Abstract
Social needs contribute to persistent diabetes disparities; thus, it is imperative to address social needs to optimize diabetes management. The purpose of this study was to determine determine the feasibility and acceptability of health system-based social care versus social care + behavioral intervention to address social needs and improve diabetes self-management among patients with type 2 diabetes. Black/African American, Hispanic/Latino, and low-income White patients with recent hemoglobin A1C (A1C) ≥ 8%, and ≥1 social need were recruited from an integrated health system. Patients were randomized to one-of-two 6-month interventions: (a) navigation to resources (NAV) facilitated by a Patient Navigator; or (b) NAV + evidence-based nine-session diabetes self-management support (DSMS) program facilitated by a community health worker (CHW). A1C was extracted from the electronic health record. We successfully recruited 110 eligible patients (54 NAV; 56 NAV + DSMS). During the trial, 78% NAV and 80% NAV + DSMS participants successfully connected to a navigator; 84% NAV + DSMS connected to a CHW. At 6-month follow-up, 33% of NAV and 34% of NAV + DSMS participants had an A1C < 8%. Mean reduction in A1C was clinically significant in NAV (−0.65%) and NAV + DSMS (−0.72%). By follow-up, 89% of NAV and 87% of NAV + DSMS were successfully connected to resources to address at least one need. Findings suggest that it is feasible to implement a health system-based social care intervention, separately or in combination, with a behavioral intervention to improve diabetes management among a high-risk, socially complex patient population. A larger, pragmatic trial is needed to test the comparative effectiveness of each approach on diabetes-related outcomes.
Keywords: Diabetes, Diabetes self-management, Social care, Social needs, Health inequities
Social care intervention, separately or combined with a behavioral intervention for diabetes self-management was considered feasible and acceptable among a high-risk, socially complex patient population.
Implications.
Practice: Healthcare settings should consider implementing social care alone or combining it with an evidence-based behavioral intervention to improve diabetes management, address patient social needs, and reduce diabetes-related inequities.
Policy: Adequate reimbursement for social care practice (e.g., screening and navigation) and delivery of evidence-based behavioral interventions for diabetes are needed to effectively reduce health inequities.
Research: Future research should examine the comparative effectiveness and sustainability of a social care only versus social care plus behavioral intervention to improve diabetes management in a high-risk, socially complex patient population.
INTRODUCTION
Despite advances in diabetes treatments, racial/ethnic and socioeconomic disparities in diabetes prevalence and management persist [1]. Black/African American, Hispanic/Latino, and individuals with low socioeconomic status are also at increased risk for diabetes complications including cardiovascular disease [2], due to challenges with achieving diabetes-related clinical targets [2–4]. Diabetes self-management interventions have been found to be efficacious for reducing A1C among Black/African American and Hispanic/Latino adults, with a mean 0.31% greater reduction in A1C compared to control across studies [5]. Unfortunately, access to these interventions [6] and ability to make health behavioral changes needed for long-term optimal diabetes management are often limited due to social needs [7, 8].
Social needs (e.g., food insecurity, unstable housing, difficulty paying for medical care, and lack of transportation) exacerbate disparities in diabetes prevalence and complications as they disproportionately affect Black/African American, Hispanic/Latino, and low-income individuals [9]. Specifically, social needs are a major barrier to adequate diabetes self-management (e.g., healthy eating, being active, monitoring blood glucose, and taking medications), resulting in suboptimal diabetes-related outcomes [10–13] and increased healthcare utilization and costs [13–15]. Healthcare systems across the USA are gradually implementing social care interventions such as navigation (NAV) to social services or community-based resources to address patient social needs [16–18]. Navigating patients to social services and/or community-based resources has been associated with reduction in homelessness [19] and food insecurity [20]; however, there have been mixed findings regarding the effectiveness of NAV on improving hemoglobin A1C [21–24]. Therefore, addressing social needs may be necessary, but not sufficient for management of a complex disease such as diabetes [25].
Several studies have tested social care interventions to address social needs as a component within a behavioral intervention for diabetes self-management. These multi-faceted interventions are often led by community health workers (CHWs). CHW-led diabetes management interventions generally reduce A1C [26–30] among Black/African American, Hispanic/Latino, and low-income patients. However, details about whether or not social needs were successfully addressed are often not reported in these combined behavioral and social care studies [25]. This limits our understanding about the association between addressing social needs and improving diabetes-related outcomes in the context of a behavioral intervention, which may have implications for whether healthcare settings decide to only implement the single-component intervention or the combined behavioral and social care intervention.
