Abstract
In children with autism spectrum disorder (ASD), joint attention is regarded as a predictor of language function, social skills, communication, adaptive function, and intelligence. However, existing information about the association between joint attention and intelligence is limited. Most such studies have examined children with low intelligence. For this study, we investigated whether joint attention is related to intelligence in young children with autism spectrum disorder (ASD) without severe intellectual disability. We analyzed 113 children with ASD aged 40–98 months. Their Kaufman Assessment Battery (K‐ABC) Mental Processing Index (MPI) scores are 60 and more (mean 93.4). We evaluated their intelligence using K‐ABC and evaluated their joint attention using ADOS‐2. After we performed simple regression analyses using K‐ABC MPI and its nine subscales as dependent variables, using joint attention as the independent variable, we identified joint attention as a positive predictor of the MPI and its two subscales. From this result, we conclude that joint attention is related to intelligence in young children with ASD without severe intellectual disability. This result suggests a beneficial effect of early intervention targeting joint attention for children with ASD.
Lay Summary
Joint attention is the ability to coordinate visual attention with another person and then shift one's gaze toward an object or event. Impairment of joint attention is regarded as an early marker of autism spectrum disorder (ASD). This study revealed impairment of joint attention as associated with lower intelligence in ASD children. These results are expected to constitute a rationale for future studies, particularly addressing beneficial effects of early intervention targeting joint attention for children with ASD.
Keywords: autism, autism diagnostic observation schedule, children, intelligence, joint attention, Kaufman Assessment Battery (K‐ABC)
INTRODUCTION
Autism spectrum disorder (ASD) is characterized classically by impairment of social interaction, social communication, and social imagination (Wing, Gould, & Gillberg, 2011). In addition, ASD is associated with lower language function (Sigman & Ruskin, 1999; Volkmar, Paul, Klin, & Cohen, 2005) and lower intelligence (Fombonne, 2002; Henninger & Taylor, 2013; Howlin, Goode, Hutton, & Rutter, 2004) Furthermore, many people with ASD need social support of some kind, even in adulthood (Howlin et al., 2004). Elucidating ASD pathophysiology is necessary because ASD strongly affects social life.
Earlier reports suggest that joint attention behavior emerges and that it can be measured in typically developing (TD) children as early as 3–6 months of age (D'Entremont, Hains, & Muir, 1997; Morales, Mundy, & Rojas, 1998). After consolidation at around 9 months of age (Bakeman & Adamson, 1984), it continues to develop to at least through 3 years of age (Adamson, Bakeman, & Deckner, 2004).
Joint attention, which is regarded as a predictor of receptive language in children (Charman et al., 2003), is also regarded as related to language acquisition, social skills, communication (Bottema‐Beutel, 2016; Charman, 2003; Poon, Watson, Baranek, & Poe, 2012), verbal/non‐verbal intelligence quotient (VIQ/NVIQ), and adaptive function (Harrison, Lu, McLean, & Sheinkopf, 2016). Some earlier studies have failed to demonstrate these relations (Stone & Yoder, 2001; Travis, Sigman, & Ruskin, 2001). Nevertheless, based on earlier positive findings, joint attention has become a target for therapeutic intervention. Intervention for joint attention improves joint attention behavior (Murza, Schwartz, Hahs‐vaughn, & Nye, 2016), and brings side benefits. Jones and Whalen demonstrated that children with ASD increased spontaneous speech after joint attention intervention (Jones, Carr, & Feeley, 2006; Whalen, Schreibman, & Ingersoll, 2006). These results are expected to constitute a rationale for future studies, particularly addressing beneficial effects of early intervention targeting on joint attention for children with ASD.
Many studies have examined relations between joint attention and language, social skills, and adaptive behaviors. Among those topics, the relation between joint attention and language function has been important. Researchers have discussed details of the relation. Numerous reports have been published (Bottema‐Beutel, 2016). However, putative relations between joint attention and intelligence itself remain under debate. Several earlier researchers have examined this relation as an outcome, but only two have primarily addressed this topic (Harrison et al., 2016; Mundy, Sigman, & Kasari, 1994). Mundy et al. recruited 30 preschool children with ASD, 30 with developmental delays, and 30 with normal development. They divided the participants into two groups (i.e., moderate to severe of intellectual disability and mild intellectual disability to average range of intelligence) based on IQ measured by the Cattell Developmental Scale (Cattell, 1960) or the Stanford–Binet Intelligence Test (Thorndike, 1972). The authors reported that lower IQ (mean IQ = 40.8) autistic children displayed a more pronounced deficit on low level joint attention (e.g., shift in eye gaze) than did higher IQ (mean IQ = 67.9) autistic children. No relation, however, was observed between cognitive impairment and high level joint attention behavior (social orienting or showing). This somewhat ambiguous finding was replicated and was elucidated further by Harrison et al. in a later study conducted with a larger sample. They assessed VIQ and NVIQ for 1061 individuals with ASD (aged 4–18 years) using the Differential Ability Scales – Second Edition (DAS‐II) and reported correlation between joint attention and both VIQ and NVIQ. It is noteworthy that the participants' intelligence was low (Mean VIQ = 53.45, NVIQ = 66.88) and that the participants' severity of ASD was high: Mean Social Responsiveness Scale total score = 81.62 (Constantino, 2005). As a result, those findings cannot be generalized for children with ASD who are more intelligent or less autistic or both, a population for which evidence of the relation remains insufficient. Aside from these two seminal studies, some other studies have evaluated the relation as a secondary outcome (Poon et al., 2012; Wetherby, Watt, Morgan, & Shumway, 2007). Results of these studies also imply possible correlation between joint attention and intelligence in children with ASD. For example, Wetherby et al. and Poon et al. reported joint attention development as positively correlated with the developmental quotient (DQ). However, one important limitation of these studies is the fact that DQ is fundamentally a comprehensive measure of development including intelligence, movement, and interpersonal relations. As such, it cannot be regarded as a specific measure of intelligence.
