Abstract
Aim
To investigate once‐weekly (OW) semaglutide, a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA), in patients with type 2 diabetes (T2D) in routine clinical practice.
Methods
The SURE Canada study was a multicenter, prospective, observational study. Adults with T2D and one or more documented HbA1c values 12 weeks or less before semaglutide initiation were enrolled. The primary endpoint was change in HbA1c from baseline to end of study (EOS; ~30 weeks). Secondary endpoints included change in body weight (BW), waist circumference and patient‐reported outcomes (PROs) and the proportion of patients achieving HbA1c of less than 7.0%, weight loss (WL) of 5% or higher, and a composite of HbA1c reduction of 1% or higher and WL of 3% or higher at EOS. Data were analysed and presented for patients on semaglutide at EOS overall and for the following baseline medication subgroups: oral antihyperglycaemic drugs (OADs) only; GLP‐1RA experienced; insulin ± OADs without GLP‐1RA.
Results
In total, 452 patients initiated semaglutide and 356 completed the study on treatment. For the 452 patients, mean baseline HbA1c was 8.1%; 86 (19.0%) patients had HbA1c of less than 7.0%. Mean dose of semaglutide at EOS was 0.76 ± 0.31 mg. Mean HbA1c was reduced by 0.9%‐point (95% confidence interval [CI]: 0.97; 0.78). Mean BW was reduced by 4.3 kg (95% CI: 4.79; 3.76). At EOS, 46.9% of patients achieved HbA1c of less than 7.0%, 40.9% achieved WL of 5% or higher and 24.1% achieved the composite endpoint. PROs improved from baseline to EOS. No new safety concerns were reported.
Conclusions
In SURE Canada, patients treated with OW semaglutide in routine clinical practice experienced clinically significant improvements in HbA1c, BW and other outcomes, supporting semaglutide use in routine clinical practice.
Keywords: body weight, GLP‐1RA, glycaemic control, routine clinical practice, semaglutide, SURE study, type 2 diabetes
1. INTRODUCTION
The prevalence of diabetes in Canada is 10.1% 1 ; around 90% of Canadian adults with diabetes have type 2 diabetes (T2D). 2 The treatment goals for T2D are to prevent or delay complications and to maintain quality of life via glycaemic control and cardiovascular (CV) risk management. 3 The 2020 update to the Canadian guidelines for T2D treatment recommend a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) or sodium‐glucose co‐transporter‐2 inhibitor (SGLT‐2i) with proven CV benefit for patients with established ischaemic CV disease and in patients aged 60 years or older with at least two CV risk factors. 4 In addition, a GLP‐1RA and/or an SGLT‐2i should be considered as the first option for second line, as add‐on to metformin therapy when weight loss is a priority. 4
Semaglutide (Novo Nordisk A/S, Denmark) is a GLP‐1RA, approved by Health Canada as once‐weekly (OW) subcutaneous (s.c.) and once‐daily oral formulations, to improve glycaemic control in adults with T2D, in addition to diet and exercise. 5 , 6
In the SUSTAIN clinical trials, OW semaglutide showed superior, clinically relevant reductions in HbA1c and body weight (BW) compared with placebo and a wide range of active comparators, including SGLT‐2is, other GLP‐1RAs and basal insulin glargine, with a safety profile similar to that of other GLP‐1RAs. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 However, randomized controlled trials (RCTs) do not fully represent the population treated in clinical practice because of strict inclusion/exclusion criteria. Real‐world (RW) studies aim to complement the findings of RCTs and are important for understanding the use of a drug in an RW patient population. 16
The SURE Canada study is part of the SURE programme, nine separate observational RW studies investigating OW semaglutide in routine clinical practice across 10 countries, in a diverse range of patients with T2D. The studies were conducted in Canada, Denmark/Sweden, France, Germany, Italy, The Netherlands, Spain, Switzerland and the UK.
2. METHODS
2.1. Study design
SURE Canada was a prospective, open‐label, observational study lasting ~30 weeks, assessing OW semaglutide in adults with T2D in routine clinical practice at 22 sites including GP and specialist centres in Canada. The decisions to initiate semaglutide and to prescribe other antihyperglycaemic treatments, diet and exercise were at the treating physician's discretion.
Patients were treated with and prescribed OW s.c. semaglutide in a prefilled pen‐injector as per routine clinical practice. The drug was not provided by the sponsor. The treating physician determined the starting dose, dose escalation and maintenance dose, as well as any subsequent changes to the maintenance dose. After visit 1 (0 weeks), patients attended visits only if applicable according to local practice. Patients who did not attend any visit between weeks 28 and 38 were considered as non‐completers.
The study was conducted in compliance with the Declaration of Helsinki 17 and the Guidelines for Good Pharmacoepidemiology Practices. 18 Study materials were approved by institutional review boards or other appropriate local bodies. Patients provided informed consent before the commencement of any study‐related activities. The study is registered with ClinicalTrials.gov (NCT03457012).
2.2. Study population
Patients aged 18 years or older diagnosed with T2D more than 12 weeks prior and with a documented HbA1c value within 12 weeks were enrolled. Exclusion criteria included previous participation in the SURE study, treatment with any investigational drug (a drug not currently approved for treatment) within 90 days prior to enrolment and hypersensitivity to semaglutide or to any of the excipients. The study was initiated with the first patient first visit on 29 March 2018 and was completed with the last patient last visit on 19 December 2019.
