Abstract
Bilateral bidirectional Glenn shunts are associated with the risk of developing pulmonary artery bifurcation stenosis, resulting in variable pulmonary blood flow to either lung. This could negatively impact the subsequent stages of the single ventricle palliation pathway. This report highlights the value of 4D flow sequence from the cardiac magnetic resonance imaging in demonstrating the pulmonary blood flow characteristics following a bilateral bidirectional Glenn procedure. Mapping the blood flow pattern and its quantification to each lung provide objective insights into the possible predisposing factors for the development of pulmonary bifurcation stenosis.
Keywords: bidirectional Glenn shunt (BDG), four‐dimensional (4D) flow, cardiac magnetic resonance imaging (CMR), pulmonary artery
4 D flow cardiac MRI is an emerging tool that will aid in follow up of patients with single ventricle pathway after the creation of Glenn shunts. This will help in early detection of pulmonary artery stenosis and planning future interventions.

1. INTRODUCTION
Patients with bilateral bidirectional Glenn (b‐BDG) shunts have a higher incidence of thrombosis and pulmonary artery confluence stenosis. It is due to a potential difference in the pulmonary blood flow pattern to each lung. 1 In these patients, the conventional calculation of pulmonary flow and vascular resistances is imprecise because it considers the lungs as a single Unit. 2 Four‐dimensional (4D) flow cardiac magnetic resonance imaging (CMR) provides an evaluation of blood flow directions at multiple orthogonal planes. It allows the derivation of various physiological parameters such as flow volume, velocity, and wall shear stress. 3 This report demonstrates the blood flow analysis by 4D flow CMR that suggests the possible mechanisms associated with pulmonary artery confluence stenosis development following b‐BDG. Informed consent was taken from the patient's parents, and the ethical committee of the Royal Hospital approved this study (SRC#CR18/2021).
2. CASE REPORT
A female neonate was diagnosed with double outlet right ventricle, bilateral superior vena cava (SVC), pulmonary atresia, ventricular septal defect with a hypoplastic left ventricle, right side aortic arch, and a large patent ductus arteriosus (PDA). She underwent urgent PDA stenting (Figure 1A,B). Then, at the age of 5 months, she developed cyanosis with oxygen saturation of 65% in room air. Cardiac catheterization showed that the right pulmonary artery (RPA) was very hypoplastic due to obstruction from the adjacent PDA stent; however, the left pulmonary artery (LPA) was of reasonable size (Figure 1C). The mean pulmonary artery (PA) pressure was 14 mmHg. The patient underwent a b‐BDG and removal of the PDA stent. The right SVC was anastomosed directly to the posterior wall of the RPA, just before the lobar branches. The RPA and PA confluence were augmented anteriorly with an autologous pericardial patch. The left SVC was anastomosed to the proximal LPA. The patient had an uneventful postoperative course.
FIGURE 1.

(A) Aortogram and selective angiography of the PDA (lateral view), (B) post PDA stent implantation with good flow in both RPA and LPA, (C) selective angiography of the PDA stent showing severe narrowing of the RPA and good flow to LPA
A follow‐up CMR was performed 2 months after surgery (Field of views full chest, scan time of approximately 8 min, normal breathing, spatial resolution 1.8 × 1.8 × 1.8 mm2, temporal resolution <40 ms, VENC 150 cm/s, flip angle 8). Analysis of the flow and other parameters were obtained using Circle Cardiovascular Imaging CVI42 post‐processing program (Calgary, AB, Canada). Parameters obtained were the flow, velocity, and wall shear stress (WSS) at both Glenn shunts, RPA and LPA. (Figure 2). The flow was higher in the left Glenn shunt than the right (2.9 ml/cycle versus 0.83 ml/cycle) with no reversal of flow in both shunts. The maximum velocity was also higher in the left Glenn shunt than the right (42 cm/s versus 30 cm/sec). Furthermore, WSS in the left Glenn shunt was higher than the right (0.25 Pa versus 0.16 Pa). The patient also underwent cardiac catheterization that showed that Glenn's pressure on both sides is the same (14 mmHg). There was good antegrade flow in both Glenn shunts with good arborization of both lungs. The interval between the two Glenn shunts showed no flow (Figure 3). This segment was crossed easily with a guide wire and was balloon dilated. The patient was discharged, and she is on regular follow‐up in our outpatient clinic.
