Introduction
The current standard of care for locally advanced rectal cancer (LARC) includes neoadjuvant long-course chemoradiotherapy (LCRT), total mesorectal excision (TME) and postoperative adjuvant chemotherapy1. However, the treatment algorithm for LARC has increased in complexity during past 10 years, balancing the goals of achieving better survival rates by preventing systemic disease spread with reducing the components of treatment that have a detrimental effects on the patient’s quality of life (QoL)2.
It is well known that a proportion of LARC patients that undergo neoadjuvant therapy (NAT) experienced a pathological complete response (pCR), i.e. the complete absence of residual tumor cells at the primary tumor site and the mesorectal nodes3. Approximately 20% of LARC patients treated with LCRT alone experience a pCR, but this can be as high as 40% in those treated with concomitant consolidative chemotherapy given after LCRT4. Patients who achieve a pCR demonstrate excellent survival rates, with less than 5% of systemic recurrence and 1% of local failure5.
TME is associated with high rates of bowel, sexual, and genitourinary dysfunction that significantly impairs the QoL of patients6,7. Given higher rates of tumor response with some NAT modalities, surgeons face the dilemma about the added value of TME in patients who achieve a pCR. Nonoperative management (NOM) for rectal cancer continues to gain acceptance as a potential treatment option for selected LARC patients given the potential benefits and avoidance of radical surgery.
This review seeks to address the principles about NOM for rectal cancer patients and the main considerations used for optimal selection and treatment of potential candidates.
Neoadjuvant Therapy for Rectal Cancer: Overview
Patients with LARC have been treated classically with two neoadjuvant modalities to improve local control: (1) a LCRT strategy described as radiotherapy (5040 cGy) administered during a 5-to-6-week period with concomitant sensitizing chemotherapeutic agents and a 6-to-10-week period of rest before TME, allowing the regression of the tumor. This strategy offers considerable advantages such as tumor-free surgical margins and higher rates of colorectal anastomosis in low rectal tumors8. Additionally, multiple studies support the use of LCRT based on a reduction in the local recurrence rate8–10 and the possibility to identify good responders11,12, and (2) short-course radiotherapy (SCRT) consisting of 25 Gy in 5 fractions (5×5 Gy) with TME in the following 7 days, has shown a significative reduction in local relapse in several phase III trials13–15.
Both neoadjuvant strategies have shown similar oncological results in terms of overall survival, local recurrence, and surgical complications16. Notably, none of these strategies have demonstrated better overall survival rates. Systemic recurrence remains the main issue facing LARC patients with 25% developing distant metastasis during follow up17–19. In consideration of these findings, the addition of systemic chemotherapy as a part of the NAT strategy has been proposed to diminish the risk of systemic recurrence.
The concept of Total Neoadjuvant Therapy (TNT) implies the use of either SCRT or LCRT and the full adjuvant dose of chemotherapy as part of NAT20–22. It has been proposed that TNT may reduce the risk of distant failure and enhanced the rate of pCR21, in addition to giving the chance for organ preservation in selected patients, but mature survival data is pending.
Tumor response after Neoadjuvant treatment
Although overall survival is not affected by any NAT strategy, the grade of tumor response after NAT is still one of the most important predictors of long-term oncological outcomes in rectal cancer23. Pathological staging of the surgical specimen post-NAT is more predictive of oncological outcome than initial clinical staging23. The presence of macroscopic tumor post-NAT is defined as incomplete response (IR), with TME being the only safe and logical treatment of choice given residual gross tumor.
More recently, the focus has been put on cCR, defined as the absence of detectable macroscopic tumor by clinical means. However, a cCR does not strictly correlate with histological pCR due to higher rates of patients who achieve a cCR after LCRT24.
Watch and wait (WW) is an organ preservation strategy for selected patients that experience a cCR after NAT and it is used interchangeably with NOM. Despite the potential benefits of a WW strategy, many surgeons are reluctant to adopt it25 because of the lack of standardization in response assessment criteria and the lack of randomized prospective data. While stricter criteria may increase the accuracy of individual patients selected for the WW strategy, more liberal criteria may risk worsening oncological outcomes. Additionally, there is an intermediate group of patients with near complete response (nCR) who demonstrate a significant tumor regression, but who fall short of achieving a true cCR26.
