ABSTRACT
Background
Traditional healthcare education typically focuses on short block clinical placements based on acute care, investigations and technical aspects of diagnosis and treatment. It may therefore fail to build the understanding, compassion and person‐centred empathy needed to help those with long‐term conditions, like dementia. Time for Dementia was developed to address this.
Method
Parallel group comparison of two cohorts of UK medical students from universities, one participating in Time for Dementia (intervention group) and one not (control group). In Time for Dementia students visit a person with dementia and their family in pairs for 2 hours three times a year for 2 years, the control group received their normal curriculum.
Results
In an adjusted multilevel model (intervention group n = 274, control n = 112), there was strong evidence supporting improvements for Time for Dementia participants in: total Approaches to Dementia Questionnaire score (coefficient: 2.19, p = 0.003) and its person‐centredness subscale (1.32, p = 0.006) and weaker evidence in its hopefulness subscale (0.78, p = 0.070). There was also strong evidence of improvement in the Dementia Knowledge Questionnaire (1.63, p < 0.001) and Dementia Attitudes Scale (total score: 6.55, p < 0.001; social comfort subscale: 4.15, p < 0.001; dementia knowledge subscale: 3.38, p = 0.001) scores. No differences were observed on the Alzheimer's Disease Knowledge Scale, the Medical Condition Regard Scale or the Jefferson Scale of Empathy.
Discussion
Time for Dementia may help improve the attitudes of medical students towards dementia promoting a person‐centred approach and increasing social comfort. Such patient‐focused programmes may be a useful complement to traditional medical education.
Keywords: Alzheimer's disease, dementia, healthcare education, Longitudinal Integrated Clerkships, long‐term conditions, multimorbidity, senior mentorship programs
Key points
Traditional healthcare education with its acute care focus may fail to build the understanding, compassion and person‐centred empathy needed to help those with long‐term conditions, such as dementia.
We conducted a scoping review of enhanced placements in dementia for healthcare students searching PubMed, SCOPUS and PsycINFO and grey literature. We found a small number of programmes, with positive, but not definitive, data on their value. They were often elective parts of curricula, so relatively few students participated. Data on comparative effectiveness were sparse.
This evaluation provides proof of concept evidence that the Time for Dementia programme where students visit a person with dementia and their family in pairs for 2 hours every term for 2 years, can be made a core part of the curriculum at medical schools; the people with dementia and their carers visited are the teachers/mentors in this relationship.
These data provide positive evidence of the value of introducing a longitudinal experience of dementia as a part of the core curriculum for medical students. Students improved in terms of their attitudes towards dementia, becoming more person‐centred and having greater social comfort with, and hopefulness for, those with dementia.
Research is needed in larger representative groups and comparative studies with other educational approaches. Its effectiveness in other healthcare student groups warrants study. This longitudinal approach might be of value in building positive attitudes and understanding for other marginalised patient groups.
1. INTRODUCTION
Traditional healthcare education, particularly for medical students, is typically based around short (6–8 week) block clinical placements often in acute settings and less frequently in primary or community care. Patients are seen opportunistically as they present under the supervision of a qualified professional. The hope is that this allows for patients to be seen at all stages of their illness and recovery, giving the students a balanced view of disorder and recovery. This may work for acute illness and trauma, but the dominance of this approach has been questioned for long‐term conditions such as dementia. 1 , 2 While necessary, it may not be sufficient, and the predominance of focus on acute care and instrumental expertise may be a factor in the finding that medical students' empathy does not increase through their training, 3 , 4 and that there are barriers to the development of compassion. 5 Curriculum innovation such as the development of Longitudinal Integrated Clerkships (LICs) which promote continuity of relationships with clinical teachers and patients has been suggested as a way to rebalance medical education towards empathy, person‐centredness and to promote generalism by relational learning. 6 , 7 They have also been promoted as a way to encourage students to take up a career in remote and rural areas where posts may be hard to fill, 8 , 9 and in primary care. 7 , 10 , 11 , 12 LICs have therefore been identified as a potential way to change current undergraduate education whose belief systems and attitudes over‐value acute care and technical aspects of diagnosis and treatment, and under‐value community‐based and long‐term care. 13 , 14
Dementia is a long‐term condition that exemplifies the challenges posed to health and social care by an ageing population with its consequent increase in the chronic and degenerative illness that are associated with ageing. Over 46 million people have dementia worldwide including over 850,000 in the United Kingdom, 15 , 16 and these numbers are set to double in the next 20 years 17 National and International policy has identified improving the treatment, care and support of those with dementia as a high priority, requiring work to address deficiencies in the knowledge, attitudes and skills of the health and care workforce at undergraduate and postgraduate level. 18 , 19 Examining the curricula of UK medical schools, Tullo and Gordon 20 found ‘widespread deficiencies in education relating to attitudes and behaviours, and a failure to ensure students had adequate exposure to patients with dementia and their carers’ and that this risked portrayal in training of a ‘narrow, and potentially misleading, view of dementia.’
