Abstract
Background
Barrier membranes and bone substitute are major tools of guided tissue regeneration (GTR) after periodontal disease. Integrity of the periodontal ligament plays a key role in periodontal health, but its functionality fails to be fully re‐established by GTR after disease or trauma. Microtissue models suggest an in vivo‐like model to develop novel GTR approaches due to its three‐dimensionality. This study aims to assess the effects of collagen membranes and bone substitute on cell viability, adhesion and gene expression of regenerative and inflammatory biomarkers by periodontal ligament cell (PDLC) microtissues.
Methods
Human PDLC microtissues and monolayers were cultured on collagen membranes or bone substitute. After 24 hours incubation, metabolic activity, focal adhesion, mRNA and protein production of collagen‐type‐I (COL1A1), periostin (POSTN), vascular endothelial growth factor (VEGF), angiogenin (ANG), interleukin (IL)6 and IL8 were measured by resazurin‐based toxicity assay, focal adhesion staining, quantitative polymerase chain reaction and enzyme‐linked immunosorbent assay, respectively.
Results
PDLC microtissues and monolayers were viable on collagen membranes and bone substitute, but microtissues were less metabolically active. Dominant staining of actin filaments was found in PDLC microtissues on collagen membranes. COL1A1, POSTN, VEGF, ANG and IL6 were modulated in PDLC microtissues on bone substitute, while there were no significant changes on collagen membranes. PDLC monolayers showed a different character of gene expression changes.
Conclusions
PDLC microtissues and monolayers react diversely to collagen membranes and bone substitute. Further descriptive and mechanistic tests will be required to clarify the potential of PDLC microtissues as in vivo‐like model for GTR.
Keywords: biocompatible materials, bone substitutes, cell culture techniques, dentistry, guided tissue regeneration, periodontal ligament
1. INTRODUCTION
Periodontal soft tissue regeneration is an essential prerequisite to successful guided tissue regeneration (GTR) for the treatment of periodontal disease. Infrabony defects, furcation defects and recessions are the main clinical indications for periodontal GTR, which are primarily caused by periodontal inflammation. 1 After successful removal of the cause of inflammation and hygienic measures, the destroyed tissue integrity of the periodontium has to be re‐established. To recover original tissue functions, periodontal tissue needs to regenerate, which is attempted by surgical GTR strategies. 2 In a standard GTR approach, a barrier membrane is used to isolate the defect site and guide the regenerating tissue, 3 while bone substitute materials are used as fillers for bone defects and serve as scaffolds for the regenerating periodontium. 4
Cells of the periodontal ligament play a key role in maintaining physiological features of the periodontium as they communicate mechanical and biological influences between tooth and alveolar bone 5 and provide regenerative capacity. 6 Therefore, complete regeneration comprises the reconstruction of the anatomical architecture and particularly requires the re‐establishment of functional features. After trauma or disease, wound healing of oral soft tissues, such as the periodontal ligament, mainly leads to tissue repair rather than regeneration. Tissue repair results in fibrotic scar tissue, which provides tissue stability but fails to exert the original tissue function. 7 Every implanted material will be recognized as a foreign material to which oral soft tissue subsequently reacts with cellular inflammation, forming a non‐functional fibrous capsule around e.g. inserted barrier membranes. 8 Besides failing to provide specialized tissue functions, the tissue will most likely undergo soft tissue recession, demanding a repetition of the invasive GTR treatment.
To avoid foreign body reactions to implanted materials, such as barrier membranes, several approaches are in evaluation to improve soft tissue integration of GTR biomaterials. 9 , 10 These biomaterials can be functionalized by coatings with different organic and inorganic compounds to improve biocompatibility and inflammatory reactions. 11 To test the potential of such modifications to biomaterials, a test model is required that safely predicts its effects in a patient. Research and development of optimized materials for GTR traditionally starts in vitro and, if successful, continues with in vivo studies and clinical trials. As the periodontal ligament is a very thin structure, it cannot be accessed in patients in vivo for technical and ethical reasons. On the other hand, traditional in vitro cell cultures represent an artificial situation that does not mimic the patient situation in all physiological aspects and particularly differs in proliferative and molecular responses to external stimuli. 12 Thus, the ideal test model for GTR biomaterials should replace or reduce animal studies and clinical trials by a three‐dimensional (3D) in vitro model with a similar morphological and functional character as the in vivo periodontal ligament in a patient.
In the past years, microtissue models like spheroids were established and were also produced with cells deriving from periodontal ligament. 13 , 14 What we know is that these periodontal ligament cell (PDLC) spheroids display different regeneration‐associated functions 15 and characteristics 16 than PDLC monolayers. Obviously, spheroid microtissues better represent the 3D aspect of an in vivo periodontal ligament tissue than cell monolayers, but what remains unknown is in which functional aspects microtissues come closer to a patient's periodontal ligament tissue compared with monolayers. Ideally, an in vitro model for GTR research that mimics patient‐side periodontal ligament should not only be a 3D construct, but also be capable to deliver information on biocompatibility, cell adhesion and production of molecular biomarkers for regeneration, angiogenesis and inflammation. During periodontal tissue regeneration, collagen‐type‐I (COL1A1) 17 and periostin (POSTN) 18 play a crucial role, while vascular endothelial growth factor (VEGF) 19 and angiogenin (ANG) 20 are known for their importance in angiogenesis and finally interleukin (IL)6 and IL8 21 mediate the periodontal inflammatory process. Thus, it is of utter importance for material development in periodontal GTR to understand which in vitro model is the most suitable to test novel or optimized membranes and bone substitute materials in terms of regeneration. Data on GTR materials like collagen membranes 22 and bone substitute 23 that have been in clinical use for many years provide a basis to compare which of the in vitro models represents a clinically relevant situation for periodontal regeneration.
Therefore, this study aims to test if the effect of collagen membranes and bone substitute on cell viability, cell adhesion and gene expression of biomarkers associated with periodontal regeneration, angiogenesis and inflammation differs in PDLC microtissues and monolayers. We hypothesize that the cellular behavior in response to collagen membranes and bone substitute will differ in PDLC microtissues and monolayers.
