Abstract
The ongoing SARS-CoV-2 pandemic continues to pose an enormous health challenge globally. The ongoing emergence of variants of concern has resulted in decreased vaccine efficacy necessitating booster immunizations. This was particularly highlighted by the recent emergence of the Omicron variant, which contains over 30 mutations in the spike protein and quickly became the dominant viral strain in global circulation. We previously demonstrated that delivery of a SARS-CoV-2 subunit vaccine via a high-density microarray patch (HD-MAP) induced potent immunity resulting in robust protection from SARS-CoV-2 challenge in mice. Here we show that serum from HD-MAP immunized animals maintained potent neutralisation against all variants tested, including Delta and Omicron. These findings highlight the advantages of HD-MAP vaccine delivery in inducing high levels of neutralising antibodies and demonstrates its potential at providing protection from emerging viral variants.
Keywords: Microarray patch, SARS-CoV-2, COVID-19, Vaccine
1. Introduction
In November of 2021, the B.1.1.529 (Omicron) variant of SARS-CoV-2 emerged, containing over 30 mutations in the spike protein, many localized to the receptor binding domain (RBD) and N-terminal domain (NTD). This variant exhibited increased transmissibility, rapidly becoming the dominant SARS-CoV-2 virus globally [1]. The large number of mutations in the spike protein raised concerns about this variant evading vaccine- and infection-induced immune responses. Indeed, initial studies showed significant decreases in antibody neutralisation and overall vaccine efficacy against the Omicron variant relative to the ancestral and Delta variant viruses [2], [3], [4], [5]. This was paralleled by a waning vaccine efficacy against symptomatic infection, necessitating booster immunizations to maintain adequate immune protection.
We previously published pre-clinical data demonstrating the efficacy of a recombinant subunit SARS-CoV-2 spike vaccine, HexaPro [6] delivered to the skin via a high-density microarray patch (HD-MAP) [7]. Complete protection in a mouse model of SARS-CoV-2 infection was demonstrated after a single vaccine dose. Serum from vaccinated mice was also able to neutralise the Alpha and Beta viral variants. Since publishing this work, several other viral variants have emerged, including the dominant Delta and Omicron variants. Here, we analyze serum from our previous study for neutralisation activity against these variants.
2. Materials and methods
2.1. Expression of HexaPro
The plasmid encoding SARS-CoV-2 S HexaPro was a gift from Jason McLellan (Addgene plasmid # 154754; https://n2t.net/addgene:154754; RRID:Addgene_154754). This plasmid was transfected into the Expi293-F™ expression system and purified via immunoaffinity purification using the monoclonal antibody 2M-10B11 as previously described [7].
2.2. Serum samples
All serum samples used in this study were collected from previous work [7] and approved by the University of Queensland animal ethics committee (approval number SCMB/322/19/AIBN). Briefly, naïve female BALB/c mice (n = 7 or 8/group) were purchased from the Animal Resources Centre (Perth, Australia) and immunized with 2 µg of HexaPro either via intradermal (i.d.) immunization or HD-MAP application. The adjuvant QS-21 (3 µg) was included in all groups used in this manuscript. All mice were immunized twice at 21-day intervals, with blood taken via tail bleed or cardiac puncture prior to each immunization or 21 days after the final dose was administered. Blood was allowed to clot overnight at 4 °C before serum harvested by centrifugation at 10,000g for 10 min at 4 °C. Serum was heat-inactivated at 56 °C for 30 min before storage at −20 °C until analysis.
2.3. Viruses
Low passage virus isolates of SARS-CoV-2 (Ancestral, Delta and Kappa variants) were kindly provided by the Queensland Health Forensic and Scientific Services, Queensland Department of Health. We acknowledge the contribution of the scientists and pathologists of Queensland Health for isolating, culturing, and sequencing clinical virus isolates. Other low passage isolates (Lambda, Gamma and Omicron variants) in this project were supplied by The Kirby Institute using methods previously described (https://doi.org/10.21203/rs.3.rs-1210846/v1). This was in collaboration with NSW Health Pathology, where diagnostics and whole genome sequencing initially identified clinical material for isolate expansion. In this setting we collectively acknowledge the contribution of the scientists and pathologists of NSW Health Pathology and the Kirby Institute to this project. Growth and use of primary isolates was under approval of the NSW Chef Health Officer following independent scientific review. Accession numbers of virus isolates used in this study were: Ancestral: hCoV-19/Australia/QLD02/2020 (GISAID Accession ID EPI_ISL_407896), Delta: hCoV-19/Australia/QLD1893C/2021 (GISAID accession ID EPI_ISL_2433928), Gamma: hCov-19/Australia/NSW4318/2021, Kappa: hCoV-19/Australia/QLD1894C/2021 and Omicron: hCov-19/Australia/NSW-RPAH-1933/2021. All viruses were passed on VeroE6-TMPRSS2 cells and titrated via immunoplaque assay as previously described [8].
