0001 |
| TNF-α, pg/mL | 3 | 14.2 (8.9-38.1) | 8.0 (4.9-34.0) | 21.1 (9.9-41.9) | 0.05 |
| IL-10, pg/mL | 3 | 50.3 (15.9-76.6) | 67.8 (20.1-143.1) | 44.2 (12.8-68.4) | 0.3 |
| IL-17A, pg/mL | 3 | 1.6 (0.2-5.2) | 0.15 (0.08-0.3) | 3.2 (0.8-6.2) | <0.0001 |
| IFN-α2, pg/mL | 3 | 0.02 (0.005-1.3) | 2.4 (0.2-15.0) | 0.013 (0.002-0.04) | 0.001 |
| IFN-β, pg/mL | 4 | 0.6 (0.6-0.6) | 0.6 (0.6-1.8) | 0.6 (0.6-0.6) | 0.2 |
| Anti-IFNα autoantibodies | 4 | 5 (15) | 1 (10) | 4 (17) | 1 |
Values are median (IQR) or n (%), unless otherwise indicated. Continuous variables are compared with Wilcoxon’s rank test; categorical variables are compared with Fisher exact test.
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; IFN = interferon; IL = interleukin; LDH = lactate dehydrogenase; TNF = tumor necrosis factor; other abbreviations as in Table 1.
Number of missing values.
The median delay between COVID-19 symptoms onset and occurrence of myocarditis was shorter in MIS-A− patients: 3 vs 8 days. Noteworthy, the delay between first COVID-19 symptoms and myocarditis was 32 days (IQR: 25-44 days) among the 12 MIS-A+ patients with prior proven symptomatic SARS-CoV-2 infection. The rate of positive serology was lower in MIS-A− patients (15% vs 96%), and their titer was also much lower than in MIS-A+ patients (P < 0.0001). Conversely, positive nasopharyngeal RT-PCR at the time of myocarditis was infrequent in MIS-A+ patients (16%), as compared with MIS-A− patients (85%).
MIS-A− patients had swifter ICU admission after myocarditis onset (1 vs 4 days) with a more severe presentation (day 0 Sequential Organ Failure Assessment score of 11 vs 6 in MIS-A+ patients). They had a lower LVEF (10% vs 30%) and LVOT-VTI (5 cm vs 13 cm) (Table 2) and were more likely to receive norepinephrine, mechanical ventilation, and renal replacement therapy. Large pericardial effusions were also more frequently observed in MIS-A− patients. The median lactate level was 5.5 vs 2.1 mmol/L in MIS-A− and MIS-A+ patients, respectively. Finally, MIS-A− patients were more likely to require VA-ECMO than MIS-A+ patients (92% vs 16%), and had a higher in-ICU mortality (31% vs 4%; P = 0.04) (Table 3). The 3-month cumulative probabilities of survival ± standard error for MIS-A− and MIS-A+ patients were, respectively, 68% ± 13% and 96% ± 4%; log-rank test P = 0.01.