2. MATERIALS AND METHODS
2.1. Ethics
This study was conducted in compliance with the Declaration of Helsinki regarding the ethical principles for medical research involving human subjects. Ethical approval for the isolation and work with primary human PDLC was obtained from the Ethics Committee of the Medical University of Vienna, Vienna, Austria (vote no. 1079/2019). Patients gave their informed and written consent before tooth donations.
2.2. Periodontal ligament cell isolation
After extraction of non‐inflamed and non‐carious wisdom teeth, periodontal ligament tissue was removed from the tooth with a scalpel. An explant culture of periodontal ligament tissue pieces was performed by placing tissue pieces of ≈1 mm in diameter onto a petri dish containing minimum essential α‐cell culture medium,* supplemented with 10% fetal calf serum (FCS)† as well as 100 U/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin (antibiotic‐antimycotic).‡ After reaching 80% to 90% confluence, adherent cells were trypsinized, expanded and afterwards stored in liquid nitrogen until experimental use. All cells that grew out from periodontal ligament tissue pieces were collected as a heterogeneous population of cells, termed human PDLC. PDLC from each donor were used separately in the experiments.
2.3. Cell culture conditions
Primary human PDLC from 6 different donors were thawed and cultured in minimum essential α‐cell culture medium§ with 10% FCS** as well as antibiotic‐antimycotic†† at 37°C, 5% CO2 and 95% atmospheric moisture. PDLC were passaged upon 80% to 90% confluence and cultured up to passage 7 for experiments. Medium was changed every 3 days.
2.4. Collagen membrane and bone substitute preparation
Collagen membranes‡‡ were cut into 1 × 1 cm pieces under sterile conditions and placed onto the well bottoms of 48‐well plates.§§ Bone substitute*** with a granule size of 0.25 to 1 mm and an approximate weight of 45 mg/well was filled into 48‐well plates††† to cover the whole bottom of a well, which has an area of ≈1 cm2.
2.5. Microtissue culture
For PDLC microtissue formation, hot liquid 1.5% agarose was poured into petri dishes for large spheroids.‡‡‡ After short cooling, solidified agarose molds were soaked with cell culture medium. We added 75 μL of 7,780,000 PDLC/mL into solid, medium‐soaked agarose molds and completely covered them with cell culture medium. After incubation for 24 hours, three spheroid‐shaped PDLC microtissues per donor were placed onto a plastic culture dish, a collagen membrane and bone substitute, respectively. This equals an approximate total cell number of 50,014 PDLC on each surface. Incubation of PDLC microtissues on the respective surfaces was performed for another 24 hours.
2.6. Monolayer culture
For traditional monolayer culture, we seeded 300 μL of 170,000 PDLC/mL into each well of a 48‐well plate§§§ onto the plastic culture dish, a collagen membrane and bone substitute, respectively. This corresponds to an approximate total cell number of 51,000 PDLC on each surface. Monolayers of PDLC were incubated on the respective surfaces for 24 hours.
2.7. TOX8 assay
For quantitative assessment of metabolic activity, a resazurin‐based toxicity assay (TOX8)**** was performed in PDLC microtissue and monolayer cultures on collagen membranes or bone substitute. PDLC microtissues and monolayers on plastic culture dishes served as control for normalization of results. Cell culture medium on cell‐free plastic culture dishes, collagen membranes or bone substitute was used as blank and subtracted from respective sample values. Resazurin was added to PDLC cultures at 10% and incubated for 6 hours. Conversion of resazurin to resorufin was measured photometrically at 540/34 nm excitation and 600/40 nm emission wavelength. The TOX8 assays were conducted with 6 biological replicates and 3 technical replicates.
2.8. Focal adhesion staining
For imaging of the cytoskeleton, the focal contacts and the nuclei of PDLC microtissues and monolayers on plastic culture dishes, collagen membranes or bone substitute, a focal adhesion staining kit†††† was used. Cell culture medium only on plastic culture dishes was used as blank control for auto‐fluorescence. Microtissues and monolayers of PDLC as well as blank controls were fixed in 4% paraformaldehyde for 15 minutes at room temperature. Afterwards, blank controls, PDLC microtissues and monolayers were permeabilized with 0.1% Triton X‐100 for 5 minutes at room temperature. After 30 minutes of blocking with 1% bovine serum albumin, the primary antibody (anti‐vinculin; 1:500) was added and incubated overnight at room temperature. Then, the secondary antibody (fluorescein isothiocyanate‐conjugated; 1:1000) and rhodamine‐conjugated phalloidin (1:1000) were incubated simultaneously with PDLC microtissues and monolayers as well as blank controls for 45 minutes. Finally, 4′, 6‐diamidino‐2‐phenylindole (1:1000) was added for 5 minutes. For fluorescence microscopy, PDLC microtissues, monolayers and blank controls were covered with phosphate‐buffered saline (PBS). Washing with 0.05% Tween‐20 in PBS was performed between each step.
Fluorescence microscopy was performed with a Revolve microscope. Images were taken in 100‐fold magnification using appropriate fluorescence filters. The focal adhesion staining was conducted with 6 biological replicates and 2 technical replicates.
2.9. RNA isolation, cDNA synthesis, and qPCR
For analysis of relative messenger ribonucleic acid (mRNA) expression of COL1A1, POSTN, VEGF, ANG, IL6 and IL8, ribonucleic acid (RNA) was isolated from PDLC microtissues and monolayers after 24 hours incubation on plastic culture dishes, collagen membranes or bone substitute. The protocol of the RNeasy Plus Mini Kit‡‡‡‡ was followed to isolate RNA from respective samples. Samples without cells were added as control samples. After isolation, RNA quality was assessed by the 260/280 ratio in a photometric measurement.
A high capacity complementary DNA (cDNA) reverse transcription kit§§§§ was used to transform total RNA into cDNA, following the protocol of the manufacturer.