2.4. Plaque reduction neutralisation tests
Serum was assessed for neutralisation activity via immunoplaque assay as previously described [7], [8]. Briefly, serum was serially diluted in DMEM + 2 % FCS and virus was added. After 30 min at 37 °C, virus-serum complexes were added to confluent monolayers of VeroE6 cells in 96-well plates. Infection was allowed to proceed for 1 h at 37 °C before overlay medium was added. Cells were fixed with 80 % acetone after 14–16 h and immunoplaques visualized using spike-specific antibody CR3022 and IRDye 800CW-conjugated goat anti-human secondary antibodies, followed by scanning using an Odyssey CLx imaging system (LI-COR).
2.5. Statistical analysis
Graphpad Prism 9 software was used for all statistical analysis and generation of figures. Statistical significance was determined via the Kruskal-Wallis test with Dunn’s multiple comparison test, with P < 0.05 considered statistically significant.
3. Results
HexaPro, a stabilized SARS-CoV-2 spike protein containing six proline substitutions and a mutated furin cleavage site was recombinantly expressed in Expi293-F™ cells as previously described [7]. BALB/c mice received two doses of HexaPro (2 µg), at three-week intervals, with or without the saponin-based adjuvant QS21 (3 µg). Mice received the vaccine via HD-MAP application or intradermal (i.d.) injection as a comparator [7]. Serum from vaccinated mice was collected 21 days after the final dose and assessed for neutralisation of SARS-CoV-2 isolates via plaque reduction neutralisation tests (PRNTs) [8]. As previously published, serum from HD-MAP groups, regardless of the inclusion of QS21, was capable of potently neutralising dominant viral variants at the time – the 614G, Alpha and Beta variants. Given the global emergence of additional variants of concern/interest we examined the neutralising potency of these sera against these newly emerged variants.
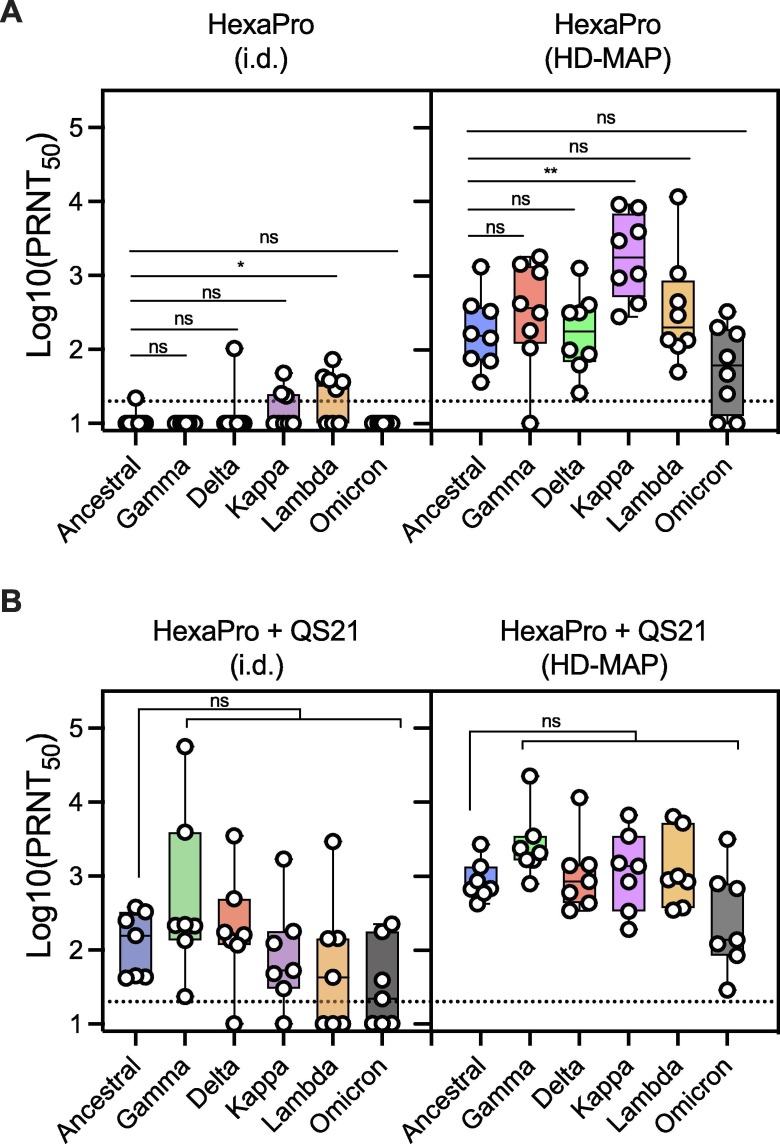
HexaPro vaccine delivery via i.d. injection, elicited almost no neutralising activity against any variant, with only limited neutralising responses seen in samples against the Delta, Kappa and Lambda variants (Fig. 1 A). Against the Omicron variant, no activity was observed. Conversely, HexaPro delivered via HD-MAP application induced a potent neutralising antibody response against all variants. These findings further demonstrate the immune-enhancing effects of HD-MAP vaccine delivery, as previously reported [9], [10], [11], [12], [13], [14], [15], [16], [17], [18].