Clear definitions of both cCR and pCR are mandatory, yet the current challenge is to accurately select patients with an apparent cCR, based on clinical assessment, who would be found to have a pCR if they were to undergo resection. This is critical for clinicians to correctly identify those would-be candidates for a WW strategy without compromising oncologic safety.
Non-operative Management Approach
Organ preservation is also a valid treatment strategy for patients who are willing to accept potentially worse oncological outcomes to achieve this goal. Gani et al27 reported that 83% of patients would consider a WW strategy if they achieved a cCR in spite of a higher rate of local regrowth during the first 2 years of follow-up. More interestingly, up to 30% of patients are willing to sacrifice rates of long-term oncological cure in comparison with clinicians28.
Initial patient evaluation should be the standard rectal cancer work-up based on the guidelines of the National Comprehensive Cancer Network1. Endoscopic image characteristics of the tumor at baseline and pre-treatment MRI are important elements for subsequent response comparisons.
Once NAT is completed, patients with a cCR or a nCR may enter a WW protocol after the patient, surgeon, and the disease management team agree upon this non-standard approach. Patients who are candidates for WW usually are associated with distal rectal adenocarcinomas that are considered for abdominoperineal resections or very low stapled/handsewn colorectal/coloanal anastomoses which may negatively impact QoL29.
When selecting patients based on pre-treatment characteristics, some features of the tumor demonstrate high-risk of local recurrence (<1mm circumferential margin, extramural venous invasion, and extensive mesorectal/pelvic lymph nodes involved) and are associated with lower rates of cCR30,31. However, it has also been reported that patients with node-positive LARC at baseline that develop a cCR after NAT are not at increased risk for local tumor regrowth or development of more advanced disease at the time of recurrence32. Ulceration and circumferential tumors may be a relative contraindication due the fact of scarring that may potentially narrow the rectal lumen, not allowing a proper endoscopic follow up. A large metanalysis found that older age, smaller tumor, shorter distance from the anal verge, and negative lymph node status are associated with higher rates of pCR33. Mutations in TP53 and KRAS, found in about 70% and 40% of rectal tumors, respectively34,35, are associated with poor response to NAT. Conversely, mismatch repair deficiency tumors are associated with good response to NAT36.
Assessment of response to NAT
The current challenge in WW is the correct selection of cCR patients. Ideally, patients with a pCR should be identified as cCR before undergoing TME to offer WW in the safest manner. However, the correlation between cCR and pCR is not perfect. Regrowth occurs in 25–30% of patients with a cCR37, most of them during the first 2 years of follow up. In contrast, up to 15% of patients with an incomplete response end up having a pCR38,39 in the surgical specimen. Three modalities are considered the pillars to assess response after NAT: digital rectal exam, magnetic resonance imaging (MRI), and endoscopy. The three modalities combined report an accuracy of 98% to predict absence of tumor39. Digital rectal exam of a cCR should be normal, but some minor mucosal abnormalities or soft scar can be palpated. Classic endoscopic features of cCR include a flat white scar, telangiectasia, and absence of both ulcer and nodularity40. MRI features of cCR include a scar not thicker than the rectal wall, only dark T2 signal, no visible lymph nodes, no restricted diffusion, and lack of or low signal on apparent diffusion coefficient (ADC) map.
One of the main difficulties in the implementation of WW is the lack of uniform and reproducible criteria for tumor response and patient selection, especially in cases of nCR. To improve the uniformity of response assessment, the Memorial Sloan Kettering three-tiered response/regression schema has been devised and was tested prospectively in the OPRA trial41 (Figure 1). Validation of these criteria in upcoming and current trials integrating a WW approach is critical for improving our ability to recognize sustained cCR patients.