Quantitative polymerase chain reaction (qPCR) was performed using the following TaqMan gene expression assays*****: COL1A1 (Hs00164004_m1), POSTN (Hs01566750_m1), VEGF (Hs00900055_m1), ANG (Hs04195574_sH), IL6 (Hs00985639_m1) and IL8 (Hs00174103_m1). GAPDH (Hs02758991_g1) was selected as reference gene. Results were generated based on ∆∆CT calculations. PDLC microtissue data from collagen membrane and bone substitute samples were normalized to PDLC microtissue data from plastic culture dish samples and PDLC monolayer data from collagen membrane and bone substitute samples to PDLC monolayer data from plastic culture dish samples. The qPCR data were obtained from 5 to 6 different donors and 2 to 3 technical replicates.
2.10. Supernatant collection and ELISA
For assessment of protein production of COL1A1, POSTN, VEGF, ANG, IL6 and IL8, enzyme‐linked immunosorbent assays (ELISA) were performed. Culture supernatant was collected after the 24 hours incubation period on plastic culture dishes, collagen membranes or bone substitute. ELISA kits for COL1A1,††††† POSTN,‡‡‡‡‡ VEGF,§§§§§ ANG,****** IL6,†††††† and IL8‡‡‡‡‡‡ were performed following the provided protocols of the respective manufacturer. The ELISA data were obtained from 5 to 6 different donors and 2 to 3 technical replicates.
2.11. Statistical analysis
Statistical analysis was performed with SPSS Statistics.§§§§§§ Quantitative data were expressed and displayed as mean + SD. Differences between groups were assessed by the Friedman test, followed by the Wilcoxon test for the pairwise comparison. Differences were considered significant at P <0.05.
3. RESULTS
3.1. Metabolic activity
Microtissues and monolayers of PDLC (Fig. 1) were both metabolically active on all surfaces. There was no significant difference in metabolic activity between PDLC cultures on collagen membranes and bone substitute or the different types of culture. On all surfaces, resorufin production was lower in PDLC microtissues than in PDLC monolayers.
FIGURE 1.
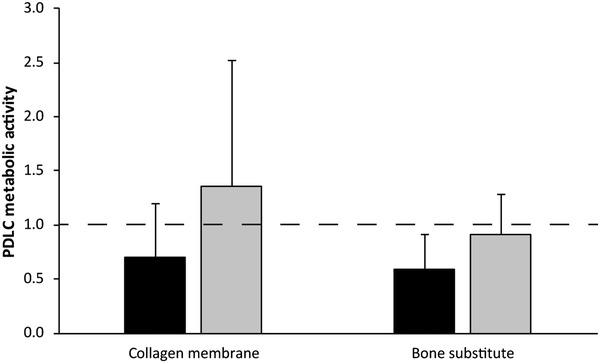
Metabolic activity of periodontal ligament cells on collagen membranes or bone substitute. The amount of metabolized resazurin by PDLC microtissues (black bars) and monolayers (gray bars) on plastic culture dishes, collagen membranes or bone substitute was measured photometrically after 24 hours. Data from 6 donors in 2 experimental repetitions are displayed as mean + SD, normalized to the control (culture dish; dashed line)
3.2. Focal adhesion
A positive staining of actin filaments, focal contacts and nuclei could be found in PDLC microtissues on plastic culture dishes (Fig. 2A). Additionally, outgrowth of single cells from the PDLC microtissue onto the plastic culture dish was observed. Outgrowing PDLC showed a characteristic elongated morphology. On collagen membranes, staining of actin filaments in PDLC microtissues was dominant and only a few nuclei were visible (Fig. 2A) while the staining was not positive for focal contacts. On bone substitute, PDLC microtissues were found between bone substitute particles and the plastic bottom of the culture dish. The focal adhesion staining was comparable with the staining on the plastic culture dish, but no cell outgrowth was observable (Fig. 2A).
FIGURE 2.

Focal adhesion molecules in PDLC on collagen membranes or bone substitute. Actin filaments (red), focal contacts (green) and nuclei (blue) of PDLC microtissues (A) and monolayers (B) on plastic culture dishes, collagen membranes or bone substitute were stained after 24 hours. Images show one representative donor and were taken at 100‐fold magnification. The white bar represents a length of 200 μm
PDLC monolayers showed positive staining for actin filaments, focal contacts and nuclei with a homogeneous distribution on the plastic culture dish surface with a characteristic elongated morphology (Fig. 2B). Similar to microtissues, PDLC monolayers on collagen membranes predominantly stained positive for actin filament (Fig. 2B). Monolayer PDLC on collagen membranes displayed a small and round morphology, which is rather typical for non‐adhering cells. Monolayers of PDLC seeded onto bone substitute settled between bone substitute particles and plastic culture dish surfaces displaying polygonal cell morphologies and showed positive staining for actin filaments, focal adhesions and nuclei (Fig. 2B). Qualitative findings of the focal adhesion staining were reproducible and are shown for all donors in the Supplementary Figure S1.
3.3. Production of molecular markers at mRNA levels
At mRNA levels (Table 1; see Supplementary Figure S2), production of COL1A1 and IL6 significantly increased in PDLC microtissues upon cultivation on bone substitute. Further, a significant difference was found in production of VEGF between PDLC microtissue culture on collagen membranes and bone substitute.
TABLE 1.