2.3. Endpoints
The primary endpoint was change from baseline to EOS in HbA1c (%‐point). Secondary endpoints included change from baseline to EOS in BW (kg) and waist circumference (cm) and the proportion of patients achieving: HbA1c less than 7%; weight loss of 5% or higher; a composite endpoint of HbA1c reduction of 1% or higher and weight loss of 3% or higher. Patient‐reported outcomes (PROs), also included as secondary outcomes, were measured by the Diabetes Treatment Satisfaction Questionnaire status version (DTSQs), the DTSQ change version (DTSQc) and the Short‐Form 36 Health Survey version 2 (SF‐36® v2). DTSQs, evaluated at visits 1 and 6, provides a measure of relative satisfaction with current diabetes treatments. DTSQc scores were collected at the EOS visit and measure the relative treatment satisfaction score compared with previous treatment. The SF‐36® v2, evaluated at baseline and EOS, measures eight domains of health‐related quality of life, which are further combined into the physical and mental component summary (PCS and MCS). Patients experiencing severe or documented hypoglycaemic events was also a secondary endpoint.
Prespecified primary exploratory data included weekly dose of semaglutide at EOS, a physician's assessment of a patient's ‘clinical success’ in relation to reason to initiate semaglutide, and the level of self‐reported treatment adherence measured by the eight‐item Morisky Medication Adherence Scale (MMAS‐8) score. Response categories for the MMAS‐8 are dichotomous yes/no for each item, except for a five‐point Likert response for the last item. MMAS‐8 scores are trichotomized into levels of adherence: high (score = 8), medium (score ≥6 to <8) and low (score <6). 19 , 20 , 21 Secondary exploratory data included reasons for initiating semaglutide. Data on insulin dose and other antihyperglycaemic medication use were also collected, although this was not a prespecified endpoint.
Safety was evaluated according to adverse event (AE) reporting by physicians; only serious adverse drug reactions, fatal events, pregnancies, AEs in foetuses or newborns and AEs leading to treatment discontinuation were systematically collected. Other AEs were collected only if reported.
2.4. Statistical analysis
Descriptive statistics (mean ± standard deviation [SD], median, interquartile range and range for continuous variables and number and proportion for categorical variables) were used to describe patient characteristics at the time of semaglutide initiation. Baseline characteristics were analysed in the full analysis set (FAS), which included all patients who initiated semaglutide treatment. Primary analyses of the primary and secondary endpoints and primary exploratory assessments were performed on the effectiveness analysis set (EAS), including all patients who completed the study (attended the EOS visit) and were receiving semaglutide at EOS. Secondary analyses of the primary and secondary endpoints, and secondary exploratory and safety assessments were performed on the FAS. Composite endpoints were analysed in both the FAS and EAS. AEs were reported in the FAS, except hypoglycaemia, which was reported in both populations.
Data were analysed and presented overall and for the following subgroups: OADs only; GLP‐1RA experienced; insulin ± OADs without GLP‐1RA. The primary analysis of the primary endpoint of change from baseline to EOS in HbA1c was performed in the EAS as a baseline‐adjusted change using an analysis of covariance (ANCOVA) model with change from baseline as the dependent variable, excluding patients with missing information on HbA1c at EOS. Sensitivity analyses were performed to explore the impact of missing data in the primary analysis, where patients were excluded if they did not complete the study, discontinued treatment or were missing information on HbA1c at EOS. The ‘in‐study’ sensitivity analysis evaluated patients in the FAS with at least one postbaseline HbA1c measurement in the in‐study observation period, and the ‘on‐treatment’ sensitivity analysis evaluated patients in the FAS in the on‐treatment observation period. A mixed model for repeated measurements was used for the sensitivity analyses of the primary endpoint in the respective observation periods and was based on the FAS population. An ANCOVA model was used for the sensitivity analysis of the continuous secondary endpoints.
The results for the primary analysis of the primary endpoint are summarized as the number of patients with available values, least‐square means estimates for change from baseline and associated two‐sided 95% confidence intervals and P values corresponding to a two‐sided test of no difference versus baseline unless otherwise specified. Analyses of the secondary endpoints were performed in the same manner as the primary analysis of the primary endpoint, using an ANCOVA model in the EAS and FAS, respectively.
3. RESULTS
3.1. Patient disposition and baseline characteristics
Of 471 patients providing informed consent, 16 did not meet the eligibility criteria and three did not initiate treatment; the FAS consisted of 452 patients. A total of 376 (83.2%) patients completed the study, attending the EOS visit between weeks 28 to 38. The EAS consisted of 356 patients who completed the study and remained on semaglutide (Figure 1). The mean study duration was 34.1 weeks (EAS). In the FAS, overall, the mean baseline HbA1c was 8.1% and 86 (19.0%) patients had HbA1c less than 7%; diabetes duration was 14.7 years; BW was 99.2 kg; waist circumference was 115.4 cm. More than 80% of patients were prescribed a starting dose of 0.25 mg semaglutide per week in all subgroups, except the GLP‐1RA–experienced subgroup, in which 47.2% patients started on 0.25 mg, 43.1% started on 0.5 mg and 9.8% went directly to the 1.0 mg dose (Table 1). The baseline characteristics for the EAS population are shown in Table S1.
FIGURE 1.
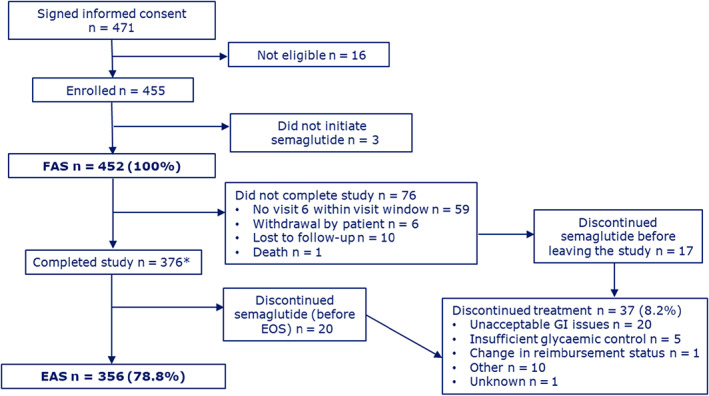
Patient disposition. *Patients who initiated semaglutide treatment and attended the end‐of‐study visit. EAS, effective analysis set; EOS, end of study; FAS, full analysis set; GI, gastrointestinal
TABLE 1.