Fig. 1.
Serum neutralization against SARS-CoV-2 viral variants. Serum from mice (n = 7 or 8/group) was collected three weeks after the second immunization with the SARS-CoV-2 HexaPro spike vaccine. The vaccine was delivered either via the intradermal (i.d.) needle-and-syringe route or by HD-MAP application, either with (A). No adjuvant or (B). The saponin adjuvant QS21. Serum was tested for neutralization against the Ancestral SARS-CoV-2 isolate, as well as the Gamma, Delta, Kappa, Lambda and Omicron variants. Dotted line indicates the assay limit of detection. *, P < 0.05, **, P < 0.01 as determined by the Kruskal-Wallis test with Dunn’s multiple comparison post hoc test.
The inclusion of the saponin-based adjuvant QS21 enhanced the neutralising activity induced by both i.d.-injected and HD-MAP delivered HexaPro, though the HD-MAP groups had higher neutralising antibody titers overall (Fig. 1B). In groups receiving HexaPro with QS21 via the HD-MAP, serum from all animals potently neutralised the Delta, Gamma, Kappa, Lambda and Omicron variants, with no significant decrease relative to the ancestral strain (Fig. 1B). While the i.d. delivered vaccine also induced neutralising activity against all variants, neutralising titres were lower and with greater variability between individual immune responses.
4. Discussion
Here we expand on our previous work demonstrating potent protection of a skin-patch delivered SARS-CoV-2 subunit protein vaccine candidate by analyzing serum responses against newly emerged viral variants of concern. The immune response to the HexaPro vaccine delivered via the HD-MAP, was shown to maintain high levels of neutralisation against all variants tested, including the now dominant Omicron variant. This data is in contrast to previous studies analyzing serum from vaccinated (with ChAdOx1-S, AstraZeneca, or BNT162b2, Pfizer) individuals, where 1 month after the second dose of the Pfizer vaccine, less than half of the samples showed neutralisation activity against the Omicron variant [19]. The ChAdOx1-S vaccine showed no detectable Omicron neutralisation 1 month after the second dose [19]. Individuals receiving the Moderna mRNA vaccine were also analyzed, though this was at 4–6 months post-second dose. At this time point, only 1 individual out of the 10 tested had detectable neutralising antibodies against the Omicron variant, and this was significantly decreased relative to other variants [19]. Convalescent serum (from individuals infected with Alpha, Beta or Delta variants) was also unable to show any significant Omicron variant neutralisation. However, prior infection combined with vaccination could induce appreciable neutralisation against Omicron [19]. Another study showed similar results, with two doses of the Pfizer mRNA vaccine unable to induce Omicron neutralisation in the majority of subjects, but previous infection followed by the two Pfizer vaccine doses improved Omicron neutralisation activity [5].
Taken together, this pre-clinical data suggests that HexaPro HD-MAP vaccination could provide a promising addition to current SARS-CoV-2 vaccine approaches. Along with improved thermal stability [7] as well as ease of application, transport and disposal, HD-MAP vaccine delivery could represent a promising step forward in achieving vaccination coverage against SARS-CoV-2, especially in resource-poor areas.
Funding
This work was funded by Advance Queensland Industry Research Fellowship 2020001511 (DAM) and the University of Queensland Research Training Scholarship (JJYC).
CRediT authorship contribution statement
Christopher L.D. McMillan: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft. Alberto A. Amarilla: Methodology, Investigation, Writing – review & editing. Naphak Modhiran: Methodology, Investigation, Writing – review & editing. Jovin J.Y. Choo: Conceptualization, Methodology, Investigation. Armira Azuar: . Kate Honeyman: Investigation. Alexander A. Khromykh: Investigation, Supervision. Paul R. Young: Conceptualization, Supervision, Writing – review & editing. Daniel Watterson: Supervision, Writing – review & editing. David A. Muller: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: David Muller reports financial support was provided by Vaxxas Pty Ltd. David Muller reports a relationship with Vaxxas that includes: consulting or advisory and funding grants. Paul Young reports a relationship with Vaxxas that includes: consulting or advisory.
