Abstract
Objective
To assess the outcomes of pre‐biopsy magnetic resonance imaging (MRI) pathways, as a tool in biopsy‐naïve men with suspicion of prostate cancer, in routine clinical practice. Secondary outcomes included a comparison of transrectal MRI‐directed biopsy (TR‐MRDB) and transperineal (TP)‐MRDB in men with suspicious MRI.
Patients and Methods
We retrospectively assessed a two‐centre cohort of consecutive biopsy‐naïve men with suspicion of prostate cancer who underwent a Prostate Imaging‐Reporting and Data System version 2 (PI‐RADS v2) compliant pre‐biopsy MRI in a single, high‐volume centre between 2015 and 2019 (Centre 1). Men with suspicious MRI scans underwent TR‐MRDB in Centre 1 and TP‐MRDB with additional random biopsies (RB) in Centre 2. The MRI and histopathology were assessed in the same institution (Centre 1). Outcomes included: (i) overall detection rates of Grade Group (GG) 1, GG ≥2, and GG ≥3 cancer in men with suspicious MRI; (ii) Biopsy‐avoidance due to non‐suspicious MRI; and (iii) Cancer detection rates and biopsy‐related complications between TR‐ and TP‐MRDB. To reduce confounding bias for MRDB comparisons, inverse probability weighting (IPW) was performed for age, digital rectal examination, prostate‐specific antigen (PSA), prostate volume, PSA density, and PI‐RADS category.
Results
Of the 2597 men included, the overall GG 1, GG ≥2, and GG ≥3 prevalence was 8% (210/2597), 27% (697/2597), and 15% (396/2597), respectively. Biopsy was avoided in 57% (1488/2597) of men. After IPW, the GG 1, GG ≥2 and GG ≥3 detection rates after TR‐ and TP‐MRDB were comparable at 24%, 57%, and 32%; and 18%, 64%, and 38%, respectively; with mean differences of −5.7% (95% confidence interval [CI] −13% to 1.4%), 6.1% (95% CI −2.1% to 14%), and 5.7% (95% CI −1.7% to 13%). Complications were similar in TR‐MRDB (0.50%) and TP‐MRDB with RB (0.62%; mean difference 0.11%, 95% CI −0.87% to 1.1%).
Conclusion
This high‐volume, two‐centre study shows pre‐biopsy MRI as a decision tool is implementable in daily clinical practice. Compared to recent trials, a substantially higher biopsy avoidance rate was achieved without compromising GG ≥2/GG ≥3 detection and coinciding with lower over detection rates of GG 1 cancer. Prostate cancer detection and complication rates were comparable for TR‐ and TP‐MRDB.
Keywords: biopsy, diagnostic imaging, multiparametric magnetic resonance imaging, Prostate Imaging‐Reporting and Data System, prostate cancer, risk stratification, transrectal, transperineal
Introduction
The incidence of prostate cancer remains significant due to opportunistic prostate‐specific antigen (PSA) testing in a growing and ageing population [1, 2]. It is estimated that the worldwide prostate cancer incidence will increase almost twofold to 2.3 million cases by 2040 [3].
One of the main concerns of (early) prostate cancer detection is the burden to biopsy all men with elevated PSA and the risk for diagnosing Grade Group (GG) 1 cancer and the related ‘overtreatment’ of these indolent cancers [4]. To minimise both biopsy‐ and treatment‐related morbidity, avoidance of redundant biopsies, and reducing overdiagnosis of indolent prostate cancer is urgently needed. In this respect, prostate multiparametric MRI (mpMRI) has proven to be effective [4, 5, 6, 7]. Pre‐biopsy MRI is currently recommended by American and European urological guidelines [4, 8]. In addition, the current prostate cancer guidelines now recommend performing biopsy only in men with a suspicious MRI and to omit systematic biopsy (SB) in men with a unsuspicious MRI within shared decision‐making between patients and healthcare providers [4, 8]. This recommendation is based on the balance between patient benefits and harms, i.e. the detection of clinically significant prostate cancers vs redundant biopsies, biopsy‐related complications, and detection of indolent cancers.
However, results of clinical trials might not always reflect clinical practice because of selective eligibility criteria (e.g. age or serum PSA level) and optimised circumstances (e.g. MRI quality or double‐reads) [9]. Realised outcomes in clinical practice are sparse, particularly with respect to realised biopsy avoidance and reduction of indolent cancers [10]. Moreover, implementing guideline recommendations may not always lead to achieved trial‐based results. Therefore, guideline recommendations on MRI‐directed biopsy (MRDB) management should be validated in routine clinical practice. For this reason, we have investigated the newly recommended biopsy management in biopsy‐naïve men in an ambulatory high‐volume, two‐centre setting.
Furthermore, MRI enables targeting of prostate biopsy cores to suspicious lesions through either a transrectal (TR)‐ or transperineal (TP)‐MRDB approach [11]. As TR‐ and TP‐MRDB under local anaesthesia have not yet been compared in clinical practice, we additionally compared different MRDB biopsy techniques (including the added value of random biopsy [RB]).
Patients and Methods
This retrospective, cohort study included 3215 consecutive men with an elevated serum PSA (≥3 ng/mL) and/or abnormal DRE who were referred to two centres. From 1 January 2017 until 31 May 2019, 663 men were included in a university centre (Centre 1). While, 2552 men were included in a non‐university centre (Centre 2), from 1 November 2015 until 30 November 2019. Figure 1 shows the study design and reasons for exclusion. Only biopsy‐naïve men were eligible. Data collection was conducted compliant with the Standards of Reporting for MRI‐targeted Biopsy Studies (START) recommendations and reported according to the Standards for Reporting Diagnostic Accuracy Studies (STARD) statement [12, 13]. The Local Ethics Committee approved the study design with a waiver of informed consent (CMO 2018‐4307/2020‐6599).
Fig. 1.