Figure 1:
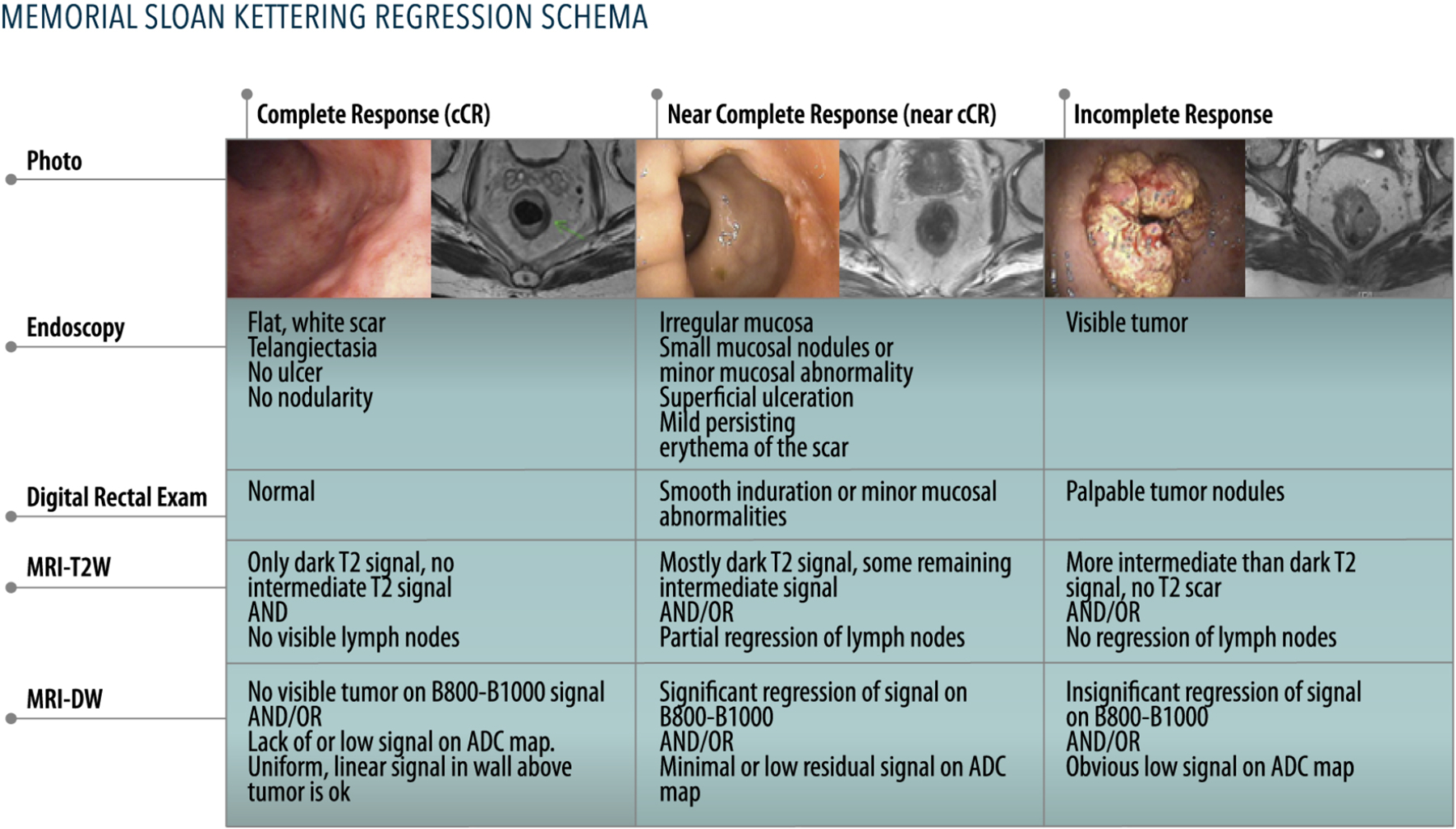
Memorial Sloan Kettering three-tiered response/regression schema
Novel tools have been tested in the search for better ways of assessing the response to NAT. Dynamic contrast-enhanced (DCE) MRI may provide improved diagnostic potential evaluating the degree of neovascularization of the tumor. A systematic review showed that DCE-MRI can identify tumors that exhibit a high pre-NAT Ktrans (representing the rate at which the contrast agent transfers from the blood to the interstitium) along with a subsequent decrease in Ktrans. Both findings appear to be predictors of a favorable response to NAT.42
Radiomics utilizes advanced imaging pattern recognition tools to extract quantitative characteristics from a large quantity of digital data to determine the relationships between the image and the underlying pathophysiology43. A recent report using 2,252 features from patient-based imaging collected both pre- and post-NAT, showed good discrimination of pCR when used in combination with tumor length (AUC 0.9756 95% CI, 0.9185–0.97)44.