Demographics and baseline characteristics of patients by previous medication subgroup (full analysis set)
| Previous medication subgroup | Total | ||||
|---|---|---|---|---|---|
| OADs only | GLP‐1RA experienced | Insulin ± OADs without GLP‐1RA | No antihyperglycaemic medication | ||
| N | 163 | 123 | 156 | 10 | 452 |
| Age, y | 58.8 (9.0) | 58.8 (9.0) | 61.3 (10.9) | 62.7 (10.7) | 59.8 (9.8) |
| Female, n (%) | 62 (38.0) | 53 (43.1) | 58 (37.2) | 5 (50.0) | 178 (39.4) |
| Diabetes duration, y | 11.4 (7.3) | 15.4 (7.4) | 17.5 (8.6) | 14.1 (13.3) | 14.7 (8.3) |
| Baseline HbA1c, % | 8.1 (1.4) | 7.9 (1.2) | 8.1 (1.3) | 7.3 (1.6) | 8.1 (1.3) |
| Starting dose of semaglutide, n (%) | |||||
| N | 163 | 123 | 156 | 10 | 452 |
| <0.25 mg | 1 (0.6) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 2 (0.4) |
| 0.25 mg | 136 (83.4) | 58 (47.2) | 129 (82.7) | 8 (80.0) | 331 (73.2) |
| 0.5 mg | 20 (12.3) | 53 (43.1) | 19 (12.2) | 1 (10.0) | 93 (20.6) |
| 1.0 mg | 6 (3.7) | 12 (9.8) | 7 (4.5) | 1 (10.0) | 26 (5.8) |
| HbA1c <7%, n (%) | 25 (15.3) | 26 (21.1) | 29 (18.6) | 6 (60.0) | 86 (19.0) |
| Fasting plasma glucose | |||||
| N | 80 | 81 | 63 | 5 | 229 |
| mmol/L | 9.4 (2.7) | 8.9 (3.6) | 8.8 (3.5) | 9.4 (3.6) | 9.1 (3.3) |
| Body weight | |||||
| N | 163 | 122 | 156 | 9 | 450 |
| kg | 96.7 (21.1) | 102.3 (20.4) | 99.2 (17.9) | 99.2 (16.5) | 99.2 (19.8) |
| Body mass index | |||||
| N | 162 | 122 | 154 | 9 | 447 |
| kg/m2 | 33.9 (6.3) | 35.8 (6.9) | 34.5 (6.0) | 34.9 (3.5) | 34.6 (6.4) |
| Waist circumference | |||||
| N | 86 | 53 | 63 | 4 | 206 |
| cm | 114.8 (13.9) | 113.8 (14.7) | 117.8 (15.9) | 110.1 (10.6) | 115.4 (14.7) |
| eGFR | |||||
| N | 114 | 100 | 115 | 8 | 337 |
| mL/min/1.73 m2 | 89.0 (18.4) | 81.6 (21.9) | 80.0 (21.6) | 64.4 (30.0) | 83.1 (21.4) |
| n (%) | |||||
| <30 mL/min/1.73 m2 | 1 (0.9) | 2 (2.0) | 0 (0.0) | 1 (12.5) | 4 (1.2) |
| 30‐59 mL/min/1.73 m2 | 7 (6.1) | 18 (18.0) | 23 (20.0) | 3 (37.5) | 51 (15.1) |
| 60‐89 mL/min/1.73 m2 | 42 (36.8) | 37 (37.0) | 49 (42.6) | 2 (25.0) | 130 (38.6) |
| ≥90 mL/min/1.73 m2 | 64 (56.1) | 43 (43.0) | 43 (37.4) | 2 (25.0) | 152 (45.1) |
| Reasons to initiate semaglutide, n (%) a | |||||
| N | 162 | 123 | 156 | 10 | 451 |
| Improve glycaemic control | 147 (90.2) | 108 (87.8) | 141 (90.4) | 7 (70.0) | 403 (89.2) |
| Weight reduction | 128 (78.5) | 99 (80.5) | 127 (81.4) | 8 (80.0) | 362 (80.1) |
| Issues with hypoglycaemia | 10 (6.1) | 4 (3.3) | 18 (11.5) | 2 (20.0) | 34 (7.5) |
| Address cardiovascular risk factors | 62 (38.0) | 49 (39.8) | 50 (32.1) | 4 (40.0) | 165 (36.5) |
| Simplify current treatment regimen | 65 (39.9) | 49 (39.8) | 63 (40.4) | 5 (50.0) | 182 (40.3) |
| Convenience | 52 (31.9) | 52 (42.3) | 59 (37.8) | 3 (30.0) | 166 (36.7) |
| Other | 1 (0.6) | 9 (7.3) | 5 (3.2) | 1 (10.0) | 16 (3.5) |
Note: Not all measurements were available for all patients; these are indicated by ‘N’, which is the number of patients for whom that particular measurement is available. Data are mean (SD) unless otherwise specified.
Abbreviations: eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; OAD, oral antihyperglycaemic drug; SD, standard deviation.
Some patients had more than one reason to initiate treatment.
In the EAS, overall, 260 (73.0%) patients were taking metformin at baseline, 222 (62.4%) were on an SGLT‐2i and 176 (49.4%) were on basal insulin (Table S2). In the FAS, overall, 345 (76.3%) patients had dyslipidaemia and 323 (71.5%) had hypertension at baseline (Table S3).