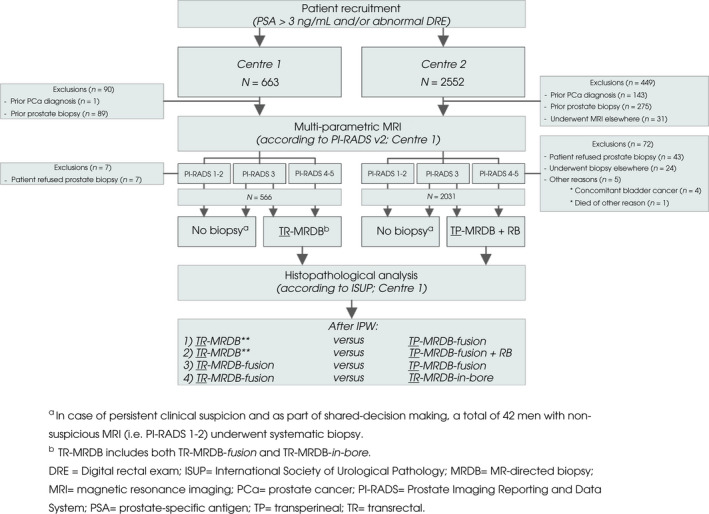
Flow diagram of the study design and participants. *In case of persistent clinical suspicion and as part of shared‐decision making, a total of 42 men with unsuspicious MRI (i.e. PI‐RADS 1–2) underwent systematic biopsy. **TR‐MRDB includes both TR‐MRDB‐fusion and TR‐MRDB‐in‐bore. PCa, prostate cancer.
Prostate MRI
According to the standard of care for both centres, all patients had a 3‐Tesla (T) MRI (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) conducted in Centre 1. The scan protocol consisted of Prostate Imaging‐Reporting and Data System version 2 (PI‐RADS v2) compliant tri‐planar T2‐weighted imaging (T2‐WI), diffusion‐weighted imaging (DWI) with calculation of apparent diffusion coefficient maps plus high b‐value images (≥b‐1400 s/mm2) and dynamic contrast‐enhanced MRI (Table S1) [14]. All MRI scans were analysed in Centre 1 in routine practice by one of six experienced prostate‐radiologists (5–20 years’ experience) according to PI‐RADS v2 criteria, radiologists were informed about the clinical findings [14]. For all patients, PSA density (PSAD; PSA divided by prostate volume) was calculated based on MRI volume. Lesions with PI‐RADS scores of 1–2 were considered unsuspicious for GG ≥2 cancer, only in case of persistent high clinical suspicion men underwent SB. Men with PI‐RADS scores of 3 were deemed equivocal, men with PI‐RADS 4 were likely and PI‐RADS 5 highly likely to have GG ≥2 cancer. All PI‐RADS 4–5 lesions were biopsied, also in case of secondary or tertiary lesions. The centre that the men were referred to was determined by the method of biopsy (i.e. TR‐MRDB or TP‐MRDB). In other words, the choice of biopsy route was not altered based on the prostate MRI results. For PI‐RADS 3 lesions, the decision to biopsy or to perform follow‐up was discussed in a multidisciplinary team (MDT) meeting and, thereafter, with the patient as part of shared‐decision making with due consideration of clinical (e.g. PSAD ≥0.12 ng/mL/mL) and MRI findings.
The TR‐MRDB
In Centre 1, MRDB was performed transrectally. The TR‐MRDB technique, either ‘in‐bore’ or ‘fusion’ was determined at the MDT‐meeting and depended on lesions’ location and size. Preferably anterior, apical, and small lesions (<8 mm) were targeted by TR‐MRDB‐in‐bore and other lesions with TR‐MRDB‐fusion. The median (interquartile range [IQR], range) time between prostate MRI and TR‐MRDB was 5 (5–6, 1–27) days. Men received 3 days of antibiotic prophylaxis (oral ciprofloxacin, 500 mg twice a day), starting the day before biopsy. TR‐MRDB‐in‐bore was performed after re‐acquiring axial T2‐WI and DWI to relocate the cancer suspicious lesions. In prone position, an MR‐compatible needle guide (Invivo, Gainsville, FL, USA) was rectally inserted, attached either to a remote‐controlled manipulator (Soteria Medical BV, Arnhem, the Netherlands) or a manual biopsy device DynaTRIM (Invivo). Verification of the final biopsy needle location was obtained by confirmation MRI and evaluated by one of the prostate‐radiologists.
TR‐MRDB‐fusion was performed using an Aplio 500 ultrasound scanner (Canon Medical Systems, Zoetermeer, the Netherlands) with rigid registration to fuse T2‐WI onto real‐time TRUS imaging, with an external magnetic field generator to perform lesion site localisation and movement tracking. Biopsies of suspicious lesions were performed using 18‐G needles. Only targeted biopsies (2–4 cores/lesion) were performed without additional RBs. Further technical details of both TR‐MRDB approaches have previously been reported [15, 16].
The TP‐MRDB
In Centre 2, men with suspicious MRI underwent TP‐MRDB‐fusion in an ambulatory setting. Prostate contours and lesions were delineated by an experienced (≥10 years) radiation‐oncologist (J.I.) using MIM SymphonyDx software (MIM, Cleveland, OH, USA). Local anaesthetic was injected (20–25 mL lidocaine 1–2%) in the dorsal lithotomy position. No periprocedural antibiotic prophylaxis was used. After perineal cleansing with chlorhexidine, the MRI contours were fused and aligned with the transrectal bkFusion‐3000 ultrasound images (BK‐Flex Focus 800 before 2018). The ultrasound probe was mounted to a tracked stepper (CIVCO, Peabody, MA, USA) with grid (holes spaced 5 mm apart). TP‐MRDB‐fusion was based on screen coordinates and using the corresponding grid holes. Two to eight cores were obtained from each suspicious lesion using an 18‐G biopsy needle. Additional RBs were performed. To decrease biopsy‐related morbidity, the RB cores were only sampled outside of the MRI suspicious regions. The number of these additional RB cores (usually four–12) depended on the prostate volume and number/size of targeted lesions.