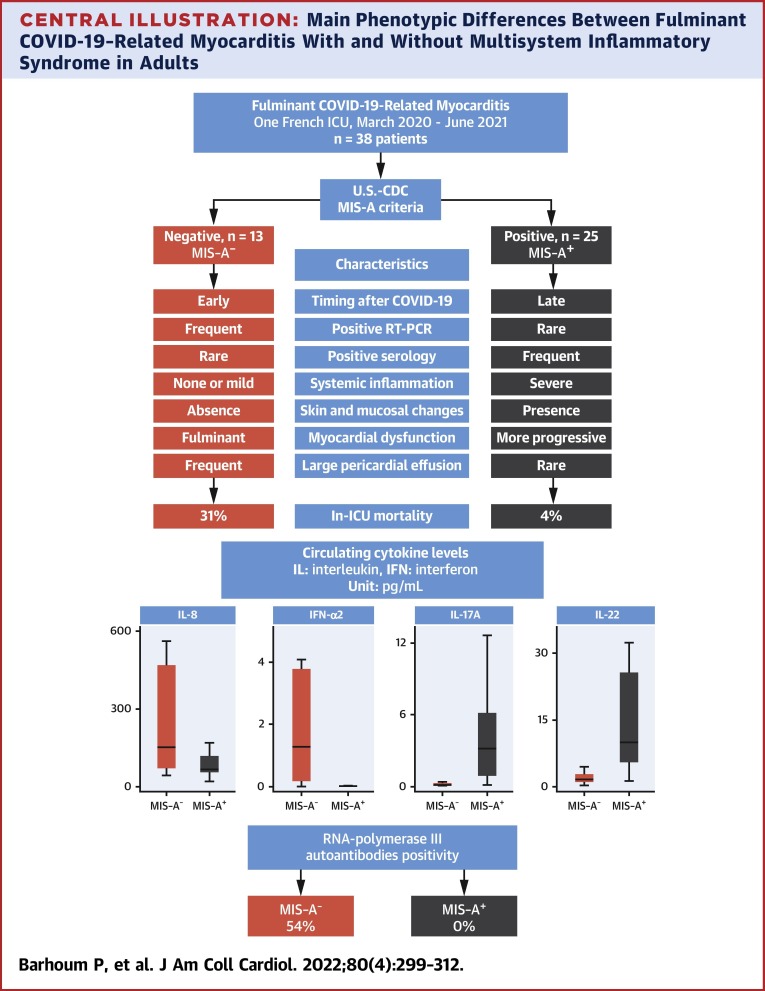
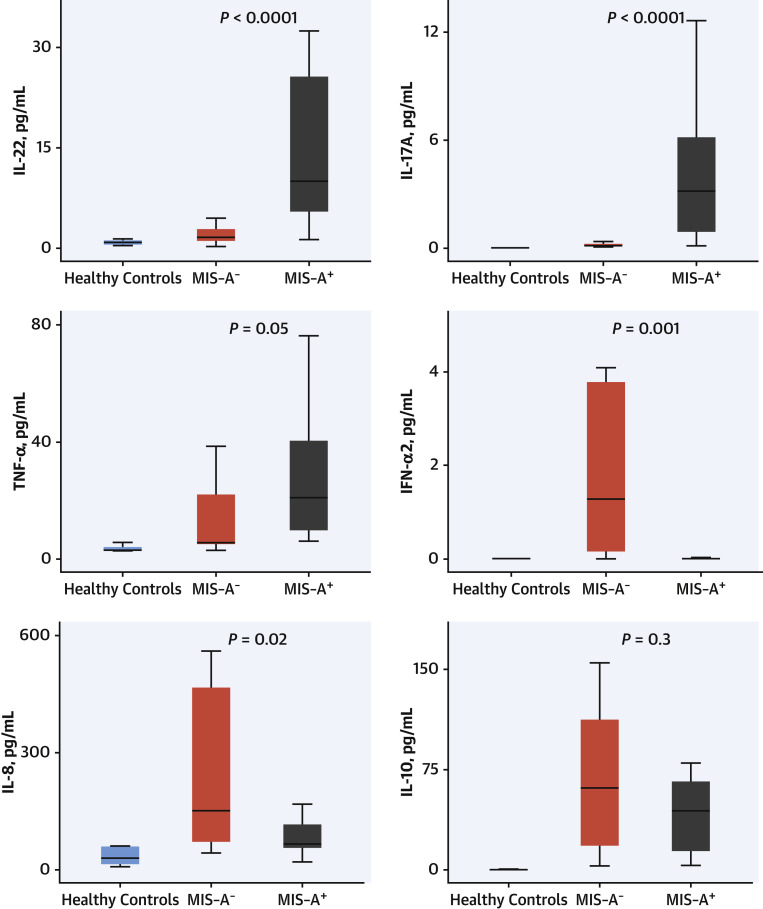
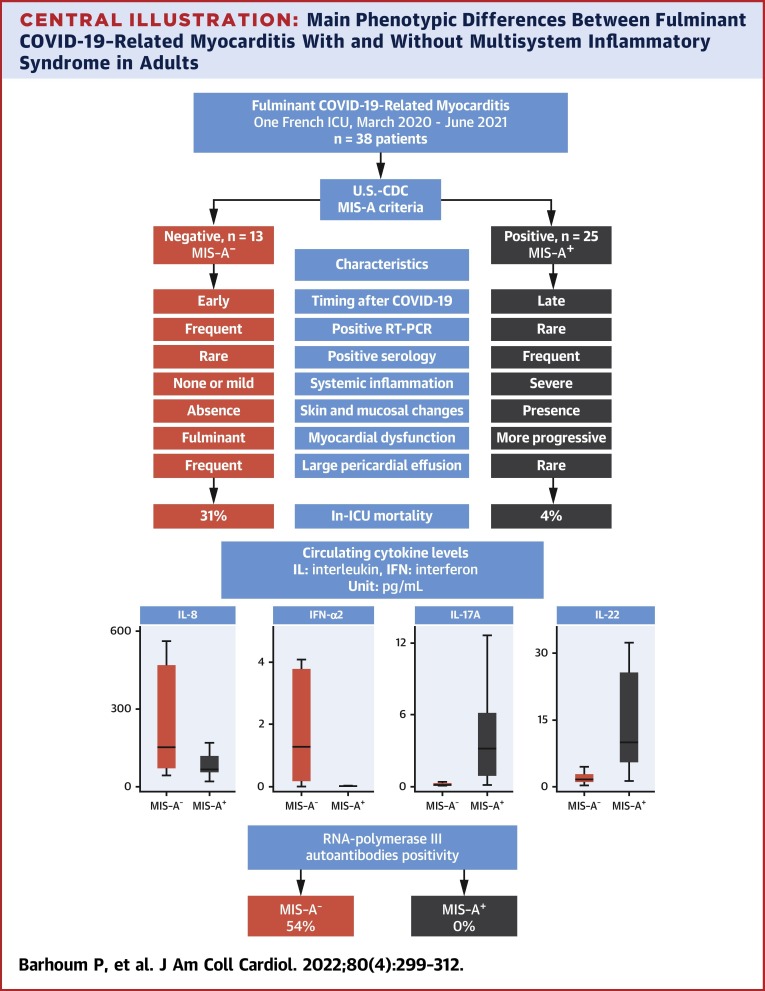
Cytokine profiling highlighted the presence of 2 distinct cytokine production profiles (Figure 1 , Table 4): MIS-A+ had higher IL-22 (9.93 vs 1.5 pg/mL; P < 0.0001), IL-17 (3.2 vs 0.15 pg/mL; P < 0.0001), and TNF-α (21.1 vs 8.0 pg/mL; P = 0.05) levels, as compared with MIS-A− patients, whereas the latter had higher IFN-α2 (2.4 vs 0.013 pg/mL; P = 0.001) and IL-8 (158.7 vs 65.7 pg/mL; P = 0.02), respectively. Moreover, RNA polymerase III autoantibodies were found in 7 MIS-A− patients (54%), 5 of them being female (Table 4).
Figure 1.