3.2. HbA1c
In the EAS, 35 of 356 patients had missing HbA1c values at EOS; therefore, 321 patients were included in the analysis. Overall, the estimated mean change from baseline in HbA1c was −0.9%‐point (95% confidence interval [CI]: −0.97; −0.78; P < .0001). The estimated mean change was slightly higher in the ‘OADs only’ subgroup (−1.1%‐point) compared with the ‘GLP‐1RA experienced’ (−0.7%‐point) or ‘insulin ± OADs without GLP‐1RA’ (−0.8%‐point) subgroups. In‐study sensitivity analysis produced similar results (Figure 2A).
FIGURE 2.
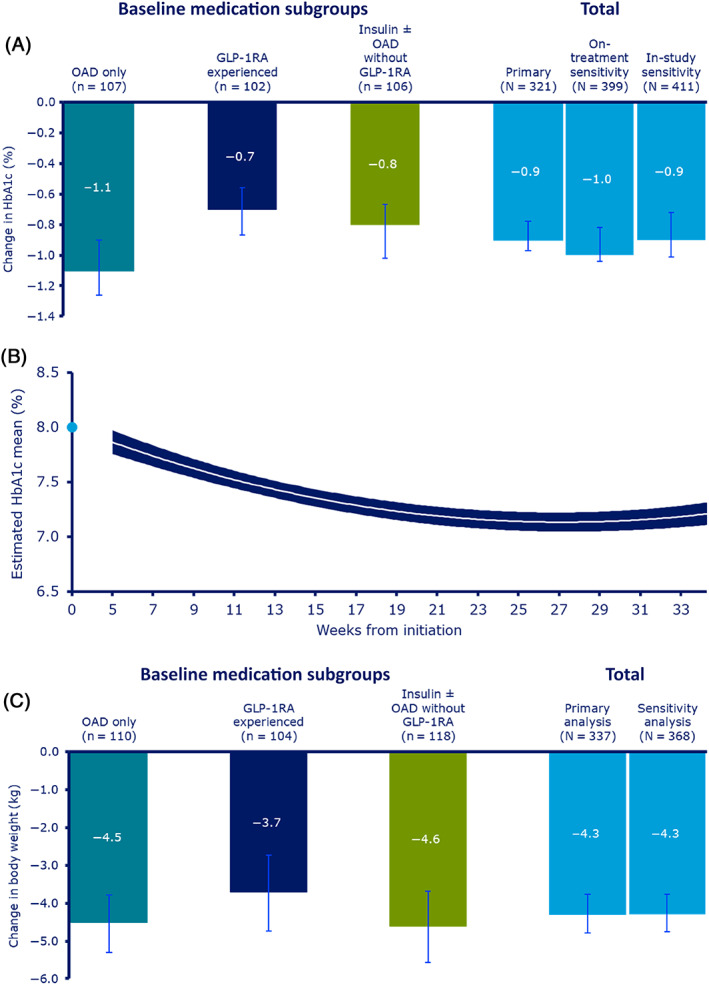
A, Change in HbA1c from baseline to end of study (EOS); B, mean HbA1c over time; and C, change in body weight from baseline to EOS. B, Based on on‐treatment observation period (full analysis set). Change in HbA1c is analysed using baseline HbA1c, type 2 diabetes (T2D) duration, age, body mass index (BMI), time and time‐squared as covariate and preinitiation use of glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) (yes/no), preinitiation use of dipeptidyl peptidase‐4 inhibitor (DPP‐4i) (yes/no), preinitiation use of insulin (yes/no), number of oral antihyperglycaemic drugs (OADs) used preinitiation (0‐1/≥2) and sex as fixed factors with random intercept and random coefficient for time (slope). The outer lines of the band represent 95% confidence interval. The light blue dot represents observed mean baseline HbA1c. A and C, All P < .0001. P value is reported for no mean change in response from baseline to EOS. Error bars indicate 95% confidence intervals. Response at EOS and change in response from baseline to EOS are analysed using baseline T2D duration, age and BMI as covariates, and preinitiation use of GLP‐1RA (yes/no), preinitiation use of DPP‐4i (yes/no), preinitiation use of insulin (yes/no), number of OADs used preinitiation (0‐1/≥2) and sex as fixed factors. A mixed model for repeated measurements was used for the sensitivity analyses of the primary endpoint in the respective observation periods and based on the full analysis set population. An analysis of covariance model was used for the sensitivity analysis of the continuous secondary endpoints
Sensitivity analysis on the FAS, including all HbA1c measurements in the on‐treatment observation period, supported the conclusions from the primary analysis (Figure 2B).
In the EAS, overall, 46.9% of patients achieved an HbA1c level of less than 7% at EOS (Figure 3A); of those with a baseline HbA1c of 7.0% or higher (n = 267), 102 (38.2%) achieved HbA1c of less than 7% at EOS. In the EAS, mean HbA1c change from baseline with semaglutide was consistent across all baseline HbA1c levels. Mean HbA1c change from baseline was higher in patients who were uptitrated to ~1.0 mg dose (−1.0%‐point; baseline 8.0%) versus those who stayed at ~0.5 mg dose (−0.7%‐point; baseline 8.1%) at EOS (Table S4).
FIGURE 3.