In sum, the relation between joint attention and intelligence has been confirmed for children with ASD having lower intelligence, but no report describes a study investigating whether the relation holds for children with higher intelligence. Extending the evidence to high‐functioning ASD is expected to be necessary from both practical and theoretical viewpoints. Practically, such a study would be helpful when one applies joint attention‐targeted intervention for high‐functioning ASD. A sizable minority of ASD having intellectual function within the normal range (e.g., reportedly 30% [Fombonne, 2002]). Theoretically, such a study would help elucidate the relation between sociality and intelligence in high‐functioning children with ASD, a population in which sociality and intelligence are reported to be mutually dependent (Hirosawa et al., 2020). Particularly, this report from an earlier study described that only a specific aspect of sociality (i.e., social cognition) relates to intelligence in children with high‐functioning ASD, but such a relation is not observed in typically developing children (Hirosawa et al., 2020). Given a specific link between a particular aspect of sociality and intelligence, it is also possible that another aspect of sociality (i.e., joint attention) also relates to intelligence in children with high‐functioning ASD. Furthermore, to identify and elucidate the intersection between intelligence and sociality, potentially interesting is whether sociality is related to intelligence itself or only to subfactors of intelligence. In this sense, it would be helpful to assess how joint attention relates differently to sub‐factors of intelligence.
Therefore, for this study, we recruited young children with ASD without severe intellectual disability and evaluated relations between intelligence and joint attention.
As reported also by Harrison et al., we evaluated joint attention using the Autism Diagnostic Observation Schedule (ADOS): a standardized scale for diagnosing and assessing ASD severity. It ensures inter‐rater consistency. Using ADOS, we can integrate results of multiple studies, construct a database, and confirm the reproducibility. We use the Kaufman Assessment Battery (K‐ABC) as a measure of intelligence. K‐ABC is used widely as an evaluation scale for intelligence. It was designed so that language plays a minimal role in the measurement of intelligence (Kaufman, O'Neal, Avant, & Long, 1987). As such, it might help control for language, which is known to be related to joint attention, and isolate intelligence more. Another difference from other IQ tests (e.g., WISC‐R) is decreased cross‐cultural score differences (Kaufman, Kaufman, & Kamphaus, 1985). In addition, using K‐ABC, one can decompose intelligence into multiple subscales for evaluation. By individually evaluating the relation between each subscale and joint attention, one can verify which component of intelligence is linked with joint attention. These facts combined to make K‐ABC particularly suitable for the current study.
Our primary hypothesis is that joint attention evaluated by ADOS is related significantly with intelligence evaluated by K‐ABC. Particularly, better joint attention corresponds to higher K‐ABC composite scores in young children having ASD without severe intellectual disability (i.e., Mental Processing Index [MPI] ≥60). Although no report of an earlier study describes investigation of the association between subscales of MPI and joint attention, as a result of the earlier demonstrated relation between non‐verbal IQ and joint attention (Harrison et al., 2016), we hypothesized that joint attention is related to each subscale of the MPI.
METHOD
Participants
From Kanazawa University and affiliated hospitals, we recruited 153 children with ASD (116 boys, 37 girls, aged 36–98 months). The ASD diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM‐IV) using the Diagnostic Interview for Social and Communication Disorders (DISCO) or the Autism Diagnostic Observation Schedule (ADOS). Nineteen children were unable to complete the psychometric evaluation. Therefore, we excluded them from statistical analyses.
The ADOS‐2 encompasses five modules, each with its own schedule of activities for participants with particular developmental and language capabilities, ranging from those who are preverbal or have minimal language skills (Module T and Module 1) or not verbally fluent children (Module 2) to verbally fluent children (Module 3), or adolescent and adults (Module 4) (Lord et al., 2012). Items are scored on a 4‐point scale, with the highest scores of 2 and 3 collapsed in the algorithm. Regarding our participants, 11 children were evaluated using Module 1, 116 children using Module 2, and 7 children using Module 3. To unify the evaluation scale of joint attention, we included only 116 children evaluated using Module 2 in statistical analyses. Additionally, we excluded three children for whom the K‐ABC MPI was below 60 to unify the intellectual function of our sample. Finally, we analyzed 113 children with ASD (85 boys, 28 girls; aged 40–98 months). Table 1 presents their characteristics. Among these 113 children, data of 84 (74%) children exceeded both ADOS‐2 and DISCO thresholds, data of 11 (10%) exceeded ADOS‐2 threshold only, and data of 15 (13%) exceeded the DISCO threshold only. Three (3%) children fell below both ADOS‐2 and DISCO thresholds, but met DSM‐IV criteria. We conducted additional analyses excluding these three children. Characteristics of participants excluding them are shown in Table S1.
TABLE 1.
Characteristics of participants
| N | 113 |
|---|---|
| Gender (%boy) | 75% |
| Age in months | 67.9 (11.1) |
| K‐ABC scores | |
| Mental processing index | 93.4 (18.2) |
| Achievement scale | 93.8 (17.2) |
| ADOS‐2 scores | |
| SA | 8.8 (3.5) |
| RRB | 1.8 (1.4) |
| Total | 10.7 (4.1) |
| CS | 5.4 (1.9) |
Note: Numbers are mean (SD) or counts.
Abbreviations: ADOS‐2, autism diagnostic observation schedule‐2; Cs, comparison score; K‐ABC, Kaufman Assessment Battery for Children; RRB, restricted and repetitive behaviors; SA, social affect.
In addition, as an exploratory analysis, we analyzed 11 children (8 boys, 3 girls; age 38–69 months) evaluated separately using Module 1. Their MPI scores were all over 60. All of them exceeded both ADOS‐2 and DISCO thresholds. Their characteristics are presented in Table S2. Written informed consent was obtained from parents before participation by the children. The Ethics Committee of Kanazawa University Hospital approved the methods and procedures, all of which were conducted in accordance with the Declaration of Helsinki. Not surprisingly, the participants had limited RRB impairment on the ADOS, possibly reflecting their higher intelligence. A report of an earlier study described greater severity in stereotypes for low‐functioning ASD compared to high‐functioning ASD (Bartak & Rutter, 1977).
Assessment of intelligence
For this study, the Japanese version of the Kaufman Assessment Battery for Children (K‐ABC) was used to evaluate the intelligence of the participants. In K‐ABC, problem‐solving ability and knowledge of things are evaluated (Kaufman & Kaufman, 1983). However, only the former is interpreted as intelligence, the latter is distinguished from intelligence. Problem‐solving ability was evaluated as the MPI. However, acquired knowledge is evaluated on the Achievement scale. It is noteworthy that language skills, which have been known to be related to joint attention, are also included in the Achievement scale. As such, it is distinguished from intelligence in K‐ABC.