Circulating Cytokines Levels in Fulminant COVID-19–Related Myocarditis
Comparison of 6 circulating serum cytokines levels (IL-8, -10, -17, -22, IFN-α2, and TNF-α) in patients with MIS-A+/MIS-A− and healthy controls. MIS-A+ had higher IL-22, IL-17, and TNF-α, whereas MIS-A− had higher IFN-α2 and IL-8. Methods: serum concentrations of IL-8, IL-10, IL-22 and TNF-α were measured on a Quanterix SP-X imaging and analysis platform using the Human CorPlex Cytokine Panel Array kit (Quanterix). Single-plex bead-based ultrasensitive immunodetection of IL-17A and IFN-α was performed by digital ELISA using the Simoa (single molecule array) HD-1 analyzer (Quanterix), according to the manufacturer’s instructions. For box and whisker plots: the center line denotes the median value (50th percentile), whereas the box contains the 25th to 75th percentiles of dataset. The whiskers mark the 5th and 95th percentiles. IFN = interferon; IL = interleukin; MIS-A = multisystem inflammatory syndrome in adults; TNF = tumor necrosis factor.
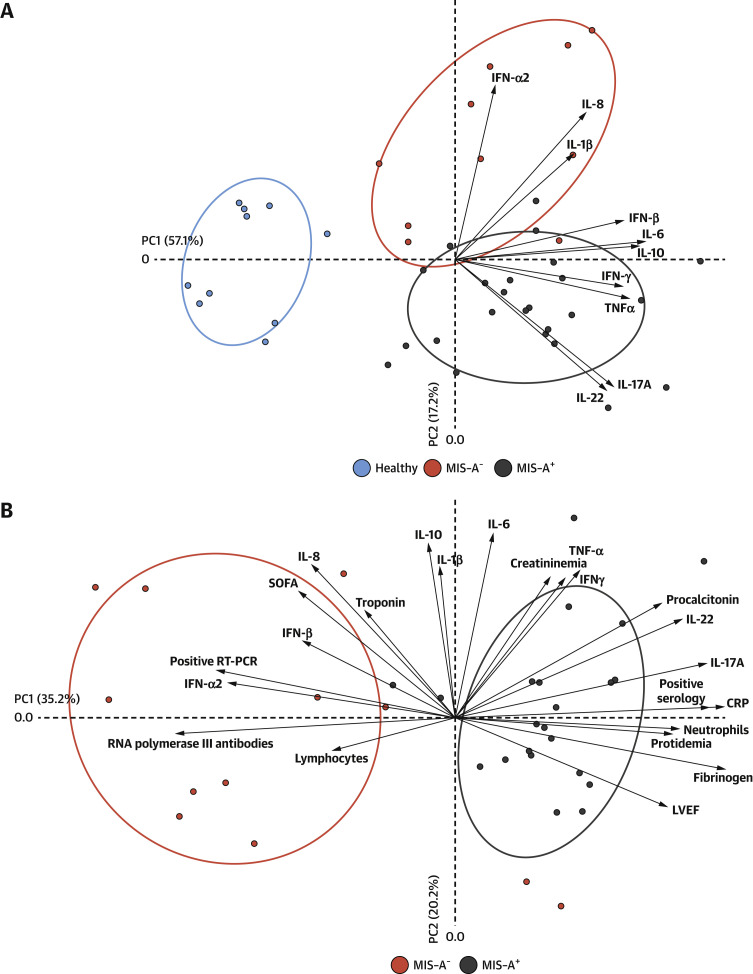
Finally, to elucidate the relative importance of the various bioclinical parameters listed in the preceding text with the clinical profile of MIS-A+ or MIS-A− patients, we performed nonsupervised PCA using study parameters contributing, in a statistically significant manner, to interpatient variation (Tables 1, 2, and 3). The results from PCA underlined important overall differences between MIS-A+ and MIS-A− patients (Figure 2 ). The data also further highlight parameters most contributing to either clinical status, that is, fibrinogen (P < 0.0001), CRP (P < 0.0001), IL-17 (P < 0.0001), IL-22 (P < 0.0001), IFN-α2 (P = 0.001) levels, SARS-CoV-2 serology (P < 0.0001), and SARS-CoV-2 RT-PCR (P < 0.0001), LVEF (P = 0.01) values on admission, and the presence of RNA polymerase III autoantibodies (P = 0.001).
Figure 2.