Alleviating the socioeconomic stressors that often supersede diabetes self-care among Black/African American, Hispanic/Latino, and low-income patients seems imperative to optimize diabetes management, reduce inequities, and decrease healthcare costs [8]. However, the best approach—social care intervention only or combined behavioral and social care intervention—to address social needs while improving diabetes-related outcomes has not been well established. Prior to establishing the effectiveness of each approach, the feasibility of implementing either approach in a large healthcare setting as well as the acceptability to patients needs to be examined as the U.S. healthcare system embarks on establishing standards for social care practice [18].
The purpose of Bridge to Health/Puente a la Salud (R34DK119853) was to determine the feasibility and acceptability of implementing a social care intervention only versus a social care plus behavioral intervention in a large, integrated health system to address social needs and improve diabetes self-management among Black/African American, Hispanic/Latino, and low-income patients with type 2 diabetes. We hypothesized that Bridge to Health/Puente a la Salud would be feasible to implement based on recruitment, retention, and other intervention-related metrics and deemed an acceptable intervention to address social needs and improve diabetes self-management by patients. For the purposes of this study and to align with current definitions used in the literature, we use the term “social risks” when referring to adverse social circumstances that are related to poor health, which a patient may endorse on a screener, and we use “social needs” when referring to the social risks that the patient identifies as priority and request assistance to address [31].
METHODS
The Bridge to Health/Puente a la Salud study protocol has been published elsewhere [32]. In summary, the trial was conducted at Kaiser Permanente Northwest (KPNW), a large, integrated healthcare system that provides comprehensive health care to its > 620,000 members in Oregon and southwest Washington. Eligible KPNW patients met the following inclusion criteria: (a) aged 18 years or older; (b) had a diagnosis of type 2 diabetes with hemoglobin A1C ≥ 8% 2 weeks prior to recruitment (this value was used as baseline A1C); (c) identified as Black/African American or Hispanic/Latino, or a Medicaid recipient of any racial or ethnic background; (d) preferred language was English or Spanish; and (e) at the time of eligibility screening endorsed one or more social risks. Patients were considered ineligible if they were already receiving NAV services to address their social needs or unable to provide verbal informed consent due to cognitive or psychiatric impairment. We used a targeted recruitment approach by first identifying potentially eligible patients in the electronic health record (EHR) and then emailing or mailing recruitment letters. Letters were followed by a phone call from study staff who described the study and further assessed eligibility by confirming race/ethnicity and health coverage and administering a social risks screener. After confirming eligibility, verbal informed consent was obtained and documented and then patients were randomized (stratified by race/ethnicity, sex, and body mass index) [3, 4] to one of two 6-month study arms—social care intervention only or social care plus behavioral intervention. Participants received a $50 gift card for completing the 6-month follow-up data collection (i.e., A1C test and a repeat social risk screener). The trial was approved by the Institutional Review Board of KPNW and was conducted from September 2018 to December 2020.
Interventions, training, and treatment fidelity
Details about each intervention arm, CHW training, and treatment fidelity have been previously published [32] and are summarized below.
Navigation only – NAV
The social care intervention consisted of navigation to resources and was designed to align and integrate with routine social care practices at KPNW. Specifically, we utilized KPNW Patient Navigators to connect participants to internal medical services and/or community-based resources to address reported social needs. The KPNW Patient Navigator addressed the participant’s social needs by providing referrals to community-based resources or assisting with enrollment in social services. During the 6-month intervention period, Patient Navigators followed up with the participant 1–3 times by phone or in person to discuss progress with referrals and to address any new social needs.
Navigation + diabetes self-management support—NAV + DSMS
Participants randomized to this study arm also received navigation to resources from a KPNW Patient Navigator as well as diabetes self-management support (DSMS) from a CHW. We leveraged existing community partnerships to identify and train six CHWs to deliver the diabetes self-management support intervention while working in tandem with the KPNW Patient Navigators to address participant social needs. The six racially and ethnically diverse CHWs were embedded in six partnering community-based organizations (CBOs) in Portland, Oregon that have a commitment to serving communities of color. Once a participant was randomized to this arm and assigned a CHW, study staff provided the CHW’s name and contact information to the clinical care team via the EHR. CHWs were provided with a list of the participant’s reported baseline social needs, and the name and contact information of the participant’s primary care provider as well as the KPNW Patient Navigator(s) working with their assigned participants to facilitate collaborative and nonredundant navigation services. CHWs did not have access or ability to document directly in the participant’s EHR.
CHWs were expected to follow-up with their assigned participants within a week of being assigned to schedule an initial meeting either in the participant’s home or a public setting (e.g., library, church, café). During the initial meeting, the CHW assessed for additional social needs, then followed up with the KPNW Patient Navigator to collaborate on how best to address the needs. CHWs applied the Pathways to Health Model [33], a three-step process (referral, check-in at 2 weeks, check-in at 30 days) to efficiently track progress towards addressing all of the social needs the participant wanted assistance with and that were not already being addressed by the KPNW Patient Navigator.