FIGURE 2.
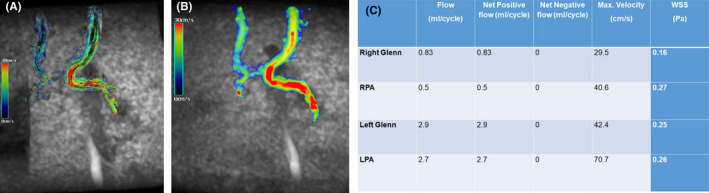
(A) Coronal 4D flow velocity map at and (B) coronal 4D flow‐based dynamic vector visualization demonstrating the direction of flow and difference in velocity between the right and left Glenn shunts. (Color coding of the velocity in cm/s). (C) Summary of the quantitative analysis of the 4D flow cardiac MRI
FIGURE 3.

(A) Angiogram of the right Glenn showing good flow to RPA with good arborization. (B) angiogram of the left Glenn after crossing of segment between the two anastomoses, showing good flow to the left lung and no flow to the RPA. (C) Post‐balloon angioplasty angiogram of the narrowed segment between the right and left Glenn shunts
3. DISCUSSION
This study illustrates the potential value of the 4D flow CMR sequence for patient‐specific evaluation of blood flow pattern following b‐BDG. It allows retrospective assessment of net flow, peak velocity, and blood flow pattern visualization throughout the heart and great arteries. Moreover, differential pulmonary blood flow to each lung can be accurately quantified and that cannot be assessed simply by cardiac catheterization. 3 , 4
Several studies highlighted the disadvantages of bilateral superior vena presence on the outcome of single ventricle palliation procedures. The creation of b‐BDG shunts increases the risk of thrombosis in the cavopulmonary circulation and is associated with higher mortality and lower conversion to Fontan circulation. The proposed mechanism is that the blood from each SVC preferentially flows to the ipsilateral pulmonary artery. This will result in sluggish flow in the intervening segment between the two cavopulmonary anastomoses and eventually inhibit its growth. 1 , 5 In this patient, the cardiac catheterization showed that both Glenn shunts had equal pressure with good patency and lung arborization. However, 4D flow MRI provided quantification of the difference in flow, velocity, and wall shear stress between the right and left Glenn shunts and clearly assessed the flow in the interval segment. Unbalanced pulmonary blood flow is a risk factor for the formation of pulmonary AV malformation due to unequal distribution of the hepatic factor, therefore, maintaining central PA segment is essential to avoid this complication.
4D flow CMR has recently gained popularity in the assessment of congenital heart disease and offer several advantages over the 2‐dimensional (2D) phase‐contrast CMR. 6 The 2D phase‐contrast CMR sequence is acquired with ECG gating during multiple phases of the cardiac cycle. It requires breath holding which could be an issue in cardiac patients. A user‐defined velocity encoding (Venc) is required for each acquisition. It represents the maximum velocity expected in imaged part of the vessel. Selection of the wrong Venc leads to aliasing artifacts which sometimes require repetition of the acquisition. The 2D image plane is pre‐selected from a single orthogonal plane across a single vessel. On the other hand, the 4D flow is a very large set of data, in volumetric acquisition, of the whole thorax and velocity encoding is acquired in all 3 dimensions (X, Y, and Z). It is acquired as ECG gated and throughout the cardiac cycle. The advantage is that the latter is acquired within no breath holding, spans the entire volume, therefore, reproducibility and multiple simultaneous flow quantification from different structures could be acquired from one set of scans with no pre‐scan orthogonal plan definition required. The 4D flow also offers the ability to study the path lines and vorticities along the postoperative intracardiac circulation which is a feature that could play a major role in the explanation of multiple physiological patterns of flowing blood and dynamics within the cardiac circulation. Quantification of the wall shear by 4D flow CMR has an additional value in the assessment of pulmonary circulation in congenital heart diseases. Reduction in wall shear stress may result in pulmonary endothelial dysfunction with a potential risk of developing pulmonary hypertension. 7 Studying of the wall shear stress of the postoperative pulmonary arteries in repaired Tetralogy of Fallot (rTOF) may add to the cumulative understanding to the flow dynamics and the structural development that affects their future growth. 8 We are hoping that as the technology evolves, the limitations of longer scan time and precision levels are improved and the 4D Flow scanning becomes a modality added to the normal diagnostic and therapeutic algorithms of the targeted patients.