Pathological Analysis
Biopsy cores of both centres were labelled individually and were analysed by dedicated uro‐pathologists in Centre 1, compliant with the most recent International Society of Urological Pathology (ISUP) guidelines [17]. The GG and the number of biopsy cores (positive for prostate cancer) were recorded.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS®) version 25 (IBM Corp., Armonk, NY, USA) and R (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). The patients’ and MRI characteristics are presented as numbers and percentages, and continuous variables as median (IQR), in accordance with the ‘Guidelines for reporting of statistics for clinical research in urology’ [18]. In men with multiple lesions on MRI, the highest PI‐RADS score was used as the index lesion. Occurrence of complications was graded by the healthcare providers, using the Clavien–Dindo classification [19]. Prostate cancer was categorised in to three groups: any core with GG ≥3 (Gleason score ≥4 + 3 = 7), with GG ≥2 (Gleason score ≥3 + 4 = 7), or only with GG 1 cancer (Gleason score 3 + 3 = 6) [20]. Primary outcomes included the overall detection rates of GG 1, GG ≥2, and GG ≥3 cancer and biopsy avoidance. Secondary outcomes included detection rates of GG ≥3, GG ≥2, and GG 1 cancers and complication rates of four different comparisons were assessed: (i) TR‐MRDB (either in‐bore or fusion) vs TP‐MRDB‐fusion, (ii) TR‐MRDB (either in‐bore or fusion) vs TP‐MRDB‐fusion + RB, (iii) TR‐MRDB‐fusion vs TP‐MRDB‐fusion, and (iv) TR‐MRDB‐fusion vs TR‐MRDB‐in‐bore. Inverse probability weighted analyses (IPW) were performed to reduce imbalance of confounders (i.e. baseline characteristics: age, DRE abnormality, PSA level, prostate volume, PSAd, and PI‐RADS score) between the groups. In all, 25 multiple imputation by chained equations (MICE) sets were used to impute missing data. Standardised mean differences of each characteristic were used to assess the cohorts’ balance, a standardised difference between −0.1 and 0.1 indicates an appropriate balance. After IPW, detection rates of GG ≥3, GG ≥2, and GG 1 cancer and complication rates between comparisons were reported as mean differences of the imputed datasets with 95% CIs.
Results
Outcome Measures of the Total Cohort
After exclusion (reasons provided in Fig. 1), 2597 consecutive men (median [IQR] age of 66 [61–71] years and PSA level of 6.3 [4.9–8.9] ng/mL) were eligible for inclusion: 566 men in Centre 1 and 2031 men in Centre 2 (Fig. 1). Patient characteristics of the total biopsy‐naïve population and per centre are presented in Table 1. Overall, prostate cancer GG ≥3, GG ≥2, and GG 1 was diagnosed with MRDB (including RB) or SB in 15% (396/2597), 27% (697/2597), and 8.1% (210/2597) of men, respectively. Seven complications were reported (0.66%), most complications of TR‐ and TP‐MRDB were infectious (five), all reported complications were Clavien–Dindo Grade ≤II (Table 1).
Table 1.
Demographic and clinical patient characteristics.
| Characteristic | Total | Centre 1 (TR‐MRDB) | Centre 2 (TP‐MRDB) |
|---|---|---|---|
| Biopsy‐naïve men, n (%) | 2597 (100) | 566 (22) | 2031 (78) |
| Age, years, median (IQR) | 66 (61–71) | 65 (60–69) | 67 (62–71) |
| DRE, n (%) | |||
| Normal* | 1205 (74) | 390 (70) | 815 (76) |
| Suspicious* | 434 (26) | 171 (30) | 262 (24) |
| Missing | 958 (37) | 5 (0.88) | 953 (47) |
| PSA level, ng/mL, median (IQR) | 6.3 (4.9–8.9) | 6.2 (4.6–9.6) | 6.4 (4.9–8.7) |
| Prostate volume MRI†, mL, median (IQR) | 62 (45–87) | 58 (42–79) | 64 (47–89) |
| Missing, n (%) | 9 (0.35) | – | 9 (0.44) |
| PSAD, ng/mL/mL, median (IQR) | 0.10 (0.07–0.15) | 0.11 (0.07–0.17) | 0.10 (0.07–0.14) |
| Missing, n (%) | 9 (0.35) | – | 9 (0.44) |
| mpMRI, n (%) | |||
| PI‐RADS 1–2 | 1467 (56) | 307 (54) | 1160 (57) |
| PI‐RADS 3 | 149 (5.7) | 30 (5.3) | 119 (5.9) |
| PI‐RADS 4 | 462 (18) | 104 (18) | 359 (18) |
| PI‐RADS 5 | 519 (20) | 125 (22) | 393 (19) |
| Method of biopsy, n (%) | |||
| No biopsy | 1488 (57) | 325 (57) | 1163 (57) |
| TR‐MRDB‐fusion | 137 (5.3) | 137 (24) | – |
| TR‐MRDB‐in‐bore | 100 (3.9) | 100 (18) | – |
| TP‐MRDB‐fusion | 830 (32) | – | 830 (41) |
| Only SB | 42 (1.6) | 4 (0.71) | 38 (1.9) |
| Biopsy cores MRDB, n, median (IQR) | 4 (3–6) | 3 (3–4) | 5 (4–7) |
| Biopsy cores, TP‐MRDB with RB, n, median (IQR) | 11 (7–12) | – | 11 (7–12) |
| Complications, n (%) | 7 (0.66) | 2 (0.84) | 5 (0.60) |
| UTI/urosepsis‡ | 5 (0.47) | 1 (0.42) | 4 (0.48) |
| Haemorrhage‡ | 1 (0.09) | 1 (0.42) | – |
| Acute urinary retention‡ | 1 (0.09) | – | 1 (0.12) |
| Positive biopsy cores, n, median (IQR) | |||
| MRDB | 3 (2–5) | 3 (2–3) | 4 (2–5) |
| RB | 2 (1–3) | – | 2 (1–3) |
| Histology, n (%) | |||
| MRDB | 1067 (41) | 237 (42) | 830 (41) |
| No MRDB | 1530 (59) | 329 (58) | 1201 (59) |
| No prostate cancer§ [+RB] | 198 (7.6) [176 (6.8)] | 40 (7.1) | 158 (7.8) [136 (6.7)] |
| Prostate cancer§ [+RB] | 869 (33) [891 (34)] | 197 (35) | 672 (33) [694 (34)] |
| GG 1 cancer§ [+RB] | 201 (7.7) [198 (7.6)] | 50 (8.8) | 151 (7.4) [148 (7.3)] |
| GG ≥2 cancer§ [+RB] | 668 (26) [693 (27)] | 147 (26) | 521 (26) [546 (27)] |
| GG ≥3 cancer§ [+RB] | 373 (14) [395 (15)] | 88 (16) | 285 (14) [307 (15)] |
| Histology, n (%) | |||
| SB | 42 (1.6) | 4 (0.71%) | 38 (1.9) |
| No prostate cancer‡ | 26 (62) | 3 (75) | 23 (61) |
| Prostate cancer‡ | 16 (38) | 1 (25) | 15 (39) |
| GG 1 cancer‡ | 12 (29) | – | 12 (32) |
| GG ≥2 cancer‡ | 4 (9.5) | 1 (25) | 3 (7.9) |
| GG ≥3 cancer‡ | 1 (2.4) | – | 1 (2.6) |
Percentages may not total 100 because of rounding. *% of available characteristic; †prostate volume was measured on MRI; ‡% of total/cohort MRDB; §numbers/% including RB cores (within square brackets).
