Abstract
Background
Coronavirus disease-2019 (COVID-19) is characterized by a dysfunctional immune response and abnormal blood rheology that contribute to endothelial dysfunction and thrombotic complications. Whole blood viscosity (WBV) is a clinically validated measure of blood rheology and an established predictor of cardiovascular risk. We hypothesize that increased WBV is associated with mortality among patients hospitalized with COVID-19.
Objectives
This study sought to determine the association between estimated BV (eBV) and mortality among hospitalized COVID-19 patients.
Methods
The study population included 5,621 hospitalized COVID-19 patients at the Mount Sinai Health System from February 27, 2020, to November 27, 2021. eBV was calculated using the Walburn-Schneck model. Multivariate Cox proportional hazards models were used to evaluate the association between eBV and mortality. Considered covariates included age, sex, race, cardiovascular and metabolic comorbidities, in-house pharmacotherapy, and baseline inflammatory biomarkers.
Results
Estimated high-shear BV (eHSBV) and estimated low-shear BV were associated with increased in-hospital mortality. One-centipoise increases in eHSBV and estimated low-shear BV were associated with a 36.0% and 7.0% increase in death, respectively (P < 0.001). Compared with participants in the lowest quartile of eHSBV, those in the highest quartile of eHSBV had higher mortality (adjusted HR: 1.53; 95% CI: 1.27-1.84). The association was consistent among multiple subgroups, notably among patients without any comorbidities (adjusted HR: 1.69; 95% CI: 1.28-2.22).
Conclusions
Among hospitalized COVID-19 patients, increased eBV is significantly associated with higher mortality. This suggests that eBV can prognosticate patient outcomes in earlier stages of COVID-19, and that future therapeutics aimed at reducing WBV should be evaluated.
Key Words: blood viscosity, cardiovascular disease, COVID-19, mortality, rheology epidemiology
Abbreviations and Acronyms: aHR, adjusted HR; COVID-19, coronavirus disease-2019; CRP, C-reactive protein; eBV, estimated blood viscosity; eHSBV, estimated high-shear blood viscosity; eLSBV, estimated low-shear blood viscosity; IL, interleukin; WBC, white blood cell count; WBV, whole blood viscosity
Central Illustration
Coronavirus disease-2019 (COVID-19) is caused by severe acute respiratory syndrome-coronavirus-2. In contrast with other beta coronaviruses, COVID-19 is accompanied by hypercoagulability, which is a significant contributor to morbidity and mortality.1 , 2 Autopsy studies examining patients with COVID-19–related acute respiratory distress syndrome have confirmed the high prevalence of thrombi in the arterial and venous vasculature.1 The significantly higher rates of vascular events and complications in COVID-19 suggest the involvement of additional pathological mechanisms.
To date, laboratory prognostication of disease severity in COVID-19 has relied on hemostatic and inflammatory biomarkers.3, 4, 5 However, progressive advancements in the understanding of COVID-19 etiopathology increasingly support the role of endothelial dysfunction and immune-mediated thrombosis as pathogenic mechanisms. In a model proposed by Bonaventura et al,6 COVID-19 coagulopathy extends beyond the activation of the coagulation cascade and involves a complex set of mediators that contribute to altered blood rheology that may not be appropriately reflected in traditional laboratory measures.
Whole blood viscosity (WBV) is a validated rheological measure for atherosclerotic cardiovascular events and mortality in a population-based study of individuals without known cardiovascular disease.7 , 8 Studies conducted in COVID-19 patients reported that WBV is significantly higher during the acute and convalescent phases of the disease.9 Furthermore, several studies in patients with acute COVID-19 have reported that increased plasma viscosity, a major component of WBV, has been associated with thrombotic complications and worsened outcomes.10 , 11 Given the derangements of inflammatory proteins and coagulation mediators in COVID-19, we aimed to investigate the prognostic value of estimated BV (eBV) in predicting all-cause mortality among patients hospitalized for COVID-19.
Methods
Data collection
The data were collected from the electronic health records of patients at 6 hospitals within the Mount Sinai Health System: Mount Sinai Beth Israel, Mount Sinai Brooklyn, Mount Sinai Hospital, Mount Sinai Morningside, Mount Sinai Queens, and Mount Sinai West. The data include demographic information (ie, age, sex, and race), comorbidities, dispensed medications during hospitalization, laboratory tests and vital signs during hospitalization, and outcomes (death or hospital discharge).
Study population
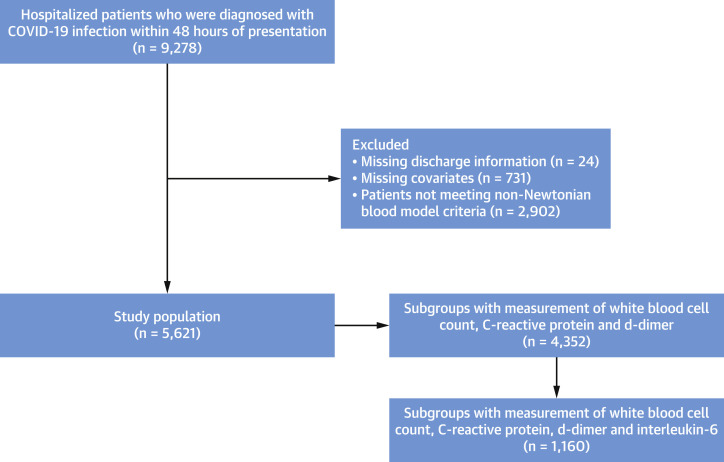
Figure 1 presents the flow diagram of the study population. The inclusion criteria of the study were patients diagnosed with COVID-19 infection within 48 hours of presentation from February 27, 2020, to November 20, 2021. Confirmation of the COVID-19 infection was defined as a positive reverse-transcriptase polymerase chain reaction assay of a specimen collected through nasopharyngeal swab or saliva. The reliability of the saliva specimens in COVID-19 infection was noted in a previous study.12 The exclusion criteria were missing values for discharge information and covariates (measurement of hemoglobin, protein, and albumin levels within 24 hours of presentation) and those who did not meet the criteria for the non-Newtonian blood model.13 The primary outcome of the study was in-hospital mortality. Estimated high-shear BV (eHSBV) and estimated low-shear BV (eLSBV) were calculated using the Walburn-Schneck model.13, 14, 15 Use of the Walburn-Schneck model to estimate WBV in patients with acute COVID-19 has been validated.9 As the ideal non-Newtonian blood model includes hematocrit dependence, the main analysis included 5,621 patients with hematocrit range between 37% and 55%.13 Additional analyses including all 4,352 participants with laboratory profiles of inflammatory markers of white blood cell count (WBC), C-reactive protein (CRP), and D-dimer were performed separately. There were 1,660 participants with measurement of interleukin (IL)-6 level, and Supplemental analysis among this subgroup was also conducted. Participants were divided into quartiles based on eHSBV and eLSBV, with the first quartile comprising those with the lowest eBV.