Production of molecular markers at mRNA levels in periodontal ligament cell microtissues and monolayers on collagen membranes or bone substitute
| PDLC microtissues | PDLC monolayers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Mean/SD | n | P value versus culture dish | P value collagen membrane versus bone substitute | Mean/SD | n | P value versus culture dish | P value collagen membrane versus bone substitute | P value microtissues versus monolayers | |
| Collagen membrane | COL1A1 | 1.60/1.16 | 6 | 0.600 | 0.463 | 0.39/0.47 | 6 | 0.046 | 0.753 | 0.345 |
| POSTN | 2.13/1.72 | 6 | 0.116 | 0.116 | 0.52/0.26 | 6 | 0.028 | 0.249 | 0.028 | |
| VEGF | 1.50/0.75 | 6 | 0.173 | 0.028 | 2.17/1.34 | 6 | 0.075 | 0.046 | 0.249 | |
| ANG | 0.68/0.86 | 6 | 0.173 | 0.345 | 1.81/1.50 | 6 | 0.173 | 0.028 | 0.028 | |
| IL6 | 2.21/2.52 | 5 | 0.893 | 0.345 | 12.92/17.16 | 6 | 0.116 | 0.116 | 0.345 | |
| IL8 | 2.31/2.47 | 5 | 0.893 | 0.225 | 81.80/53.37 | 5 | 0.043 | 0.080 | 0.068 | |
| Bone substitute | COL1A1 | 2.39/1.60 | 6 | 0.046 | 0.463 | 0.28/0.07 | 6 | 0.028 | 0.753 | 0.028 |
| POSTN | 0.99/0.83 | 6 | 0.600 | 0.116 | 0.84/0.72 | 6 | 0.173 | 0.249 | 0.917 | |
| VEGF | 0.94/0.81 | 6 | 0.249 | 0.028 | 5.16/2.31 | 6 | 0.028 | 0.046 | 0.028 | |
| ANG | 1.20/1.21 | 6 | 0.600 | 0.345 | 0.73/0.26 | 6 | 0.028 | 0.028 | 0.917 | |
| IL6 | 3.98/4.24 | 5 | 0.043 | 0.345 | 16.83/17.51 | 6 | 0.028 | 0.116 | 0.080 | |
| IL8 | 81.18/115.61 | 5 | 0.345 | 0.225 | 430.17/400.71 | 5 | 0.043 | 0.080 | 0.068 | |
Production of collagen type I (COL1A1), periostin (POSTN), vascular endothelial growth factor (VEGF), angiogenin (ANG), interleukin 6 (IL6) and interleukin 8 (IL8) mRNA levels were assessed by qPCR in PDLC microtissues and monolayers on plastic culture dishes, collagen membranes or bone substitute.
Data from five to six donors (n) and two to three technical replicates are displayed as mean + SD mRNA data are calculated by the ∆∆CT method, relative to the reference gene GAPDH.
Data from PDLC microtissues on collagen membranes or bone substitute were normalized to PDLC microtissues on plastic culture dishes and data from PDLC monolayers on collagen membranes or bone substitute to PDLC monolayers on plastic culture dishes (culture dish; equals a value of 1).
P values demonstrate results of the pairwise comparison by the Wilcoxon test.
In PDLC monolayers (Table 1; see Supplementary Figure S2), production of COL1A1 and POSTN was significantly decreased, while production of IL8 was significantly increased upon culture on collagen membranes. On bone substitute, production of COL1A1 and ANG significantly decreased, while production of VEGF, IL6 and IL8 increased significantly. The difference of VEGF and ANG production was significant between PDLC monolayer cultures on collagen membranes and bone substitute.
On collagen membranes, there was a significant difference of POSTN and ANG production between the two culture models, PDLC microtissues and monolayers (Table 1; see Supplementary Figure S2). On bone substitute, production of COL1A1 and VEGF was significantly different in PDLC microtissues and monolayers (Table 1; see Supplementary Figure S2).
3.4. Production of molecular markers at protein levels
At protein levels (Table 2; see Supplementary Figure S2), ANG and IL6 production was found to be significantly increased in PDLC microtissues on bone substitute, while VEGF production significantly decreased in the same group. A significant difference was further found in IL6 production by PDLC microtissues between culture on collagen membranes and bone substitute.
TABLE 2.
Production of molecular markers at protein levels in periodontal ligament cell microtissues and monolayers on collagen membranes or bone substitute
| PDLC microtissues | PDLC monolayers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean/SD | n | P value versus culture dish | P value collagen membrane versus bone substitute | Mean/SD | n | P value versus culture dish | P value collagen membrane versus bone substitute | P value microtissues versus monolayers | ||
| Culture dish | COL1A1 [ng/mL] | 395.35/264.81 | 6 | 1368.96/381.10 | 6 | 0.345 | ||||
| POSTN [ng/mL] | 4.19/1.62 | 6 | 23.62/11.30 | 6 | 0.028 | |||||
| VEGF [ng/mL] | 0.75/0.43 | 6 | 0.38/0.36 | 6 | 0.026 | |||||
| ANG [pg/mL] | 72.79/71.78 | 6 | 49.00/17.47 | 6 | 0.136 | |||||
| IL6 [pg/mL] | 322.00/988.97 | 6 | 626.67/560.56 | 6 | 0.480 | |||||
| IL8 [pg/mL] | 26.60/46.12 | 6 | 25.42/39.41 | 6 | 0.735 | |||||
| Collagen membrane | COL1A1 [ng/mL] | 269.12/126.91 | 6 | 0.116 | 0.345 | 826.03/604.29 | 6 | 0.249 | 0.173 | 0.028 |
| POSTN [ng/mL] | 6.39/15.11 | 6 | 0.917 | 0.465 | 4.95/3.21 | 6 | 0.028 | 0.463 | 0.345 | |
| VEGF [ng/mL] | 0.72/0.61 | 6 | 0.893 | 0.068 | 0.29/0.46 | 6 | 0.465 | 0.285 | 0.068 | |
| ANG [pg/mL] | 46.76/51.52 | 6 | 0.075 | 0.075 | 14.60/12.66 | 6 | 0.075 | 0.463 | 0.173 | |
| IL6 [pg/mL] | 407.18/432.59 | 6 | 0.173 | 0.028 | 588.43/633.04 | 6 | 0.345 | 0.345 | 0.249 | |
| IL8 [pg/mL] | 21.88/40.32 | 6 | 0.109 | 0.893 | 150.42/129.27 | 6 | 0.116 | 0.463 | 0.043 | |
| Bone substitute | COL1A1 [ng/mL] | 213.96/127.63 | 6 | 0.075 | 0.345 | 517.99/699.65 | 6 | 0.116 | 0.173 | 0.345 |
| POSTN [ng/mL] | 0.29/0.45 | 6 | 0.345 | 0.465 | 11.10/12.96 | 6 | 0.249 | 0.463 | 0.028 | |
| VEGF [ng/mL] | 0.09/0.23 | 6 | 0.043 | 0.068 | 0.08/0.20 | 6 | 0.068 | 0.285 | 0.655 | |
| ANG [pg/mL] | 39.99/50.39 | 6 | 0.028 | 0.075 | 9.68/4.61 | 6 | 0.046 | 0.463 | 0.345 | |
| IL6 [pg/mL] | 114.68/134.34 | 6 | 0.043 | 0.028 | 285.87/336.85 | 6 | 0.753 | 0.345 | 0.345 | |
| IL8 [pg/mL] | 27.45/34.95 | 6 | 0.285 | 0.893 | 86.09/82.31 | 6 | 0.043 | 0.463 | 0.249 | |
Production of collagen type I (COL1A1), periostin (POSTN), vascular endothelial growth factor (VEGF), angiogenin (ANG), interleukin 6 (IL6) and interleukin 8 (IL8) protein were assessed by ELISA in the supernatant of PDLC microtissues and monolayers on plastic culture dishes, collagen membranes or bone substitute.