Finally, molecular markers that can individually predict the risk of disease relapse may significantly aid in the identification and selection of patients who are safe candidates for WW45. The use of ctDNA is not well-explored in this setting and there are no prospective data, but exploratory analyses from OPRA and the next generation of WW trials could provide some insight into ctDNA clearance and sustained cCR.
Strategies to Optimize Tumor Response
Improving rates of response to NAT may be associated with better overall outcome of patients who undergo WW. Among the potential strategies that can be utilized alone or in combination, includes (1) dose escalation of preoperative radiation therapy, (2) adding systemic chemotherapy to radiotherapy in the neoadjuvant setting, (3) increasing the interval period between NAT and surgery, and (4) administering consolidation chemotherapy after LCRT or SCRT rather than induction chemotherapy followed by LCRT.
Dose escalation strategies have utilized various techniques such as external beam radiotherapy, brachytherapy, contact radiotherapy, and proton/iron beam radiotherapy. A pooled analysis of nearly 3300 patients showed that the dose of NAT was a significant predictor of pCR in a multivariate analysis46. On the other hand, neoadjuvant brachytherapy boost did not add a significant benefit in survival or local recurrence when given after standard NAT and TME47. In a recent report, dose escalation with an external radiation therapy boost to the tumor as part of NAT did not increase the pCR or sustained cCR in LARC (odds ratio [OR] = 0.94; 95% CI, 0.46–1.92)48. Currently, it is unknown whether a dose escalation approach will lead to a higher rate of organ preservation and would need to be studied prospectively.
The use of systemic chemotherapy to improve pCR to NAT has been proposed based on a single-institution phase II trial with 32 patients49 that demonstrated the potential for selective elimination of preoperative LCRT might be feasible in patients with LARC using only 6 cycles of FOLFOX plus bevacizumab. The CAO/ARO/AIO-04 German randomized phase III trial showed higher rates of pCR in LARC patients when oxaliplatin was added to fluorouracil-based NA treatment50. However, other reports assessing the addition of oxaliplatin to chemotherapy regimens for rectal cancer patients demonstrated a considerable increase in toxicity with no improvement in the rates of pCR51. Notably, the Timing of Rectal Cancer Response to Chemoradiation Consortium trial showed a 38% rate of pCR if 6 cycles of FOLFOX were given (after LCRT) in an extended interval period compared with an 18% pCR rate in the LCRT alone group52. Using a chemo-intensification approach (triplet vs. doublet chemotherapy), the PRODIGE 23 trial53 randomized patients with LARC to either three months of neoadjuvant mFOLFIRINOX followed by LCRT and surgery and three months of adjuvant chemotherapy or standard of care (LCRT + surgery + six months of adjuvant FOLFOX). Patients in the mFOLFIRINOX arm had a higher rate of pathological complete response (27.5% vs 11.7%, p-value<0.001) and better 3-year rates of disease-free survival (75.7% vs 68–5%, p-value=0.034) but no significant change in overall survival. Compliance with FOLFOX was low in the adjuvant setting, so the control arm was relatively under-treated compared to the FOLFIRINOX arm.
A longer interval between NAT and surgery is associated with higher rates of pCR. The Stockholm III trial showed that waiting 4 to 8 weeks after SCRT was associated with similar tumor regression rates compared to LCRT54, without adding more surgical morbidity. An analysis of a National Cancer Database that included all stage II and III rectal cancer patients undergoing LCRT suggested that any surgery interval longer than 8 weeks had higher odds of pCR (odd ratio 1.12, 95% CI 1.01 to 1.25)55.
Finally, it seems that using a consolidation chemotherapy based-TNT approach is associated with higher rates of response and organ preservation than using induction chemotherapy. The phase II German trial CAO/ARO/AIO-12 compared 4 cycles of FOLFOX before (induction) or after (consolidation) LCRT. They reported 17% (induction) and 25% (consolidation) pathological complete response rates, respectively56. Long term follow-up of this cohort showed similar results in favor of LCRT followed by consolidation chemotherapy as the preferred TNT sequence, especially if organ preservation is a priority57. Although no WW was offered, patients in the experimental arm the RAPIDO Trial (SCRT followed by consolidation systemic chemotherapy) had higher rate of pCR (27.7% vs 13.8%, p-value<0,001)58. The preliminary results of the OPRA trial, in which patients were randomized to induction or consolidation TNT and then proceeded to surgery or WW depending on response, showed higher rates of organ preservation in the consolidation arm (58% vs 43%; P 0.01), with no difference in disease-free survival or distant-metastasis-free survival59. These data suggest that higher rates of organ preservation can be achieved in patients managed with a WW approach using consolidation chemotherapy and LCRT compared to induction chemotherapy followed by LCRT.