CHWs also delivered the ADA-recognized diabetes support program (https://www.professional.diabetes.org/content-page/diabetes-support-directory), Decision-making Education for Choices In Diabetes Everyday (DECIDE) [34, 35]. DECIDE is a nine-module, low-literacy-adapted program that uses problem-solving training as an evidence-based behavior change skill to facilitate identifying and managing barriers to diabetes self-management [36]. The first session covers diabetes and cardiovascular disease education and self-management behaviors. The remaining eight sessions focus on the five steps of problem-solving within the context of diabetes self-management.
Since DECIDE and accompanying materials were initially developed and delivered in English, for Bridge to Health/Puente a la Salud, the participant-facing materials were professionally translated into Spanish and culturally adapted by one of the co-investigators (N.L.) and an external consultant to ensure they were linguistically and culturally appropriate for participants whose origins were from various Spanish-speaking countries. Specifically, N.L. and the external consultant conducted a thorough overview of the content, paying close attention to the terminology referenced and readability levels. Adaptations were made to make sure terms and concepts resonated with Hispanic/Latino participants, including the use of culturally specific references, for example, referring to tortillas and rice when discussing carbohydrate intake. Similar to the original DECIDE materials, text was translated to accommodate Spanish-speaking participants with limited literacy, avoiding, when possible, complex or clinical jargon, long sentences, and low-frequency words.
CHWs delivered the DECIDE program in English or Spanish, based on the participant’s preference, on a weekly or bi-weekly basis primarily in-person, but sometimes over the phone depending on the participant’s needs. During DECIDE visits, CHWs addressed social needs with participants. When social need crises arose (e.g., eviction), priority was given to addressing the social need(s), either in addition to or in place of discussing the DECIDE content.
Interventionist training and treatment fidelity
All CHWs completed a 20-hour training on the study design, research ethics, diabetes 101, delivering DECIDE, and addressing social needs. CHWs received supervision and case management twice a month from the principal investigator (S.L.F.). A random selection of CHW visits were audio-recorded and reviewed by S.L.F. as another check for fidelity.
Study measures
All diabetes-related clinical data (e.g., labs, vitals, anthropometric measures, comorbid diagnoses) and demographics (i.e., sex, age, race and ethnicity, health coverage) were extracted from the EHR. Median household income was pulled from the 2013 to 2017 American Community Survey 5-year estimate data at the block level. During eligibility screening/baseline and at the 6-month follow-up, study staff administered the Your Current Life Situation (YCLS), a 9-item social risks screener [37], over the phone in either English or Spanish. At the end of the screener, participants were asked which of the social risks reported that they wanted assistance with to address (i.e., social needs). Participant responses to each item was documented in the EHR to be viewed later by the KPNW Patient Navigator as well as the participant’s care team. In addition to the YCLS, for participants in the NAV + DSMS arm, CHWs documented their progress with Pathways (e.g., Food Access, Employment, Health Insurance Coverage, Housing, Transportation) during the 6-month intervention period. CHWs documented which Pathways were opened, which referrals were placed, and if the Pathway was “closed” or “resolved.” All KPNW Patient Navigator contacts were obtained from the EHR, whereas CHW contacts including DECIDE sessions and Pathways were obtained from a web-based case management tracking tool used by the CHWs.
Feasibility
The primary outcome for this pilot trial was feasibility, which was determined by the following: number successfully enrolled (recruitment); proportion in each arm with an A1C test at follow-up (retention); proportion of participants in each arm successfully connected to a CBO CHW and/or KPNW Patient Navigator; mean number of days to connect participants to a CHW and/or KPNW Patient Navigator; and number of DECIDE sessions completed. Specific goals for each feasibility metric and how each was calculated is provided in Table 1. Proportion of participants successfully connected (i.e., at least 50%) as well as time to connect to a CHW and/or KPNW Patient Navigator (i.e., 7–14 days) were included as feasibility metrics based on a previous quality improvement project at KPNW in which only 50% of the patients referred were successfully connected to a navigator and among those who connected within 7 days of the referral were more likely to have their social needs addressed and re-engaged in care. We also examined the percent of participants with at least one social need addressed during the 6-month intervention period in each study arm. For both study arms, social needs that participants requested help with on the YCLS at baseline, but no longer requested assistance at follow-up were considered “successfully addressed.” Social needs among participants in the NAV + DSMS arm were also considered “successfully addressed” if the CHW indicated that a Pathway was “closed” or “resolved.” We did not set a specific goal for percent of participants with a social need addressed as this was often dependent on the specific need and availability of community resources.
Table 1.