Subscales of MPI are the following:
For Hand Movements, the child must imitate the exact sequence of taps on the table made with the fist, palm, or side of the hand as performed by the examiner. For Number Recall, the child must repeat, in sequence, a series of numbers presented orally by the examiner. For Word Order, the child must point to silhouettes of objects in the same sequence as those objects named by the examiner. The Simultaneous Processing Scale × seven subtests: For Magic Window, the child must name a picture that is Exposed a section at a time through rotation behind a narrow slit. For Face Recognition, a picture showing one or two faces is presented briefly. The child must then select the correct face (s) from a subsequently presented group picture. For Gestalt Closure, after the child is shown a partially completed inkblot, the child must then draw and name or describe the drawing. For Triangles, a timed task, the child is provided with a set of blue and yellow rubber triangles and is required to duplicate an abstract design presented by the examiner. For Matrix Analogies, the child is shown a 2 × 2 visual analogy from which the last element is missing: one which completes the abstract analogy correctly. For Spatial Memory, after being shown a set of randomly arranged pictures, the child must recall and indicate their locations on a subsequently presented grid.
In each subscale of MPI, each participant is assigned multiple questions according to the participant's age. The total number of correct answers is regarded as a raw score. This raw score is converted to an evaluation score with an average of 10 and a SD of 3 using a conversion table. Some subscales are given at all ages. Others are given to selected age groups. MPI is calculated by converting the sum of the evaluation scores of each subscale performed by the participant to a standard score with an average of 100 and a SD of 15 using a conversion table.
Assessment of joint attention
Traditionally, specific paradigms designed to elicit and quantify joint attention have been used, but those are very time‐consuming (Mundy, Sigman, Ungerer, & Sherman, 1986; Seibert, Hogan, & Mundy, 1982). Those measures have great merit when one describes how joint attention is impaired in the subject in detail. However, as a result of the great deal of labor involved in using them, their efficacy is limited. They are infrequently used in studies with large samples. Therefore, we applied an alternative approach. Particularly, we used a joint attention measure derived from the ADOS. In this way, because ADOS is used widely as a gold standard for ASD diagnosis and because it is regarded as an appropriate evaluation method for large samples, it enables us to assess the relation between joint attention and intelligence in large samples of children with ASD.
Research has been undertaken recently to evaluate joint attention using ADOS‐derived measures (e.g., Gotham et al., 2008; Gotham, Risi, Pickles, & Lord, 2007; Harrison et al., 2016; Maljaars, Noens, Scholte, & Van Berckelaer‐Onnes, 2012; Oosterling et al., 2010; Thurm, Lord, Lee, & Newschaffer, 2007). Among those, we used the joint attention factor identified by Gotham et al. (2008, 2007), which is replicated by Oosterling et al. (2010). In those studies, Pointing, Gesture, Unusual Eye Contact, Showing, and Initiation of Joint Attention were factors of joint attention (Gotham et al., 2007). Accordingly, we use the sum of the algorithm scores of these five items (i.e., JA sum). In ADOS‐2, possible raw score of pointing is 0–3, that of gesture is 0–3 and 8 (not applicable for evaluating), that of unusual eye contact is 0 or 2, and that of showing and initiation of joint attention is 0–2. Each raw score is converted to algorithm scores which are 0–2 (i.e., 0–2 are converted to the value as they are. 3 is converted to 2. 8 is converted to 0). As such, JA sum is 0–10.
Statistical analysis
First, we conducted a simple regression analysis to evaluate the joint attention effects on MPI using MPI as the dependent variable and JA sum as the independent variable. Technically, JA sum is a categorical variable. However, treating it as a categorical variable with 11 possible outcomes and doing a multinomial logistic regression might require a very large sample. Using this conservative approach, we can be sure that we are not overstating any effects. A downside of this approach, however, is that we lose the information in the ordering and might fail to answer our research question. Therefore, we reasonably assume that the numerical distance between each set of subsequent categories is equal, and treat JA sum as a continuous variable. Statistical significance was inferred for p < 0.05. We used the standard score of MPI and treated it as a continuous variable. Statistical significance was inferred for p < 0.05. We used the standard score of MPI and treated it as a continuous variable. Actually, JA sum was treated as a continuous variable ranging from 0 to 10.
Next, we performed nine simple regression analyses using the MPI subscales for hand movements, number recall, word order, magic window, face recognition, gestalt closure, triangles, matrix analogies, and spatial memory, individually as the dependent variables. The independent variable was JA sum as before. We calculated nine analyses simultaneously performing the Benjamini–Hochberg procedure to decrease the False discovery rate. Statistical significance was inferred for p < 0.05. In this method, the p‐values are ranked in ascending order, with the smallest p‐value representing the rank of 1. Each p‐value is then multiplied by the number of tests and divided by its rank. The largest corrected p‐value that is less than 0.05 as well as all p‐values of higher rank are considered statistically significant (Benjamini & Hochberg, 1995).
We used the evaluation scores of each K‐ABC scale and treated them as a continuous variable. Before we applied linear regression, we verified that our data meet the assumptions for regression analysis. Specifically, we used standard methods to verify linearity, normality, homogeneity of variance, model specifications, influence, and collinearity.
RESULTS
We conducted a simple linear regression analysis to predict MPI based on JA sum.
A significant regression equation was found as [t(104) = −3.25, p = 0.0015)], with R 2 of 0.10. Participants' predicted MPI is equal to 109.26–2.97 (JA sum). Details are presented in Table 2.
TABLE 2.
Association between K‐ABC mental processing index and ADOS‐2 joint attention
| N | Coeff. | SE | β | t | p > t | 95%CI | p | R2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Versus mental processing index | ||||||||||
| JA sum | 106 | −2.97 | 0.91 | −0.30 | −3.25 | 0.0015 | −4.78 | −1.16 | 0.0015 | 0.10 |
Note: JA sum, the total score of ADOS‐2 module2 items, pointing, gesture, unusual eye contact, showing, and initiation of joint attention.
Abbreviations: CI, confidence interval; Coeff., regression coefficient; SE, robust standard error.
Next, nine simple regression analyses were done to predict each subscale of MPI based on JA sum. Significant results were obtained for hand movements [t(104) = −3.70, Benjamini–Hochberg corrected p = 0.0036] and matrix analogies [t(84) = −2.81, Benjamini–Hochberg corrected p = 0.0274].