Data from six donors (n) and two to three technical replicates are displayed as mean + SD. Measured concentration units are displayed next to the protein target names.
Data were calculated based on respective standards provided by the ELISA kit manufacturers.
P values demonstrate results of the pairwise comparison by the Wilcoxon test.
PDLC monolayers (Table 2; see Supplementary Figure S3) showed a significantly increased protein production of POSTN upon culture on collagen membranes and of ANG and IL8 upon culture on bone substitute.
In control groups on plastic culture dishes, a significant difference between PDLC microtissues and monolayers was detected in production of POSTN and VEGF (Table 2; see Supplementary Figure S3). On collagen membranes, COL1A1 and IL8 production was significantly different in PDLC microtissues compared with monolayers (Table 2; see Supplementary Figure S3). On bone substitute, only POSTN production differed significantly in both culture models (Table 2; see Supplementary Figure S3).
4. DISCUSSION
Periodontal soft tissue recession and formation of fibrotic capsules instead of full regeneration of the tissue remain the major drawbacks after periodontal GTR. Collagen membranes and bone substitute help to guide the direction of regeneration and fill the defect, but they fail to support the periodontal soft tissue to regain its original molecular composition and function. Biocompatibility should be a key feature of a functional GTR system consisting of a biomaterial together with the biological tissue. 24 A newly developed or improved biomaterial for periodontal GTR should be validated for its desired features in a system that is ethically acceptable, technically feasible and particularly reflects in vivo conditions in a patient. Therefore, it was suggested that 3D cell cultures could be suitable to study characteristics of novel and potentially improved GTR biomaterials in a way that better corresponds to expected reactions in an organism. 25 In this study, we compared the reactions of PDLC microtissues and monolayers to clinically applied collagen membranes and bone substitute, regarding the most important aspects of an optimal biomaterial for GTR. 26
One of these aspects is cell viability, which we assessed by quantification of metabolic activity in this study. PDLC microtissues and monolayers remained metabolically active on collagen membranes and bone substitute, which supports the fact that collagen membranes and bone substitutes can be safely used in patients as they have been in clinical use for over 20 years. 27 Although PDLC microtissues seem to be less metabolically active than monolayers, the difference was not statistically significant (Fig. 1). Spheroids of a hepatoma cell line showed enhanced metabolic gene expression profiles as response to 3D culturing, 28 which does not match the observations made in the present study. The cell number for PDLC microtissues that was used in this study was higher than the reported hepatoma cell spheroids. High cell numbers lead to the formation of larger spheroids, which causes the formation of a dense core with reduced cell viability. 29 Future studies will have to assess if PDLC microtissue size is associated with cell viability and corresponding pathways. As microtissues consist of many cell layers, it should be considered that cells in the dense core and cells on the surface might behave different from each other. To reveal the contributions of the heterogeneously reacting cells in a microtissue, microtissue responses will have to be studied at a single‐cell level in future. 30 In the end, a compromise in cell numbers must be found that is feasible in handling and more importantly shows similarity to original tissue behavior, which could be confirmed for kidney cell spheroids in contrast to monolayers, 31 but remains to be determined for the periodontal ligament. This will be important to determine the ideal PDLC microtissue size that mirrors the cellular responsiveness to external stimuli of human in vivo periodontal ligament.
Foreign body reactions form the source of a major pitfall in periodontal soft tissue integration. After implantation of a biomaterial into the defect site, the surrounding soft tissue will react with an inflammatory response, driven by macrophages and other immune cells, finally forming a fibrous capsule at the surface of the inserted material, lacking functional and mechanical requirements of the original soft tissue. 32 This fibrous capsule consists of different types of collagen 33 and collagen production is in turn linked to the assembly of the actin cytoskeleton. 34 In our study, positive staining for actin filaments was shown in PDLC microtissues on collagen membranes, while there was no staining for focal contacts (Fig. 2, see Supplementary Figure S1). This finding was reproducible in other cell donors as well as experimental repetitions (see Supplementary Figure S1), although staining intensities and tones of color showed some variability due to rather fast bleaching of the staining and high sensitivity to environmental conditions, like temperature, under which experiments are performed. The focal adhesion staining was performed 24 hours after seeding PDLC microtissues onto collagen membranes. Probably, the time point of focal contact production was missed, since the formation of focal contacts is a dynamic process. 35 This aspect will be addressed in future by testing different time points. The same aspect is also of considerable importance for mRNA and protein production as transcription and translation differ in duration and timing, which is to be investigated in a following study. Another possibility is that collagen membranes have a functional effect on PDLC microtissues by means of increasing actin filament production, which is currently unknown. Actin filaments are closely connected to focal adhesion molecules, mediating cellular attachment, 36 which points to a high disposition for attachment in the analyzed PDLC microtissues on collagen membranes in this study. A previous study on attachment of gingiva cell microtissues supports the hypothesis that periodontal soft tissue‐derived cells favor attachment onto collagen membranes rather than on plastic culture dishes or bone substitute. 37 The underlying biological mechanisms and the meaning for foreign body reactions remain undiscovered.