Bridge to Health/Puente a la Salud feasibility metrics and results
| Goal | Definition/calculation | Navigation only | Navigation + DSMS |
|---|---|---|---|
| Recruit 100 eligible participants over 6 months | # of eligible participants enrolled/ total # of eligible patients | Recruited 110 participants over 8 months | |
| Successfully connect 50% or more participants to CHW and/or Patient Navigator | # of participants with at least one encounter (via phone or in person) with a CHW and/or Patient Navigator/total number of participants enrolled in each arm | 78% connected to Patient Navigator |
80% connected to Patient Navigator 84% connected to CHW |
| Connect participants to CHW and/or Patient Navigator in 7–14 days, M (SD) | Mean # of days between date of assignment to date of first encounter with a CHW and/or Patient Navigator across participants per study arm | 13.5 (15.4) days to connect to Patient Navigator |
14.3 (18) days to connect to Patient Navigator 9 (7.3) days to connect to CHW |
| Participants complete at least 8/9 DECIDE sessions | Number of DECIDE sessions the CHW successfully delivers to a participant | Not applicable | 40 participants completed a mean of 7.4 sessions (30 [75%] completed all 9 sessions) |
| At least 80% of participants with 6-month A1C at the end of study | # of participants with an A1C test at 6-month follow-up/ # of enrolled participants | 67% (91% for A1C or YCLS) | 80% (91% for A1C or YCLS) |
CHW community health worker; DECIDE Decision-making Education for Choices in Diabetes Everyday; DSMS diabetes self-management support; YCLS Your Current Life Situation.
Acceptability
Semi-structured qualitative interviews were conducted by phone with a small subset of randomly selected participants to assess acceptability of each intervention arm. We intentionally aimed for a greater number of interviews with the NAV + DSMS participants to reach a saturation point in understanding the acceptability of working with a CHW regarding social needs and diabetes self-management. We felt that interviewing a smaller number of NAV participants would be sufficient for understanding experiences in this intervention arm.
A1C
A1C obtained during routine care and documented in the EHR was used to determine the proportion of participants in each study arm with an A1C < 8%, A1C test at follow-up, and mean change in A1C. Because routine A1C testing schedule was not always aligned with participants’ 6-month post-randomization date, A1C tests that occurred during the 60 days prior or 80 days after the 6-month post-randomization date were used to determine A1C at follow-up.
Statistical and qualitative analysis
For A1C-related analyses, we used an intention-to-treat (ITT) approach (missing A1C follow-up data was handled using multiple imputation) as well as a per protocol approach. Specifically, we ran a chi-square test to compare the two study arms on proportion of randomized participants with an A1C < 8% at follow-up and examined mean change in A1C over 6 months in each arm separately. For the per protocol analysis, we examined the proportion of participants with an A1C < 8% at follow-up as well as mean change in A1C in each arm among participants with an A1C test at follow-up and who received their assigned intervention (at least one encounter with the KPNW Patient Navigator in the NAV arm or completed at least one DECIDE session with the CHW in the NAV + DSMS arm). Lastly, using logistic regression, we explored the association between number of social needs addressed and A1C < 8% in each study arm, controlling for age (centered) and sex, among participants with an A1C test at follow-up and separately among participants that received the intervention. All quantitative analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC).
For the qualitative analysis, study staff trained in qualitative methods (D.P.-T. and J.L.S.) conducted a content analysis aided by a qualitative software program, Dedoose. First, a coding dictionary was developed based on review of the interview questions and a subset of transcripts. During the coding process, D.P.-T. and J.L.S. met regularly to discuss and refine the codebook and coding process, and any differences in coding were resolved through discussion with changes subsequently applied to transcripts. Once all transcripts were coded within Dedoose, text retrieval and grouping functions were used to produce reports on specific codes and combinations of codes for a particular topic. These topical reports were then iteratively reviewed, and the content summarized. This process resulted in a list of themes related to intervention acceptability.