Long term follow-up
Local and distant failure
One of the mayor uncertainties of a WW strategy is the long-term oncologic results23. Habr-Gama et al have reported local regrowth rates ranging from 2.8% to 30%60,61. Systematic reviews showed a local regrowth rate between 15.7 to 30% and that surgical salvage was feasible in almost 93–95.4%% of the cases62,63. The International Watch and Wait Database (IWWD)37 reported a 2-year cumulative incidence of local regrowth of 25.2%, among 880 patients who underwent WW after a cCR, with all regrowth cases diagnosed within the first 2 years of surveillance. The 5-year overall survival and disease-specific survival were 85% and 94%, respectively. All this evidence provides an estimation for the risks of local regrowth and of distant failure across multiple centers (Table 1).
Table 1.
Summary of important WW studies for patients with locally advanced rectal cancer treated with neoadjuvant therapy
| Study | N | NAT Strategy | Regrowth, n (%) | Salvage Therapy, n (%) | Overall Survival, % |
|---|---|---|---|---|---|
| Habr-Gama et al,37 2004 | 71 | LCRT | 2 (3%) | 2 (100%) | OS: 100%, DFS: 92% |
| Smith et al,65 2012 | 32 | LCRT | 6 (18.8%) | 6 (100%) | OS: 96%, DFS: 88% |
| Habr-Gama et al,23 2014 | 90 | LCRT | 28 (31%) | 26 (92.8%) | OS: 91%, DFS: 68% |
| Appelt et al66, 2015 | 40 | LCRT | 9 (25.9%) | 9 (100%) | OS: 100% |
| Lai et al67, 2016 | 18 | LCRT | 2(11%) | 2 (100%) | OS: 100%, DFS: 69.78 |
| Martens et al68, 2016 | 100 | LRCT: 95% SCRT: 5% |
15(15%) | 15 (100%) | OS: 96.6%, DFS: 80.6% |
| OnCore Project69, 2016 | 129 | 45 Gy w/5-FU | 44(34%) | 41 (93.2%) | OS: 96%, DFS:88% |
| IWWD Consortium,36 2019 | 880 | LCRT: 91% | 213 (25.3%) | 148 (69.5%) | OS: 85%, DFS: 94% |
| Smith et al, 201959 | 113 | LCRT: 31(27%) Induction: 47(42%) Consolidation: 33 (29%) Chemotherapy alone: 2(2%) |
22 (19.5%) | 22 (100%) | OS: 73%, DFS: 75% |
| Jimenez-Rodriguez et al,60 2021 | 33 | Induction TNT (FOLFOX) | 2 (6%) | 2(100%) | OS:97%, DFS:94% |
| Garcia-Aguilar et al (OPRA Trial),56 TBD | 307 (Total study accrual) | TNT (Induction & Consolidation chemotherapy) | N/A | N/A | DFS: 78% (Induction) vs 77% (Consolidation) |
Abbreviations: DFS, disease-free survival; IWWD, International Watch and Wait Database; LCRT, long-course radiotherapy; OS, overall survival; SCRT, short-course radiotherapy; TNT, total neoadjuvant therapy.
A concern that remains even in patients with apparent cCR, is a higher rate of distant metastasis after tumor regrowth. Data from retrospective evaluation of a 10-year experience at Memorial hospital suggest a higher rate of distant metastases in patients with local regrowth when compared to those without local regrowth64. Data from the IWWD suggests the same pattern between local regrowth and distant metastases37. Recently, Jimenez-Rodriguez et al reported a lower rate of local regrowth (6%) in patients treated with TNT by a single surgeon that actively performed WW65. Interestingly, only half of the patients that experienced local regrowth developed distant metastasis. One of the reasons that may explain this result is the strict surveillance commitment of patient and surgeon using a standardized method to evaluate response (MSK Regression Schema). Still, whether removing the primary tumor after completion of NAT would have mitigated the risk of distant metastases is unknown (and impossible to ascertain given the small numbers), but this current finding using strict surveillance is encouraging as WW is contemplated in the context of a standardized clinical protocol.