Biopsy Avoidance
Overall, 56% (1467/2597) of men had a unsuspicious MRI (PI‐RADS 1–2). Prostate biopsy was avoided in a total of 57% of men (1488/2597); in 97% (1425/1467) of men with PI‐RADS 1–2, and 42% (63/149) of men with PI‐RADS 3.
Cancer Detection Rates
A total of 44% (1130/2597) of men had an equivocal or suspicious MRI, of whom 5.7% (149) had a PI‐RADS score of 3, 18% (462) had a PI‐RADS score of 4, and the remaining 20% (519) had a score of 5 (Table 1).
In 1067 men who underwent MRDB, prostate cancer was detected in 81% of men. The GG ≥3, GG ≥2 and GG 1 detection rates were 35% (373/1067), 63% (668/1067), and 19% (201/1067), respectively. Prostate cancer detection rates of MRDB per PI‐RADS score are reported in Fig. 2. In 42 men (1.6%) SB was performed after an unsuspicious MRI (PI‐RADS 1–2) because of persistent clinical suspicion. This resulted in the detection of one GG3, three GG2, and 12 GG 1 cancers.
Fig. 2.
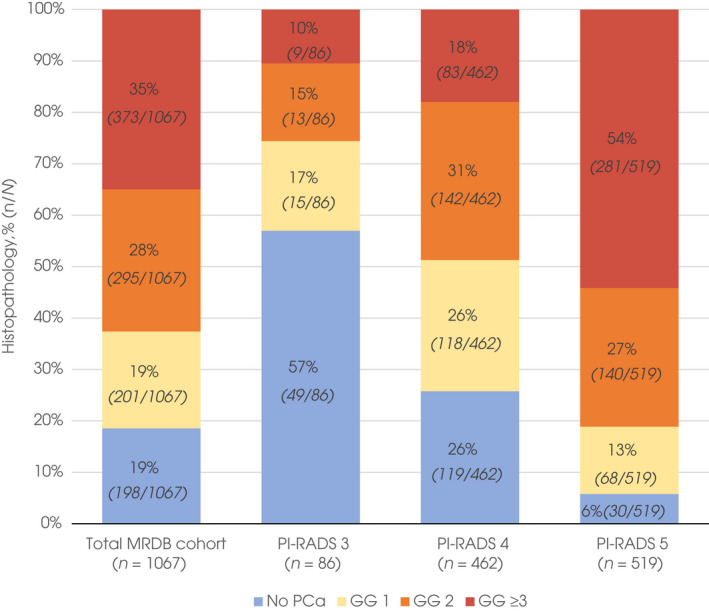
Histopathology of MRDB results per PI‐RADS group. Histopathological outcomes of the total number of men who underwent MRDB due to suspicious MRI (1067/2597 men) and per PI‐RADS category. In total, 81% of men (869/1067) who underwent MRDB had prostate cancer, 63% (668/1067) and 35% (373/1067) had GG ≥2 and GG ≥3 cancer, respectively, in other words, the positive predictive value of MRDB. Outcomes of separate PI‐RADS scores show higher PI‐RADS scores (i.e. greater likelihood of clinically significant prostate cancer) result in increasing rates of GG ≥2 and GG ≥3 cancer.
MRDB Comparisons (With IPW)
After IPW, baseline characteristics of men were similar for both MRDB approaches (Table 2 and Fig. S1). Due to the IPW analysis, the cancer detection rates were slightly different from the results before IPW. Prostate cancer detection rates for GG ≥3, GG ≥2 and GG 1 of both biopsy techniques (comparison 1) were comparable with detection rates of 32%, 57%, and 24% for TR‐MRDB (either in‐bore or fusion) and 38%, 64%, and 18% for TP‐MRDB‐fusion, respectively (mean [95% CI] differences of 5.7% [−1.7% to 13%], 6.1% [−2.1% to 14%] and −5.7% [−13% to 1.4%], respectively; Table 2 and Fig. 3). Compared to TR‐MRDB (either in‐bore or fusion), TP‐MRDB‐fusion + RB had a slightly higher detection rate for GG ≥2 cancer (57% vs 67%; mean [95%CI] difference 9.2% [1.0–17%]), but not for GG ≥3 and GG 1 cancers (comparison 2). Complications occurred in 0.50% and 0.62% of men for TR‐MRDB and TP‐MRDB‐fusion with RB, respectively (mean difference [95% CI] 0.11% [−0.87% to 1.1%]). TR‐MRDB‐fusion compared to TP‐MRDB‐fusion showed similar prostate cancer detection rates (comparison 3). Finally, TR‐MRDB‐fusion compared to TR‐MRDB‐in‐bore (comparison 4) showed a higher percentage of GG ≥3, and a lower percentage of GG 1 detection: 43% vs 19% (mean [95% CI] difference −23% [−37% to 9.7%]) and 14% vs 29% (mean [95% CI] difference 15% [2.7–27%]), respectively (Table 2 and Fig. 3).
Table 2.
Comparison of prostate biopsy: TR‐MRDB (‘fusion’ and ‘in‐bore’) and TP‐MRDB ‘fusion’ (with and without RB), before and after IPW.