The predicted value of hand movements was equal to 12.63–0.65 (JA sum), with R 2 of 0.12. The predicted value of matrix analogies was equal to 12.01–0.62 (JA sum), with R 2 was 0.09. For the other subscales, no significant regression equation was obtained after adjusting the false discovery rate using the Benjamini–Hochberg procedure. Details are given in Table 3.
TABLE 3.
Associations between mental processing index subscales and ADOS‐2 joint attention
| Dependent variable: JA sum | |||||||
|---|---|---|---|---|---|---|---|
| N | Coeff. | SE | β | t | p | R2 | |
| Hand movements | 106 | −0.65 | 0.18 | −0.34 | −3.70 | 0.0004 a | 0.12 |
| Number recall | 106 | −0.16 | 0.17 | −0.09 | −0.95 | 0.3428 | 0.01 |
| Word order | 99 | −0.24 | 0.21 | −0.11 | −1.12 | 0.2668 | 0.01 |
| Magic window | 20 | −0.77 | 0.41 | −0.40 | −1.87 | 0.0783 | 0.16 |
| Face recognition | 20 | −1.00 | 0.46 | −0.46 | −2.18 | 0.0426 | 0.21 |
| Gestalt closure | 106 | −0.28 | 0.16 | −0.16 | −1.67 | 0.0972 | 0.03 |
| Triangles | 99 | −0.34 | 0.20 | −0.17 | −1.67 | 0.0984 | 0.03 |
| Matrix analogies | 86 | −0.62 | 0.22 | −0.29 | −2.81 | 0.0061 a | 0.09 |
| Spatial memory | 86 | −0.49 | 0.22 | −0.24 | −2.23 | 0.0281 | 0.06 |
Note: JA sum, the total score of ADOS‐2 module2 items, pointing, gesture, unusual eye contact, showing, and initiation of joint attention.
Abbreviations: CI, confidence interval; Coeff., regression coefficient; SE, robust standard error.
Significant result following Benjamini–Hochberg FDR correction for all regression tests.
For reference purposes, we provide scatter plots and draw regression lines to visualize the associations between joint attention and MPI, hand movements, matrix analogies (Figures 1, 2, and 3).
FIGURE 1.
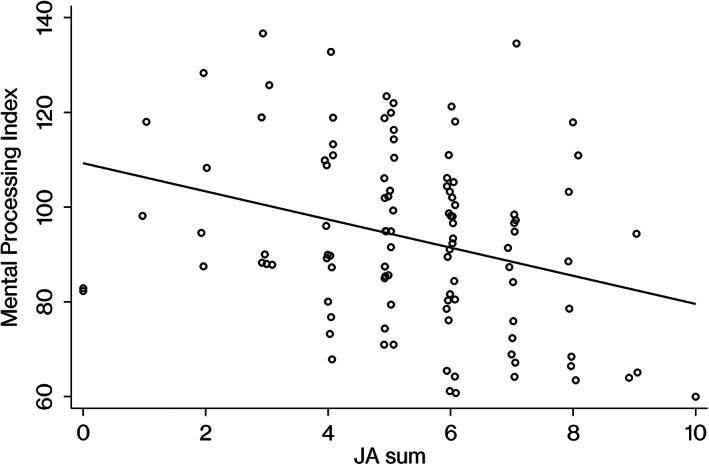
Association between mental processing index scores on the Kaufman Assessment Battery for Children and ADOS‐2 joint attention. JA sum, the total score of ADOS‐2 module 2 items, pointing, gesture, unusual eye contact, showing, and initiation of joint attention
FIGURE 2.
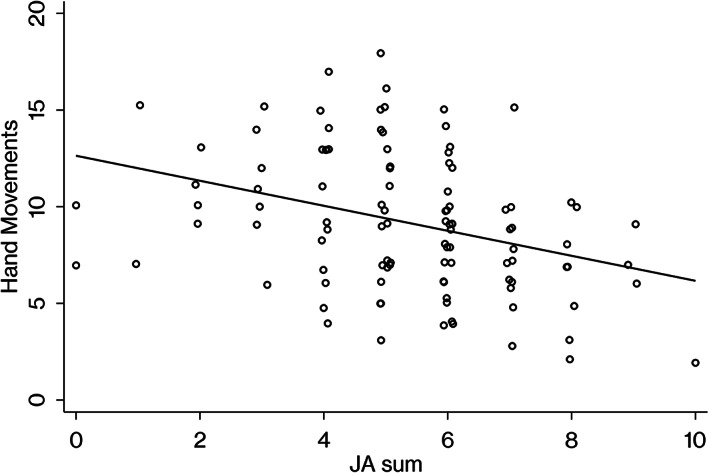
Association between hand movements subscale scores on the Kaufman Assessment Battery for Children and ADOS‐2 joint attention. JA sum, the total score of ADOS‐2 module 2 items, pointing, gesture, unusual eye contact, showing, and initiation of joint attention
FIGURE 3.
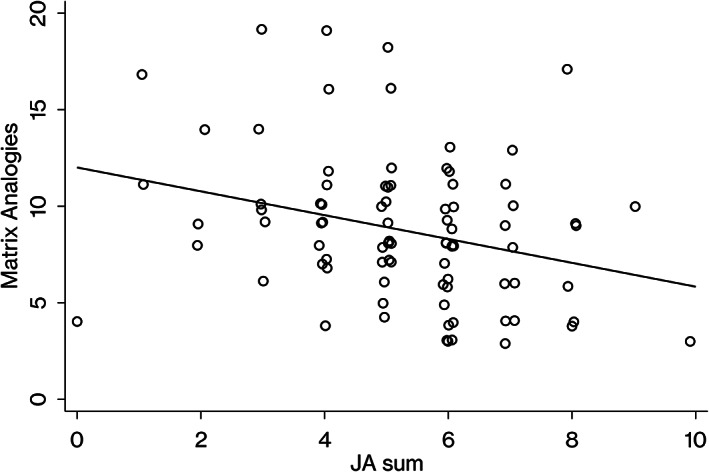
Association between matrix analogies subscale scores on the Kaufman Assessment Battery for Children and ADOS‐2 joint attention. JA sum, the total score of ADOS‐2 module 2 items, pointing, gesture, unusual eye contact, showing, and initiation of joint attention
Results from analyses limiting the sample to those who exceeded thresholds for ADOS‐2 or DISCO were found to be almost identical to those before exclusion. These results are presented in Tables S3 and S4. Results from analysis for children evaluated by ADOS‐2 Module 1 are presented in Table S5.