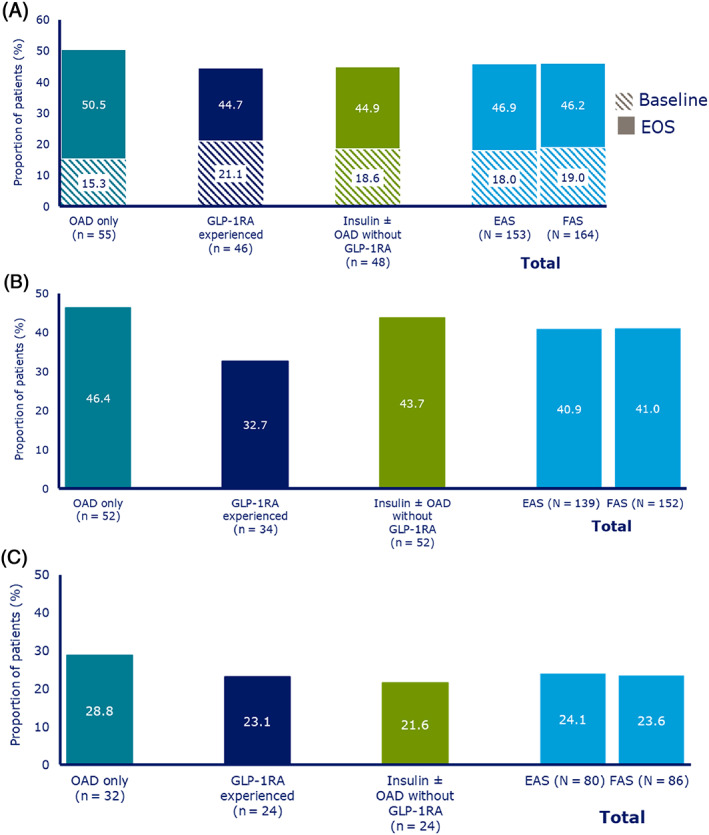
Proportions of patients achieving: A, HbA1c <7.0%; B, Weight loss of ≥5%; and C, Composite endpoint of HbA1c reduction of ≥1% and weight loss of ≥3%. Numbers below bars indicate the number of patients achieving targets. EAS, effective analysis set; EOS, end of study; FAS, full analysis set; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; OAD, oral antihyperglycaemic drug
3.3. BW and waist circumference
Overall, in the EAS, the estimated mean change from baseline in BW was −4.3 kg (95% CI: −4.79; −3.76; P < .0001) and was statistically significant in all three subgroups (P < .0001; Figure 2C). For patients taking semaglutide at doses of 0.5 mg or less at EOS, the estimated mean change from baseline in BW was −3.8 kg (95% CI: −4.53; −3.00; P < .0001); for doses of more than 0.5 mg, estimated mean BW change was −4.6 kg (95% CI: −5.29; −3.91; P < .0001). Individual patient‐level data for baseline change in HbA1c and BW are shown in Figure S1.
Overall, 40.9% patients in the EAS and 41.0% patients in the FAS achieved 5% or higher weight loss (Figure 3B).
In the EAS, the estimated mean change from baseline in waist circumference (n = 206) was −4.7 cm (95% CI: −5.70; −3.77; P < .0001). This was similar in the ‘OADs only’ subgroup (−5.2 cm [95% CI: −6.23; −4.18]; P < .0001) and the ‘insulin ± OADs without GLP‐1RA’ subgroup (−5.0 cm [95% CI: −7.18; −2.76]; P < .0001); however, a smaller change was observed in the ‘GLP‐1RA experienced’ subgroup (−3.8 cm [95% CI: −6.16; −1.42]; P = .0028).
3.4. Composite endpoint
Overall, 24.1% of patients in the EAS and 23.6% in the FAS achieved both an HbA1c reduction of 1% or higher and a weight loss of 3% or higher (Figure 3C). The more stringent endpoint (assessed post hoc) of an HbA1c reduction of 1% or higher and a weight loss of 5% or higher was achieved by 18.5% of the EAS.
3.5. Patient‐reported outcomes
Overall, in the EAS, the observed mean DTSQs score at baseline was 27.7, and the estimated mean score at EOS was 31.4, resulting in an estimated change of 3.7 (95% CI: 3.21; 4.29; P < .0001; Figure S2A).
In the EAS, the estimated change from baseline to EOS in SF‐36® v2 PCS score and SF‐36® v2 MCS score was 1.1 (95% CI: 0.35; 1.76; P = .0035) and 0.5 (95% CI: −0.35; 1.41; P = .2363), respectively (Figure S2B,C).
In the EAS, the mean MMAS‐8 score increased from 6.5 at baseline to 6.8 at EOS, with the proportion of patients with high adherence (score = 8) increasing from 31.5% to 42.2% (Table 2).
TABLE 2.
MMAS‐8 scores at baseline and EOS (effective analysis set)
| MMAS‐8 score | Baseline (n = 355) | EOS (n = 341) |
|---|---|---|
| Estimated mean (SD) | 6.5 (1.46) | 6.8 (1.47) |
| Level of adherence, n (%) | ||
| High adherence (8) | 112 (31.5) | 144 (42.2) |
| Medium adherence (6 to <8) | 132 (37.2) | 126 (37.0) |
| Low adherence (<6) | 111 (31.3) | 71 (20.8) |
Note: MMAS‐8 is an eight‐item, structured, self‐reported medication adherence measure with scores ranging from 0 (no adherence) to 8 (high adherence). 19 , 20 , 21 Use of the ©MMAS is protected by US Copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, MMAS Research LLC 14725 NE 20th St. Bellevue WA 98007 or from dmorisky@gmail.com.
Abbreviations: EOS, end of study; MMAS‐8, 8‐item Morisky Medication Adherence Scale; SD, standard deviation.
In total, 81.1% of patients in the EAS achieved ‘clinical success’ in relation to the reason for semaglutide initiation, as assessed by the physician. The results were comparable across the previous medication subgroups.