| Before IPW | After IPW | |||||
|---|---|---|---|---|---|---|
| Variables* | TR‐MRDB | TP‐MRDB | Standardised mean difference (range*) | TR‐MRDB | TP‐MRDB | Standardised mean difference (range*) |
| Age, years, mean (sd) | 65 (6.8) | 68 (7.2) | −0.43 (−0.43 to −0.43) | 67 (6.2) | 67 (7.5) | −0.071 (−0.076 to −0.066) |
| Abnormal DRE, % | 50 | 35 | 0.31 (0.27–0.36) | 40 | 39 | 0.041 (0.022–0.051) |
| PSA, ng/mL, mean (sd) | 17 (32) | 10 (13) | 0.30 (0.30–0.30) | 12 (19) | 12 (17) | 0.026 (0.023–0.032) |
| Prostate volume MRI, mL, mean (sd) | 54 (26) | 60 (29) | −0.23 (−0.23 to −0.22) | 59 (28) | 58 (28) | 0.010 (0.0001– 0.016) |
| PSAD, ng/mL/mL, mean (sd) | 0.37 (0.79) | 0.20 (0.27) | 0.30 (0.30–0.30) | 0.25 (0.44) | 0.23 (0.38) | 0.028 (0.025–0.034) |
| mpMRI, % | ||||||
| PI‐RADS 3 | 3.4 | 9.4 | −0.25 (−0.25 to −0.25) | 9.0 | 8.1 | 0.037 (0.027–0.054) |
| PI‐RADS 4 | 44 | 43 | 0.015 (0.015–0.015) | 45 | 43 | 0.039 (0.035–0.043) |
| PI‐RADS 5 | 53 | 47 | 0.11 (0.11–0.11) | 46 | 49 | −0.057 (−0.064 to −0.051) |
| Comparison 1 and 2: TR‐MRDB vs TP‐MRDB (with RB) | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Before IPW | After IPW | Mean difference, %† | |||
| TR‐MRDB | TP‐MRDB | TR‐MRDB | TP‐MRDB‐fusion | TP‐MRDB with RB | ||
| Complications, % (95% CI) | 0.35 | 0.25‡ | 0.50 (0–1.3) | NA | 0.62 (0.07–1.2) | 0.11 (−0.87 to 1.1) |
| Histology, % (95% CI) | ||||||
| No prostate cancer | 17 | 19 | 19 (13–25) | 18 (16–21) | 16 (13–18) | −0.42 (−7.3 to 6.4) |
| GG 1 cancer | 21 | 18 | 24 (17–30) | 18 (15–21) | 17 (15–20) | −5.7 (−13 to 1.4) |
| GG≥2 cancer | 62 | 63 | 57 (50–65) | 64 (60–67) | 67 (63–70) | 6.1 (−2.1 to 14) |
| GG ≥3 cancer | 37 | 34 | 32 (25–39) | 38 (34–41) | 38 (35–41) | 5.7 (−1.7 to 13) |
| Comparison 3: TR‐MRDB‐fusion vs TP‐MRDB‐fusion | |||||
|---|---|---|---|---|---|
| Outcomes | Before IPW | After IPW | Mean difference, % | ||
| TR‐MRDB‐fusion, % | TP‐MRDB‐fusion, % | TR‐MRDB‐fusion | TP‐MRDB‐fusion | ||
| Histology, % (95% CI) | |||||
| No prostate cancer | 12 | 19 | 20 (9–31) | 18 (16–21) | −1.9 (−13 to 9.7) |
| GG 1 cancer | 12 | 18 | 15 (7–22) | 18 (15–20) | 3.0 (−5.1 to 11) |
| GG≥2 cancer | 75 | 63 | 65 (54–77) | 64 (61–68) | −1.1 (−13 to 11) |
| GG ≥3 cancer | 53 | 34 | 39 (29–50) | 39 (35–42) | −0.04 (−12 to 11) |
| Comparison 4: TR‐MRDB‐fusion vs TR‐MRDB‐in‐bore | |||||
|---|---|---|---|---|---|
| Outcomes | Before IPW | After IPW | Mean difference, % | ||
| TR‐MRDB‐fusion, % | TR‐MRDB‐in‐bore, % | TR‐MRDB‐fusion (%) | TR‐MRDB‐in‐bore | ||
| Histology, % (95% CI) | |||||
| No prostate cancer | 12 | 23 | 17 (9.1–25) | 16 (9.3–22) | −0.98 (−11 to 9.1) |
| GG 1 cancer | 12 | 33 | 14 (7.5–21) | 29 (19–39) | 15 (2.7–27%) |
| GG ≥2 cancer | 75 | 44 | 69 (60–78) | 55 (44–66) | −14 (−28 to 0.57) |
| GG ≥3 cancer | 53 | 15 | 43 (34–51) | 19 (8.8–29) | −23 (−37 to −9.7) |
Percentages may not total 100 because of rounding. Due to the IPW, histology outcomes presented in this table can be different from numbers in the article before IPW. *Balance across 25 imputation sets. †Mean differences of complications between TR‐ and TP‐MRDB with RB, histological mean differences between TR‐ and TP‐MRDB‐fusion. ‡Complications before IPW include RB.
Fig. 3.
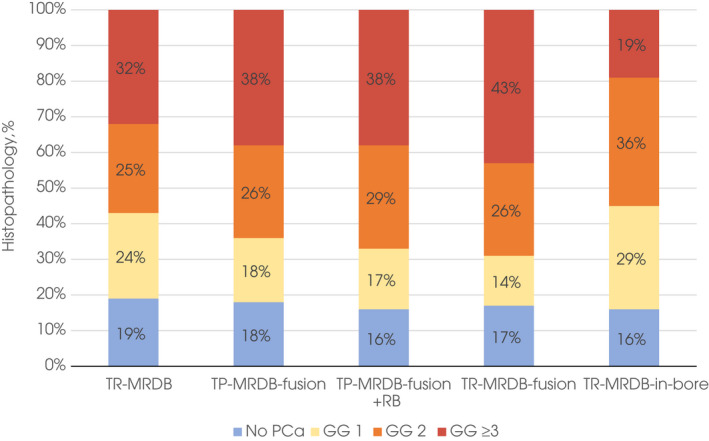
Histopathology of MRDB results per MRDB technique after IPW. Histopathological outcomes of different MRDB techniques: TR‐MRDB (‘fusion’ and ‘in‐bore’) and TP‐MRDB ‘fusion’ (with and without RB). Due to the IPW, histology outcomes presented in this figure can be different from numbers in this article before IPW. Comparable prostate cancer rates were obtained with TR‐MRDB (either fusion or in‐bore) and TP‐MRDB‐fusion. Adding RB to TP‐MRDB‐fusion slightly improved the detection rate of GG 2 cancer. TR‐MRDB‐fusion showed higher detection rates of GG ≥3 and lower rates of GG 1 cancer compared to TR‐MRDB‐in‐bore probably due to a selection bias. TR‐MRDB‐fusion and TR‐MRDB‐in‐bore were assessed after IPW for this particular comparison (i.e. Comparison 4). Therefore, the outcomes of TR‐MRDB‐fusion are slightly different when compared to TP‐MRDB‐fusion (Comparison 3 in Table 2) and TR‐MRDB‐in‐bore (Comparison 4 in Table 2). *TR‐MRDB‐fusion and TR‐MRDB‐in‐bore were assessed after IPW for this particular comparison (i.e. Comparison 4). Therefore, the outcomes of TR‐MRDB‐fusion are slightly different when compared to TP‐MRDB‐fusion (Comparison 3 in Table 2) and TR‐MRDB‐in‐bore (Comparison 4 in Table 2). PCa, prostate cancer.
