INTRODUCTION
Severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) infection can cause a wide array of symptoms ranging from mild to severe or fatal forms of coronavirus disease 2019 (COVID‐19). 1 Furthermore, it has been observed that in a proportion of patients a variable range of symptoms may persist for a long time. 2
An increasing number of studies have been focused on long COVID, but they have mainly been concentrated on previously hospitalized severe COVID‐19 patients reporting symptoms up to 6‐months after illness. 3 , 4 The main aim of this study was to evaluate the prevalence of COVID‐related symptoms 12‐months after the onset of mild‐to‐moderate disease.
METHODS
We conducted a prospective study on mild‐to‐moderate symptomatic patients consecutively assessed between March 1 and March 31, 2020, who tested positive for SARS‐CoV‐2 RNA by polymerase chain reaction (PCR) on nasopharyngeal and throat swabs performed according to World Health Organization recommendation. An interdisciplinary task force of medical doctors and nurses was created in our region in response to the COVID‐19 pandemic in order to monitor all isolated patients with PCR‐confirmed SARS‐CoV‐2 infection. The task force provided us with the names and telephone contacts of the home‐isolated COVID‐19 patients. The study was approved by the Ethics Committee of the Friuli Venezia Giulia Region (CEUR‐2020‐Os‐156). Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
In order to have a homogeneous population of patients who could be contacted by telephone, only mild‐to‐moderately symptomatic subjects were included in the study. Patients were considered mild‐to‐moderately symptomatic if they had less severe clinical symptoms, oxygen saturation (SpO2) ≥ 94%, not requiring hospitalization, and therefore considered suitable for being treated at home. Asymptomatic patients were not included in the study.
Of the 496 eligible patients, 354 (71.4%) completed the baseline telephone interview, which was conducted within 3 weeks after the first positive swab performed from March 1, 2020 to March 31, 2020. The median time from onset of symptoms to SARS‐CoV‐2 testing was 7 days (interquartile range [IQR], 4–12 days). All patients completing the baseline interview were phoned from March 5, 2021 to March 22, 2021, so that all patients were re‐contacted 12‐months after the onset of symptoms; in case of a nonresponse, patients were re‐contacted twice.
Finally, of the 354 patients who completed the survey at baseline, 304 completed the 12‐months follow‐up survey (85.9%) as well; no differences emerged between responders and nonresponders in terms of baseline sociodemographic and clinical characteristics, including the number and the severity of symptoms (data not shown).
Differences in prevalence were evaluated through Fisher's exact test and odds ratio (OR) for symptoms persistence was calculated according to multivariable unconditional logistic regression model.
RESULTS
Baseline sociodemographic and clinical characteristics of 304 patients are reported in Table 1. The median age of the study cohort was 47 years (range, 18–76 years).
TABLE 1.
Sociodemographic and clinical characteristics of 304 patients positive for COVID‐19
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 119 (39.1) |
| Female | 185 (60.9) |
| Age (years) a | |
| <40 | 93 (30.6) |
| 40–54 | 119 (39.1) |
| ≥55 | 92 (30.3) |
| Smoking habits | |
| Never | 179 (58.9) |
| Former | 74 (24.3) |
| Current | 51 (16.8) |
| Drinking habits | |
| Never | 202 (66.5) |
| Former | 30 (9.9) |
| Current | 72 (23.7) |
| BMI (kg/m2) | |
| <25 | 170 (55.9) |
| ≥25 | 134 (44.1) |
| Comorbidity | |
| None | 206 (67.8) |
| Any | 98 (32.2) |
| Obesity | 36 (11.8) |
| Cardiovascular disease | 26 (8.6) |
| Chronic respiratory disease | 21 (6.9) |
| Immunosuppression | 18 (5.9) |
| Diabetes | 10 (3.3) |
| Cancer | 5 (1.6) |
| Liver disease | 5 (1.6) |
| Kidney disease | 2 (0.7) |
| Time for a negative swab (days) | |
| ≤23 | 157 (51.6) |
| >23 | 147 (48.4) |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Median age (IQR): 47 years (37–56 years).
During the acute phase of COVID‐19 the most frequently reported symptoms were fever (n = 232, 76.3%), felt tired (n = 216, 71.1%), smell impairment (n = 201, 66.1%), taste impairment (n = 198, 65.1%), and joint pain (n = 181, 59.5%) (Figure 1).
FIGURE 1.
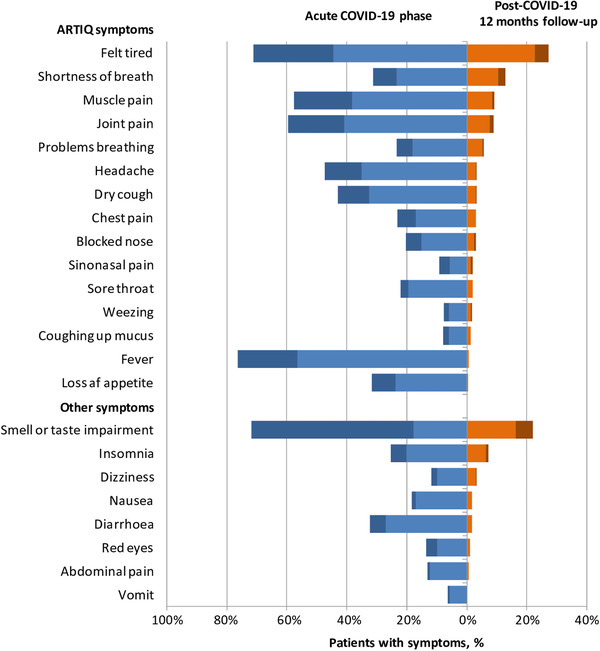
COVID‐19‐related symptoms. The percentages of patients with COVID‐19‐related symptoms during the acute phase of illness (blue) and at 12‐months follow‐up (orange) according to severity (light, mild; dark, severe). Abbreviations: ARTIQ, Acute Respiratory Tract Infection Questionnaire; COVID‐19, coronavirus disease 2019.