RESULTS
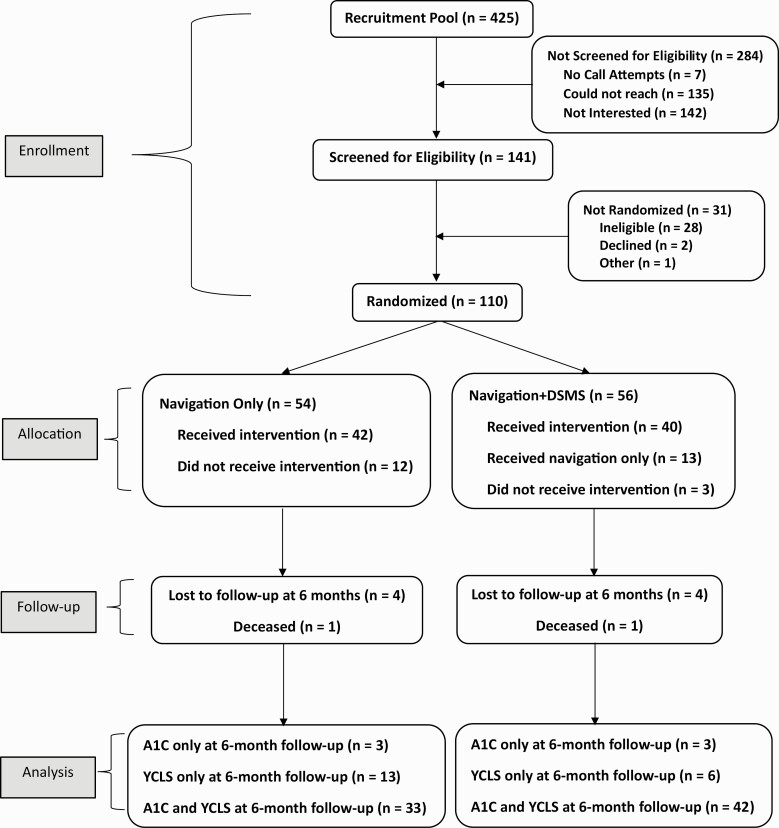
Figure 1 presents the Consort Diagram. Out of 425 potentially eligible patients, 141 were screened, and 110 were randomized (54 NAV; 56 NAV + DSMS). There were 12 participants in the NAV arm and 3 participants in the NAV + DSMS arm who did not receive their assigned intervention because they either changed their mind about participation or were hard to reach by phone after randomization. Thirteen participants in the NAV + DSMS arm only received NAV as they were experiencing an active social needs crisis (i.e., housing insecurity or homelessness) throughout the intervention period and were not able to start DECIDE sessions. Two participants (one in each study arm) died during the study period for reasons unrelated to participating in the trial.
Fig. 1.
CONSORT diagram.
Table 2 presents the baseline characteristics of the 110 participants enrolled in Bridge to Health/Puente a la Salud. Mean age across both study arms was 53 years and 70% of patients were female. The sample was racially and ethnically diverse, including 14 participants total whose primary language was Spanish. Forty percent of the participants had Medicaid coverage. Participants had been living with diabetes an average of 9 years, with 42% prescribed oral medications, 16% insulin, and 40% both oral and insulin to manage their diabetes. Mean baseline A1C was 9.9%. In terms of social risks, over 60% of participants in each arm reported three or more social risks at baseline on the YCLS with financial hardship (e.g., trouble paying for utilities, medical supplies) and food insecurity as top reported risks. More specifically, 27 (50%) NAV participants and 22 (39%) NAV + DSMS participants reported having trouble paying for medical care/supplies in the past 3 months (data not shown in table).
Table 2.
Bridge to Health/ Puente a la Salud participant characteristics
| Total | Navigation only | Navigation + DSMS | |
|---|---|---|---|
| (n = 54) | (n = 56) | ||
| Age in years, M (SD) | 53.3 (12) | 52.9 (12.1) | 53.7 (12.1) |
| Female, n (%) | 77 (70) | 38 (70) | 39 (70) |
| Race/ethnicity, n (%) | 40 (36) | 19 (35) | 21 (38) |
| Black/African American | 29 (26) | 16 (30) | 13 (23) |
| Hispanic/Latino | 3 (3) | 1 (2) | 2 (4) |
| Multiple races | 38 (35) | 18 (33) | 20 (36) |
| White | |||
| Primary Language, n (%) | 96 (87) | 46 (85) | 50 (89) |
| English | 14 (13) | 8 (15) | 6 (11) |
| Spanish | |||
| Medical coverage, n (%) | 37 (34) | 18 (33) | 19 (34) |
| Private/commercial | 16 (15) | 8 (15) | 8 (14) |
| Medicare | 44 (40) | 21 (39) | 23 (41) |
| Medicaid | 13 (12) | 7 (13) | 6 (11) |
| Dual Coverage | |||
| Median household income within neighborhood (ACS 2013–2017), M (SD) | 44,844 (13,594) | 44,135 (14,133) | 45,529 (13,144) |
| A1C, M (SD) | 9.9 (1.7) | 9.9 (1.7) | 9.8 (1.6) |
| Diabetes medications, n (%) | 46 (42) | 21 (39) | 25 (45) |
| Oral | 18 (16) | 7 (13) | 11 (20) |
| Insulin | 44 (40) | 24 (44) | 20 (36) |
| Both | 2 (2) | 2 (4) | 0 (0) |
| None | |||
| Number of years with diabetes, M (SD) | 9.0 (7.7) | 9.4 (7.5) | 8.7 (7.9) |
| Body mass index (kg/m2), M (SD) | 37.7 (10.6) | 38 (11) | 37 (10) |
| Diagnosis of hypertension, n (%) | 73 (66) | 35 (65) | 38 (68) |
| Diagnosis of dyslipidemia, n (%) | 66 (60) | 35 (65) | 31 (55) |
| Social needs reported, n (%) | |||
| 1 | 12 (11) | 2 (4) | 10 (18) |
| 2 | 27 (25) | 15 (28) | 12 (21) |
| 3 or more | 71 (65) | 37 (69) | 34 (61) |
| Types of social needs, n (%) | |||
| Insufficient housing | 33 (30) | 15 (28) | 18 (32) |
| Financial hardship | 96 (87) | 51 (94) | 45 (80) |
| Food insecurity | 67 (61) | 34 (63) | 33 (59) |
| Lack of transportation | 37 (34) | 18 (33) | 19 (34) |
ACS American Community Survey; DSMS diabetes self-management support.