In response to these challenges in 2015 the UK government mandated that Health Education England should ensure that all undergraduate and preregistration courses for health and social care workers include training in dementia. 21 In this context we developed the Time for Dementia programme, a patient and carer‐focussed programme drawing on LIC models, as a complement to more traditional healthcare education. 22 In the Time for Dementia programme students are introduced to a family with dementia in their second year of training. Pairs of students then visit that family for at least 2 hours every 3–4 months for 2 years, learning from them, discussing the experiences of the family and developing a relationship with them. Detail of the content of the Time for Dementia programme is presented in our protocol paper 22 and in Section 2.3 below. The purpose of Time for Dementia is that, through this interaction, the students build an understanding of dementia, ageing, the role of family carers in long term care and the response of health and social care systems to their problems. The families are recruited and supported in a network run by the Alzheimer's Society. In Time for Dementia people with dementia and their carers are the students' teachers and mentors, delivering the potential for longitudinal patient contact to develop skills and positive understanding and attitudes. 2 We built on foundational work carried out in the United States which has shown, in small selected groups of students, that this approach might work for dementia. 23 , 24 We took this approach, modified it and extended it by making it a compulsory part of the curriculum for all students, not just those already interested in dementia. 22 In a qualitative evaluation of the impact of the programme we identified that building this relationship between the students and family was most impactful in supporting student learning, and that there were consequent improvements in knowledge, attitudes and practice. 25 Data on the comparative effectiveness of such interventions is sparse, 22 so here we report quantitative analyses of the first cohorts of medical students who received the Time for Dementia programme compared with medical students at another university who did not.
2. METHODS
2.1. Study design
The aims of the Time for Dementia evaluation were as stated in our protocol paper 22 : (i) to evaluate the feasibility of delivering the Time for Dementia programme; (ii) to evaluate Time for Dementia in terms of process and its impact on student attitudes, understanding and knowledge towards dementia; and (iii) to evaluate the satisfaction and views of the people with dementia and their carers enrolled in Time for Dementia and to assess its impact on patient quality of life and carer burden. The first two aims are addressed for medical students in this paper. We completed a parallel group comparison, over 2 years, of medical students at the same stage of training from each of two medical schools, one participating in the Time for Dementia programme (Brighton and Sussex Medical School [BSMS], the intervention group) and one which did not (Norwich Medical School [NMS], the control group). We measured dementia knowledge, attitudes and empathy at baseline (before they started the programme) and repeated these measures 1 year after (while the programme was active for the intervention group), and 2 years after (when the programme was completed).
2.2. Participants and consent
The intervention group consisted of all medical students at BSMS who entered their second year of medical training in 2014 and 2015. The control group included all medical students at NMS who entered their second year of medical training in 2015. Participation in Time for Dementia was a compulsory part of curriculum at BSMS but participation in the evaluation was not. All students were offered the opportunity to participate in the evaluation at a plenary session at the beginning of the academic year at each medical school. They were given an information sheet and those who gave written consent were recruited into the evaluation. The study was approved by the NHS Health Research Authority London Queen Square Research Ethics Committee (15/LO/0046).
2.3. Time for Dementia programme intervention
The 2‐year programme starts in the second year of study for medical students with an initiation meeting where the programme is introduced. This includes information about the aims of the programme, student expectations, safeguarding and the role of healthcare professionals with vulnerable adults and communicating with a person with dementia. After this pairs of students visit a person with dementia and their family in pairs for 2 hours every 3–4 months. It is a core and therefore compulsory element of the curriculum. During the visits, students follow a visit guideline that includes: (i) conversation where they discuss the person with dementia's life and the impact of dementia including their experiences of health and social services; (ii) life story work reviewing and discussing the individual's life involving reminiscence and storytelling as an enjoyable and empowering activity; and (iii) completion of ‘This is Me’ a simple practical tool created by the Royal College of Nursing and the Alzheimer's Society for individuals with dementia to inform health and social care professionals about their needs, interests, preferences, likes and dislikes. Time for Dementia is not designed as a therapeutic intervention and students do not give health information. Students are required to complete a reflective practice assignment about their visits for assessment. A final conference brings together all students along with people with dementia and carers from the programme.
2.4. Study assessments
All students completed the same battery of instruments at baseline and 12 and 24 months later, in all higher scores indicate a higher, or more positive, level of the attribute.
Approaches to Dementia Questionnaire (ADQ), 26 19 items assessing attitudes towards dementia, each scored 1–5 (total: 19–95) with two subscales ‘hopefulness’ and ‘person‐centeredness’, the total score was our primary outcome;
Alzheimer's Disease Knowledge Scale (ADKS), 27 30 true/false items (total: 0–30) assessing students' knowledge of Alzheimer's disease;
Dementia Knowledge Questionnaire (DKQ), 28 20 true/false items (total: 0–20) assessing dementia knowledge;
Dementia Attitude Scale (DAS), 29 20 items assessing attitudes toward dementia scored on a 7‐point Likert‐type scale (total: 20–140) with two subscales ‘dementia knowledge’ and ‘social comfort’;
Medical Condition Regard Scale (MCRS), 30 11 items scored on a 6‐point Likert‐type scale (total: 11–66) assessing the extent students find patients with a given condition to be enjoyable, treatable and worthy of medical resources; and
Jefferson Scale of Empathy (JSE): Medical Student Version, 31 20 items scored on a 7‐point Likert‐type scale (total: 20–140) measuring student empathy.