The main component of the extracellular matrix in periodontal soft tissue is COL1A1. Our results in PDLC monolayers confirm the previously published fact that bone substitute can decrease COL1A1 production 38 whereas PDLC microtissues on bone substitute show the opposite effect with increased levels of COL1A1 (Table 1, see Supplementary Figure S2). This could be of interest regarding the production of a fibrous capsule, consisting of collagens, 33 in response to a foreign material. POSTN is a relevant molecule that indicates bone formation and periodontal fibroblast migration. 39 The use of collagen membranes supports POSTN production more than bone substitute in vivo. 40 , 41 While at mRNA levels the reported effect was only significant in PDLC monolayers on collagen membranes (Table 1, see Supplementary Figure S2), at protein levels no significant modulation was found (Table 2, see Supplementary Figure S3).
Proper angiogenesis enables transport of nutrients and oxygen to regenerated tissues with VEGF and ANG as known molecular mediators to promote this process. In our study, VEGF production in PDLC monolayers was increased by culture on bone substitute (Table 1,Supplementary Figure S2), which is in parallel with current literature in two‐dimensional in vitro models. 42 Experiments in vivo demonstrated the opposite effect where various bone substitute materials downregulated VEGF 43 as it was also the case in PDLC microtissues (Table 2, Supplementary Figure S3). It is known that ANG acts as an enhancer of angiogenesis, for example in skin when loaded onto a collagen‐chitosan scaffold 44 and that it is produced by cells from the periodontal ligament. 14 Its role in the human periodontal ligament has not been clarified yet. In our study, ANG protein levels were increased in both, PDLC microtissues and monolayers on bone substitute (Table 2, Supplementary Figure S3).
Production of IL6 is upregulated at protein levels in PDLC microtissues on bone substitute (Table 2, Supplementary Figure S3) and at mRNA levels in PDLC microtissues and monolayers on bone substitute (Table 1, Supplementary Figure S2), unlike osteoblast‐like cells on bone substitute. 45 The chemokine IL8 was found significant altered in monolayers of PDLC on bone substitute only, while all standard deviations of IL8 levels were relatively high. This is due to the fact that this inflammatory response shows high differences in production level intensities between individual donors, which is visible in Table 1 and Table 2. As proinflammatory cytokines, IL6 and IL8 are involved in the mediation of inflammatory reactions and are therefore relevant in the process of foreign body reactions. 46 Interestingly, PDLC microtissues produced lower concentrations of IL6 and IL8 than PDLC monolayers upon culture with collagen membranes and bone substitute. The meaning of this observation has to be addressed in future studies since in vivo data on IL6 and IL8 in response of the human periodontal ligament to implantation of collagen membranes or bone substitute is still missing, but would contribute to an important aspect of GTR.
Overall, protein data of this study represent measurements of proteins that were secreted into the supernatant. Parallel data from total protein could help to gain deeper insights into functional behavior of PDLC microtissues compared to monolayers on different surfaces. Established protocols for total protein isolation were not compatible with the surfaces used in this study, presumably because of partial protein absorption by the biomaterials. Thus, existing protocols are to be adapted in future in order to be able to deepen knowledge on protein production by PDLC microtissues. This study focused on molecular markers for regeneration, angiogenesis and inflammation, which are important processes during GTR. Besides, influence of biomechanics is of utter importance for the regeneration of periodontal ligament as this oral tissue has mechanosensing properties. Currently, our team is evaluating the effect of mechanical stretch on a range of regenerative molecular markers in PDLC microtissues and monolayers, which will be reported in future. Taken together, many of these results underline a discrepancy between literature in in vitro and in vivo studies that could be bridged with an in vivo‐like in vitro model.
The main limitations of this study are the difficulty in choosing the right size of microtissues as well as the missing clinical data for proper validation of results. Human periodontal ligament is hard to study in vivo due to technical limitations and for ethical reasons. The size of PDLC microtissues in this study was chosen to be quantitatively comparable to PDLC monolayers as this makes quantitative results easier to interpret. Nevertheless, this choice bears a compromise between comparability, feasibility and current literature reporting functional alterations with increasing microtissue size as reported above. 29 Large spheroids are easy to handle and process for experiments, thus, suitable for first insights into their characteristics and functional behavior. To circumvent the zoning in microtissues due to large size and keep the handy size at the same time, other microtissue shapes could be tested that allow more diffusion of nutrients 47 or co‐culture with endothelial cells could be developed to mimic vasculature, 48 which becomes essential with growing cell density.
5. CONCLUSIONS
Improvement of biomaterials for GTR is of great importance for periodontal regeneration and requires suitable testing models. The results of this study demonstrate how diverse PDLC microtissues and monolayers react to materials used for GTR in terms of metabolic activity, adhesion as well as mRNA and protein production of molecular biomarkers for regeneration, angiogenesis and inflammation. The statistical differences between measured effects in PDLC microtissues and monolayers were not robust enough to confirm a superior suitability of PDLC microtissues to better reflect in vivo periodontal ligament tissue. Therefore, more descriptive as well as mechanistic studies are required to fully understand the similarities and differences of microtissues to develop an optimal in vitro testing system that could hopefully replace animal studies and make clinical trials safer in the future.