Feasibility metrics
Table 1 presents results of the feasibility metrics. In terms of recruitment, we successfully enrolled 110 eligible patients over an 8-month period, recruiting and enrolling 1–4 patients per week. In both study arms, over 70% of participants were successfully connected to a KPNW Patient Navigator (78% NAV; 80% NAV + DSMS) and CHW (84% NAV + DSMS). Connecting participants to a KPNW Patient Navigator took a little longer on average (13.5 days NAV; 14.3 days NAV + DSMS) compared to connecting participants to a CHW (9 days NAV + DSMS). Of the 40 participants in the NAV+DSMS arm, mean number of completed DECIDE sessions was 7.4. Ten (25%) participants completed less than eight DECIDE sessions and the remaining 30 (75%) completed all nine sessions. We did not reach our retention goal in terms of follow-up A1C test for the NAV arm (67% with follow-up A1C test), but we did for the NAV + DSMS arm (80% with follow-up A1C test). Over 90% of participants in each study arm provided either an A1C or completed the YCLS over the phone with study staff at follow-up.
Social needs
There were 46 participants in the NAV arm (85%) and 48 in the NAV + DSMS arm (86%) who completed the YCLS at follow-up. Based on comparing social needs reported at baseline to needs reported at follow-up on the YCLS, 41 participants in each study arm, 89% in NAV and 87% in NAV+DSMS, had at least one social need addressed during the 6-month intervention period. Supplemental Table S1 presents the proportion of participants with specific social needs addressed by study arm. CHWs opened 55 Pathways across 20 different Pathway categories (e.g., Dental Care-Urgent, Food Access, Essentials, Mental Health treatment, Transportation, Housing) for participants in the NAV + DSMS arm. Of the 55 Pathways opened, 39 (71%) were closed by 6-month follow-up (data not shown).
A1C
Based on ITT analysis, 33% of NAV participants versus 34% of NAV + DSMS participants had an A1C < 8% (p = .73). Mean reduction in A1C was clinically significant in both study arms, 0.65% (1.76) and 0.72% (1.88) in the NAV and NAV + DSMS arms, respectively. In the per protocol analyses, with participants who had a follow-up A1C test and at least one encounter with the KPNW Patient Navigator in the NAV arm (n = 30) or completed at least one DECIDE session with the CHW in the NAV + DSMS arm (n = 33), there were 37% in the NAV arm and 42% in the NAV + DSMS arm with an A1C < 8% at follow-up (p = .64). Mean reduction in A1C was also clinically significant in each study arm among participants with a follow-up A1C and exposed to their respective interventions (−0.67% (1.88) NAV, −1.01% (1.93) NAV + DSMS, p = .49). In exploratory analysis, there was no association between number of needs addressed (based on YCLS at follow-up) and having an A1C < 8% among participants in the NAV arm with a follow-up A1C test (n = 36; b = −0.26; p = .31) nor participants exposed to the NAV intervention (n = 30; b = −0.25; p = .37). On the other hand, the relationship between number of needs addressed and A1C < 8% was marginal for NAV + DSMS participants with a follow-up A1C test (n = 50; b = 0.33; p = .07) and participants that received at least one DECIDE session (n = 33; b = 0.39, p = .11)
Qualitative findings
A total of 23 participants (6 NAV; 17 NAV+DSMS) completed semi-structured qualitative interviews. Interviewees were primarily female (74%), and racially and ethnically diverse (35% Black/African American, 39% Hispanic/Latino, and 22% White) with 17% Spanish-speaking only. The thematic findings presented in Supplemental Tables S2 and 3 included acceptability of interventions, helpfulness of the interventions, experience working with the KPNW Patient Navigators and CBO CHWs, and areas for improvement. Among the NAV participants interviewed (n = 6), working with the KPNW Patient Navigator (n = 3) and receiving a list of community resources to address their social needs (n = 4) were perceived as helpful. Furthermore, receiving the ADA handouts monthly reportedly led to increased knowledge (n = 3), healthier eating (n = 2), and increased engagement with their provider (n = 2). In terms of improvements, NAV participants felt that there could be better and more frequent follow-up from the KPNW Patient Navigator after the initial contact (n = 4).