PCA of Parameters Associated With COVID-19–Related Myocarditis
Unsupervised PCA was performed using R software v3.6.2 with the FactoExtra and FactoMineR functions, on z-scaled log10-transformed cytokine concentrations. Samples with missing data were excluded from the PCA analysis for 1 MIS-A+ patient and 2 MIS-A− patients. Ellipses with 66% CI are drawn for each group. (A) The principal component analysis of 10 circulating serum cytokines. (B) The principal component analysis including clinical findings, laboratory findings and immunological profiles highlight the main features of MIS-A− and MIS-A+ fulminant COVID-19–related myocarditis phenotypes. CRP = C-reactive protein; LVEF = left ventricular ejection fraction; PCA = principal component analysis; RT-PCR = reverse transcription polymerase chain reaction; SOFA = Sequential Organ Failure Assessment; other abbreviations as in Figure 1.
Discussion
In this retrospective monocenter cohort of fulminant COVID-19–related myocarditis, we applied the MIS-A criteria case definition, and we identified 2 subsets of patients with very different clinical/biological presentations, outcomes, and immunological profiles. This phenotypic heterogeneity being likely explained by important differences in pathophysiological mechanisms.
The patients in this cohort were mostly young men with severely impaired cardiac function, frequently requiring VA-ECMO, with infrequent concomitant COVID-19–associated pneumonia. All survivors recovered a near normal cardiac function at distant follow-up. To our knowledge, this study is the largest cohort of COVID-19–related fulminant myocarditis and extends prior reports of COVID-19–related myocarditis9 , 12, 13, 14, 15 and fulminant non–COVID-19 myocarditis.7 , 16, 17, 18, 19
This analysis underscores the major clinical and immunological differences between patients with fulminant COVID-19–related myocarditis fulfilling or not MIS-A criteria. The original description of MIS-C was reported in May 2020,8 and MIS-A, a few months afterwards.9 , 20 , 21 This somewhat delayed description, together with the rarity of the disease, may have participated to an under-recognition of MIS in the adult population. Furthermore, whereas MIS-C is now well-defined with classification criteria established by the World Health Organization and the Centers for Disease Control and Prevention,22 , 23 only the latter has adapted its criteria to the adult population.10
The main differences between the phenotypes of MIS-A+ and MIS-A− patients are summarized in the Central Illustration . MIS-A+ COVID-19–related myocarditis appears to be a postinfectious complication of SARS-CoV-2 infection, as suggested by the higher delay between COVID-19 symptoms and myocarditis, as well as by frequently positive serology and negative (or slightly positive) RT-PCR. Mucocutaneous manifestations are frequent in addition to laboratory evidence of severe systemic inflammation. Heart failure is more progressive, leading to fewer accounts of refractory cardiogenic shock, and is associated with a lower mortality rate. Conversely, MIS-A− fulminant COVID-19–related myocarditis occurred at the early phase of SARS-CoV-2 infection (negative or slightly positive serology and positive RT-PCR) with an explosive and refractory cardiogenic shock in nearly all patients leading to high morbidity and mortality.
Central Illustration.
Main Phenotypic Differences Between Fulminant COVID-19–Related Myocarditis With and Without Multisystem Inflammatory Syndrome in Adults
MIS-A+ and MIS-A− fulminant COVID-19–related myocarditis patients have 2 distinct phenotypes with different clinical presentations, prognosis and immunological profiles. For box and whisker plots: the center line denotes the median value (50th percentile), whereas the box contains the 25th to 75th percentiles of dataset. The whiskers mark the 5th and 95th percentiles. ICU = intensive care unit; MIS-A = multisystem inflammatory syndrome in adults; RT-PCR = reverse transcription polymerase chain reaction; U.S.-CDC = U.S. Centers for Disease Control and Protection.