Figure 1.
Flow Diagram of the Study Population
Flow chart presenting study population. COVID-19 = coronavirus disease-2019.
Statistical analysis
Categorical variables were reported as count and percentage, and continuous variables were reported as mean ± SD. Chi-square tests for categorical variables and analysis of variance tests for continuous variables were performed to evaluate the statistical significances between study groups. Cox proportional hazards regressions were conducted to evaluate the adjusted HR (aHR) and 95% CI of the in-house mortality according to the quartile eBV. The proportional hazards assumption was graphically tested and verified with the Schoenfeld residual method. Considered covariates were age (continuous: years); sex (categorical: male or female); race (categorical: White, Black, Asian, Hispanic, and other); history of hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease (all categorical: yes or no); admission date (categorical: March 2020 to August 2020, September 2020 to February 2021, and March 2021 to November 2021); oxygen support device at the time of presentation (categorical: none, nasal cannula or nonrebreather mask or high-flow nasal cannula, bilevel positive airway pressure or continuous positive airway pressure, and mechanical ventilator); in-hospital statin therapy (categorical: none, low- to moderate-intensity statin, and high-intensity statin); and in-hospital anticoagulation therapy (categorical: none, prophylactic anticoagulation, and therapeutic anticoagulation). Demographic factors were retrieved from the electronic health records. In hospital, anticoagulation therapy and statin therapy were considered as major covariates based on previous studies on hospitalized COVID-19 patients.16 , 17 We also included the date of admission as a categorical variable, as treatment options, vaccination status, and variants have been evolving and might affect mortality. Additional analysis was performed on subgroup populations who had measured of inflammatory markers of WBC, CRP, D-dimer, and IL-6 within 24 hours of presentation. Kaplan-Meier curves of survival rate according to the quartiles of eHSBV and eLSBV were plotted. Stratified analyses were conducted according to subgroups of age, sex, race, history of comorbidities, admission date, in-hospital therapies (statin and anticoagulation), oxygen support device at presentation, and inflammatory markers (WBC, CRP, and D-dimer) and plotted as a forest plot. Continuous linear association between eHSBV and in-hospital mortality was analyzed, and restricted cubic splines were fitted with Cox proportional hazards regression models. A median value for eHSBV (4.30 cP) was used as a reference value in this analysis, and 4 knots were placed at the fifth, 35th, 65th, and 95th percentiles of eHSBV in accordance with previous studies.18 , 19 A histogram was drawn to display the distribution of eHSBV. Supplemental analysis among 1,660 participants with measurement of all inflammatory markers (WBC, CRP, D-dimer, and IL-6) was performed to compare the aHRs of biomarkers. WBC, CRP, D-dimer, and IL-6 were all considered as categorical variables. WBC, CRP, and D-dimer were divided into higher one-half and lower one-half, and the median value was used for the cutoff range. For IL-6, a cutoff value of 86 pg/mL was used to divide into 2 groups, based on a previous study. Analyses including IL-6 excluded CRP as a covariate, owing to collinearity.5 Last, internal validity was assessed by bootstrap analysis with 1,000 resampling datasets.
Statistical significances were tested in a 2-sided manner, with a P value of <0.05. Data collection and statistical analyses were performed using SAS Enterprise Guideline 8.3 (SAS Institute) and RStudio version 4.0.5 (RStudio, PBC).
Ethical consideration
This study was approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board (number 20-03558). The requirement for informed consent was waived, as the individual data were strictly anonymized and deidentified prior to distribution to researchers.
Results
Table 1 shows the descriptive characteristics of the study participants. The range of eHSBV was 3.01 to 4.00 cP, 4.00 to 4.24 cP, 4.24 to 4.53 cP, and 4.53 to 9.86 cP for the first, second, third, and fourth quartiles of eHSBV, respectively. Participants in the highest (fourth) eHSBV quartile were more likely to be men, be Black or Hispanic, have a history of diabetes, and require oxygen support at the time of presentation. Analysis of 4,352 participants who had laboratory data for inflammatory markers within 24 hours of presentation noted that participants with higher eHSBV tended to have higher inflammatory markers (WBC, CRP, and D-dimer) at baseline. Supplemental Table 1 shows the descriptive characteristics of the study participants based on eLSBV. Participants with higher eLSBV had similar characteristics to those with eHSBV, and were more likely to be men, be Hispanic, and require oxygen support at the time of presentation, but there was no significant difference in comorbidities according to eLSBV.
Table 1.
Descriptive Characteristics of the Study Population
| High-Shear BV Quartile 1 (Lowest) (n = 1,405) | High-Shear BV Quartile 2 (n = 1,406) | High-Shear BV Quartile 3 (n = 1,405) | High-Shear BV Quartile 4 (Highest) (n = 1,405) | P Value | |
|---|---|---|---|---|---|
| BV range, cP | 3.01-4.00 | 4.00-4.24 | 4.24-4.53 | 4.53-9.86 | |
| Sex | <0.001 | ||||
| Men | 663 (46.2) | 819 (58.3) | 929 (66.1) | 1,048 (74.6) | |
| Women | 742 (52.8) | 587 (41.8) | 476 (33.9) | 357 (25.4) | |
| Age, y | 64.9 ± 17.4 | 63.2 ± 17.0 | 62.8 ± 16.5 | 62.0 ± 17.3 | <0.001 |
| Race | <0.001 | ||||
| White | 536 (38.2) | 359 (24.9) | 314 (22.4) | 279 (19.9) | |
| Black | 242 (17.2) | 312 (22.2) | 308 (21.9) | 333 (23.7) | |
| Asian | 92 (6.6) | 95 (6.8) | 81 (5.8) | 69 (4.9) | |
| Hispanic | 311 (22.1) | 387 (27.5) | 429 (30.5) | 443 (31.5) | |
| Other | 224 (15.9) | 262 (18.6) | 273 (19.4) | 281 (20.0) | |
| Comorbidity | |||||
| HTN | 458 (32.6) | 472 (33.6) | 484 (33.7) | 437 (31.1) | 0.424 |
| DM | 239 (17.0) | 292 (20.8) | 285 (20.3) | 296 (21.1) | 0.025 |
| CKD | 76 (5.4) | 79 (5.6) | 92 (6.6) | 85 (6.1) | 0.588 |
| CAD | 172 (12.2) | 175 (12.5) | 154 (11.0) | 170 (12.1) | 0.619 |
| Admission date | <0.001 | ||||
| 3/1/2020-8/31/2020 | 622 (44.3) | 689 (49.0) | 742 (52.8) | 771 (54.9) | |
| 9/1/2020-2/28/2021 | 568 (40.4) | 523 (37.2) | 479 (34.1) | 429 (30.5) | |
| 3/1/2021-11/20/2021 | 215 (15.3) | 194 (13.8) | 184 (13.1) | 205 (14.6) | |
| Initial oxygen support device | <0.001 | ||||
| Room air | 487 (34.7) | 401 (28.5) | 345 (24.6) | 322 (22.9) | |
| NC or NRB or HFNC | 827 (58.9) | 890 (63.3) | 888 (63.2) | 888 (63.2) | |
| BiPAP or CPAP | 59 (4.2) | 84 (6.0) | 112 (8.0) | 141 (10.0) | |
| Intubated | 32 (2.3) | 31 (2.2) | 60 (4.3) | 54 (3.8) | |
| Anticoagulation | 0.072 | ||||
| No anticoagulation | 93 (6.6) | 59 (4.2) | 81 (5.8) | 76 (5.4) | |
| Prophylactic dose | 578 (41.1) | 601 (42.8) | 613 (42.6) | 569 (40.5) | |
| Therapeutic dose | 734 (52.2) | 746 (53.1) | 711 (50.6) | 760 (54.1) | |
| No. of participants with lab data | 1,006 | 1,094 | 1,116 | 1,136 | |
| WBC, × 103/μL | 7.5 ± 4.1 | 8.0 ± 4.4 | 8.5 ± 3.9 | 9.4 ± 5.4 | <0.001 |
| CRP, mg/L | 94.0 ± 84.5 | 113.1 ± 87.1 | 124.1 ± 93.6 | 126.3 ± 96.9 | <0.001 |
| D-dimer, μg/mL | 1.9 ± 3.1 | 2.5 ± 4.0 | 2.6 ± 4.2 | 4.2 ± 6.2 | <0.001 |
Values are n (%) or mean ± SD, unless otherwise indicated.