Additional Prostate Cancer Detection with RBs (Without IPW)
Additional RBs to TP‐MRDB‐fusion resulted in the extra detection of 3.0% (25/830) GG ≥2 cancers (20 GG2, two GG3, and three GG4; Fig. S2) in men with either GG 1 (21/25) or no prostate cancer (four of 25) at TP‐MRDB‐fusion. In 72% (18/25) of these GG ≥2 cancers, the detected GG ≥2 cancer was ipsilateral to the MRI lesion. Any GG upgrading by adding RB occurred in 8.2% (68/830) (Fig. S2).
Discussion
Recent guidelines recommend MRI over TRUS SB as a triage test based on prospective and controlled studies [4, 5, 8, 21]. However, to assess the external validity and applicability in daily practice, results in a contemporary routine clinical setting should be evaluated. The present report is to our knowledge the largest study assessing the outcomes of MRI‐directed biopsy pathways including 2597 consecutive, clinical biopsy‐naïve patients from two ambulatory, high‐volume setting centres but based on a single‐centre consistent high‐quality MRI and pathology analysis. The strength of the present study, therefore, is its consecutive recruitment in a routine ‘real‐world’ setting.
Compared to a recent Cochrane systematic review and meta‐analysis by Drost et al. [7] the present study shows a comparable GG ≥2 overall cancer detection rate: 26% (95% CI 24–27%) in the present study vs 23% (95% CI 19–28%) in the pooled MRI pathway (for GG ≥3: 16% vs 13%). Yet, in contrast to their meta‐analysis, the number of men with an unsuspicious MRI (PI‐RADS 1–2) in which (immediate) biopsy was avoided was significantly higher in the present study: 56% (95% CI 55–58%) vs 33% (95% CI 26–41%).
To utilise MRI pathways to the fullest, avoidance of ‘unnecessary’ biopsies is critical. Not only to prevent biopsy‐related morbidity and anxiety but also to reduce diagnosis (and potential overtreatment) of indolent prostate cancer [22, 23]. The latter is of particular interest in the ongoing debate on (opportunistic) prostate cancer screening [2, 24]. Recently, the Imperial Prostate 1 Prostate Cancer Screening Trial Using Imaging (IP1‐PROSTAGRAM) was the first study to show MRI as a screening test could improve detection of clinically significant prostate cancer compared with initial PSA testing [25]. Furthermore, it is worth highlighting that the total prevalence of men with GG 1 cancers in our present study was low (8.1%) compared to the MRI pathway of the previously mentioned meta‐analysis (11%), possibly because of higher biopsy avoidance [7]. Our higher percentage of biopsy avoidance, a lower detection rate of GG 1 cancers, without compromising the detection rate of GG ≥2 and GG ≥3 cancers may be explained by the ‘state‐of‐the‐art’ acquisition and reporting of MRI by dedicated radiologists with strict adherence to PI‐RADS v2 recommendations [26]. Despite the routine clinical setting in which the MRIs were performed and assessed, the number of ‘equivocal’ or ‘uncertain’ PI‐RADS 3 scores was low (5.7%). The positive predictive value of suspicious MRI (i.e. PI‐RADS scores 3–5) for GG ≥2 cancer was markedly higher than reported in the previously mentioned meta‐analysis, and in a recent large multicentre study: 63% vs 44% vs 35%, respectively [7, 27]. However, variability between centres is wide, this emphasises the importance of high‐quality MRI acquisition and reporting, in addition to accurate MRDB [26, 27].
After IPW to reduce confounding by indication, the comparison between TR‐MRDB and TP‐MRDB did not result in significantly different detection rates for GG 1, GG ≥2, and GG ≥3 cancers, indicating equal accuracy. Also, there was no difference in (infectious) complications. However, some differences between TP‐ and TR‐MRDB strategies should be discussed. Men who underwent TP‐MRDB had additional RB, whereas in the TR‐MRDB cohort only targeted cores were taken. We suggest that the equally (very) low complication rate in men who underwent TR‐MRDB could be the result of omitting additional RB cores. This accords with a previous study in men with prior negative biopsies, where a correlation was reported between the number of transrectal biopsies and infectious complications [28]. Also, the TP‐MRDB patients did not receive antibiotic prophylaxis, a novelty in prostate cancer diagnostics in an era of increasing prophylactic antibiotic resistance [29]. Nonetheless, under‐reporting of complications cannot be excluded. Studies using patient‐reported biopsy complications report higher compilation rates compared to data from electronic patient files registered by the healthcare providers [30, 31].
The current American and European urological guidelines recommend combined MRDB and SB in biopsy‐naïve men with a suspicious MRI, as SB has been shown to identify an additional 4.9% (95% CI 2.8–8.3%) of GG ≥2 cancer in men undergoing MRDB [4, 7, 8]. An important finding of our present study was that a comparable percentage of extra GG ≥2 cancers (3%; 95% CI 1.9–4.4%) was detected with RB, predominantly on the same side as the MRI lesion. These findings add to a growing body of research supporting a ‘target/focal saturation’ approach (i.e. extra perilesional biopsy cores), instead of SB [32, 33]. Focal saturation instead of SB minimises the number of cores and thus biopsy‐related morbidity, and also reduces the detection of GG 1 cancers without affecting the GG ≥2 cancer detection rate [34]. Prior studies showed the effectiveness of such approaches, although SB could still be preferred if nerve‐sparing surgery or focal therapy is considered [35, 36]. However, as previously stated, adding additional biopsy cores to TR‐MRDB might increase the risk of infections as opposed to TP‐MRDB where this risk remains low. In a recent study, Ahdoot et al. [37] presented their data of combined MRDB and SB in 2103 men with MRI‐visible lesions. They reported that GG ≥2 cancer was detected in an extra 5.8% (95% CI 4.9–6.9%) of men by performing additional SB to MRDB, which is higher compared to 3% for additional RB to TP‐MRDB in the present study [37]. This slightly higher percentage can be explained by their mixed population: one‐third were positive for prostate cancer at prior biopsy. These men have a higher re‐classification risk compared to biopsy‐naïve men [38].