Persistence of at least one symptom at 12 months was reported by 161 patients (53.0%) with the most frequent being "felt tired" (n = 83, 27.3%), followed by "smell or taste impairment" (n = 67, 22.0%), "shortness of breath" (n = 39, 12.8%), and "muscle pain" (n = 28, 9.2%).
The risk of symptom's persistence at 12‐months follow‐up was trending upward in females (OR = 1.64; 95% confidence interval [CI], 1.00–2.70; p = 0.051), and significantly higher in subjects aged between 40 and 54 years (OR = 1.92; 95% CI, 1.07–3.44; p = 0.029) and in those with a body mass index BMI ≥ 25 k/mg2 (OR = 1.67; 95% CI, 1.00–2.78; p = 0.049). The presence of three to seven symptoms during the acute phase of the disease was associated with a significantly higher chance of symptoms persistence after 12 months (OR = 3.22; 95% CI, 1.01–10.24; p = 0.048), with this association being stronger in those having eight or more symptoms (OR = 8.71; 95% CI, 2.73–27.76; p < 0.001).
DISCUSSION
Twelve months after the onset of illness, 53.0% of patients with mild‐to‐moderate disease endorsed at least one persistent symptom. A previous investigation in a large cohort of previously hospitalized patients reported at least one symptom in 76% of cases 6 months after acute infection, 4 whereas in another study, including outpatients with mild disease, ∼30% reported persistent symptoms at 6 months. 5 Thus, taking into account that our series included only outpatients with mild‐to‐moderate disease representing the overwhelming majority of COVID‐19 patients, 6 the burden of long haulers on the healthcare systems will be pressing and urged researchers to identify strategies for prevention and treatment and to plan education and rehabilitation services in order to face with the considerable health and economic concerns. 2
In agreement with other evidence, the most commonly reported symptom was fatigue followed by chemosensory dysfunction. 2 Chemosensory dysfunction has been consistently reported as one of the most predominant symptom during the acute phase of the disease as well as among the most frequent long‐lasting symptoms in patients with COVID‐19, with a higher prevalence in patients with mild‐to‐moderate disease. 7 Some authors have speculated that mild‐to‐moderate COVID‐19 patients may be affected by nasal‐centric viral spread, whereas patients requiring hospitalization may be experiencing a more pulmonary‐centric and systemic viral infection, consequently impacting the prevalence of different symptoms both in acute phase and in the context of long COVID syndrome. 8 However, although all patients in the study were not formally diagnosed with pneumonia having an SpO2 ≥ 94%, about 30% reported shortness of breath at the onset and 13% still complained shortness of breath at 12‐months follow‐up interview. Interestingly, an abnormal pulmonary function consisting in an impaired diffusing‐capacity was observed in 30% of patients after the acute phase of mild COVID‐19 without pneumonia. 9 However, shortness of breath can be a manifestation of underlying acute stress or anxiety with these conditions being reported in patients with COVID‐19. 4
Experiencing more symptoms at baseline was the most significant factor associated with long COVID. Moreover, the prevalence of long haulers was higher in females, patients in their middle age, and those with BMI ≥ 25. Thus, models to identify patients at risk for long COVID may be developed.
This study has several limitations. First, the absence of a control group may have an impact on the interpretation of results. Hospitalized patients were not included in the study. Although this made our cohort more homogeneous, studies comparing the long‐term outcomes in inpatients and outpatients are definitely needed. Symptoms were self‐reported and based on telephone interview. Though we tried to perform a comprehensive symptoms assessment, some symptoms may have been undetected. Furthermore, a more precise evaluation of the chemosensory function by psychophysical assessment was lacking. Finally, a total of 14.1% of responders at baseline did not complete the follow‐up interview, thus introducing a potential bias in the results. Nonetheless, nonresponders were comparable to responders in terms of symptoms frequency and severity, thus limiting selection bias.
In conclusion, our study indicates that persistent symptoms of SARS‐CoV‐2 infection can be detected beyond 12 months from the onset of the illness in more than half of outpatients. Identifying patients at risk for prevention and treatment will be critical to improving outcomes and reducing health costs. Finally, a structured and validated questionnaire for the assessment of symptoms in COVID‐19 patients is highly desirable to characterize the full clinical spectrum of long COVID and improve the reliability and reproducibility of clinical studies.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Trieste within the CRUI‐CARE Agreement.
Boscolo‐Rizzo P, Guida F, Polesel J, et al. Sequelae in adults at 12 months after mild‐to‐moderate coronavirus disease 2019 (COVID‐19). Int Forum Allergy Rhinol. 2021;11:1685–1688. 10.1002/alr.22832
[Correction added on 16 May 2022, after first online publication: CRUI funding statement has been added.]
REFERENCES
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324:782‐793. [DOI] [PubMed] [Google Scholar]
- 2. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carfì A, Bernabei R, Landi F. Gemelli against COVID‐19 post‐acute care study group. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324:603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID‐19 infection. JAMA Netw Open. 2021;4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383:1757‐1766. [DOI] [PubMed] [Google Scholar]
- 7. Boscolo‐Rizzo P, Polesel J, Spinato G, et al. Predominance of an altered sense of smell or taste among long‐lasting symptoms in patients with mildly symptomatic COVID‐19. Rhinology. 2020;58:524‐525. [DOI] [PubMed] [Google Scholar]
- 8. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]