Interestingly, these different clinical phenotypes are supported by immunological findings. The frequency of RNA polymerase III autoantibodies is high in MIS-A−, whereas it is absent in MIS-A+ patients. The presence of these rare autoantibodies, usually associated with severe systemic sclerosis, has been previously reported by Pineton de Chambrun et al24 in patients with severe recurrent myocarditis and/or pericarditis, especially related to influenza virus. The role of these autoantibodies in the susceptibility to viral myocarditis is not yet elucidated. Their presence might reflect altered immune defenses toward viral infections or, alternatively, exaggerated antiviral responses leading to organ damage. Another patient with recurrent viral myocarditis, including COVID-19–related myocarditis, has been recently reported.25
The cytokine profiles of these patients were also found definitely different in the 2 clinical phenotypes (Figure 2). In MIS-A− patients, high levels of systemic circulating antiviral IFN-α2 likely arise from the ongoing viral infection, in relation to detectable viral replication and yet undetectable anti–SARS-CoV-2 IgG humoral responses. Levels of IL-8, a proinflammatory cytokine, were also more elevated in MIS-A−, as compared with MIS-A+ patients, further underlining the dominance of an innate type of immune response in the former group. Conversely, elevated IL-17 and IL-22 levels were found particularly associated with the MIS-A+ phenotype, in agreement with the mucocutaneous manifestations observed in these patients. IL-17 and IL-22 shape innate defenses at mucosal and epithelial surfaces, IL-17 being proinflammatory and involved in the pathogenesis of several autoimmune diseases, whereas the latter cytokine is playing an important role in tissue regeneration.26 Of note, the extremely high serum IL-10 levels observed in both MIS-A− and MIS-A+ patients have been previously associated with severe myocardial injury27 and increased risk of death in severe COVID-19 patients.28
MIS pathogenesis is not fully understood, but the delay between SARS-CoV-2 infection and disease onset, and overexpression of mucosal T cell cytokines (IL-22 and IL-17) suggest a role for the adaptive immune response in MIS-A+ patients. Conversely, in MIS-A− cases, innate antiviral immunity and/or direct toxicity of the virus are more likely involved in heart tissue injury. In our series, SARS-CoV-2 RT-PCR on 2 MIS-A− endomyocardial biopsies (EMBs) were negative. This is in line with previous cases reports,2 , 29 even if positive SARS-CoV-2 RT-PCR in myocardial samples has also been sporadically reported.3 , 30 To the best of our knowledge, only 1 study demonstrated the presence of viral particles in cardiomyocytes by electronical microscopy,31 with only mild interstitial inflammatory infiltrate and no necrosis or microthrombosis, thereby suggesting that the underlying mechanism of myocarditis development was mainly related to a virus-mediated immune response. The EMBs published results from fulminant COVID-19–related myocarditis often reported important myocardial edema with no or mild inflammatory infiltrate or necrosis,2 , 32 a finding that is also consistent with CMR observations.2 , 5
The phenotypic clustering of patients with fulminant COVID-19–related myocarditis seems relevant for their management. Indeed, MIS-A− cases, owing to the high risk of evolution toward refractory cardiogenic shock, should be urgently referred to a center with VA-ECMO capability and closely monitored to avoid a too-late cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcome.33 The 5 patients who died in our series had late VA-ECMO implantation, while having multiple organ failure or under resuscitation. Conversely, the risk of evolution toward refractory cardiogenic shock is lower in MIS-A+ cases. Our results are consistent with those of a large series of 186 MIS-C from the United States, where only 8 patients required VA-ECMO and 4 died.34 MIS-A+ patient identification is all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C.35 Best treatment regimen is yet to be determined because conflicting results have been reported with standalone or combination treatment.36 , 37 However, one should take with caution the results of nonrandomized/nonblinded therapeutic intervention in a disease where spontaneous recovery occurs in most patients in a few days.