DISCUSSION
We found significant association between MPI and joint attention in children with ASD without severe intellectual disability. Among this population, severe joint attention deficits were found to be associated significantly with low intelligence. From the subsequent subscale analyses, significant associations were found only for hand movements and matrix analogies subscales. Greater severity in joint attention deficit corresponded to lower scores in these subscales, implying that these specific relations might drive the relation between joint attention and overall intelligence.
We found the relation between joint attention and intelligence to be significant. The result is consistent with findings from earlier studies (Harrison et al., 2016; Mundy et al., 1994), but we extended their findings to children with ASD having no severe intellectual disability or potentially milder autistic symptom. Harrison et al. demonstrated from their larger scale study that better joint attention is related to higher intelligence in children with ASD. The average intelligence of their participants; however, was lower than that found in this study. Although comparison of the characteristics directly is difficult because the intelligence was derived from different measures, the average intelligence findings for these participants were VIQ 53.45 and NVIQ 66.88 (measured using the differential ability scales – second edition (Elliott, 2007) or the Mullen scales of early learning (Mullen, 1995)). By contrast, we analyzed more intelligent participants with MPI over 60 (mean MPI 93.4 measured using K‐ABC), and ADOS‐2 module 2 level language function with moderate ASD severity (CS 5.4 ± 1.9). We used K‐ABC because its measures for intelligence help us control for language, which is known already to be related to joint attention, thereby isolating intelligence somewhat more. Moreover, we limited the participants to Japanese, which contributes to maintenance of cultural background homogeneity. In this sense, we controlled many factors such as language skills, race, and cultural areas. Moreover, we showed that joint attention and intelligence are related even in ASD children who have higher intelligence and potentially milder autistic symptoms than those evaluated in earlier studies. Results of this study can provide a rational basis for future clinical trials to apply joint attention‐based intervention for higher‐functioning ASD. Application for this specific population of ASD is expected to be beneficial because higher‐functioning individuals account for a sizable portion of those with ASD (as many as 30% [Fombonne, 2002]). Moreover, under the heterogeneity of ASD, it would help practitioners to have better prediction of whether the intervention is appropriate for their clients.
In subsequent analyses for nine subscales of MPI, only two subscales were found to be related significantly to joint attention: hand movements and matrix analogies. Face recognition and spatial memory also tended to show a relation to joint attention (p < 0.05), although they were not found to be significant after Bonferroni correction. By contrast, we found no tendency for triangle, magic window, gestalt closure, number recall, or word order. The ability unique to matrix analogies is analogical reasoning (Kaufman et al., 1987). Our results therefore suggest that impaired joint attention, a characteristic of ASD, corresponds to a lower level of analogical reasoning in children with ASD. Most reports of earlier studies describe comparable performance in analogical reasoning between ASD and control groups when IQ scores were matched (Morsanyi & Holyoak, 2010; Scott & Baron‐Cohen, 1996; Tan et al., 2018). If joint attention deficits are a general marker of autistic pathology, those studies contradict the present results because individuals with ASD are then expected to have a lower level of analogical reasoning. It might therefore be true that joint attention deficit is a marker of a circumscribed aspect of this heterogeneous syndrome. In this sense, it would be beneficial for future studies to investigate relations between subscales of intelligence and other aspects of ASD, which is potentially independent of deficits in joint attention (e.g., impairment in theory of mind [Shaw, Bryant, Malle, Povinelli, & Pruett, 2017]). Potentially different relations between subscales of intelligence and each aspect of autistic pathology might help us describe and interpret ASD heterogeneity. On the other hand, an ability that is unique to hand movements, face recognition, and spatial memory is visual short‐term memory (Kaufman et al., 1987). Few studies have investigated the relation between visual short‐term memory and joint attention (Gregory & Jackson, 2017; Gregory & Jackson, 2019), but the results suggest consistently that joint attention enhances visual short‐term memory in TD individuals. Particularly, Gregory and Jackson (Gregory & Jackson, 2017) combined a “cue” face with a traditional visual short‐term memory task, based on which they reported short‐term memory as better for jointly attended (validly cued) versus invalidly cued targets. Furthermore, for a subsequent study, they added open and closed barriers that do or do not obstruct the cue face view. After analyzing healthy individuals, they concluded that the gaze influence is driven by observers sharing a perspective rather than simply by increased attention to the cued location (Gregory, 2019). Given that joint attention enhances short‐term memory, children with ASD in this study who had better joint attention might use it during short‐term memory tasks, and might consequently have higher scores for Hand Movements, Face Recognition, and Spatial Memory subscales. In this sense, the present results extend findings from results of earlier studies suggesting the influence of joint attention on visual short‐term memory in children with ASD and in TD individuals. This report is the first of a study investigating relations between joint attention and sub‐factors of intelligence. As such, the present study might extend results reported by Harrison et al. (Harrison et al., 2016) and by Mundy et al. (Mundy et al., 1994) in that the reported relation between joint attention and intelligence might have been driven by the influence of joint attention on some specific sub‐factors of intelligence. It is noteworthy that not every component of intelligence is influenced by joint attention. According to the results, it is unlikely that joint attention influences the unique abilities measured by triangle, magic window, gestalt closure, number recall, or word order such as nonverbal concept formation (triangle), integration of sequentially presented visual stimuli (magic window), perceptual inference (gestalt closure), automatic auditory vocal memory (number recall), or auditory–motor memory (word order) (Kaufman et al., 1987). In this sense, the present study provides a new insight into the relation between sociality and intelligence in high‐function ASD. It is expected to be an important step to clarify and elucidate the intersection between intelligence and sociality in this population.