Selected students in the intervention group were also invited to participate in qualitative individual interviews and focus groups which have been reported elsewhere. 25
2.5. Statistical analyses
Outcomes were modelled using three‐level multilevel linear regression models as outcomes were measured at two further time points after baseline for each student and students performed visits in pairs. Independent variables included in the models were: the outcome at baseline (continuous), time point (12/24 months vs. baseline), student age (continuous), student gender (female/male), student previous experience of dementia (yes/no) and intervention group (intervention/control). These models control for baseline scores, time point, age, gender and previous experience of dementia. Analyses were performed in Stata 15.1 (Stata Statistical Software: Release 15, StataCorp LLC).
2.6. Patient and public involvement
People with dementia and their carers were involved throughout the research process though a dementia advisory group, including designing the study, interpreting findings and helping to disseminate the intervention and findings. Family members, people with dementia and the Alzheimer's Society also actively participated in the Time for Dementia programme management board.
3. RESULTS
3.1. Student recruitment and demographics
In the intervention group, 280 students were approached and 274 (98%) consented to participate in the evaluation. In the control group 179 students were approached and 112 (63%) consented. At baseline data were obtained from 260 (95%) of the consenting intervention group and 66 (59%) of the consenting control group. At 12 months, 234 in the intervention group (85%) and 89 (79%) in the control group were followed‐up, and at 2‐year follow‐up the numbers were 142 (52%) and 70 (63%), respectively. Details of the demographic characteristics and past experience of dementia are presented in Table 1.
TABLE 1.
Demographic characteristics and experience with dementia of student group
| Control group (n = 112) | Intervention group (n = 274) | Total (n = 386) | ||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Age (years) | 20.0 | 19.0–21.0 | 20.0 | 19.0–23.0 | 20.0 | 19.0–22.0 |
| n | % | n | % | N | % | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 39 | 37.1 | 108 | 39.4 | 147 | 38.8 |
| Female | 66 | 62.9 | 166 | 60.6 | 232 | 61.2 |
| Student ethnicity | ||||||
| White British/European | 51 | 52.6 | 186 | 69.4 | 237 | 64.9 |
| Mixed/multiple ethnic groups | 5 | 5.2 | 16 | 6.0 | 21 | 5.8 |
| Asian/Asian British | 24 | 24.7 | 39 | 14.6 | 63 | 17.3 |
| Black/African/Caribbean/black British | 12 | 12.4 | 12 | 4.5 | 24 | 6.6 |
| Other | 5 | 5.2 | 15 | 5.6 | 20 | 5.5 |
| Experience of knowing someone with dementia | ||||||
| Yes | 40 | 41.2 | 128 | 47.9 | 168 | 46.2 |
| No | 57 | 58.8 | 139 | 52.1 | 196 | 53.8 |
| Student experience with dementia | ||||||
| Family member or friend | 18 | 45.0 | 68 | 53.1 | 86 | 51.2 |
| Paid or unpaid work | 9 | 22.5 | 37 | 28.9 | 46 | 27.4 |
| Both | 13 | 32.5 | 23 | 18.0 | 36 | 21.4 |
Abbreviation: IQR, interquartile range.
3.2. Measures of outcome
The scores on the study outcome measures are presented in Tables 2 (baseline), 3 (12‐months follow‐up) and 4 (24‐months follow‐up). Table 5 presents the results of the adjusted multilevel models comparing medical student 2‐year outcomes in Time for Dementia recipients compared with controls. There was strong evidence supporting improvements for those receiving the Time for Dementia in attitudes to dementia including in our primary outcome the ADQ total score (coefficient: 2.19, 95% confidence interval [95% CI]: 0.75–3.64, p = 0.003) and its person‐centredness subscale (1.32, 0.39–2.25, p = 0.006) and weak evidence for improvement in its hopefulness subscale (0.78, −0.06 to 1.62, p = 0.070). Similarly, there were more positive scores on the DAS total score (6.55, 95% CI: 3.91–9.19, p < 0.001) and its social comfort (4.15, 95% CI: 2.26–6.06, p < 0.001) and dementia knowledge subscales (2.27, 95% CI: 0.95–3.59, p = 0.001) for those participating in the Time for Dementia programme. Evidence of impact on knowledge was less clear with positive changes in the DKQ (1.63, 95% CI: 1.04–2.23, p < 0.001) but not in the ADKS (0.19, 95% CI: ‐0.42 to 0.81, p = 0.539). There were no changes between the intervention and control groups in the MCRS, an attitudinal measure (0.39, 95% CI: ‐1.05 to 1.83, p = 0.593) or empathy as measured by the JSE (1.33, 95% CI: ‐1.08 to 3.74, p = 0.278). To illustrate the magnitude of the differences Figure 1 presents the adjusted regression coefficients for the Time for Dementia group versus the control group as a percentage of each scale.