Study limitations
First, the external validity is limited by its monocentric and retrospective nature. Notably, as an ECMO center, there might be a selection bias toward the inclusion of the most severe patients. Second, although being the largest series of fulminant COVID-19–related myocarditis, the sample size remains small, limiting the power of the study. Lastly, EMBs were performed in only 3 patients, whereas expert consensus and guidelines recommend to consider EMB in fulminant presentation for its diagnostic and therapeutic implications.38, 39, 40 However, coagulation disorders are frequent in COVID-19–related myocarditis and VA-ECMO patients. The benefit/risk ratio was evaluated against EMB in all but 3 cases, especially given the known diagnosis of SARS-CoV-2 infection. It is nevertheless unfortunate that we cannot provide a more extensive characterization of COVID-19–related myocarditis histopathological findings in MIS-A+ and MIS-A− patients.
Conclusions
We identified 2 phenotypes of fulminant COVID-19–related myocarditis harboring distinct clinical and laboratory manifestations, evolutions, and outcomes. Differentiating these patients seems relevant for their management and for further pathophysiological studies. The role of RNA polymerase III autoantibodies in fulminant myocarditis requires further investigation.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: MIS-A criteria distinguish phenotypes of patients who develop fulminant myocarditis related to COVID-19, with different clinical presentations, immunological profiles, and outcomes. Those with MIS-A criteria more often have elevated serum levels of IL-17 and IL-22 while those without are frequently positive for serum RNA polymerase III autoantibodies, have high serum level of IFN-α and more often require ECMO.
TRANSLATIONAL OUTLOOK: Further investigation is needed to characterize the role of RNA-polymerase-III antibodies in the pathophysiology of MIS-A COVID-19–related fulminant myocarditis.
Funding Support and Author Disclosures
This study was supported by the Fondation de France, ‘‘Tous unis contre le virus’’ framework Alliance (Fondation de France, AP-HP, Institut Pasteur) in collaboration with Agence Nationale de la Recherche (ANR Flash COVID19 program), by the SARS-CoV-2 Program of the Faculty of Medicine from Sorbonne University ICOViD programs, by the Programme Hospitalier de Recherche Clinique PHRC-20-0375 COVID-19 (principal investigator D Gorochov). The authors declare that a patent application has been filed on these results. Dr Pineton de Chambrun was supported for this study by a grant from la Société Française Nationale de Médecine Interne (SNFMI-2021). Dr Pineton de Chambrun has received a research grant from Octapharma; and has received lecture fees from Sanofi. Dr Kerneis has received research grants from the Fédération Française de Cardiologie, French Ministry of Health; and has received consulting fees from Bayer, Sanofi, and Kiniksa. Dr Montalescot has received research grants from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cell Prothera, CSL Behring, Europa, Idorsia, IRIS-Servier, Medtronic, Merck Sharp and Dohme, Novartis, Pfizer, Quantum Genomics, and Sanofi. Dr Combes has received grants and personal fees from Maquet, Xenios, and Baxter outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
Andrew Civitello, MD, served as Guest Associate Editor for this paper. Christie M. Ballantyne, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42(2):206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J.-H., Liu Y.-X., Yuan J., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48(5):773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garot J., Amour J., Pezel T., et al. SARS-CoV-2 fulminant myocarditis. J Am Coll Cardiol Case Rep. 2020;2:1342–1346. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesici S., Aykan H.H., Orhan D., Bayrakci B. Fulminant COVID-19-related myocarditis in an infant. Eur Heart J. 2020;41 doi: 10.1093/eurheartj/ehaa515. 3021-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirabel M., Luyt C.-E., Leprince P., et al. Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med. 2011;39:1029–1035. doi: 10.1097/CCM.0b013e31820ead45. [DOI] [PubMed] [Google Scholar]
- 8.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hékimian G., Kerneis M., Zeitouni M., et al. COVID-19 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159(2):657–662. doi: 10.1016/j.chest.2020.08.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in adults (MIS-A) case definition information for healthcare providers. https://www.cdc.gov/mis/mis-a/hcp.html Accessed November 1, 2021.