All of the NAV+DSMS participants felt that the DECIDE sessions and material were helpful as they served as a reminder or refresher on diabetes self-management (n = 17). Support from the CHWs in terms of working through the DECIDE sessions, setting goals, and having someone to discuss their diabetes with was also described as helpful (n = 11). Furthermore, NAV+DSMS participants reported that participating in Bridge to Health/Puente a la Salud led to improved eating habits (n = 15), increased engagement in physical activity (n = 10), improved consistency in managing blood sugar (n = 10), medication adherence (n = 8), and a sense of empowerment in terms of feeling more in control of managing their diabetes (n = 10). A few participants also mentioned an increased awareness of the association between diabetes and mental health (n = 8). All participants from the NAV + DSMS arm stated that “nothing was least helpful” (n = 17).
Discussion
Based on our metrics and qualitative interviews, overall, implementation of the two interventions to address both social needs and diabetes self-management, NAV and NAV + DSMS, were considered feasible and acceptable. Although it took us a little longer to recruit the 110 eligible patients as intended (eight instead of 6 months), this was expected given that we were targeting a high-risk, socially complex patient population. Study staff were particularly instrumental in connecting almost 80% or higher of participants to a CHW and/or KPNW Navigator within an average of 14 days or less, likely because they maintained close follow-up with our CBO and health system partners. This successful connection rate certainly had implications for engagement throughout the intervention period and retention at follow-up. For future trials, we may consider setting the connection goal to be much higher than 50% given the success in this trial. Time to connect the participant to a CHW was shorter, on average, compared to connecting to a KPNW Patient Navigator because the caseload for the CBO-embedded CHWs primarily consisted of Bridge to Health/Puente a la Salud participants (up to 10 participants maximum), whereas KPNW Patient Navigators were responsible for seeing study participants as well as their regular patient caseload (up to 200 patient referrals per week).
In terms of retention, it should be noted that 50 (45%) participants (23 (43%) NAV, 27 (48%) NAV + DSMS) had their 6-month follow-up occur between February and April 2020. This time period was the beginning of the COVID-19 pandemic with an implementation of stay-at-home orders and an increase in telehealth care, which had detrimental effects on patients with diabetes [38]. However, we would expect poor follow-up in both arms if it was primarily due to the pandemic. Perhaps the lack of follow-up from KPNW Patient Navigators in the NAV arm versus regular contact and a sense of accountability to the CHW in the NAV + DSMS arm, as described in the qualitative interviews, contributed to the difference in retention.
Several studies have established that long-term glycemic control is associated with lower healthcare costs over time [39, 40]. Follow-up A1C results in both study arms are quite promising as these findings suggest that an intervention that addresses social needs may help improve glycemic control among more than one-third of participants, which may have significant healthcare costs implications. Furthermore, participants in each study arm had a clinically significant mean reduction in A1C that was greater than what has been found in previous diabetes self-management interventions with Black/African American and Hispanic/Latino participants (i.e., −0.31%) [5], suggesting the importance of addressing social needs to improve diabetes-related outcomes. This is further supported by the exploratory analysis suggesting a marginal association between an increase in number of needs successfully addressed and having an A1C < 8%, at least among NAV + DSMS participants. The greater mean reduction in A1C among NAV + DSMS participants (−0.72%), especially among those who completed at least one DECIDE session (−1.01%), compared to NAV participants (−0.65%) is similar to previous studies [41, 42] and highlights the importance of not only addressing social needs, but also empowering patients to implement recommended diabetes self-care behaviors.
Regarding acceptability, participants in both study arms reported that components of both interventions were helpful in terms of addressing social needs and improving diabetes self-management. The concern among NAV participants regarding lack of follow-up from the KPNW Patient Navigator is important to consider for any navigation program in terms of maintaining patient engagement or re-engagement in care. The overall acceptability of the NAV + DSMS arm was not surprising given the demonstrated effectiveness of CHWs in the literature [26] and that the DECIDE program met all of the components for an effective diabetes self-management intervention for Black/African American, Hispanic/Latino, and low-income participants: problem-solving training; an empowerment approach; social support; and had undergone both surface and deep cultural adaption [43, 44].