TABLE 2.
Scores on outcome measures at baseline
| Control | Intervention | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | N | Mean | SD | n | |
| ADQ total (19–95) | 77.8 | 5.6 | 66 | 76.8 | 5.6 | 260 | 77.0 | 5.6 | 326 |
| ADQ—hopeful (8–40) | 28.2 | 3.3 | 66 | 27.4 | 3.6 | 260 | 27.6 | 3.5 | 326 |
| ADQ—person‐centredness (11–55) | 49.9 | 3.6 | 66 | 49.4 | 3.6 | 260 | 49.4 | 3.6 | 326 |
| ADKS (0–30) | 23.6 | 2.5 | 66 | 23.4 | 2.9 | 261 | 23.5 | 2.9 | 327 |
| DKQ (0–20) | 15.0 | 3.2 | 66 | 15.6 | 2.5 | 225 | 15.4 | 2.7 | 291 |
| DAS total (20–140) | 108.7 | 12.0 | 66 | 109.3 | 12.2 | 262 | 109.1 | 12.1 | 328 |
| DAS—comfort (10–70) | 46.8 | 9.1 | 66 | 48.4 | 9.1 | 262 | 48.1 | 9.1 | 328 |
| DAS—knowledge (10–70) | 61.9 | 5.2 | 66 | 60.9 | 5.5 | 262 | 61.1 | 5.5 | 328 |
| MCRS (11–66) | 52.0 | 6.2 | 65 | 52.8 | 6.4 | 263 | 52.6 | 6.4 | 328 |
| JSE (20–140) | 118.3 | 9.0 | 64 | 117.5 | 10.0 | 260 | 117.7 | 9.8 | 324 |
Abbreviations: ADKS, Alzheimer's Disease Knowledge Scale; ADQ, Approaches to Dementia Questionnaire; DAS, Dementia Attitude Scale; DKQ, Dementia Knowledge Questionnaire; JSE, Jefferson Scale of Empathy; MCRS, Medical Condition Regard Scale; SD, standard deviation.
TABLE 3.
Scores on outcome measures at 1‐year follow‐up
| Control | Intervention | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | N | Mean | SD | n | |
| ADQ total | 77.2 | 6.3 | 89 | 79.5 | 5.7 | 234 | 78.8 | 6.0 | 323 |
| ADQ—hopefulness | 28.2 | 3.5 | 89 | 29.0 | 3.5 | 234 | 28.8 | 3.5 | 323 |
| ADQ—person‐ centredness | 49.0 | 4.1 | 89 | 50.4 | 3.6 | 234 | 50.0 | 3.8 | 323 |
| ADKS | 23.7 | 3.1 | 89 | 24.7 | 2.6 | 232 | 24.4 | 2.8 | 321 |
| DKQ | 14.5 | 3.5 | 89 | 16.8 | 2.0 | 231 | 16.2 | 2.7 | 320 |
| DAS total | 106.6 | 13.1 | 89 | 113.9 | 11.4 | 234 | 111.9 | 12.3 | 323 |
| DAS—comfort | 46.6 | 8.8 | 89 | 51.3 | 8.4 | 234 | 50.0 | 8.7 | 323 |
| DAS—knowledge | 60.1 | 6.5 | 89 | 62.6 | 5.2 | 234 | 61.9 | 5.7 | 323 |
| MCRS | 51.6 | 6.7 | 89 | 53.6 | 6.3 | 232 | 53.0 | 6.5 | 321 |
| JSE | 116.4 | 11.4 | 87 | 117.7 | 10.4 | 232 | 117.3 | 10.7 | 319 |
Abbreviations: ADKS, Alzheimer's Disease Knowledge Scale; ADQ, Approaches to Dementia Questionnaire; DAS, Dementia Attitude Scale; DKQ, Dementia Knowledge Questionnaire; JSE, Jefferson Scale of Empathy; MCRS, Medical Condition Regard Scale; SD, standard deviation.
TABLE 4.