The present study is not without limitations. The main limitation is the lack of histopathological confirmation in men with a unsuspicious MRI. The negative predictive values of MRI for GG ≥2 cancer in two recent systematic reviews were 91% and 92%, whereas a previous trial in our centre reported a negative predictive value of 96% (including 1‐year of follow‐up) [6, 7, 39]. Most GG ≥2 cancers that are overlooked by MRI are GG2 cancers [7]. A recent sub‐analysis of the template‐verification biopsies in the PROstate MRI Imaging Study (PROMIS) study in men with an unsuspicious MRI confirmed that MRI missed few clinically significant prostate cancers and that all undetected prostate cancers were GG ≤2 [40]. More importantly, omitting biopsy in men with an unsuspicious MRI can safely be realised, provided that there is an effective safety net to enable finding ‘MRI pathway missed’ GG ≥2/GG ≥3 cancers [41]. This safety net consists of at least 6‐monthly testing of the serum PSA level [6, 42]. In men with prostate cancer, unavailability of a reference standard (i.e. radical prostatectomy specimen or template‐mapping biopsy) disallows a comparison with the ‘gold standard’.
Furthermore, even though IPW was used to correct for the most relevant confounders, due to the retrospective nature of the present study, and biopsy operator variability, residual confounding and a possible centre effect cannot fully be excluded. Although reflecting clinical practice, the use of two TR‐MRDB methods, including the time‐consuming and costly MRDB‐in‐bore, might have induced a selection bias and could influence the generalisability of our present findings. Reproducibility could further be limited by optimised acquisition using 3‐T MRI scanners and assessment by experienced radiologists.
Finally, dedicated radiologists and pathologists assessed the images and the histopathology, but a central review was not performed. Nonetheless, prior studies have shown a MRI inter‐reader agreement rate of 93% regarding whether the MRI was suspicious (PI‐RADS 3–5) or not (PI‐RADS 1–2) [43]. This large practice‐based study confirms the validity and feasibility to implement pre‐biopsy MRI pathways in biopsy‐naïve men with suspicion of prostate cancer, as recommended by the current prostate cancer guidelines.
Conclusion
The present high‐volume, two‐centre study confirms the value of a pre‐biopsy MRI‐based decision tool in routine clinical practice. Compared to recent trials, a substantially higher biopsy avoidance rate was achieved without compromising GG ≥2/GG ≥3 detection and coinciding with lower over detection rates of GG 1 cancer. For the TR‐ and TP‐MRDB approaches, prostate cancer detection and complication rates were comparable.
Disclosures of Interests
All authors reported to have no conflict of interest.
Author Contributions
Jelle O. Barentsz had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Israël, Immerzeel, Debruyne, Sedelaar and Barentsz. Acquisition of data: Israël and Immerzeel. Analysis and interpretation of data: Israël, Immerzeel, Hannink, Schoots and Barentsz. Drafting of the manuscript: Israël, Immerzeel and Barentsz. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Israël and Hannink. Obtaining funding: None. Supervision: Barentsz. Other: None.
Abbreviations
- DWI
diffusion‐weighted imaging
- GG
Grade Group
- IPW
Inverse probability weighting
- ISUP
International Society of Urological Pathology
- mpMRI
multiparametric MRI
- MRDB
MRI‐directed biopsy
- PI‐RADS
Prostate Imaging‐Reporting And Data System
- PSAD
PSA density
- RB
random biopsy
- T
Tesla
- T2‐WI
T2‐weighted imaging
- TP
transperineal
- TR
transrectal
Supporting information
Fig. S1. Standardised mean differences of patient characteristics before and after IPW of TR‐MRDB vs TP‐MRDB (including RB).
Fig. S2. Upgrading of TP‐MRDB‐fusion by RBs (n = 830).
Table S1. The mpMRI acquisition protocol on 3‐T MRI‐scanner.
References
- 1. Ferlay J, Ervik M, Lam F et al. Global cancer observatory: cancer tomorrow. 2018. Available at: https://gco.iarc.fr/tomorrow. Accessed September 2020
- 2. Grossman DC, Curry SJ, Owens DK et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA 2018; 319: 1901–13 [DOI] [PubMed] [Google Scholar]
- 3. Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 2020; 77: 38–52 [DOI] [PubMed] [Google Scholar]
- 4. Mottet N, van den Bergh RCN, Briers E et al. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer 2020. European Association of Urology Guidelines 2020 Edition. Arnhem, t: he Netherlands: European Association of Urology Guidelines Office, 2020. [Google Scholar]
- 5. Rouvière O, Puech P, Renard‐Penna R et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy‐naive patients (MRI‐FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019; 20: 100–9 [DOI] [PubMed] [Google Scholar]
- 6. van der Leest M, Cornel E, Israël B et al. Head‐to‐head comparison of transrectal ultrasound‐guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance‐guided biopsy in biopsy‐naive men with elevated prostate‐specific antigen: a large prospective multicenter clinical study. Eur Urol 2019; 75: 570–8 [DOI] [PubMed] [Google Scholar]
- 7. Drost FJ, Osses DF, Nieboer D et al. Prostate MRI, with or without MRI‐targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev 2019; 4: CD012663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjurlin MA, Carroll PR, Eggener S et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J Urol 2020; 203: 706–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high‐impact general medical journals: a systematic sampling review. JAMA 2007; 297: 1233–40 [DOI] [PubMed] [Google Scholar]
- 10. Barrett T, Slough R, Sushentsev N et al. Three‐year experience of a dedicated prostate mpMRI pre‐biopsy programme and effect on timed cancer diagnostic pathways. Clin Radiol 2019; 74: 894.e1–9 [DOI] [PubMed] [Google Scholar]
- 11. Grummet J, Pepdjonovic L, Huang S, Anderson E, Hadaschik B. Transperineal vs. transrectal biopsy in MRI targeting. Transl Androl Urol 2017; 6: 368–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore CM, Kasivisvanathan V, Eggener S et al. Standards of reporting for MRI‐targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013; 64: 544–52 [DOI] [PubMed] [Google Scholar]
- 13. Bossuyt PM, Reitsma JB, Bruns DE et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 2015; 277: 826–32 [DOI] [PubMed] [Google Scholar]
- 14. Weinreb JC, Barentsz JO, Choyke PL et al. PI‐RADS prostate imaging – reporting and data system: 2015, version 2. Eur Urol 2016; 69: 16–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bomers JG, Bosboom DG, Tigelaar GH, Sabisch J, Fütterer JJ, Yakar D. Feasibility of a 2(nd) generation MR‐compatible manipulator for transrectal prostate biopsy guidance. Eur Radiol 2017; 27: 1776–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hambrock T, Fütterer JJ, Huisman HJ et al. Thirty‐two‐channel coil 3T magnetic resonance‐guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol 2008; 43: 686–94 [DOI] [PubMed] [Google Scholar]