Acknowledgments
We would like to thank Alyssa Pyke and Frederick Moore from the Queensland Health Forensic and Scientific Services, Queensland Department of Health, as well as A/Prof. Stuart Turville from the Kirby Institute, the University of New South Wales, New South Wales, Australia, and the scientists and pathologists of NSW Health Pathology for providing SARS-CoV-2 isolates. This work was supported by Advance Queensland Industry Research Fellowship sponsored by Vaxxas and Technovalia. HD-MAPs were kindly provided by Vaxxas Pty Ltd.
Data availability
Data will be made available on request.
References
- 1.Lambrou A.S., Shirk P., Steele M.K., Paul P., Paden C.R., Cadwell B., et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants — United States, June 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(6):206–211. doi: 10.15585/mmwr.mm7106a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta infection. medRxiv. 2022:2021.12.30.21268565. [DOI] [PMC free article] [PubMed]
- 3.Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022;386(5):494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt F., Muecksch F., Weisblum Y., Da Silva J., Bednarski E., Cho A., et al. Plasma Neutralisation of the SARS-CoV-2 Omicron Variant. N Engl J Med. 2021;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralisation. Nature. 2021 doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh C.-L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.-C., Javanmardi K., et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369(6510):1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillan C.L.D., Choo J.J.Y., Idris A., Supramaniam A., Modhiran N., Amarilla A.A., et al. Complete protection by a single-dose skin patch-delivered SARS-CoV-2 spike vaccine. Sci Adv. 2021;7(44) doi: 10.1126/sciadv.abj8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarilla A.A., Modhiran N., Setoh Y.X., Peng N.Y.G., Sng J.D.J., Liang B., et al. An Optimized High-Throughput Immuno-Plaque Assay for SARS-CoV-2. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.625136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbett H.J., Fernando G.J.P., Chen X., Frazer I.H., Kendall M.A.F., Koutsopoulos S. Skin Vaccination against Cervical Cancer Associated Human Papillomavirus with a Novel Micro-Projection Array in a Mouse Model. PLoS ONE. 2010;5(10):e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando G.J.P., Chen X., Primiero C.A., Yukiko S.R., Fairmaid E.J., Corbett H.J., et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release. 2012;159(2):215–221. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Fernando G.J.P., Chen X., Prow T.W., Crichton M.L., Fairmaid E.J., Roberts M.S., et al. Potent Immunity to Low Doses of Influenza Vaccine by Probabilistic Guided Micro-Targeted Skin Delivery in a Mouse Model. PLoS ONE. 2010;5(4):e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernando G.J.P., Hickling J., Jayashi Flores C.M., Griffin P., Anderson C.D., Skinner S.R., et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™) Vaccine. 2018;36(26):3779–3788. doi: 10.1016/j.vaccine.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 13.Fernando G.J.P., Zhang J., Ng H.-I., Haigh O.L., Yukiko S.R., Kendall M.A.F. Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8+ T cell responses. J Control Release. 2016;237:35–41. doi: 10.1016/j.jconrel.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 14.Forster A.H., Witham K., Depelsenaire A.C.I., Veitch M., Wells J.W., Wheatley A., et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase I clinical trial. PLoS Med. 2020;17(3):e1003024. doi: 10.1371/journal.pmed.1003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller D.A., Depelsenaire A.C.I., Shannon A.E., Watterson D., Corrie S.R., Owens N.S., et al. Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays. Vaccines (Basel) 2019;7(4):189. doi: 10.3390/vaccines7040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller D.A., Fernando G.J.P., Owens N.S., Agyei-Yeboah C., Wei J.C.J., Depelsenaire A.C.I., et al. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-13011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller D.A., Pearson F.E., Fernando G.J.P., Agyei-Yeboah C., Owens N.S., Corrie S.R., et al. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci Rep. 2016;6(1) doi: 10.1038/srep22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choo J.J.Y., Vet L.J., McMillan C.L.D., Harrison J.J., Scott C.A.P., Depelsenaire A.C.I., et al. A chimeric dengue virus vaccine candidate delivered by high density microarray patches protects against infection in mice. NPJ Vaccines. 2021;6(1) doi: 10.1038/s41541-021-00328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rössler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 Omicron Variant Neutralisation in Serum from Vaccinated and Convalescent Persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.