3.6. Semaglutide dose at EOS
At EOS, 12.6%, 25.6% and 57.3% of patients were taking 0.25, 0.5 and 1.0 mg doses of semaglutide, respectively, with a mean dose of 0.76 ± 0.31 mg. The remaining 4.5% of patients were on intermediate doses made possible via click‐counting; such dosing is off‐label and is not encouraged by the sponsor (Table S5).
In the FAS, most patients were titrated from a less than 0.5 mg dose to higher doses during the first 2 months of the study (Figure S3).
3.7. Insulin dose and antihyperglycaemic medication use
The mean bolus insulin dose for insulin‐using patients in the EAS was 41.7 (35.4) IU/day at baseline (n = 80) and 42.4 (39.2) IU/day at EOS (n = 85); the mean basal insulin dose was 57.7 (42.8) IU/day at baseline (n = 176) and 56.0 (40.9) IU/day at EOS (n = 191).
The mean number of antihyperglycaemic drugs used by patients in the EAS was 2.9 at baseline and 3.6 at EOS; the number at EOS also included OW semaglutide. The difference between EOS and baseline was comparable in the ‘OADs only’ subgroup (3.1‐2.2 = 0.9) and ‘insulin ± OADs without GLP‐1RA’ subgroup (4.0‐3.1 = 0.9) and smaller in the ‘GLP‐1RA experienced’ subgroup (3.9‐3.7 = 0.2).
3.8. Safety
There were no pregnancies reported during the study and thus no AEs in foetuses or newborns. A total of 83 AEs were reported for 53 patients during the study. This included nine serious AEs (SAEs) in eight patients. Four SAEs (two severe events [hypoglycaemic seizure and hypoglycaemia] and two moderate events [diabetic ketoacidosis and dizziness leading to hospitalization]) were considered as possibly related to OW semaglutide. One patient with an SAE died during the study as a result of sepsis, which was judged as not related to semaglutide. A total of 15 AEs in 10 patients led to treatment discontinuation. The most frequent AEs were gastrointestinal. In the EAS, severe or documented hypoglycaemic episodes were reported by 10.7% of patients. One hypoglycaemic episode was severe, with the remaining categorized as ‘documented’ (Table S6).
4. DISCUSSION
SURE Canada, part of the SURE programme, was one of the first prospective RW studies to investigate the use of OW semaglutide in a diverse population of adults with T2D in routine clinical practice.
Patients treated with OW semaglutide during this study experienced clinically significant reductions in HbA1c, BW and waist circumference and an improvement in PROs. The mean HbA1c reduction observed in this study was lower than that seen in the SUSTAIN phase 3 trials; there may be several reasons for this. The semaglutide maintenance dose was not maximized at EOS, as would generally have been carried out in a clinical trial. For the 55% of participants who reached the maximum semaglutide dose of 1.0 mg per week, the mean HbA1c reduction was 1.1%, while those receiving 0.5 mg had a mean HbA1c reduction of 0.85%. At baseline, 19% of patients were already at target HbA1c (<7%); larger reductions would not be expected in these patients. This contrasts with the SUSTAIN trials, where no patients had baseline HbA1c of less than 7%. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 For the 27% of patients who were already on a GLP‐1RA, switching to another GLP‐1RA may not have resulted in large reductions in HbA1c. This is consistent with an RW study of dulaglutide showing that GLP‐1RA–naïve patients had greater reductions in HbA1c versus GLP‐1RA–experienced patients, 22 and again contrasts with the SUSTAIN programme, which did not include any GLP‐1RA–experienced patients. The EXPERT study showed that a switch to semaglutide from another GLP‐1RA was associated with significant reductions in HbA1c. 23 Additionally, 19% of patients were taking a DPP‐4 inhibitor at initiation, decreasing to 5.6% at EOS; this shows that the majority of patients stopped DPP‐4 inhibition having initiated semaglutide as recommended in the protocol. The greater presence of co‐morbidities in the population in SURE Canada versus RCTs 16 may have affected achieving and maintaining glycaemic control. 24 Patients enrolled in RCTs usually have access to additional healthcare provider support, which is not as extensive for patients in routine clinical practice, and the ‘trial effect’ may be greater in RCTs than in RW studies. 25 Although overall adherence improved during this study, poorer adherence to treatment is more common in routine clinical practice than in clinical trials, and this has been suggested to be one reason for substantially lower HbA1c reductions in RW evidence studies than in RCTs. 26 In RCTs, medications are generally added on to the current ones rather than being switched; switching, rather than adding, may have impacted the magnitude of reductions in the SURE Canada study. The patients in SURE Canada had advanced disease, indicated by a long mean diabetes duration (~15 years), with almost 50% using basal insulin and the majority using more than three antihyperglycaemic drugs. Therefore, these patients may be considered hard‐to‐treat, and the data should be compared with studies with a similar population.
The HbA1c and BW reductions observed in the SURE Canada study are similar to those observed in the SPARE retrospective observational study of data from the Canadian LMC Diabetes Registry, which investigated outcomes in patients using OW semaglutide for 1 year. In a cohort of 937 adult patients with T2D, HbA1c was reduced from baseline by 1.0% and BW by 3.9 kg (both P < .0001) at 4.9 months of follow‐up. 25 The dose‐dependent effects on BW observed in the current study should be interpreted with caution, as patients recruited into the study were not randomized to different doses, and observed treatment effects may have affected decisions on dose escalation.