- 17. Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason grading of prostatic carcinoma. Am J Surg Pathol 2017; 41: e1–7 [DOI] [PubMed] [Google Scholar]
- 18. Assel M, Sjoberg D, Elders A et al. Guidelines for reporting of statistics for clinical research in urology. BJU Int 2019; 123: 401–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitropoulos D, Artibani W, Biyani CS, Bjerggaard Jensen J, Rouprêt M, Truss M. Validation of the Clavien‐Dindo grading system in urology by the European association of urology guidelines ad hoc panel. Eur Urol Focus 2018; 4: 608–13 [DOI] [PubMed] [Google Scholar]
- 20. Briganti A, Fossati N, Catto JW et al. Active surveillance for low‐risk prostate cancer: the European Association of Urology position in 2018. Eur Urol 2018; 74: 357–68 [DOI] [PubMed] [Google Scholar]
- 21. Kasivisvanathan V, Rannikko AS, Borghi M et al. MRI‐targeted or standard biopsy for prostate‐cancer diagnosis. N Engl J Med 2018; 378: 1767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wade J, Rosario DJ, Macefield RC et al. Psychological impact of prostate biopsy: physical symptoms, anxiety, and depression. J Clin Oncol 2013; 31: 4235–41 [DOI] [PubMed] [Google Scholar]
- 23. Loeb S, Bjurlin MA, Nicholson J et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014; 65: 1046–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gandaglia G, Albers P, Abrahamsson P‐A et al. Structured population‐based prostate‐specific antigen screening for prostate cancer: the European Association of Urology position in 2019. Eur Urol 2019; 76: 142–50 [DOI] [PubMed] [Google Scholar]
- 25. Eldred‐Evans D, Burak P, Connor MJ et al. Population‐based prostate cancer screening with magnetic resonance imaging or ultrasonography: the IP1‐PROSTAGRAM study. JAMA Oncol 2021; 7: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Rooij M, Israël B, Tummers M et al. ESUR/ESUI consensus statements on multi‐parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists' training. Eur Radiol 2020; 30: 5404–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westphalen AC, McCulloch CE, Anaokar JM et al. Variability of the positive predictive value of PI‐RADS for prostate MRI across 26 centers: experience of the society of abdominal radiology prostate cancer disease‐focused panel. Radiology 2020; 296: 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wegelin O, Exterkate L, van der Leest M et al. Complications and adverse events of three magnetic resonance imaging‐based target biopsy techniques in the diagnosis of prostate cancer among men with prior negative biopsies: results from the FUTURE trial, a multicentre randomised controlled trial. Eur Urol Oncol 2019; 2: 617–24 [DOI] [PubMed] [Google Scholar]
- 29. Pilatz A, Dimitropoulos K, Veeratterapillay R et al. Antibiotic prophylaxis for the prevention of infectious complications following prostate biopsy: a systematic review and meta‐analysis. J Urol 2020; 204: 224–30 [DOI] [PubMed] [Google Scholar]
- 30. Rosario DJ, Lane JA, Metcalfe C et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012; 344: d7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamhankar AS, El‐Taji O, Vasdev N, Foley C, Popert R, Adshead J. The clinical and financial implications of a decade of prostate biopsies in the NHS: analysis of Hospital Episode Statistics data 2008–2019. BJU Int 2020; 126: 133–41 [DOI] [PubMed] [Google Scholar]
- 32. Lahoud J, Doan P, Kim L, Patel MI. Perilesional biopsies increase detection of significant prostate cancer in men with PI‐RADS 4/5 lesions: validation of the PI‐RADS steering committee recommendation. Eur Urol 2021; 80: 260–1 [DOI] [PubMed] [Google Scholar]
- 33. Hansen NL, Barrett T, Lloyd T et al. Optimising the number of cores for magnetic resonance imaging‐guided targeted and systematic transperineal prostate biopsy. BJU Int 2020; 125: 260–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldberg H, Ahmad AE, Chandrasekar T et al. Comparison of magnetic resonance imaging and transrectal ultrasound informed prostate biopsy for prostate cancer diagnosis in biopsy naive men: a systematic review and meta‐analysis. J Urol 2020; 203: 1085–93 [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Milot L, Khalvati F et al. Value of increasing biopsy cores per target with cognitive MRI‐targeted transrectal US prostate biopsy. Radiology 2019; 291: 83–9 [DOI] [PubMed] [Google Scholar]
- 36. Tschirdewahn S, Wiesenfarth M, Bonekamp D et al. Detection of significant prostate cancer using target saturation in transperineal magnetic resonance imaging/transrectal ultrasonography‐fusion biopsy. Eur Urol Focus 2020. (Online ahead of print). 10.1016/j.euf.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 37. Ahdoot M, Wilbur AR, Reese SE et al. MRI‐targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 2020; 382: 917–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chesnut GT, Vertosick EA, Benfante N et al. Role of changes in magnetic resonance imaging or clinical stage in evaluation of disease progression for men with prostate cancer on active surveillance. Eur Urol 2020; 77: 501–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sathianathen NJ, Omer A, Harriss E et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta‐analysis. Eur Urol 2020; 78: 402–14 [DOI] [PubMed] [Google Scholar]
- 40. Norris JM, Carmona Echeverria LM, Bott SR et al. What type of prostate cancer is systematically overlooked by multiparametric magnetic resonance imaging? An analysis from the PROMIS cohort. Eur Urol 2020; 78: 163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Venderink W, van Luijtelaar A, van der Leest M et al. Multiparametric magnetic resonance imaging and follow‐up to avoid prostate biopsy in 4259 men. BJU Int 2019; 124: 775–84 [DOI] [PubMed] [Google Scholar]
- 42. Israël B, van der Leest M, Sedelaar M, Padhani AR, Zámecnik P, Barentsz JO. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 2: interpretation. Eur Urol 2020; 77: 469–80 [DOI] [PubMed] [Google Scholar]
- 43. van der Leest M, Israël B, Cornel EB et al. High diagnostic performance of short magnetic resonance imaging protocols for prostate cancer detection in biopsy‐naive men: the next step in magnetic resonance imaging accessibility. Eur Urol 2019; 76: 574–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Standardised mean differences of patient characteristics before and after IPW of TR‐MRDB vs TP‐MRDB (including RB).
Fig. S2. Upgrading of TP‐MRDB‐fusion by RBs (n = 830).
Table S1. The mpMRI acquisition protocol on 3‐T MRI‐scanner.