Scores on outcome measures at 2‐year follow‐up
| Control | Intervention | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | N | Mean | SD | n | |
| ADQ | 79.1 | 7.4 | 70 | 80.8 | 7.0 | 142 | 80.2 | 7.2 | 212 |
| ADQ—hopefulness | 29.7 | 3.6 | 70 | 29.8 | 4.1 | 142 | 29.8 | 3.9 | 212 |
| ADQ—person‐ centredness | 49.4 | 4.9 | 70 | 50.9 | 4.3 | 142 | 50.4 | 4.6 | 212 |
| ADKS | 24.5 | 2.9 | 71 | 25.2 | 2.8 | 144 | 24.9 | 2.8 | 215 |
| DKQ | 14.9 | 3.3 | 70 | 17.0 | 2.2 | 143 | 16.3 | 2.8 | 213 |
| DAS total | 110.0 | 13.7 | 69 | 118.8 | 12.0 | 142 | 115.9 | 13.2 | 211 |
| DAS—comfort | 48.7 | 8.8 | 69 | 55.4 | 8.2 | 142 | 53.2 | 9.0 | 211 |
| DAS—knowledge | 61.3 | 7.8 | 69 | 63.3 | 5.8 | 142 | 62.7 | 6.6 | 211 |
| MCRS | 52.9 | 6.9 | 70 | 54.8 | 7.1 | 142 | 54.2 | 7.1 | 212 |
| JSE | 116.3 | 13.8 | 68 | 119.4 | 11.0 | 141 | 118.4 | 12.0 | 209 |
Abbreviations: ADKS, Alzheimer's Disease Knowledge Scale; ADQ, Approaches to Dementia Questionnaire; DAS, Dementia Attitude Scale; DKQ, Dementia Knowledge Questionnaire; JSE, Jefferson Scale of Empathy, MCRS, Medical Condition Regard Scale; SD, standard deviation.
TABLE 5.
Adjusted multilevel models comparing medical student 2‐year outcomes in Time for Dementia recipients compared with controls
| Variable | Coefficient | 95% Confidence interval | p‐Value |
|---|---|---|---|
| ADQ total | 2.19 | 0.75–3.64 | 0.003 |
| ADQ—hopefulness | 0.78 | −0.06 to 1.62 | 0.070 |
| ADQ—person‐centredness | 1.32 | 0.39–2.25 | 0.006 |
| ADKS | 0.19 | −0.42 to 0.81 | 0.539 |
| DKQ | 1.63 | 1.04–2.23 | <0.001 |
| DAS total | 6.55 | 3.91–9.19 | <0.001 |
| DAS—social comfort | 4.16 | 2.26–6.06 | <0.001 |
| DAS—dementia knowledge | 2.27 | 0.95–3.59 | 0.001 |
| MCRS | 0.39 | −1.05 to 1.83 | 0.593 |
| JSE | 1.33 | −1.08 to 3.74 | 0.278 |
Abbreviations: ADKS, Alzheimer's Disease Knowledge Scale; ADQ, Approaches to Dementia Questionnaire; DAS, Dementia Attitude Scale; DKQ, Dementia Knowledge Questionnaire; JSE, Jefferson Scale of Empathy; MCRS, Medical Condition Regard Scale.
FIGURE 1.
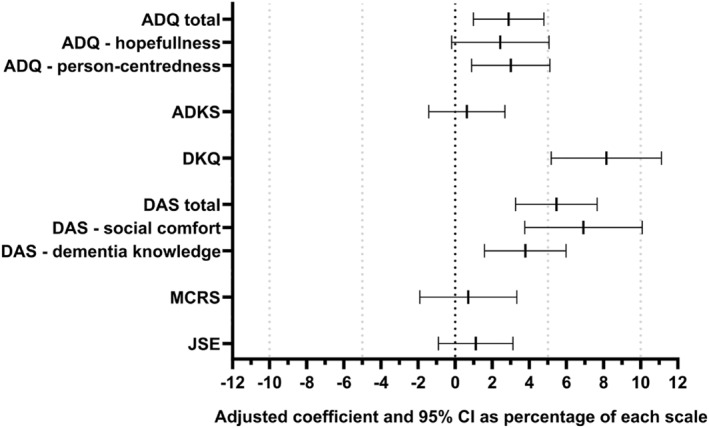
Adjusted regression coefficients for the Time for Dementia group versus the control group as a percentage of each scale (positive values favour Time for Dementia)
4. DISCUSSION
This is the first quantitative evaluation to provide positive evidence of the value of introducing a 2‐year patient and carer‐led longitudinal experience of dementia to a whole year group of medical students. We found that the medical students receiving Time for Dementia improved in terms of their attitudes towards dementia, becoming more person‐centred and having greater social comfort with and hopefulness for those with dementia. We are not aware of any other undergraduate educational intervention that has achieved this. We did not find an increase in empathy or regard, but these concepts are difficult to measure quantitatively. Taken with the positive findings from our qualitative evaluation of students participating in the programme, 25 these findings suggest that there may well be beneficial outcomes of the programme for students and provide a proof of concept for this approach. Time for Dementia addresses the three main recommendations made by Tullo and Gordon 20 to improve the quality of dementia education in medical schools: (i) establishing dementia as a core element of the curriculum; (ii) using dementia training to encourage professional development by exploring attitudes and behaviours; and (iii) involving people with dementia and carers in teaching.