BiPAP = bilevel positive airway pressure; BV = blood viscosity; CPAP = continuous positive airway pressure; CAD = coronary artery disease; CKD = chronic kidney disease; CRP = C-reactive protein; DM = diabetes mellitus; HFNC = high-flow nasal cannula; HTN = hypertension; NC = nasal cannula; NRB = nonrebreather mask; SBP = systolic blood pressure; WBC = white blood cell count.
The association of eHSBV and in-hospital mortality of COVID-19 patients is depicted in Table 2 . Compared with participants with the lowest eHSBV (quartile 1), those with the eHSBV had a significantly higher risk of death (aHR: 1.53; 95% CI: 1.27-1.84). This association was consistent (aHR: 1.50; 95% CI: 1.20-1.87) with additional adjustment for initial laboratory data including WBC, CRP, and D-dimer. An increase in eBV quartiles was also associated with higher mortality (P for trend < 0.001). A 1-cP increase in eHSBV was associated with a 36.0% (P < 0.001) increase in death.
Table 2.
Association of High-Shear BV and Mortality Among COVID-19 Patients
| High-Shear BV Quartile 1 (Lowest) (n = 1,405) | High-Shear BV Quartile 2 (n = 1,406) | High-Shear BV Quartile 3 (n = 1,405) | High-Shear BV Quartile 4 (Highest) (n = 1,405) | P for Trend | |
|---|---|---|---|---|---|
| BV range, cP | 3.01-4.00 | 4.00-4.24 | 4.24-4.53 | 4.53-9.86 | |
| Person-days | 12,833 | 13,769 | 13,364 | 14,105 | |
| In-hospital deaths | 193 | 238 | 260 | 332 | |
| aHR (95% CI)a | 1.00 (reference) | 1.27 (1.04-1.54) | 1.23 (1.02-1.49) | 1.53 (1.27-1.84) | <0.001 |
| aHR (95% CI)b | 1.00 (reference) | 1.21 (0.97-1.52) | 1.16 (0.92-1.45) | 1.50 (1.20-1.87) | <0.001 |
| aHR (95% CI)c | 1.00 (reference) | 1.17 (0.79-1.72) | 1.14 (0.77-1.68) | 1.60 (1.09-2.35) | 0.020 |
The linear modeling results were an aHR of 1.36 (95% CI: 1.19-1.55; P < 0.001) per 1-cP increase and an aHR of 1.18 (95% CI: 1.10-1.26; P < 0.001) per IQR (0.53 cP) increase.
aHR = adjusted HR; COVID-19 = coronavirus disease-2019; other abbreviations as in Table 1.
aHRs calculated by Cox proportional hazards regression after adjustments for age, sex; hospital site; race; history of HTN, DM, CKD, and CAD; in-hospital statin use; anticoagulation therapy; date of admission; and measure of initial oxygen support.
aHRs calculated by Cox proportional hazards regression after adjustments for age; sex; hospital site; race; history of HTN, DM, CKD, and CAD; in-hospital statin use; anticoagulation therapy; date of admission; measure of initial oxygen support; and initial lab data (WBC, CRP, and D-dimer).
Additionally adjusted for interleukin-6 and excluded CRP due to collinearity.
Table 3 shows the association of eLSBV and death in hospitalized patients for COVID-19. Similar to the high-shear BV analysis, COVID-19 patients in the highest quartile of eLSBV had higher mortality (aHR: 1.36; 95% CI: 1.14-1.64) compared with those in the lowest quartile of eLSBV, and this association was also consistent after additional adjustment for inflammatory markers. A 1-cP increase in eLSBV was associated with a 7.0% (P < 0.001) increase in mortality.
Table 3.
Association of Low-Shear BV and Mortality Among COVID-19 Patients
| Low-Shear BV Quartile 1 (Lowest) (n = 1,405) | Low-Shear BV Quartile 2 (n = 1,406) | Low-Shear BV Quartile 3 (n =1,405) | Low-Shear BV Quartile 4 (Highest) (n = 1,405) | P for Trend | |
|---|---|---|---|---|---|
| Blood viscosity range, cP | 6.49-9.05 | 9.05-10.01 | 10.01-11.29 | 11.29-25.50 | |
| Person-days | 13,016 | 13,456 | 13,547 | 14,052 | |
| In-hospital deaths | 207 | 247 | 257 | 312 | |
| aHR (95% CI)a | 1.00 (reference) | 1.24 (1.03-1.50) | 1.13 (0.93-1.36) | 1.36 (1.14-1.64) | 0.004 |
| aHR (95% CI)b | 1.00 (reference) | 1.24 (0.99-1.54) | 1.08 (0.87-1.35) | 1.32 (1.06-1.64) | 0.045 |
| aHR (95% CI)c | 1.00 (reference) | 1.39 (0.96-2.01) | 1.09 (0.74-1.59) | 1.32 (0.91-1.91) | 0.349 |
The linear modeling results were an aHR of 1.07 (95% CI: 1.03-1.10; P < 0.001) per 1-cP increase and an aHR of 1.15 (95% CI: 1.07-1.24; P < 0.001) per IQR (2.24 cP) increase.
aHRs calculated by Cox proportional hazards regression after adjustments for age; sex; hospital site; race; history of HTN, DM, CKD, and CAD; in-hospital statin use; anticoagulation therapy; date of admission; and measure of initial oxygen support.
aHRs calculated by Cox proportional hazards regression after adjustments for age; sex; hospital site; race; history of HTN, DM, CKD, and CAD; in-hospital statin use; anticoagulation therapy; date of admission; measure of initial oxygen support; and initial lab data (WBC, CRP, and D-dimer).