AUTHOR CONTRIBUTIONS
Klara Janjić contributed to conceptualization of the project and manuscript, performance of experiments, data evaluation and interpretation as well as manuscript writing. Hermann Agis contributed to conceptualization of the project and manuscript preparation. Andreas Moritz and Xiaohui Rausch‐Fan contributed to data interpretation and manuscript preparation. Oleh Andrukhov contributed to conceptualization of the project and manuscript, data evaluation and interpretation as well as manuscript preparation.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Bio‐Gide collagen membranes and Bio‐Oss bone substitute were kindly provided by Geistlich Pharma, Wolhusen, LU, Switzerland for this study. The study was supported by a Young Researcher Grant (grant no. 15‐184) of the Osteology Foundation, Lucerne, Switzerland. The authors report no conflicts of interest related to this study.
Janjić K, Agis H, Moritz A, Rausch‐Fan X, Andrukhov O. Effects of collagen membranes and bone substitute differ in periodontal ligament cell microtissues and monolayers. J Periodontol. 2022;93:697–708. 10.1002/JPER.21-0225.
Footnotes
Thermo Fisher Scientific, Waltham, MA
Thermo Fisher Scientific, Waltham, MA
Thermo Fisher Scientific, Waltham, MA
Thermo Fisher Scientific, Waltham, MA
Thermo Fisher Scientific, Waltham, MA
Thermo Fisher Scientific, Waltham, MA
Bio‐Gide; Geistlich Biomaterials Vertriebsgesellschaft, Baden‐Baden, Germany
TPP Techno Plastic Products, Trasadingen, Switzerland
Small Geistlich Bio‐Oss granules; Geistlich Biomaterials Vertriebsgesellschaft, Baden‐Baden, Germany
TPP Techno Plastic Products, Trasadingen, Switzerland
Microtissues, Providence, RI
TPP Techno Plastic Products, Trasadingen, Switzerland
Sigma‐Aldrich, St. Louis, MO
Sigma‐Aldrich, St. Louis, MO
Qiagen, Hilden, Germany
Thermo Fisher Scientific, Waltham, MA
Thermo Fisher Scientific, Waltham, MA
R&D Systems, Minneapolis, MN
R&D Systems, Minneapolis, MN
PeproTech Austria, Vienna, Austria
R&D Systems, Minneapolis, MN
PeproTech Austria, Vienna, Austria
PeproTech Austria, Vienna, Austria
IBM, Armonk, NY
REFERENCES
- 1. Majzoub J, Barootchi S, Tavelli L, Wang C‐W, Travan S, Wang H‐L. Treatment effect of guided tissue regeneration on the horizontal and vertical components of furcation defects: a retrospective study. J Periodontol. 2020;91:1148‐1158. [DOI] [PubMed] [Google Scholar]
- 2. Periodontology AAO. Position paper: periodontal regeneration. J Periodontol, 2005;76:1601‐1622. [DOI] [PubMed] [Google Scholar]
- 3. Bottino MC, Thomas V, Schmidt G et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration–A materials perspective. Dent Mater, 2012;28(3):703‐721. 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 4. Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple ILC, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol 2000, 2015;68:182‐216. [DOI] [PubMed] [Google Scholar]
- 5. Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci, 2007;115:355‐362. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, An Y, Gao L‐N, Zhang Y‐J, Jin Y, Chen F‐M. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials, 2012;33:6974‐6986. [DOI] [PubMed] [Google Scholar]
- 7. Smith PC, Martínez C, Martínez J, McCulloch CA. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front Physiol, 2019;10:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donath K, Laass M, Günzl HJ. The histopathology of different foreign‐body reactions in oral soft tissue and bone tissue. Virchows Arch A, Pathol Anat Histopathol, 1992;420:131‐137. [DOI] [PubMed] [Google Scholar]
- 9. Rothamel D, Benner M, Fienitz T et al. Biodegradation pattern and tissue integration of native and cross‐linked porcine collagen soft tissue augmentation matrices—An experimental study in the rat. Head Face Med, 2014;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghanaati S, Schlee M, Webber MJ et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater, 2011;6:015010. [DOI] [PubMed] [Google Scholar]
- 11. Florjanski W, Orzeszek S, Olchowy A et al. Modifications of polymeric membranes used in guided tissue and bone regeneration. Polymers (Basel), 2019;11(5):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edmondson R, Broglie JJ, Adcock AF, Yang L. Three‐dimensional cell culture systems and their applications in drug discovery and cell‐based biosensors. Assay Drug Dev Technol, 2014;12:207‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berahim Z, Moharamzadeh K, Rawlinson A, Jowett AK. Biologic interaction of three‐dimensional periodontal fibroblast spheroids with collagen‐based and synthetic membranes. J Periodontol, 2011;82:790‐797. [DOI] [PubMed] [Google Scholar]
- 14. Janjić K, Bauer P, Edelmayer M et al. Angiogenin production in response to hypoxia and l‐mimosine in periodontal fibroblasts. J Periodontol, 2019;90:674‐681. [DOI] [PubMed] [Google Scholar]
- 15. Moritani Y, Usui M, Sano K et al. Spheroid culture enhances osteogenic potential of periodontal ligament mesenchymal stem cells. J Periodontal Res, 2018;53:870‐882. [DOI] [PubMed] [Google Scholar]
- 16. Iwasaki K, Nagata M, Akazawa K, Watabe T, Morita I. Changes in characteristics of periodontal ligament stem cells in spheroid culture. J Periodontal Res, 2019;54:364‐373. [DOI] [PubMed] [Google Scholar]
- 17. Özcan E, Saygun I, Kantarcı A, Özarslantürk S, Serdar MA, Özgürtaş T. The effects of a novel non‐invasive application of platelet‐rich fibrin on periodontal clinical parameters and gingival crevicular fluid transforming growth factor‐β and collagen‐1 levels: a randomized controlled clinical study. J Periodontol, 2020; 10.1002/JPER.20-0713. [DOI] [PubMed] [Google Scholar]