There are important limitations to the design of this study. First, it is a simple parallel group comparison of two medical schools, not a randomised controlled trial; while the schools were similar in size, ethos and age, the design does not allow for direct inference that the changes seen over the 2 years of study are attributable to the Time for Dementia Programme. There may have been other elements of the curriculum or external environment that were responsible for the differences. It is however positive that the comparison was carried out at the same time and that the statistical modelling used controlled for potential confounders such as past experience of dementia. Second, the participation rate in the control group was low, only 112/179 (63%) consented to participate in the evaluation. This may have led to a higher level of response bias and a lower level of generalisability than in the intervention group where 274/280 (98%) consented. A further source of response bias may have come from the attrition in responses over the study time points. However, the prospective nature of the study, the possibility that differential response in the control group will have included people more interested in dementia where positive outcomes might have been predicted, and the statistical methods that allow all data points to be included in the analyses, give some support to the longitudinal findings. Third, it is a limitation that there is only one medical school in the intervention and one in the control group. Different medical schools have different curricula and students, and better or worse performance might have been observed in other schools. This also limits the generalisability of the data generated in this study. Nonetheless, it is positive that we were able to implement the curriculum change in the intervention school over two whole year groups and to follow each group up over 2 years. Finally, there are limitations to quantitative evaluation of complex interventions and outcomes such as those considered here. We have collected qualitative data from the intervention group and the data reported support the findings in this study. 25
While informed by the ethos of LICs, Time for Dementia is patient‐focussed rather than service‐focussed. Students are ‘placed’ with families rather than with a particular clinical team, they learn from those families not from clinicians, and students do not engage in the care of those patients. As such it does not meet the definition of a LIC coined by the Consortium of LICs which requires that ‘(i) medical students participate in the comprehensive care of patients over time, (ii) medical students have continuing learning relationships with these patients' clinicians and (iii) through these experiences, medical students meet the majority of the academic year's core clinical competencies across multiple disciplines simultaneously’. 32 Instead Time for Dementia is a pragmatic and patient‐focussed response to attempt to derive much of the value of a LIC (building positive attitudes and person‐centredness) without the need for wholescale curricular revision and time needed to deliver LICs to a whole year. The practicality of LICs as a solution to deliver person‐centredness to the medical workforce in training as a whole has been questioned. 33 It is striking that because of their cost and complexity, few LICs have been offered as a compulsory element of the curriculum, with important exceptions, such as that at the Northern Ontario School of Medicine 34 where the focus is serving a particular remote rural community. LICs typically include only a small number of interested students in any year, with an average of 20 (range: 2–85). 32 Time for Dementia is designed to be delivered to all students, and is perhaps a rediscovery of longitudinal patient/student contact experiences which were more common in past decades. 35 , 36 The data from this study suggest that Time for Dementia, a relatively simple and inexpensive (financially and in tuition time) addition to medical education, could help create a workforce that is better equipped to meet the needs of the populations we serve.
These data from Time for Dementia add to an emerging evidence base that shows the potential for improving the knowledge, confidence and attitudes of healthcare students towards those affected by dementia, such novel experiential programmes can help build ‘a culture of positive attitudes among future healthcare professionals’. 37 Providing person‐centred care is a national priority, and a fundamental standard of care. 38 It supports a holistic approach to care where the psychological needs of the individual are central and the focus is on the individuality of the person, not on their impairments. 39 This allows care staff to move away from focussing on tasks, to the wishes and experiences of the person with dementia, providing opportunities for staff to understand and meet the psychological needs of the person with dementia to enhance their well‐being. In this study we found an improvement in person‐centredness in the Time for Dementia group compared with controls. This has similarities to those completing the elective Harvard Medical School Cambridge Integrated Clerkship which reported that immediately after the LIC students held more person‐centred attitudes, and that these were sustained over time. 40 Further research is needed to follow up the Time for Dementia and control groups to see if these changes persist into practice and if it affects career preferences.
The data presented from this study are unique and encouraging but not definitive. Further research is needed in larger representative groups and comparative studies with medical schools running formal LICs would be of interest. The purpose of the programme was not just to improve attitudes and person‐centredness for dementia, but also to help students more generally to understand the role of families, long‐term conditions, frailty and what is like to be old and ill in society. The data presented here along with our qualitative data suggest that Time for Dementia succeeds in this. 25 Such approaches which facilitate the healthcare staff of the future to be informed and positive about working with people with dementia are a vital component in generating dementia‐friendly communities and societies. 41 We have now introduced Time for Dementia to other undergraduate healthcare professional courses in the southeast of England including adult nursing, mental health nursing, paramedic and occupational therapy students. We will evaluate its acceptability and value in these groups. Programmes such as Time for Dementia present no barriers (other than practicalities) to the possibility of inter‐professional delivery and therefore true interprofessional learning as preparation for the teamwork that 21st century medicine requires. We are working to pilot and evaluate such approaches in the southwest of England. Finally, even without definitive evidence this proof of concept raises the possibility that this longitudinal approach might be of value in building positive attitudes and understanding for other marginalised patient groups. It is possible to imagine a number of different patient groups being followed through undergraduate training allowing students to carry into practice a set of more balanced and inclusive attitudes, understandings and behaviour towards people with long term and complex conditions such as dementia. In this way we can create healthcare systems that are truly inclusive of and effective for the people that most need them and who are most often failed now.