Additionally adjusted for interleukin-6 and excluded CRP due to collinearity.
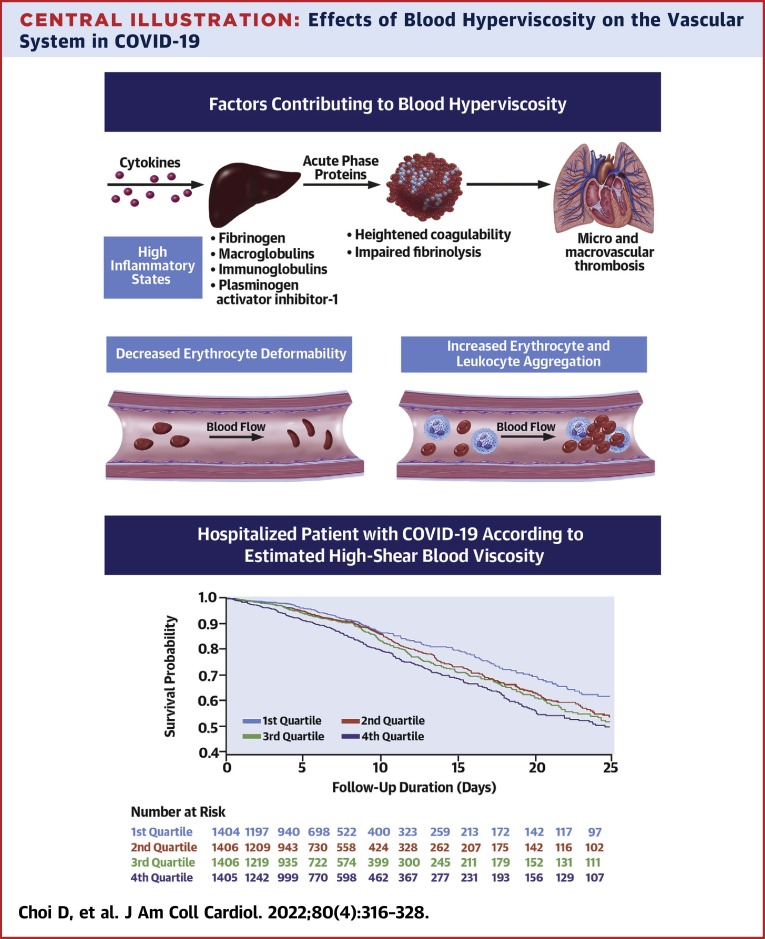
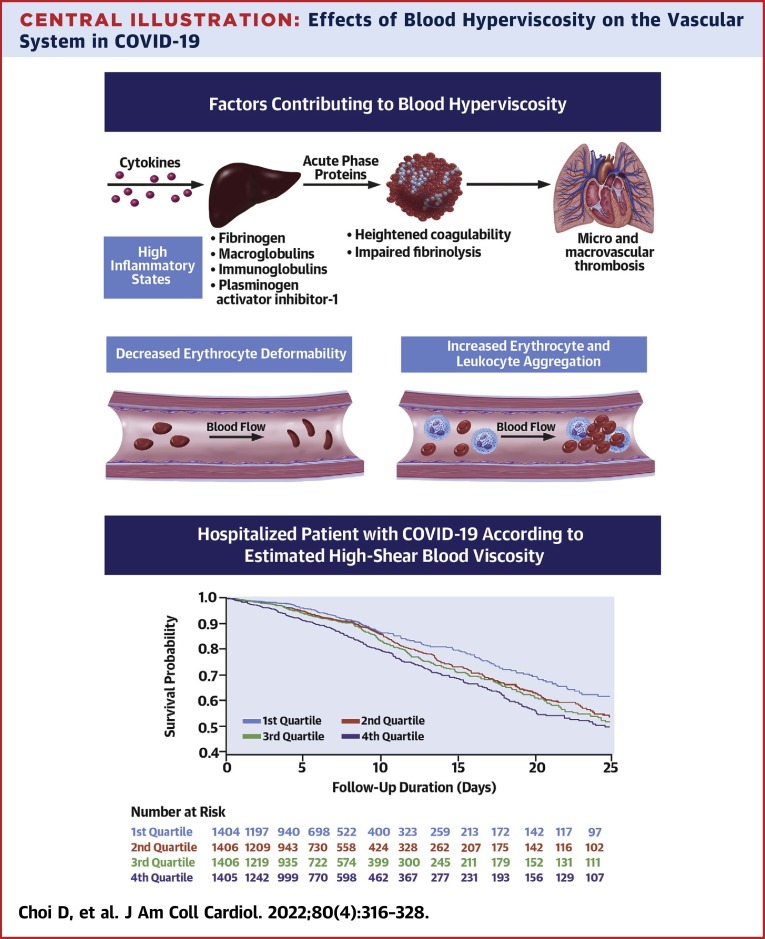
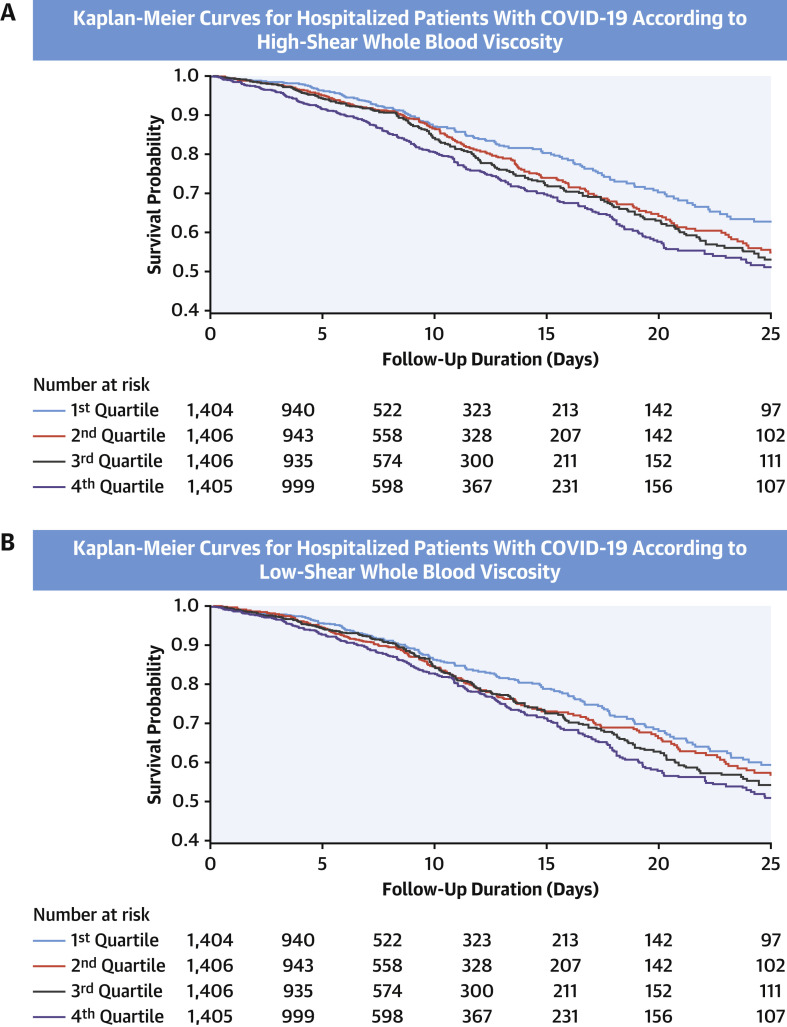
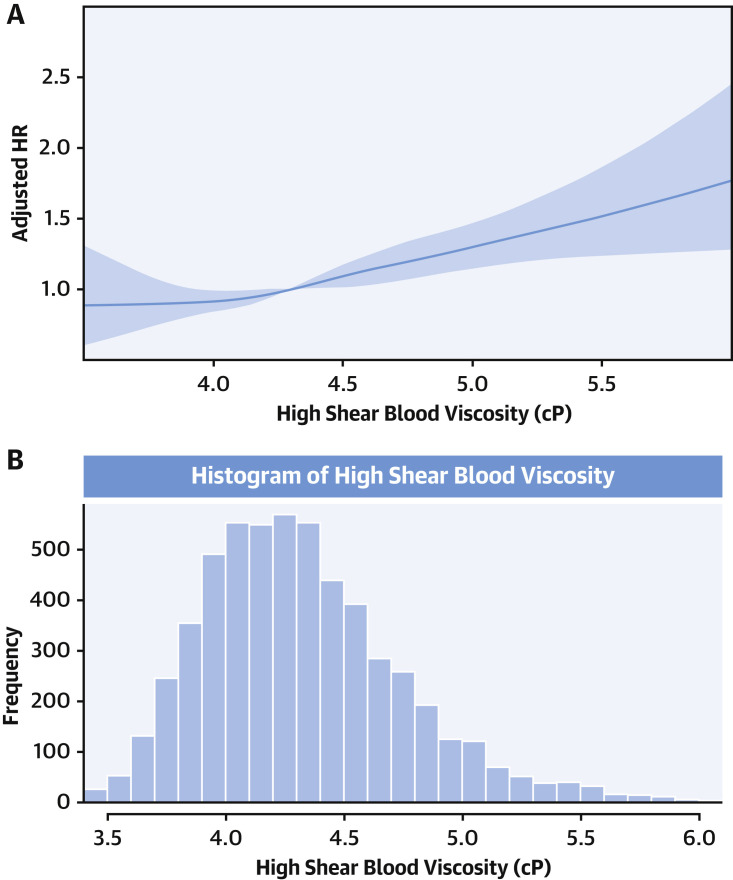
The unadjusted Kaplan-Meier survival curves according to the quartiles of eHSBV are shown in the Central Illustration and Figure 2 . Participants with higher eHSBV and eLSBV both had reduced survival rates. The linear association between eHSBV and in-hospital mortality is depicted in Figure 3 . An increase in eHSBV was associated with an increased risk of mortality in hospitalized patients with COVID-19.
Central Illustration.
Effects of Blood Hyperviscosity on the Vascular System in COVID-19
Adjusted HRs (aHRs) calculated by Cox proportional hazards regression after adjustments for age; sex; hospital site; race; history of hypertension, diabetes mellitus, chronic kidney disease, and coronary artery disease; in-hospital statin use; anticoagulation therapy; date of admission; measure of initial oxygen support; and initial lab data (white blood cell count, C-reactive protein, and D-dimer). COVID-19 = coronavirus disease-2019.
Figure 2.
Kaplan-Meier Curves for Hospitalized Patients With COVID-19 According to Estimated BV
Kaplan-Meier curves for in-hospital mortality among patients with coronavirus disease-2019 (COVID-19) according to (A) estimated high-shear blood viscosity (BV) and (B) estimated low-shear BV.
Figure 3.
Restricted Cubic Spline Showing Association Between eHSBV and In-Hospital Mortality
(A) Restricted cubic spline showing association between estimated high-shear blood viscosity (eHSBV) and in-hospital mortality. (B) Histogram showing the distribution of eHSBV of the study participants. Models are adjusted for age; sex; hospital site; race; history of hypertension, diabetes, chronic kidney disease, and coronary artery disease; in-hospital statin use; anticoagulation therapy; date of admission; and measure of initial oxygen support. The solid line indicates HRs and shaded areas indicate 95% CIs. Four knots were placed at the 5th, 35th, 65th, and 95th percentiles of BV.