- 18. Albuquerque‐Souza E, Schulte F, Chen T et al. Maresin‐1 and Resolvin E1 promote regenerative properties of periodontal ligament stem cells under inflammatory conditions. Front Immunol, 2020;11:585530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwasaki K, Akazawa K, Nagata M et al. Angiogenic effects of secreted factors from periodontal ligament stem cells. Dent J (Basel), 2021;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morelli T, Neiva R, Nevins ML et al. Angiogenic biomarkers and healing of living cellular constructs. J Dent Res, 2011;90:456‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamato H, Sanui T, Yotsumoto K et al. Combined application of geranylgeranylacetone and amelogenin promotes angiogenesis and wound healing in human periodontal ligament cells. J Cell Biochem, 2021;122(7):716–730. [DOI] [PubMed] [Google Scholar]
- 22. AlGhamdi AS, Ciancio SG. Guided tissue regeneration membranes for periodontal regeneration–A literature review. J Int Acad Periodontol, 2009;11:226‐231. [PubMed] [Google Scholar]
- 23. Fukuba S, Okada M, Nohara K, Iwata T. Alloplastic bone substitutes for periodontal and bone regeneration in dentistry: current status and prospects. Materials (Basel), 2021;14:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams DF. There is no such thing as a biocompatible material. Biomaterials, 2014;35:10009‐10014. [DOI] [PubMed] [Google Scholar]
- 25. Park Y, Huh KM, Kang S‐W. Applications of biomaterials in 3D cell culture and contributions of 3D cell culture to drug development and basic biomedical research. Int J Mol Sci, 2021;22:2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res, 2013;57:3‐14. [DOI] [PubMed] [Google Scholar]
- 27. Camelo M, Nevins ML, Schenk RK et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio‐Oss and Bio‐Gide. Int J Periodontics Restorative Dent, 1998;18:321‐331. [PubMed] [Google Scholar]
- 28. Takahashi Y, Hori Y, Yamamoto T, Urashima T, Ohara Y, Tanaka H. 3D spheroid cultures improve the metabolic gene expression profiles of HepaRG cells. Biosci Rep, 2015;35:e00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gong X, Lin C, Cheng J et al. Generation of multicellular tumor spheroids with microwell‐based agarose scaffolds for drug testing. Plos One, 2015;10:e0130348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muciño‐Olmos EA, Vázquez‐Jiménez A, Avila‐Ponce de León U et al. Unveiling functional heterogeneity in breast cancer multicellular tumor spheroids through single‐cell RNA‐seq. Sci Rep, 2020;10:12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lagies S, Schlimpert M, Neumann S et al. Cells grown in three‐dimensional spheroids mirror in vivo metabolic response of epithelial cells. Commun Biol, 2020;3:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol, 2008;20:86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akilbekova D, Bratlie KM. Quantitative characterization of collagen in the fibrotic capsule surrounding implanted polymeric microparticles through second harmonic generation imaging. Plos One, 2015;10:e0130386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin Z, Fisher GJ, Voorhees JJ, Quan T. Actin cytoskeleton assembly regulates collagen production via TGF‐β type II receptor in human skin fibroblasts. J Cell Mol Med, 2018;22:4085‐4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaidel‐Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell‐matrix adhesion complexes. Biochem Soc Trans, 2004;32:416‐420. [DOI] [PubMed] [Google Scholar]
- 36. Seetharaman S, Etienne‐Manneville S. Microtubules at focal adhesions—A double‐edged sword. J Cell Sci, 2019;132:jcs232843. [DOI] [PubMed] [Google Scholar]
- 37. Janjić K, Cvikl B, Schädl B, Moritz A, Agis H. The impact of collagen membranes on 3D gingival fibroblast toroids. BMC Oral Health, 2019;19:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sollazzo V, Palmieri A, Scapoli L et al. Bio‐Oss® acts on stem cells derived from peripheral blood. Oman Med J, 2010;25:26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du J, Li M. Functions of Periostin in dental tissues and its role in periodontal tissues’ regeneration. Cell Mol Life Sci, 2017;74:4279‐4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kojima T, Amizuka N, Suzuki A et al. Histological examination of bone regeneration achieved by combining grafting with hydroxyapatite and thermoplastic bioresorbable plates. J Bone Miner Metab, 2007;25:361‐373. [DOI] [PubMed] [Google Scholar]
- 41. Elgali I, Turri A, Xia W et al. Guided bone regeneration using resorbable membrane and different bone substitutes: early histological and molecular events. Acta Biomater, 2016;29:409‐423. [DOI] [PubMed] [Google Scholar]
- 42. Rombouts C, Jeanneau C, Camilleri J, Laurent P, About I. Characterization and angiogenic potential of xenogeneic bone grafting materials: role of periodontal ligament cells. Dent Mater J, 2016;35:900‐907. [DOI] [PubMed] [Google Scholar]
- 43. Blatt S, Thiem DGE, Pabst A, Al‐Nawas B, Kämmerer PW. Does platelet‐rich fibrin enhance the early angiogenetic potential of different bone substitute materials? An in vitro and in vivo analysis. Biomedicines, 2021;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi H, Han C, Mao Z, Ma L, Gao C. Enhanced angiogenesis in porous collagen‐chitosan scaffolds loaded with angiogenin. Tissue Engineering Part A, 2008;14:1775‐1785. [DOI] [PubMed] [Google Scholar]
- 45. Torricelli P, Fini M, Giavaresi G, Botter R, Beruto D, Giardino R. Biomimetic PMMA‐based bone substitutes: a comparative in vitro evaluation of the effects of pulsed electromagnetic field exposure. J Biomed Mater Res A, 2003;64:182‐188. [DOI] [PubMed] [Google Scholar]
- 46. Lock A, Cornish J, Musson DS. The role of in vitro immune response assessment for biomaterials. J Funct Biomater, 2019;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen Y, Nguyen DT, Kokil GR, Wong YX, Dang TT. Microencapsulated islet‐like microtissues with toroid geometry for enhanced cellular viability. Acta Biomater, 2019;97:260‐271. [DOI] [PubMed] [Google Scholar]
- 48. Yang F, Cohen RN, Brey EM. Optimization of co‐culture conditions for a human vascularized adipose tissue model. Bioengineering (Basel), 2020;7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