CONFLICT OF INTERESTS
Sube Banerjee is the guarantor for the paper. He developed the Time for Dementia concept, designed the study with Stephanie Daley and Juliet Wright and wrote the first draft of this paper. All authors participated in the conduct of the study and in all stages of writing up and revision. All meet the journal criteria for authorship. Sube Banerjee, the manuscript's guarantor, affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained. The Time for Dementia programme and its evaluation was funded by Health Education England but the views reported here are those of the aurthors, not those of Health Education England; the authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
ACKNOWLEDGMENTS
The authors would like to thank all the families and students that have taken part in Time for Dementia and for the Alzheimer's Society and NHS mental health trusts in Surrey and Sussex for their help in recruiting the families into the programme. Special thanks are due to Rob and Pippa Stanley, Christine Maddocks, Eddie and Mary Woods, Ellen Jones, Christine Wyatt, and Gillian Sleater. The views reported here are those of the authors and may not reflect those of the funder or sponsor. This project was funded by Health Education England as part of its Dementia Skills Development Programme but the views expressed are those of the authors. The funders and the sponsor (the University of Sussex) had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We confirm the independence of researchers from funders and that all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
DATA AVAILABILITY STATEMENT
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hirsh DA, Ogur B, Thibault GE, Cox M. “Continuity” as an organizing principle for clinical education reform. N Engl J Med. 2007;356:858‐866. [DOI] [PubMed] [Google Scholar]
- 2. Norris TE, Schaad DC, DeWitt D, Ogur B, Hunt DD. Consortium of Longitudinal Integrated Clerkships . Longitudinal integrated clerkships for medical students: an innovation adopted by medical schools in Australia, Canada, South Africa, and the United States. Acad Med. 2009;84:902‐907. [DOI] [PubMed] [Google Scholar]
- 3. Quince TA, Parker RA, Wood DF, Benson JA. Stability of empathy among undergraduate medical students: a longitudinal study at one UK medical school. BMC Med Educ. 2011;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newton BW, Barber L, Clardy J, Cleveland E, O'Sullivan P. Is there hardening of the heart during medical school? Acad Med. 2008;83:244‐249. [DOI] [PubMed] [Google Scholar]
- 5. Dev V, Fernando AT, 3rd , Kirby JN, Consedine NS. Variation in the barriers to compassion across healthcare training and disciplines: a cross‐sectional study of doctors, nurses, and medical students. Int J Nurs Stud. 2018;90:1‐10. [DOI] [PubMed] [Google Scholar]
- 6. Hudson JN, Poncelet AN, Weston KM, Bushnell JA, A Farmer E. Longitudinal Integrated Clerkships. Med Teach. 2016;39:7. 10.1080/0142159X.2017.1245855 [DOI] [PubMed] [Google Scholar]
- 7. Bartlett M, Muir F. A new model of undergraduate clinical education? Br J Gen Pract. 2018;68:216‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walters L, Greenhill J, Richards J, et al. Outcomes of longitudinal integrated clinical placements for students, clinicians and society. Med Educ. 2012;46:1028‐1041. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan B, McGrail M, Russell D, et al. Duration and setting of rural immersion during the medical degree relates to work outcomes. Med Educ. 2018;52:803‐815. [DOI] [PubMed] [Google Scholar]
- 10. Brown M, Anderson K, Finn GM. A narrative literature review considering the development and implementation of Longitudinal Integrated Clerkships, including a practical guide for application. J Med Educ Curric Dev. 2019;6:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McKeown A, Mollaney J, Ahuja N, Parekh R, Kumar S. UK Longitudinal Integrated Clerkships: where are we now? Educ Prim Care. 2019;30:270‐274. 10.1080/14739879.2019.1653228 [DOI] [PubMed] [Google Scholar]
- 12. Bartlett M, Dowell J, Graham F, et al. Dundee's Longitudinal Integrated Clerkship: drivers, implementation and early evaluation. Educ Prim Care. 2019;30(2):72‐79. [DOI] [PubMed] [Google Scholar]
- 13. Banerjee S. Multimorbidity‐older adults need health care that can count past one. Lancet. 2015;385:587‐589. [DOI] [PubMed] [Google Scholar]
- 14. Gillespie M. Student nurse perceptions of client groups and clinical placement areas. Br J Nurs. 2013;22:340‐345. [DOI] [PubMed] [Google Scholar]
- 15. Prince M, Guerchet M, Prina M, Alzheimer's Disease International . The Global Impact of Dementia 2013‐2050. London: Alzheimer's Disease International; 2013. [Google Scholar]
- 16. Prince M, Knapp M, Guerchet M, et al. Dementia UK: Update. 2nd ed. London: Alzheimer's Society; 2014. [Google Scholar]