In summary, 4D flow CMR added new insights into the understanding and visualization of the blood flow distribution in patients with b‐BDG. It provides excellent qualitative images and important quantitative parameters to help in clinical decisions, assessment, follow‐up, and surgical planning.
AUTHOR CONTRIBUTIONS
Faiza A. L. Kindi did the 4D flow analysis and wrote the manuscript. Madan Maddali wrote the manuscript. Asim Al Balushi edited the manuscript. Hamood Al Kindi is the operating surgeon, idealized and conceptualized the manuscript, and wrote the manuscript. He also submitted the manuscript to the journal and the corresponding author.
CONFLICT OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in the accordance with the journal's patient consent policy.
ACKNOWLEDGMENT
None.
Al Kindi F, Maddali MM, Al Balushi A, Al Kindi H. Evaluation of pulmonary blood flow in bilateral bidirectional Glenn shunts: value of 4‐D flow cardiac magnetic resonance in the evaluation of pulmonary artery confluence stenosis. Clin Case Rep. 2022;10:e06038. doi: 10.1002/ccr3.6038
Funding information
None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iyer GK, Van Arsdell GS, Dicke FP, McCrindle BW, Coles JG, Williams WG. Are bilateral superior vena cavae a risk factor for single ventricle palliation? Ann Thorac Surg. 2000;70(3):711‐716. [DOI] [PubMed] [Google Scholar]
- 2. Gorback MS. Problems associated with the determination of pulmonary vascular resistance. J Clin Monit. 1990;6(2):118‐127. [DOI] [PubMed] [Google Scholar]
- 3. Lawley CM, Broadhouse KM, Callaghan FM, Winlaw DS, Figtree GA, Grieve SM. 4D flow magnetic resonance imaging: role in pediatric congenital heart disease. Asian Cardiovasc Thorac Ann. 2018;26(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 4. Gabbour M, Schnell S, Jarvis K, Robinson JD, Markl M, Rigsby CK. 4‐D flow magnetic resonance imaging: blood flow quantification compared to 2‐D phase‐contrast magnetic resonance imaging and doppler echocardiography. Pediatr Radiol. 2015;45(6):804‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mendelsohn AM, Bove EL, Lupinetti FM, Crowley DC, Lloyd TR, Beekman RH 3rd. Central pulmonary artery growth patterns after the bidirectional Glenn procedure. J Thorac Cardiovasc Surg. 1994;107(5):1284‐1290. [PubMed] [Google Scholar]
- 6. Itatani K, Miyazaki S, Furusawa T, et al. New imaging tools in cardiovascular medicine: computational fluid dynamics and 4D flow MRI. Gen Thorac Cardiovasc Surg. 2017;65(11):611‐621. [DOI] [PubMed] [Google Scholar]
- 7. Tang BT, Pickard SS, Chan FP, Tsao PS, Taylor CA, Feinstein JA. Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: an image‐based, computational fluid dynamics study. Pulmonary Circulation. 2012;2(4):470‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frydrychowicz A, Berger A, Russe MF, et al. Time‐resolved magnetic resonance angiography and flow‐sensitive 4‐dimensional magnetic resonance imaging at 3 tesla for blood flow and wall shear stress analysis. J Thorac Cardiovasc Surg. 2008;136(2):400‐407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