The treatment discontinuation rate in the SURE Canada study (8%) was lower than that observed in the SUSTAIN clinical trials (11%), 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 suggesting that OW semaglutide is well tolerated in routine clinical practice. This might be attributed to the different trial durations and/or to the slower uptitration of semaglutide observed in the SURE study compared with RCTs, which might also contribute to the lower HbA1c reduction. The discontinuation rate is also lower than was observed in the SPARE study (17%). 25 This may be because SURE had a shorter duration than SPARE (1 year) or because SURE was based on primary data collection, whereas SPARE was based on secondary data. Patients participating in SURE could be more compliant/adherent, knowing that they were being monitored. The SURE study also had some RCT features not present in SPARE, for example, availability of a research assistant and more discussions between patients and healthcare providers, which may have contributed to the lower discontinuation rate.
The SUSTAIN RCTs did not include data on patients switching to OW semaglutide from another GLP‐1RA. In SURE Canada, patients switching to OW semaglutide from another GLP‐1RA had additional reductions in HbA1c (0.7%) and BW (3.7 kg), despite previous treatment with an agent from the same class. There may be several reasons for people to switch, including not meeting HbA1c targets, need for additional weight loss, progressing to an increased CV risk status and poor adherence. 27 This has been observed in other RW studies of people with T2D switching to OW semaglutide from another GLP‐1RA. A retrospective study involving 40 patients in an endocrine practice in Canada switching from liraglutide to semaglutide observed an HbA1c reduction of 0.8% and a BW reduction of 4.6 kg after the switch. 28 The REALISE‐DM study, also a retrospective chart review of 164 adult patients with T2D in an endocrine practice in Canada, showed that switching to OW semaglutide from dulaglutide or liraglutide resulted in further significant reductions in HbA1c of 0.7% and in BW of 1.58 kg at 6 months. 29
The reasons for the dose not being maximized in SURE Canada may have been because of patients reaching their target HbA1c, AEs or clinical inertia. Patients who were uptitrated to the maximum semaglutide dose had a 0.3% greater reduction in HbA1c compared with those who were not uptitrated. The rate and timing of dose titration also varied between the different study sites and physicians. Titration outside of the standard dosages (0.25, 0.5 and 1.0 mg) was not uncommon in the SURE study because of the option of fine titration by ‘click counting’ and investigator experience, suggesting that this may reduce the risk of GI AEs. Interestingly, despite the need for an injection, adherence increased with semaglutide compared with the previous medication regimen. Contributing factors may include the OW administration, the small needle used with the pen‐injector, or efficacy or tolerance.
In the SURE Canada study, the mean number of antihyperglycaemic medications used by patients was 2.9 at baseline and 3.6 at EOS. As the number at EOS included OW semaglutide, this indicates that the number of concomitant antihyperglycaemic drugs remained similar. Mean insulin dose and use of OADs did not decrease after adding or switching to semaglutide; this may be attributable in part to the comparatively short timeline of the study. Additionally, it may be a result of poor adherence to antihyperglycaemic drugs and relatively low dose maximalization of semaglutide compared with RCTs, which have a fixed dose escalation scheme. These may indicate clinical inertia in RW clinical practice that could also have contributed. Safety observations in SURE Canada were consistent with those seen in the SUSTAIN RCTs.
Overall, the SURE data had some unique features that are not seen in RCTs: included patients were taking different antihyperglycaemic medications and had a wide range of baseline characteristics, including those with a baseline HbA1c of less than 7%; the population included patients who switched from another GLP‐1RA to semaglutide; patients had a longer diabetes duration compared with RCTs in general. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15
A strength of SURE Canada is the close‐to‐RW monitoring of a T2D population in routine clinical practice that was more diverse than those typically enrolled in RCTs. Several limitations are attributable to the observational nature of this study: confounding factors could not be ruled out; and data were collected as part of routine clinical practice rather than through mandatory assessments at prespecified time points, which may have affected the robustness and completeness of data. Also, the study did not include a comparator arm, meaning that the proportion of the observed changes because of semaglutide, spontaneous variation or study effect could not be evaluated. The primary analysis was based on patients continuing treatment to EOS, which could lead to overestimated reductions in HbA1c and BW. Sensitivity analyses based on FAS, however, produced estimates similar to the primary analyses.
In conclusion, in a routine clinical practice population in Canada, patients with T2D treated with OW semaglutide experienced clinically significant improvements in glycaemic control, BW and PROs. Patients switching from another GLP‐1RA to OW semaglutide had reductions in HbA1c and BW from baseline, despite previous treatment with an agent from the same class. These results may support the use of OW semaglutide in routine clinical practice as an aspect of the overall management of T2D.
CONFLICT OF INTEREST
JFY reports receiving grants from Novo Nordisk during the conduct of the study; and grants and personal fees from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Merck, Janssen and AstraZeneca, all outside the submitted work. AMC, KG and AK‐A are employees of Novo Nordisk and own stock in the company. SH reports receiving grants from Novo Nordisk during the conduct of the study; grants, personal and other fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen and Sanofi; grants and other fees from Applied Therapeutics; personal and other fees from Bayer Inc. and Novo Nordisk; grants from Health Canada/First Nations and Inuit Health Branch, Canadian Institutes for Health Research, Juvenile Diabetes Research Foundation and The Lawson Foundation; and personal fees from HLS Therapeutics, all outside the submitted work. RR‐L reports receiving grants from Diabetes Canada, Cystic Fibrosis Canada, CIHR, NIH, JDRF, Prometic and SFD; grants and personal fees from AstraZeneca, Janssen, Merck, Novo Nordisk, Sanofi Avantis and Vertex Pharmaceutical; grants, personal fees and non‐financial support from Eli Lilly; personal fees from Abbott, Amgen, Boehringer Ingelheim, CPD Network, Dexcom, HSL therapeutics, Insulet, Neomed and Roche; personal fees and non‐financial support from Medtronic; and non‐financial support from Animas, all outside the submitted work. LR reports receiving consulting fees and serving on the advisory board for Novo Nordisk and Bioscript Solutions; speakers bureau and honoraria from Bausch and Novo Nordisk; and grants and research support from Novo Nordisk, all outside the submitted work. VW reports receiving personal fees from Novo Nordisk outside the submitted work. JL reports receiving personal fees, grants and non‐financial support from Novo Nordisk, outside the submitted work.