WW in young patients
WW has been frequently associated with older patients, but in a recent publication from the IWWD group no differences were observed between patients younger versus older of 50 years old that underwent WW in terms disease-specific survival, local regrowth, and cumulative risk of distant metastasis66. WW should also be discussed with younger patients as a viable treatment option.
Functional outcomes
The rates of bowel, sexual, and genitourinary dysfunction after NAT and WW remain unknown. Organ preservation in the presence of pelvic radiation may also significantly affect the QoL of patients. A case-matched study comparing 47 WW patients with 41 patients after NAT and TME, showed that QoL was better in the WW group67. Notably, a third of the WW patients experienced major low anterior resection syndrome (LARS) as measured by the LARS Score. In the Memorial Sloan Kettering published experience, WW patients usually report better bowel function when measured by the Memorial Sloan Kettering Cancer Center Bowel Function Instrument68. However, prospective evaluation of these patient-reported outcomes is needed and is ongoing in the OPRA trial.
Summary and Future Directions
Although in some patients with a cCR after NAT TME may no be longer necessary, the challenge is still to identify true responders by clinical assessment. Most of the current evidence for WW is retrospective, making prospectively collected data of great value. The OPRA trial was the first to integrate WW into a TNT strategy aimed at increasing response rates. Building off OPRA and RAPIDO, Fokas et al. have an ongoing trial which randomizes SCRT versus LCRT each followed by consolidative chemotherapy and is using cCR and organ preservation rates as an endpoint69. NOM or WW strategies should be part of the treatment discussion of LARC, considering patient interest, patient preference, and acceptance of risk along with the possible reduction in morbidity with avoidance of TME. Balancing physician concerns over recurrent disease versus patient values to sacrifice some degree of survival in favor of organ preservation should also be carefully considered. Currently, the best way for patients to utilize WW strategies is in the context of a prospective trial, if possible, with a strict protocol and objective assessment standards.
CLINICS CARE POINTS.
Pathological complete response in rectal cancer treatment has become a more frequent phenomenon after the introduction of novel neadjuvant treatment strategies.
The current challenge is to corrcly identify patients that will experience a pathological complete response after neoadyuvant treatment to safetly offer a nonoperative management.
Nonoperative management should be part of the treatment options discussion with rectal cancer patients and must be always be offer as an standardize protocol until more prospectives studies will validated this approach.
Key Points.
Neoadjuvant treatment can potentially eliminate tumoral cells in rectal cancer patients, a phenomenon known as pathological complete response, with excellent long-term oncological outcomes.
The idea behind non-operative management (NOM) is to correctly identify patients who will develop a pathological complete response after neoadjuvant treatment, replacing surgical resection with safe surveillance.
Correlation between a clinical complete response and a pathological complete response is not sufficiently accurate, which is the reason why a standardized non-operative management protocol is needed.
Although non-operative management data are promising, most of the current evidence corresponds to retrospective series. Prospectively collected data from trials suggests safety and high rates of organ preservation (i.e., the OPRA trial) after optimal neoadjuvant therapy approaches in early readouts, but long-term follow-up and additional trials integrating NOM will be critical to validate these findings.
Synopsis.
The treatment algorithm for locally advanced rectal cancer (LARC) has increased in complexity over the past ten years. Nonoperative management (NOM) for rectal cancer in patients with a clinical complete response (cCR) after neoadjuvant therapy has been gaining acceptance as a potential treatment option for selected LARC patients. The current challenge is to accurately select the patients with an apparent cCR, thereby correctly identifying those would-be appropriate candidates for a NOM strategy without compromising oncologic safety. NOM should be part of the treatment discussion of LARC, considering increasing rates of cCR, patient preference, potential quality of life gains, and the potential avoidance of surgical morbidity.
Abbreviation/Glossary list
- LARC
Locally Advanced Rectal Cancer
- TME
Total Mesorectal Excision
- NAT
Neoadjuvant Therapy
- TNT
Total Neoadjuvant Therapy
- NOM
Non-operative management
- WW
watch-and-wait
- pCR
pathological complete response
- cCR
clinical complete response
- LCRT
Long course chemoradiation
- SCRT
Short course radiotherapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The Authors have nothing to disclose
Contributor Information
Felipe F. Quezada-Diaz, Colorectal Unit, Department of Surgery, Complejo Asistencial Doctor Sótero del Río, Santiago, RM, Chile.