These results show correlation between joint attention and intelligence rather than a causal relation. Confounders that might influence both joint attention and intelligence should be considered. For example, the parents' financial situation and educational attainment might be related to their child's learning opportunities and might affect their child's development of both joint attention and intelligence (Abels & Hutman, 2015). It is noteworthy that the K‐ABC test format itself is a task that requires joint attention. Impairment of joint attention in ASD might also affect the score. In many parts of K‐ABC test, the examiner shows his participant some objects and directs him to do a task about the objects. Because joint attention behaviors include the ability to follow the direction of the gaze and gestures of others to share a common point of reference (Mundy & Newell, 2007), impairment of participant's joint attention can hinder this process, resulting in underestimated intelligence. To verify this hypothesis, it is desirable to apply an intelligence test that requires no communication using joint attention between the examiner and the participant. However, most commonly performed intelligence tests such as Wechsler intelligence scale for children (WISC), differential ability scales (DAS) require the examiner to sit in front of a participant and present the task using objects. Therefore, it might be difficult to evaluate intelligence without the effects of joint attention.
In this paragraph, we divide joint attention into possible sub‐elements and discuss their possible relations to intelligence. First, joint attention includes visual disengagement and spontaneous orienting to social stimuli. Visual disengagement is the ability to take attention away from a first visual stimulus and then specifically examine a new visual stimulus. Visual disengagement is reported to be necessary to develop joint attention (Keehn, Müller, & Townsend, 2013), but it is not the same as joint attention. Visual disengagement differs from joint attention in that it can occur in the absence of any other person. Visual disengagement is well known to be atypical in children with ASD, similarly to joint attention (Sacrey, Armstrong, Bryson, & Zwaigenbaum, 2014). Importantly, although we have demonstrated a significant relation between intelligence and joint attention, association between intelligence and visual disengagement was not demonstrated. For example, in their extensive review, Sacrey et al. discussed earlier studies of that relation and concluded that no clear association exists between visual disengagement and intelligence (Sacrey et al., 2014). Therefore, spontaneous orienting to social stimuli, the other fundamentally important factor for the development of joint attention, might drive the present result. Second, spontaneously orienting to social stimuli includes “social orienting” and “dyadic engagement” (Franchini, Armstrong, Schaer, & Smith, 2019). Social orienting is a tendency to prefer social stimuli (e.g., human face) to non‐social stimuli (e.g., geometric pattern); dyadic engagement is an interaction between the child and another person. Typically, dyadic engagement is measured as the amount of time a child maintains eye contact on the mother's eyes divided by the time spent looking at the mouth (Merin, Young, Ozonoff, & Rogers, 2007; Young, Merin, Rogers, & Ozonoff, 2009). Dyadic engagement differs from joint attention in a sense that dyadic engagement occurs purely between a child and another person in which any third object is required. From the only reported study to have specifically examined the association between social orienting and intelligence (Pierce et al., 2016) Pierce et al. found that higher social orienting (i.e., tendency to prefer social stimuli to non‐social stimuli) corresponded to better cognitive function and milder symptoms of ASD. In contrast, Young et al. reported higher dyadic engagement (i.e., longer duration looking at one's mother's eyes) was associated with lower verbal intelligence, but its effects on visual reception and fine motor were not significant (Young et al., 2009). Combining all of those results, social orienting, purely “social” component of joint attention, might drive the association between joint attention and intelligence. Additional study employing traditional paradigms to elicit and describe joint attention (e.g., Mundy et al., 1986; Seibert et al., 1982) might have merit in further clarification of this somewhat ambiguous influence of joint attention on intelligence.
This report is the first of a study demonstrating that joint attention evaluated by ADOS is related closely with intelligence evaluated by K‐ABC in young children without severe intellectual disability (i.e., mental processing scale ≥60). Therefore, we conclude that joint attention is related to intelligence in young children with ASD without severe intellectual disability.
LIMITATIONS
It remains unclear whether the relation between joint attention and intelligence described herein is characteristic of ASD children or whether a similar relation is apparent in TD children. To address the uniqueness, further verification is necessary. For such studies of verification, ADOS derived measures for joint attention might be inappropriate considering possible floor effects of this variable in TD children. In fact, Maljaars et al. reported the distribution of their ADOS‐derived joint attention measure as too skewed in TD children (Maljaars et al., 2012).
For current study, we analyzed only participants of ADOS‐2 module 2. Therefore, similar relations might not be seen in children at higher languages or development levels. Studies for this population might be helpful for additional elucidation of the complex association between joint attention and intelligence in ASD.
Another limitation was the version of K‐ABC we used. The K‐ABC second edition (K‐ABCII, Kaufman & Kaufman, 2004) came out in 2004, yet we used the original version of K‐ABC (Kaufman & Kaufman, 1983) because Japanese version of K‐ABC second edition had not been verified at the time we started this study. In any case, the use of that version did not align with best practice standards.
Although correlation between joint attention and intelligence can be confirmed using this test method, the causal relation could not be verified. Another agent such as visual information processing can affect both joint attention and intelligence. Therefore, in future studies, it will be necessary to perform therapeutic intervention particularly addressing joint attention and to evaluate visual information processing and intelligence as outcomes.
Supporting information
Appendix S1: Supporting information.