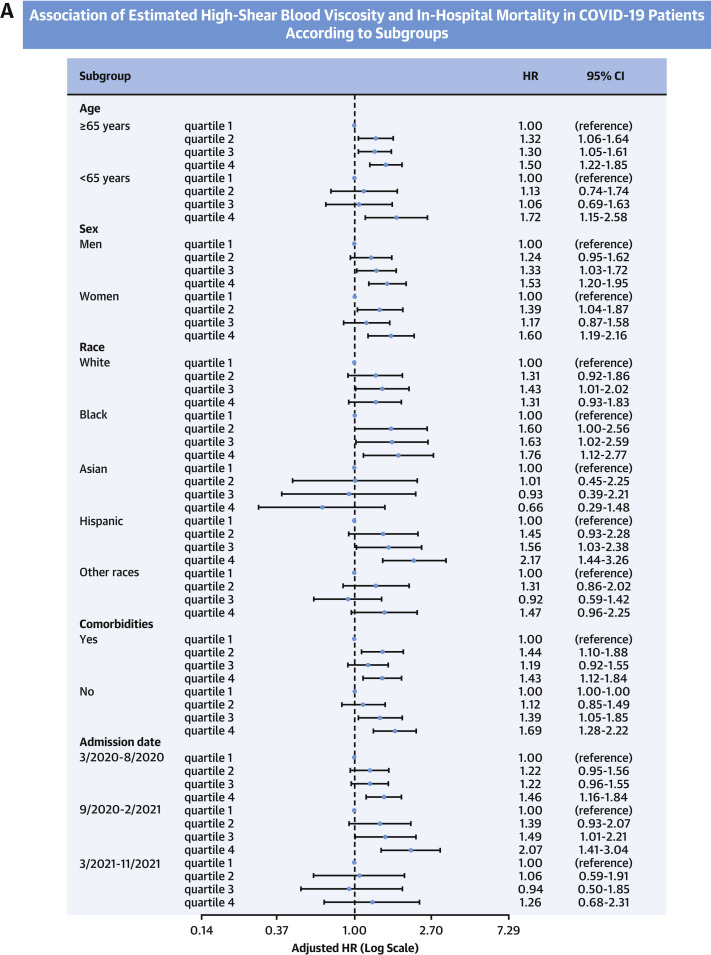
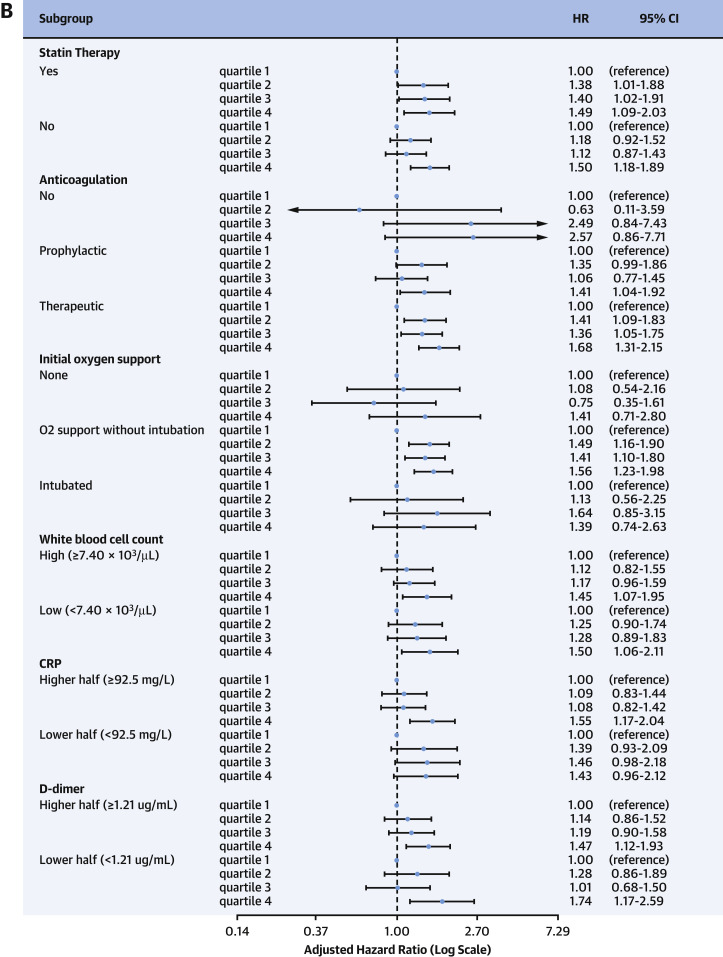
Results from the subgroup analyses are depicted in Figure 4 . Participants in the highest quartile of eHSBV were consistently associated with a higher risk of death compared with those in the lowest quartile of eHSBV in multiple subgroups of age, sex, and comorbidities. This association was more prominent among participants who were Hispanic (aHR: 2.17; 95% CI: 1.44-3.26), diabetic (aHR: 1.85; 95% CI: 1.22-2.82), or without any comorbidities (aHR: 1.69; 95% CI: 1.28-2.22). The Asian population (aHR: 0.66; 95% CI: 0.29-1.48) was the only exception and did not show a significant difference in mortality between participants within the highest and the lowest quartiles of eBV.
Figure 4.
Association of eHSBV Mortality Patients According to Subgroups
(A,B) Adjusted HRs calculated by Cox proportional hazards regression after adjustments for age; sex; hospital site; race; history of hypertension, diabetes, chronic kidney disease, and coronary artery disease; in-hospital statin use; anticoagulation therapy; date of admission; and measure of initial oxygen support. COVID-19 = coronavirus disease-2019; CRP = C-reactive protein; eHSBV = estimated high-shear blood viscosity.
Supplemental Table 2 shows the aHRs for the in-hospital mortality of major covariates and inflammatory biomarkers. Higher age (aHR: 1.04; 95% CI: 1.04-1.05), CRP level (aHR: 1.41; 95% CI: 1.19-1.67), and IL-6 level (aHR: 1.50; 95% CI: 1.15-1.96) were associated with increased mortality. Higher levels of WBC (P = 0.158) and D-dimer level (P = 0.070) did not significantly alter the risk of in-hospital death after adjustment for other inflammatory markers. Last, bootstrap aHRs and 95% CIs are shown in Supplemental Table 3. Similar results were observed, as participants in the highest quartile of eHSBV had higher mortality compared with those in the lowest quartile of eHSBV.
Discussion
In this multihospital retrospective cohort study of 5,621 patients hospitalized for acute COVID-19, we found that increased eHSBV and eLSBV were both associated with higher in-hospital mortality. The mortality associated risk per 1-cP rise in eHSBV was reflected by a 36.0% increased risk of death (P < 0.001). Similarly, 1-cP rise in eLSBV demonstrated a 7% increased risk of death (P = 0.045). The associations between elevated eBV and mortality remained consistent among various subgroups of age, sex, the presence of cardiovascular or metabolic comorbidities, values of inflammatory laboratory markers, and in-house pharmacotherapy.
To date, previous trials investigating the association of blood hyperviscosity and COVID-19 have been limited by small sample size and limited data on clinical outcomes.9, 10, 11 , 20 Our study is the first large-scale, multicenter investigation aimed at determining the prognostic value of WBV in predicting all-cause mortality among patients hospitalized for COVID-19. To our knowledge, there is only 1 previous study that investigated the association of WBV and mortality, but this was a long-term prospective population-based study.8 In the Scottish Heart Study, a higher WBV predicted a higher mortality, but this association was not statistically significant after adjustment of multiple covariates. In contrast, our study was conducted among hospitalized patients with acute viral illness and evaluated in-hospital mortality, which has not been investigated before.