J. Joshua Smith, Colorectal Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States.
References
- 1.National Comprehensive Cancer Network. NCCN guidelines: rectal cancer. Available at https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed January 11, 2019. www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 2.Smith JJ, Chow OS, Gollub MJ, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. [DOI] [PubMed] [Google Scholar]
- 6.Ho VP, Lee Y, Stein SL, Temple LKF. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum. 2011;54:113–125. [DOI] [PubMed] [Google Scholar]
- 7.Chen TYT, Wiltink LM, Nout RA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106–114. [DOI] [PubMed] [Google Scholar]
- 8.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 9.Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J hong, Yoon SM, Yu CS Kim JH, Kim TW Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer. 2011;117:3703–3712. [DOI] [PubMed] [Google Scholar]
- 11.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. [DOI] [PubMed] [Google Scholar]
- 12.Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554–1562. [DOI] [PubMed] [Google Scholar]
- 13.Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. [DOI] [PubMed] [Google Scholar]
- 14.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 15.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 17.Schmoll HJ, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine +/− oxaliplatin in locally advanced rectal cancer: Final results of PETACC-6. JCO. 2018;36(15_suppl):3500–3500. [Google Scholar]
- 18.Banwell VC, Phillips HA, Duff MJ, et al. Five-year oncological outcomes after selective neoadjuvant radiotherapy for resectable rectal cancer. Acta Oncol. 2019;58:1267–1272. [DOI] [PubMed] [Google Scholar]
- 19.Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169–4182. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26:1722–1728. [DOI] [PubMed] [Google Scholar]
- 21.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahma OE, Yothers G, Hong TS, et al. Use of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Initial Results From the Pembrolizumab Arm of a Phase 2 Randomized Clinical Trial. JAMA Oncol. Published online July 1, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822–828. [DOI] [PubMed] [Google Scholar]
- 25.Caycedo-Marulanda A, Patel SV, Verschoor CP, et al. A Snapshot of the International Views of the Treatment of Rectal Cancer Patients, a Multi-regional Survey: International Tendencies in Rectal Cancer. World J Surg. 2021;45:302–312. [DOI] [PubMed] [Google Scholar]
- 26.Lynn PB, Strombom P, Garcia-Aguilar J. Organ-Preserving Strategies for the Management of Near-Complete Responses in Rectal Cancer after Neoadjuvant Chemoradiation. Clin Colon Rectal Surg. 2017;30:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gani C, Gani N, Zschaeck S, et al. Organ Preservation in Rectal Cancer: The Patients’ Perspective. Front Oncol. 2019;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy ED, Borowiec AM, Schmocker S, et al. Patient and Physician Preferences for Nonoperative Management for Low Rectal Cancer: Is It a Reasonable Treatment Option? Dis Colon Rectum. 2018;61:1281–1289. [DOI] [PubMed] [Google Scholar]
- 29.Emmertsen KJ, Laurberg S, Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013;100:1377–1387. [DOI] [PubMed] [Google Scholar]
- 30.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20–31. [DOI] [PubMed] [Google Scholar]
- 31.Kim YI, Jang JK, Park IJ, et al. Lateral lymph node and its association with distant recurrence in rectal cancer: A clue of systemic disease. Surg Oncol. 2020;35:174–181. [DOI] [PubMed] [Google Scholar]
- 32.Habr-Gama A, São Julião GP, Vailati BB, et al. Organ Preservation Among Patients With Clinically Node-Positive Rectal Cancer: Is It Really More Dangerous? Dis Colon Rectum. 2019;62:675–683. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Lee D, Young C. Predictors for complete pathological response for stage II and III rectal cancer following neoadjuvant therapy - A systematic review and meta-analysis. Am J Surg. 2020;220:300–308. [DOI] [PubMed] [Google Scholar]
- 34.Chen MB, Wu XY, Yu R, et al. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: a meta-analysis in rectal cancer. PLoS ONE. 2012;7:e45388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Aguilar J, Chen Z, Smith DD, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg. 2011;254:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Rosa N, Rodriguez-Bigas MA, Chang GJ, et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol. 2016;34:3039–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. The Lancet. 2018;391:2537–2545. [DOI] [PubMed] [Google Scholar]