Sano, M. , Yoshimura, Y. , Hirosawa, T. , Hasegawa, C. , An, K. , Tanaka, S. , Naitou, N. , & Kikuchi, M. (2021). Joint attention and intelligence in children with autism spectrum disorder without severe intellectual disability. Autism Research, 14(12), 2603–2612. 10.1002/aur.2600
REFERENCES
- Abels, M. , & Hutman, T. (2015). Infants' behavioral styles in joint attention situations and parents' socio‐economic status. Infant Behavior and Development, 40, 139–150. 10.1016/j.infbeh.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson, L. B. , Bakeman, R. , & Deckner, D. F. (2004). The development of symbol‐infused joint engagement. Child Development, 75(4), 1171–1187. 10.1111/j.1467-8624.2004.00732.x [DOI] [PubMed] [Google Scholar]
- Bakeman, R. , & Adamson, L. B. (1984). Coordinating attention to people and objects in mother‐infant and peer‐infant interaction. Child Development, 55(4), 1278–1289. 10.2307/1129997 [DOI] [PubMed] [Google Scholar]
- Bartak, L. , & Rutter, M. (1977). Differences between mentally retarded and normally intelligent autistic children. Journal of Autism and Childhood Schizophrenia, 7(2), 205. 10.1007/BF01537732 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate : A practical and powerful approach to multiple testing Yoav Benjamini; Yosef Hochberg controlling the false discovery rate : A practical and powerful approach to multiple testing. Society, 57(1), 289–300. [Google Scholar]
- Bottema‐Beutel, K. (2016). Associations between joint attention and language in autism spectrum disorder and typical development: A systematic review and meta‐regression analysis. Autism Research, 9(10), 1021–1035. 10.1002/aur.1624 [DOI] [PubMed] [Google Scholar]
- Cattell, P. (1960). The measurement of intelligence of infants and young children. Psychological Corp. [Google Scholar]
- Charman, T. (2003). Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society, B: Biological Sciences, 358(1430), 315–324. 10.1098/rstb.2002.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman, T. , Baron‐Cohen, S. , Swettenham, J. , Baird, G. , Drew, A. , & Cox, A. (2003). Predicting language outcome in infants with autism and pervasive developmental disorder. International Journal of Language and Communication Disorders, 38(3), 265–285. 10.1080/136820310000104830 [DOI] [PubMed] [Google Scholar]
- Constantino, J. (2005). Social responsiveness scale (SRS) manual. Western Psychological Services. [Google Scholar]
- D'Entremont, B. , Hains, S. M. J. , & Muir, D. W. (1997). A demonstration of gaze following in 3‐ to 6‐month‐olds. Infant Behavior and Development, 20(4), 569–572. 10.1016/S0163-6383 [DOI] [Google Scholar]
- Elliott, C. D. (2007). Differential ability scales (2nd ed.). Harcourt Assessment. [Google Scholar]
- Fombonne, E. (2002). Epidemiological trends in rates of autism. Molecular Psychiatry, 7, 4–6. 10.1038/sj.mp.4001162 [DOI] [PubMed] [Google Scholar]
- Franchini, M. , Armstrong, V. L. , Schaer, M. , & Smith, I. M. (2019). Initiation of joint attention and related visual attention processes in infants with autism spectrum disorder: Literature review. Child Neuropsychology, 25(3), 287–317. 10.1080/09297049.2018.1490706 [DOI] [PubMed] [Google Scholar]
- Gotham, K. , Risi, S. , Dawson, G. , Tager‐Flusberg, H. , Joseph, R. , Carter, A. , & Lord, C. (2008). A replication of the autism diagnostic observation schedule (ADOS) revised algorithms. Journal of the American Academy of Child and Adolescent Psychiatry, 47(6), 642–651. 10.1097/CHI.0b013e31816bffb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham, K. , Risi, S. , Pickles, A. , & Lord, C. (2007). The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627. 10.1007/s10803-006-0280-1 [DOI] [PubMed] [Google Scholar]
- Gregory, S. E. A. , & Jackson, M. C. (2017). Joint attention enhances visual working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43(2), 237–249. 10.1037/xlm0000294 [DOI] [PubMed] [Google Scholar]
- Gregory, S. E. A. , & Jackson, M. C. (2019). Barriers block the effect of joint attention on working memory: Perspective taking matters. Journal of Experimental Psychology, Learning, Memory, and Cognition, 45(5), 795–806. 10.1037/xlm0000622 [DOI] [PubMed] [Google Scholar]
- Harrison, A. J. , Lu, Z. , McLean, R. L. , & Sheinkopf, S. J. (2016). Cognitive and adaptive correlates of an ADOS‐derived joint attention composite. Research in Autism Spectrum Disorders, 29, 66–78. 10.1016/j.rasd.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger, N. A. , & Taylor, J. L. (2013). Outcomes in adults with autism spectrum disorders: A historical perspective. Autism, 17(1), 103–116. 10.1177/1362361312441266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosawa, T. , Kontani, K. , Fukai, M. , Kameya, M. , Soma, D. , Hino, S. , Kitamura, T. , Hasegawa, C. , An, K. M. , Takahashi, T. , Yoshimura, Y. , & Kikuchi, M. (2020). Different associations between intelligence and social cognition in children with and without autism spectrum disorders. PLoS One, 15, 1–18. 10.1371/journal.pone.0235380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin, P. , Goode, S. , Hutton, J. , & Rutter, M. (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45(2), 212–229. 10.1111/j.1469-7610.2004.00215.x [DOI] [PubMed] [Google Scholar]
- Jones, E. A. , Carr, E. G. , & Feeley, K. M. (2006). Multiple effects of joint attention intervention for children with autism. Behavior Modification, 30(6), 782–834. 10.1177/0145445506289392 [DOI] [PubMed] [Google Scholar]
- Kaufman, A. S. , & Kaufman, N. L. (1983). Kaufman assessment battery for children. American Guidance Service. [Google Scholar]
- Kaufman, A. S. , & Kaufman, N. L. (2004). Kaufman Assessment Battery for children (2nd ed.). American Guidance Service. [Google Scholar]
- Kaufman, A. S. , Kaufman, N. L. , & Kamphaus, R. W. (1985). The Kaufman Assessment Battery for children (K‐ABC), in Newmark CS(ed): Major psychological assessment instruments. Allyn & Bacon. [Google Scholar]
- Kaufman, A. S. , O'Neal, M. R. , Avant, A. H. , & Long, S. W. (1987). Review article: Introduction to the Kaufman Assessment Battery for Children (K‐ABC) for pediatric neuroclinicians. Journal of Child Neurology, 2(1), 3–16. 10.1177/088307388700200102 [DOI] [PubMed] [Google Scholar]
- Keehn, B. , Müller, R. A. , & Townsend, J. (2013). Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews, 37(2), 164–183. 10.1016/j.neubiorev.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P. C. , Risi, S. , Gotham, K. , & Bishop, S. (2012). Autism diagnostic observation schedule, second edition (ADOS‐2) manual (part I): Modules 1–4. Western Psychological Services. [Google Scholar]