WBV is a clinically validated measure of blood rheology7 contributing to endothelial damage, atherogenesis, plaque growth, and plaque rupture.21 WBV is chiefly determined by hematocrit, plasma viscosity, erythrocyte aggregation, and erythrocyte deformability.7 As whole blood is a non-Newtonian fluid, WBV varies as a function of shear rate.7 , 22 , 23 Low shear rates (5 seconds-1) contribute to erythrocyte aggregation and microvascular ischemia, while high shear rates (300 seconds-1) induce endothelial damage to the vessel wall and promote plaque instability.24 In both circumstances, hyperviscosity is directly implicated with impaired microcirculatory perfusion and vascular damage.23 , 25 , 26
In the setting of COVID-19, inflammation contributes to increases in WBV. High concentrations of acute-phase proteins increase plasma viscosity through their large molecular mass, and in the case of fibrinogen, asymmetry.27 , 28 Additionally, as inflammatory proteins are positively charged, interactions of erythrocytes are altered, resulting in impaired red blood cell deformation and increased aggregation.29 A recent study of rheological profiles of 172 hospitalized COVID-19 patients in Lyon, France, found that shear rates in excess of 500 seconds−1 were needed to disrupt red blood cell aggregates in this cohort.20 Last, activation and overexpression of tissue factor activated by proinflammatory cytokines has been implicated in further coagulopathy and alterations of blood flow.
Our study found that BV predicted mortality in models that adjusted for IL-6, CRP, D-dimer, and other biomarkers routinely used for risk stratification of hospitalized patients with COVID-19. While CRP and IL-6 levels were associated with increased mortality (aHR: 1.41 and 1.50, respectively), participants in the highest quartiles of high-shear eBV had an aHR of 1.50 (95% CI: 1.15-1.96) even after adjustment for these biomarkers (Supplemental Table 2).
Higher eHSBV and eLSBV were both associated with increased inpatient COVID-19 mortality in our study (Tables 2 and 3), but the result of eLSBV was not statistically significant after adjustment for inflammatory biomarkers including IL-6 (Table 3). Although this statistical insignificance can be attributed to the decrease in the number of study participants, the result from eHSBV was still significant and showed a stronger association with mortality compared with eLSBV. Similarly, high-shear viscosity was more associated with mortality, with a 1-cP increase in eHSBV reflecting a 36.0% increased risk of death (P < 0.001) when compared with 7.0% for eLSBV (P = 0.045). Future investigations may be warranted into the pathological mechanisms and differences of high- and low-shear blood hyperviscosity in the setting of COVID-19. Nevertheless, the association of eBV with mortality remained consistent despite differences in age, sex, presence of cardiovascular or metabolic comorbidities, values of inflammatory laboratory markers, use of statins, and administration of heparins. Based on these findings, we report that blood hyperviscosity at both high and low shear contribute to mortality independently from other established COVID-19 risk factors. We suggest that BV may be more physiologically relevant with respect to immune-mediated thrombosis, which characterizes acute and subacute COVID-19 infections.
Among variables associated with increased WBV, our study found that male sex accounted for 74.6% of the subjects in the top quartile of eBV but only 46.2% of the subjects in the bottom quartile of eBV, which is likely due to the increased hematocrit of men as compared with women.30 As WBV increases logarithmically with rising hematocrit, small variations of this variable can have profound rheological differences.31 The impact of increased hematocrit on hyperviscosity may explain, in part, why men carry a higher risk of severe disease and death from COVID-19 than women.32 While men were more likely to have an elevated eBV, the risk of mortality with rising eBV remained increased for all genders. Additionally, we found a strong correlation between eBV and mortality among patients without cardiovascular or metabolic comorbidities (aHR: 1.69; 95% CI: 1.28-2.22). This finding supports the use of WBV to prognosticate the outcome, especially among those without comorbidities or with traditionally low risk factors for in-hospital death.
We failed to observe the same association between WBV and mortality among the Asian population. The Asian population accounted for the least number of COVID-19 patients hospitalized in our study (6.0%), and we may not have enough events to observe a statistical difference. Additionally, as the Asian population has the lowest incidence of thromboembolic disease,33 we hypothesize that the Asian population may have intrinsic protective mechanisms against the effect of blood hyperviscosity. Future studies evaluating the impact of racial difference on COVID-19 clinical outcomes are merited.
Study limitations and study strengths
First, eBV was calculated and not directly measured. In our analysis, we employed the Walburn-Schneck model to determine eBV, which has been demonstrated to have an R 2 statistic of 91% when validated on a Wells-Brookfield viscometer.9 , 15 In a separate study conducted on 58 COVID-19 patients, a Hemathix scanning capillary viscometer (Health Onvector) was utilized to measure WBV across a complete range of shear rates. Using these data, investigators found that although eBV calculated by the Walburn-Schneck model tends to underestimate WBV, particularly at a low shear rate, there was a moderate-to-high correlation between WBV and eBV.9 Despite these validation studies, the use of eBV, rather than measured BV, introduces variability, reduces accuracy, and may fail to capture variables that otherwise contribute to rheology. Future investigations may explore improved algorithms or may seek to determine the association of directly measured WBV and COVID-19 mortality. Second, owing to the evolving nature of the pandemic, our study included patients who differed with regard to viral variants, vaccination status, hospital protocol, and disease severity. We attempted to take this into account by using initial measure of oxygen support as a major covariate to represent disease severity and admission date to reflect changes in viral variants or vaccination status. Third, as a nature of observational retrospective, there might be unrecognized confounders that potentially impact the association. Although we tried to consider multiple possible covariates, future prospective studies will be needed to confirm the findings.
Despite the previous limitations, our study has multiple strengths. This is the first study to investigate the impact of blood hyperviscosity on COVID-19 mortality. We included a large number of hospitalized COVID-19 patients and took account of multiple comorbidities, demographic factors and in-house treatment. Laboratory data including major inflammatory markers were considered, and the result was consistent among multiple subgroups.
Conclusions
While COVID-19 is chiefly recognized as a respiratory illness, there is a growing body of evidence demonstrating the burden of endothelial dysfunction and vasculopathy on disease outcome. Overall, our findings demonstrate a significant association between inflammation, WBV, and the risk of COVID-19–associated mortality. As new emerging antiviral agents suggest benefits in patients at high risk of progressing to severe illness,34 , 35 identifying high-risk populations in the earlier stage of the disease becomes crucial. From a translational perspective, the variables to calculate eBV (hematocrit, albumin, and total protein) are readily available to practitioners and are easily obtained from most admission labs, suggesting a possible use of eBV as an efficient and simple risk assessment of patients with COVID-19 to offer proper preventive therapy. Additionally, further studies investigating the impact of targeted reduction of WBV are merited given the association between eBV and mortality.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Elevated whole blood viscosity is associated with increased in-hospital mortality in patients with COVID-19.
TRANSLATIONAL OUTLOOK: Additional research is needed to understand the mechanisms responsible for the association of BV with clinical outcomes in patients with COVID-19 and evaluate the efficacy and safety of therapeutic interventions aimed at reducing WBV.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The CURA Foundation provided support for data retrieval from electronic medical records.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
Aldo Bonaventura, MD, PhD, served as Guest Associate Editor for this paper.