AUTHOR CONTRIBUTIONS
All authors contributed to the analysis and interpretation of data, the writing and critical revision of the manuscript at all stages of development. All authors approved the final version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14468.
Supporting information
Appendix S1. Supplementary Information
ACKNOWLEDGEMENTS
This study was funded by Novo Nordisk A/S. Investigating physicians were reimbursed for time spent on documentation and to support data collections and the running of the study. We thank all the participants, investigators and trial‐site staff, as well as Umut Erhan, Michael Lyng Wolden, Andreas Ross Kirk and Trine Stougaard from Novo Nordisk for their review and input to the manuscript, and Priya Talluri (AXON Communications) for medical writing and editorial assistance (funded by Novo Nordisk A/S). Use of the ©MMAS is protected by US Copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, MMAS Research LLC 14725 NE 20th St. Bellevue WA 98007 or from dmorisky@gmail.com.
Yale J‐F, Catarig A‐M, Grau K, et al. Use of once‐weekly semaglutide in patients with type 2 diabetes in routine clinical practice: Results from the SURE Canada multicentre, prospective, observational study. Diabetes Obes Metab. 2021;23(10):2269‐2278. 10.1111/dom.14468
Funding information Novo Nordisk
DATA AVAILABILITY STATEMENT
The data sets analysed during the current study are available on reasonable request.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. [Google Scholar]
- 2. Public Health Agency of Canada . Diabetes in Canada: Highlights from the Canadian Chronic Disease Surveillance System . http://publications.gc.ca/collections/collection_2018/aspc-phac/HP35-94-2017-eng.pdf. Accessed 21 December 2020.
- 3. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes. 2020;44(7):575‐591. [DOI] [PubMed] [Google Scholar]
- 5. Novo Nordisk Canada Inc . Health Canada approves RYBELSUS® (semaglutide tablets) the first and only GLP‐1 analogue in a pill for the treatment of adults with type 2 diabetes. https://www.newswire.ca/news-releases/health-canada-approves-rybelsus-r-semaglutide-tablets-the-first-and-only-glp-1-analogue-in-a-pill-for-the-treatment-of-adults-with-type-2-diabetes-814386455.html. Accessed 21 December 2020.
- 6. Novo Nordisk Canada Inc . Ozempic® approved in Canada for the treatment of adults with type 2 diabetes. https://www.newswire.ca/news-releases/ozempic-approved-in-canada-for-the-treatment-of-adults-with-type-2-diabetes-668432133.html. Accessed 21 December 2020.
- 7. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 8. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 9. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 10. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 11. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 13. Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once‐weekly semaglutide versus daily canagliflozin as add‐on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double‐blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834‐844. [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add‐on to SGLT‐2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356‐367. [DOI] [PubMed] [Google Scholar]
- 15. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once‐weekly semaglutide 1.0 mg vs once‐daily liraglutide 1.2 mg as add‐on to 1‐3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100‐109. [DOI] [PubMed] [Google Scholar]
- 16. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real‐world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Medical Association . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 18. International Society for Pharmacoepidemiology . Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf. 2008;17(2):200‐208. [DOI] [PubMed] [Google Scholar]
- 19. Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348‐354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Krousel‐Wood MA, Islam T, Webber LS, Re RS, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009;15(1):59‐66. [PMC free article] [PubMed] [Google Scholar]
- 21. Morisky DE, DiMatteo MR. Improving the measurement of self‐reported medication nonadherence: Final response. J Clin Epidemi 2011;64:258‐263. PMID: 21144706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mody R, Grabner M, Yu M, et al. Real‐world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34(6):995‐1003. [DOI] [PubMed] [Google Scholar]
- 23. Lingvay I, Kirk A, Lophaven S, Wolden ML, Shubrook J. GLP‐1–experienced patients switching to once‐weekly semaglutide in a real‐world setting (EXPERT study). Poster 954‐P presented at the 81st American Diabetes Association Scientific Sessions, virtual event; 25‐29 June 2020.
- 24. Luijks H, Biermans M, Bor H, et al. The effect of comorbidity on glycemic control and systolic blood pressure in type 2 diabetes: a cohort study with 5 year follow‐up in primary care. PLoS One. 2015;10(10):e0138662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown RE, Bech PG, Aronson R. Semaglutide once weekly in people with type 2 diabetes: real‐world analysis of the Canadian LMC diabetes registry (SPARE study). Diabetes Obes Metab. 2020;22(11):2013‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425‐1432. [DOI] [PubMed] [Google Scholar]
- 27. Jain AB, Ali A, Gorgojo Martínez JJ, et al. Switching between GLP‐1 receptor agonists in clinical practice: expert consensus and practical guidance. Int J Clin Pract. 2021;75(2):e13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goncalves E, Bell DS. Efficacy of semaglutide versus liraglutide in clinical practice. Diabetes Metab. 2020;46(6):515‐517. [DOI] [PubMed] [Google Scholar]
- 29. Jain AB, Kanters S, Khurana R, Kissock J, Severin N, Stafford S. Real world efficacy analysis of switch from liraglutide or dulaglutide to semaglutide in type 2 diabetes mellitus: REALiSe‐DM study. Poster OP 0073 presented at the International Diabetes Federation Congress, Busan, Korea; 2‐7 December 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Information
Data Availability Statement
The data sets analysed during the current study are available on reasonable request.