- Maljaars, J. , Noens, I. , Scholte, E. , & Van Berckelaer‐Onnes, I. (2012). Language in low‐functioning children with autistic disorder: Differences between receptive and expressive skills and concurrent predictors of language. Journal of Autism and Developmental Disorders, 42(10), 2181–2191. 10.1007/s10803-012-1476-1 [DOI] [PubMed] [Google Scholar]
- Merin, N. , Young, G. S. , Ozonoff, S. , & Rogers, S. J. (2007). Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6‐month‐old infants at‐risk for autism from comparison infants. Journal of Autism and Developmental Disorders, 37(1), 108–121. 10.1007/s10803-006-0342-4 [DOI] [PubMed] [Google Scholar]
- Morales, M. , Mundy, P. , & Rojas, J. (1998). Following the direction of gaze and language development in 6‐month‐olds. Infant Behavior and Development, 21(2), 373–377. 10.1016/S0163-6383 [DOI] [Google Scholar]
- Morsanyi, K. , & Holyoak, K. J. (2010). Analogical reasoning ability in autistic and typically developing children. Developmental Science, 13(4), 578–587. 10.1111/j.1467-7687.2009.00915.x [DOI] [PubMed] [Google Scholar]
- Mullen, E. M. (1995). Mullen scales of early learning. American Guidance Service. [Google Scholar]
- Mundy, P. , & Newell, L. (2007). Attention, joint attention & social cognition. Social Cognition, 16, 269–274. 10.1111/j.1467-8721.2007.00518.x.Attention [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy, P. , Sigman, M. , & Kasari, C. (1994). Joint attention, developmental level, and symptom presentation in autism. Development and Psychopathology, 6(3), 389–401. 10.1017/S0954579400006003 [DOI] [Google Scholar]
- Mundy, P. , Sigman, M. , Ungerer, J. , & Sherman, T. (1986). Defining the social deficits of autism: The contribution of non‐verbal communication measures. Journal of Child Psychology and Psychiatry, 27(5), 657–669. 10.1111/j.1469-7610.1986.tb00190.x [DOI] [PubMed] [Google Scholar]
- Murza, K. A. , Schwartz, J. B. , Hahs‐vaughn, D. L. , & Nye, C. (2016). Joint attention interventions for children with autism spectrum disorder: A systematic review and meta‐analysis. International Journal of Language & Communication Disorders, 51(3), 236–251. 10.1111/1460-6984.12212 [DOI] [PubMed] [Google Scholar]
- Oosterling, I. , Roos, S. , De Bildt, A. , Rommelse, N. , De Jonge, M. , Visser, J. , & Buitelaar, J. (2010). Improved diagnostic validity of the ADOS revised algorithms: A replication study in an independent sample. Journal of Autism and Developmental Disorders, 40(6), 689–703. 10.1007/s10803-009-0915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, K. , Marinero, S. , Hazin, R. , McKenna, B. , Barnes, C. C. , & Malige, A. (2016). Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biological Psychiatry, 79(8), 657–666. 10.1016/j.biopsych.2015.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, K. K. , Watson, L. R. , Baranek, G. T. , & Poe, M. D. (2012). To what extent do joint attention, imitation, and object play behaviors in infancy predict later communication and intellectual functioning in ASD? Journal of Autism and Developmental Disorders, 42(6), 1064–1074. 10.1007/s10803-011-1349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey, L. A. R. , Armstrong, V. L. , Bryson, S. E. , & Zwaigenbaum, L. (2014). Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neuroscience and Biobehavioral Reviews, 47, 559–577. 10.1016/j.neubiorev.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Scott, F. J. , & Baron‐Cohen, S. (1996). Logical, analogical, and psychological reasoning in autism: A test of the Cosmides theory. Development and Psychopathology, 8(1), 235–245. 10.1017/s0954579400007069 [DOI] [Google Scholar]
- Seibert, J. M. , Hogan, A. E. , & Mundy, P. C. (1982). Assessing interactional competencies: The early social‐communication scales. Infant Mental Health Journal, 3(4), 244–258. 10.1002/1097-0355 [DOI] [Google Scholar]
- Shaw, J. A. , Bryant, L. K. , Malle, B. F. , Povinelli, D. J. , & Pruett, J. R. (2017). The relationship between joint attention and theory of mind in neurotypical adults. Consciousness and Cognition, 51, 268–278. 10.1016/j.concog.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Sigman, M. , & Ruskin, E. (1999). Chapter I: Background and goals of this study. Monographs of the Society for Research in Child Development, 64(1), 1–10. 10.1111/1540-5834.00002 [DOI] [PubMed] [Google Scholar]
- Stone, W. L. , & Yoder, P. J. (2001). Predicting spoken language level in children with autism spectrum disorders. Autism, 5, 341–361. 10.1177/1362361301005004002 [DOI] [PubMed] [Google Scholar]
- Tan, E. , Wu, X. , Nishida, T. , Huang, D. , Chen, Z. , & Yi, L. (2018). Analogical reasoning in children with autism spectrum disorder: Evidence from an eye‐tracking approach. Frontiers in Psychology, 9, 1–12. 10.3389/fpsyg.2018.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike, R. L. (1972). Stanford Binet intelligence scale: Norms and tables. Houghton Mifflin. [Google Scholar]
- Thurm, A. , Lord, C. , Lee, L. C. , & Newschaffer, C. (2007). Predictors of language acquisition in preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(9), 1721–1734. 10.1007/s10803-006-0300-1 [DOI] [PubMed] [Google Scholar]
- Travis, L. , Sigman, M. , & Ruskin, E. (2001). Links between social understanding and social behavior in verbally able children with autism. Journal of Autism and Developmental Disorders, 31(2), 119–130. 10.1023/A:1010705912731 [DOI] [PubMed] [Google Scholar]
- Volkmar, F. R. , Paul, R. , Klin, A. , & Cohen, D. (2005). In Volkmar F. R., Paul R., Klin A., & Cohen D. (Eds.), Handbook of autism and pervasive developmental disorders. Wiley. 10.1002/9780470939345 [DOI] [Google Scholar]
- Wetherby, A. M. , Watt, N. , Morgan, L. , & Shumway, S. (2007). Social communication profiles of children with autism spectrum disorders late in the second year of life. Journal of Autism and Developmental Disorders, 37(5), 960–975. 10.1007/s10803-006-0237-4 [DOI] [PubMed] [Google Scholar]
- Whalen, C. , Schreibman, L. , & Ingersoll, B. (2006). The collateral effects of joint attention training on social initiations, positive affect, imitation, and spontaneous speech for young children with autism. Journal of Autism and Developmental Disorders, 36(5), 655–664. 10.1007/s10803-006-0108-z [DOI] [PubMed] [Google Scholar]
- Wing, L. , Gould, J. , & Gillberg, C. (2011). Autism spectrum disorders in the DSM‐V: Better or worse than the DSM‐IV? Research in Developmental Disabilities, 32(2), 768–773. 10.1016/j.ridd.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Young, G. S. , Merin, N. , Rogers, S. J. , & Ozonoff, S. (2009). Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science, 12(5), 798–814. 10.1111/j.1467-7687.2009.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.