- 17. Alzheimer's Disease International . World Alzheimer's Report 2015 The Global Impact of Dementia. London: Alzheimer's Disease International; 2015. [Google Scholar]
- 18. Department of Health . Living Well with Dementia: A National Dementia Strategy. London: Department of Health; 2009. [Google Scholar]
- 19. World Health Organisation . Global Action Plan on the Public Health Response to Dementia 2017–2025. Geneva: WHO; 2017. [Google Scholar]
- 20. Tullo ES, Gordon AL. Teaching and learning about dementia in UK medical schools: a national survey. BMC Geriatr. 2013;13:29. 10.1186/1471-2318-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Department of Health . Delivering High Quality, Effective, Compassionate Care: Developing the Right People with the Right Skills and the Right Values. A Mandate from the Government to Health Education England. London: Department of Health; 2015. [Google Scholar]
- 22. Banerjee S, Farina N, Daley S, et al. How do we enhance undergraduate healthcare education in dementia? A review of the role of innovative approaches and development of the time for dementia programme. Int J Geriatr Psychiatry. 2016;32:68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morhardt D. Educating medical students on Alzheimer's disease and related disorders: an overview of the Northwestern University Buddy Program. Dementia. 2006;5:448‐456. [Google Scholar]
- 24. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer's disease: the PAIRS Program. BMC Med Educ. 2012;12:80. 10.1186/1472-6920-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daley S, Feeney Y, Grosvenor W, et al. A qualitative evaluation of the effect of a longitudinal dementia education programme on healthcare student knowledge and attitudes. Age Ageing. 2020;49(6):1080‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lintern T, Woods B, Phair L. Before and after training: a case study of intervention. J Dementia Care. 2000;8:15‐17. [Google Scholar]
- 27. Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer's Disease Knowledge Scale: development and psychometric properties. Gerontologist. 2009;49:236‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shanahan N, Orrell M, Schepers AK, Spector A. The development and evaluation of the DK‐20: a knowledge of dementia measure. Int Psychogeriatr. 2013;25:1899‐1907. [DOI] [PubMed] [Google Scholar]
- 29. O'Connor ML, McFadden SH. Development and psychometric validation of the dementia attitudes Scale. Int J Alz Dis. 2010;2010:454218. 10.4061/2010/454218 [DOI] [Google Scholar]
- 30. Christison GW, Haviland MG, Riggs ML. The Medical Condition Regard Scale. Acad Med. 2002;77:257‐262. [DOI] [PubMed] [Google Scholar]
- 31. Hojat M, Mangione S, Nasca TJ, et al. The Jefferson Scale of physician empathy: development and preliminary psychometric data. Educ Psychol Meas. 2001;61:349‐365. [Google Scholar]
- 32. Worley P, Couper I, Strasser R, et al. A typology of Longitudinal Integrated Clerkships. Med Educ. 2016;50:922‐932. [DOI] [PubMed] [Google Scholar]
- 33. Mol SS, Chen HC, Steerneman AH, de Groot E, Zwart DL. The feasibility of longitudinal patient contacts in a large medical School. Teach Learn Med. 2019;31:178‐185. [DOI] [PubMed] [Google Scholar]
- 34. Dubé TV, Schinke RJ, Strasser R. It takes a community to train a future physician: social support experienced by medical students during a community‐engaged Longitudinal Integrated Clerkship. Can Med Educ J. 2019;10(3):e5‐e16. [PMC free article] [PubMed] [Google Scholar]
- 35. Geyman C, Smith L, Hadac R, Smith CK. Benefits of early predoctoral experiences in longitudinal patient care. J Fam Pract. 1984;18(6):911‐914. [PubMed] [Google Scholar]
- 36. Kumagai AK, Murphy EA, Ross PT. Diabetes stories: use of patient narratives of diabetes to teach patient‐centered care. Adv Health Sci Educ. 2009;14(3):315‐326. [DOI] [PubMed] [Google Scholar]
- 37. Williams M, Daley S. Innovation in dementia education within undergraduate healthcare programmes: a scoping review. Nurse Educ Today. 2021;98:104742. 10.1016/j.nedt.2020.104742 [DOI] [PubMed] [Google Scholar]
- 38. Care Quality Commission . The Fundamental Standards. https://www.cqc.org.uk/what‐we‐do/how‐we‐do‐our‐job/fundamental‐standards [Google Scholar]
- 39. Brooker D. What is person‐centred care in dementia? Rev Clin Gerontol. 2003;13(03):215‐222. [Google Scholar]
- 40. Gaufberg E, Hirsh D, Krupat E, et al. Into the future: patient‐centredness endures in Longitudinal Integrated Clerkship graduates. Med Educ. 2014;48(6):572‐582. [DOI] [PubMed] [Google Scholar]
- 41. Buckner S, Darlington N, Woodward M, et al. Dementia friendly communities in England: a scoping study. Int J Geriatr Psychiatry. 2019;34:1235‐1243. 10.1002/gps.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.