- 11.Bonaca M.P., Olenchock B.A., Salem J.-E., et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi M., Moretti F., Bassi E.M., et al. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. Lancet. 2020;395:e116. doi: 10.1016/S0140-6736(20)31307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castiello T., Georgiopoulos G., Finocchiaro G., et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27(1):251–261. doi: 10.1007/s10741-021-10087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho J.S., Sia C.-H., Chan M.Y., Lin W., Wong R.C. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung J Crit Care. 2020;49:681–685. doi: 10.1016/j.hrtlng.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ammirati E., Veronese G., Brambatti M., et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 17.Lorusso R., Centofanti P., Gelsomino S., et al. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: a 5-year multi-institutional experience. Ann Thorac Surg. 2016;101:919–926. doi: 10.1016/j.athoracsur.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Hékimian G., Jovanovic T., Bréchot N., et al. When the heart gets the flu: fulminant influenza B myocarditis: a case-series report and review of the literature. J Crit Care. 2018;47:61–64. doi: 10.1016/j.jcrc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Ammirati E., Cipriani M., Lilliu M., et al. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation. 2017;136:529–545. doi: 10.1161/CIRCULATIONAHA.117.026386. [DOI] [PubMed] [Google Scholar]
- 20.Shaigany S., Gnirke M., Guttmann A., et al. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396:e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Accessed November 1, 2021.
- 23.Centers for Disease Control and Prevention. Case Definition for MIS-C https://www.cdc.gov/mis/mis-c/hcp/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fmis%2Fhcp%2Findex.html Accessed November 1, 2021.
- 24.Pineton de Chambrun M., Charuel J.-L., Hékimian G., et al. Severe viral myopericarditis with autoantibodies directed against RNA polymerase III. Ann Intern Med. 2020;172:502–504. doi: 10.7326/M19-3074. [DOI] [PubMed] [Google Scholar]
- 25.Caraffa R., Marcolongo R., Bottio T., et al. Recurrent autoimmune myocarditis in a young woman during the coronavirus disease 2019 pandemic. ESC Heart Fail. 2021;8:756–760. doi: 10.1002/ehf2.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyerich S., Eyerich K., Cavani A., Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Nishii M., Inomata T., Takehana H., et al. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol. 2004;44:1292–1297. doi: 10.1016/j.jacc.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Dorgham K., Quentric P., Gökkaya M., et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J Allergy Clin Immunol. 2021;147:2098–2107. doi: 10.1016/j.jaci.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weckbach L.T., Curta A., Bieber S., et al. Myocardial inflammation and dysfunction in COVID-19-associated myocardial injury. Circ Cardiovasc Imaging. 2021;14 doi: 10.1161/CIRCIMAGING.120.011713. [DOI] [PubMed] [Google Scholar]
- 30.Escher F., Pietsch H., Aleshcheva G., et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert C.L., Carmona-Rubio A.E., Weiss A.J., Procop G.G., Starling R.C., Rodriguez E.R. The enemy within: sudden-onset reversible cardiogenic shock with biopsy-proven cardiac myocyte infection by severe acute respiratory syndrome coronavirus 2. Circulation. 2020;142:1865–1870. doi: 10.1161/CIRCULATIONAHA.120.050097. [DOI] [PubMed] [Google Scholar]
- 32.Salamanca J., Díez-Villanueva P., Martínez P., et al. COVID-19 “fulminant myocarditis” successfully treated with temporary mechanical circulatory support. J Am Coll Cardiol Img. 2020;13:2457–2459. doi: 10.1016/j.jcmg.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combes A., Leprince P., Luyt C.-E., et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 34.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McArdle A.J., Vito O., Patel H., et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son M.B.F., Murray N., Friedman K., et al. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caforio A.L.P., Pankuweit S., Arbustini E., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 39.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 40.Ammirati E., Frigerio M., Adler E.D., et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.