- 38.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maas M, Lambregts DMJ, Nelemans PJ, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol. 2015;22:3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53:1692–1698. [DOI] [PubMed] [Google Scholar]
- 41.Smith JJ, Chow OS, Gollub MJ, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dijkhoff RAP, Beets-Tan RGH, Lambregts DMJ, Beets GL, Maas M. Value of DCE-MRI for staging and response evaluation in rectal cancer: A systematic review. Eur J Radiol. 2017;95:155–168. [DOI] [PubMed] [Google Scholar]
- 43.Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30:1234–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Zhang XY, Shi YJ, et al. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin Cancer Res. 2017;23:7253–7262. [DOI] [PubMed] [Google Scholar]
- 45.Koyama FC, Lopes Ramos CM, Ledesma F, et al. Effect of Akt activation and experimental pharmacological inhibition on responses to neoadjuvant chemoradiotherapy in rectal cancer. Br J Surg. 2018;105:e192–e203. [DOI] [PubMed] [Google Scholar]
- 46.Hall MD, Schultheiss TE, Smith DD, Fakih MG, Wong JYC, Chen YJ. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol. 2016;55:1392–1399. [DOI] [PubMed] [Google Scholar]
- 47.Appelt AL, Vogelius IR, Pløen J, et al. Long-term results of a randomized trial in locally advanced rectal cancer: no benefit from adding a brachytherapy boost. Int J Radiat Oncol Biol Phys. 2014;90:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Couwenberg AM, Burbach JPM, Berbee M, et al. Efficacy of Dose-Escalated Chemoradiation on Complete Tumor Response in Patients with Locally Advanced Rectal Cancer (RECTAL-BOOST): A Phase 2 Randomized Controlled Trial. Int J Radiat Oncol Biol Phys. 2020;108:1008–1018. [DOI] [PubMed] [Google Scholar]
- 49.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. [DOI] [PubMed] [Google Scholar]
- 51.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology. 2021;22:702–715. [DOI] [PubMed] [Google Scholar]
- 54.Pettersson D, Lörinc E, Holm T, et al. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg. 2015;102:972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Probst CP, Becerra AZ, Aquina CT, et al. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg. 2015;221:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fokas E, Allgäuer M, Polat B, et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37:3212–3222. [DOI] [PubMed] [Google Scholar]
- 57.Fokas E, Schlenska-Lange A, Polat B, et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncology. Published online November 18, 2021:e215445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Aguilar J, Patil S, Jin Kim, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. Journal of Clinical Oncology 2020. 38:15_suppl, 4008–4008 [Google Scholar]
- 60.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822–828. [DOI] [PubMed] [Google Scholar]
- 62.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501–513. [DOI] [PubMed] [Google Scholar]
- 63.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–928. [DOI] [PubMed] [Google Scholar]
- 64.Smith JJ, Strombom P, Chow OS, et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019;5:e185896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jimenez-Rodriguez RM, Quezada-Diaz F, Hameed I, et al. Organ Preservation in Patients with Rectal Cancer Treated with Total Neoadjuvant Therapy. Dis Colon Rectum. 2021;64:1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bahadoer RR, Peeters KCMJ, Beets GL, et al. Watch and wait after a clinical complete response in rectal cancer patients younger than 50 years. Br J Surg. Published online November 5, 2021:znab372. [DOI] [PubMed] [Google Scholar]
- 67.Hupkens BJP, Martens MH, Stoot JH, et al. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection - A Matched-Controlled Study. Dis Colon Rectum. 2017;60:1032–1040. [DOI] [PubMed] [Google Scholar]
- 68.Quezada-Diaz FF, Smith JJ, Jimenez-Rodriguez RM, et al. Patient-Reported Bowel Function in Patients With Rectal Cancer Managed by a Watch-and-Wait Strategy After Neoadjuvant Therapy: A Case-Control Study. Dis Colon Rectum. 2020;63:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rödel C PD med. Short-Course Radiotherapy Versus Chemoradiotherapy, Followed by Consolidation Chemotherapy, and Selective Organ Preservation for MRI-Defined Intermediate and High-Risk Rectal Cancer Patients. Available at https://clinicaltrials.gov/ct2/show/NCT04246684. Accessed November 27, 2021.