Athena Poppas, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Wichmann D., Sperhake J.-P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 4.Manocha K.K., Kirzner J., Ying X., et al. Troponin and other biomarker levels and outcomes among patients hospitalized with COVID-19: derivation and validation of the HA 2 T 2 COVID-19 Mortality Risk Score. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laguna-Goya R., Utrero-Rico A., Talayero P., et al. IL-6–based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:799–807. doi: 10.1016/j.jaci.2020.07.009. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonaventura A., Vecchié A., Dagna L., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong S.-K., Cho Y.I., Duey M., Rosenson R.S. Cardiovascular risks of anemia correction with erythrocyte stimulating agents: should blood viscosity be monitored for risk assessment? Cardiovasc Drugs Ther. 2010;24:151–160. doi: 10.1007/s10557-010-6239-7. [DOI] [PubMed] [Google Scholar]
- 8.Peters S.A., Woodward M., Rumley A., Tunstall-Pedoe H.D., Lowe G.D. Plasma and blood viscosity in the prediction of cardiovascular disease and mortality in the Scottish Heart Health Extended Cohort Study. Eur J Prev Cardiol. 2017;24:161–167. doi: 10.1177/2047487316672004. [DOI] [PubMed] [Google Scholar]
- 9.Shaik A, Chen Q, Mar P, et al. Blood hyperviscosity in acute and recent COVID-19 infection. Clin Hemorheol Microcirc. Published online April 18, 2022. https://doi.org/10.3233/CH-221429. [DOI] [PMC free article] [PubMed]
- 10.Truong A.D., Auld S.C., Barker N.A., et al. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion. 2021;61:1029–1034. doi: 10.1111/trf.16218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier C.L., Truong A.D., Auld S.C., Polly D.M., Tanksley C.-L., Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395:1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan C., Ditelberg S., Dutta S., et al. Saliva is comparable to nasopharyngeal swabs for molecular detection of SARS-CoV-2. Microbiol Spectr. 2021;9 doi: 10.1128/spectrum.00162-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goubergrits L., Wellnhofer E., Kertzscher U. In: 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics. Katashev A., Dekhtyar Y., Spigulis J., editors. Springer; 2008. Choice and impact of a non-Newtonian blood model for wall shear stress profiling of coronary arteries; pp. 111–114. [Google Scholar]
- 14.Soulis J.V., Giannoglou G.D., Chatzizisis Y.S., Seralidou K.V., Parcharidis G.E., Louridas G.E. Non-Newtonian models for molecular viscosity and wall shear stress in a 3D reconstructed human left coronary artery. Med Eng Phys. 2008;30:9–19. doi: 10.1016/j.medengphy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Walburn F.J., Schneck D.J. A constitutive equation for whole human blood. Biorheology. 1976;13:201–210. doi: 10.3233/bir-1976-13307. [DOI] [PubMed] [Google Scholar]
- 16.Choi D, Chen Q, Goonewardena SN, et al. Efficacy of statin therapy in patients with hospital admission for COVID-19. Cardiovasc Drugs Ther. Published online September 15, 2021. https://doi.org/10.1007/s10557-021-07263-2 [DOI] [PMC free article] [PubMed]
- 17.Paranjpe I., Fuster V., Lala A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell F.E., Jr. Springer; 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- 19.Son J.S., Choi S., Kim K., et al. Association of Blood Pressure Classification in Korean Young Adults According to the 2017 American College of Cardiology/American Heart Association Guidelines With Subsequent Cardiovascular Disease Events. JAMA. 2018;320:1783–1792. doi: 10.1001/jama.2018.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nader E., Nougier C., Boisson C., et al. Increased blood viscosity and red blood cell aggregation in patients with COVID-19. Am J Hematol. 2021;97:283–292. doi: 10.1002/ajh.26440. [DOI] [PubMed] [Google Scholar]
- 21.Tschöpe C., Sherif M., Anker M.S., Geisel D., Kuehne T., Kelle S. COVID-19-convalescence phase unmasks a silent myocardial infarction due to coronary plaque rupture. ESC Heart Fail. 2021;8:971–973. doi: 10.1002/ehf2.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pop G.A., Duncker D.J., Gardien M., et al. The clinical significance of whole blood viscosity in (cardio)vascular medicine. Neth Heart J. 2002;10:512–516. [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenson R.S., Shott S., Tangney C.C. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: triglycerides and blood viscosity. Atherosclerosis. 2002;161:433–439. doi: 10.1016/s0021-9150(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 24.Dhawan S.S., Avati Nanjundappa R.P., Branch J.R., et al. Shear stress and plaque development. Expert Rev Cardiovasc Ther. 2010;8:545–556. doi: 10.1586/erc.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho Y.I., Cho D.J., Rosenson R.S. Endothelial shear stress and blood viscosity in peripheral arterial disease. Curr Atheroscler Rep. 2014;16:404. doi: 10.1007/s11883-014-0404-6. [DOI] [PubMed] [Google Scholar]
- 26.Cowan A.Q., Cho D.J., Rosenson R.S. Importance of blood rheology in the pathophysiology of atherothrombosis. Cardiovasc Drugs Ther. 2012;26:339–348. doi: 10.1007/s10557-012-6402-4. [DOI] [PubMed] [Google Scholar]
- 27.Blann A., Bignell A., McCollum C. von Willebrand factor, fibrinogen and other plasma proteins as determinants of plasma viscosity. Atherosclerosis. 1998;139:317–322. doi: 10.1016/s0021-9150(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 28.Junker R., Heinrich J., Ulbrich H., et al. Relationship between plasma viscosity and the severity of coronary heart disease. Arterioscler Thromb Vasc Biol. 1998;18:870–875. doi: 10.1161/01.atv.18.6.870. [DOI] [PubMed] [Google Scholar]
- 29.Chien S., Jan K. Ultrastructural basis of the mechanism of rouleaux formation. Microvasc Res. 1973;5:155–166. doi: 10.1016/0026-2862(73)90068-x. [DOI] [PubMed] [Google Scholar]
- 30.Billett H.H. In: Clinical Methods: The History, Physical, and Laboratory Examinations. Walker H.K., Hall W.D., Hurst J.W., editors. Butterworths; 1990. Hemoglobin and hematocrit. Chapter 151. [PubMed] [Google Scholar]
- 31.Rosenson R.S., Shott S., Katz R. Elevated blood viscosity in systemic lupus erythematosus. Semin Arthritis Rheum. 2001;31:52–57. doi: 10.1053/sarh.2001.24876. [DOI] [PubMed] [Google Scholar]
- 32.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White R.H., Keenan C.R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 34.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2020;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.