Limitations
There were several limitations in this pilot trial. First, we were not able to detect statistically significant differences in proportion of participants with A1C < 8% at follow-up between the two study arms. However, the findings from this pilot are promising and warrant a larger, pragmatic clinical trial to test the comparative effectiveness of these two approaches. Second, we used A1C values taken during routine clinical care. Although this approach was less burdensome to the participant, this approach may have contributed to our missing A1C data at follow-up due to barriers such as lack of transportation to the clinic lab, inability to cover co-pay, limited lab hours, and clinical care interruptions due to COVID. Third, given that some social needs are ongoing (e.g., housing insecurity) or recurring (e.g., food insecurity), our definition of social needs being “successfully addressed” or “resolved” may not have been accurate based on the lived experience of many of our participants. There is a need for longitudinal studies to understand the effect of ongoing or recurring social needs on the sustainability of clinical benefits from diabetes self-management interventions. Lastly, the generalizability of our findings may be limited given that we did not have any participants from the Native American, Asian, or Pacific Islander communities, which are also disproportionately burdened by diabetes prevalence and complications [1].
Lessons learned
From both a clinical research and practice perspective, it is important to keep in mind the social crises, mistrust, and discrimination patients with both poor diabetes management and social needs may be experiencing. Therefore, our study team learned that it was important to meet participants where they were and apply an equity lens throughout the trial, from recruitment to retention. Furthermore, we realized that our success with implementing this pilot trial relied on strong, reciprocal partnerships among the study team, health system, and CBOs. As found in previous research [45], the health system-community partnership was particularly strengthened by the CHWs who served as a bridge between the patients and the health system. However, this relationship could be strengthened by further integrating CHWs on the care team by providing them with access to document directly in the EHR. Lastly, as with any pragmatic trial that also utilizes a community-based participatory approach, there needs to be a balance of rigor in terms of clinical trial design and respect for the community in which we are trying to support.
Supplementary Material
Acknowledgments:
We would like to acknowledge our participants and community-based organization partners on this study: Project Access NOW, Familias en Acción, Northwest Family Services, Latino Network, Volunteers of America, Portland Opportunities Industrialization Center, Immigrant and Refugee Community Organization, El Programa Hispano, and Impact Northwest. We would also like to acknowledge Dr. Ana Grinberg, PhD for her work on translating the DECIDE materials.
Contributor Information
Stephanie L Fitzpatrick, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Dea Papajorgji-Taylor, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Jennifer L Schneider, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Nangel Lindberg, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Melanie Francisco, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Ning Smith, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Katie Vaughn, Kaiser Permanente Center for Health Research, Portland, OR 97227, USA.
Elizabeth A Vrany, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY 11030, USA.
Felicia Hill-Briggs, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY 11030, USA.
Funding
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R34DK119853).
Compliance With Ethical Standards
Conflicts of Interest: Dr. Hill-Briggs has a financial conflict of interest for DECIDE. All other authors declare that they have no conflicts of interest.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all participants included in the study.
Welfare of Animals: This article does not contain any studies with animals.
Transparency Statements
This study was pre-registered at clinicaltrials.gov (NCT03802825). The analysis plan was not formally pre-registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author. Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author. Materials used to conduct the study are not publicly available.
References
- 1. CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 2. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of Medicare coverage. Ann Intern Med. 2009;150(8):505–515. [DOI] [PubMed] [Google Scholar]
- 4. Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009-2011. J Diabetes Complications. 201630(2):212–220. [DOI] [PubMed] [Google Scholar]
- 5. Ricci-Cabello I, Ruiz-Perez I, Rojas-Garcia A, Pastor G, Rodriguez-Barranco M, Goncalves DC. Characteristics and effectiveness of diabetes self-management educational programs targeted to racial/ethnic minority groups: A systematic review, meta-analysis and meta-regression. BMC Endocr Disord. 2014;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chomko ME, Odegard PS, Evert AB. Enhancing access to diabetes self-management education in primary care. Diabetes Educ. 2016;42(5):635–645. [DOI] [PubMed] [Google Scholar]
- 7. Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci. 2016;351(4):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodridge D, Bandara T, Marciniuk D, et al. Promoting chronic disease management in persons with complex social needs: A qualitative descriptive study. Chron Respir Dis. 2019;16:1479973119832025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258–279. doi: 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnard LS, Wexler DJ, DeWalt D, Berkowitz SA. Material need support interventions for diabetes prevention and control: A systematic review. Curr Diab Rep. 2015;15(2):574. [DOI] [PubMed] [Google Scholar]
- 11. Gucciardi E, Vahabi M, Norris N, Del Monte JP, Farnum C. The intersection between food insecurity and diabetes: A review. Curr Nut Rep. 2014;3(4):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knight CK, Probst JC, Liese AD, Sercye E, Jones SJ. Household food insecurity and medication “scrimping” among US adults with diabetes. Prev Med. 2016;83:41–45. [DOI] [PubMed] [Google Scholar]
- 13. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becerra MB, Allen NL, Becerra BJ. Food insecurity and low self-efficacy are associated with increased healthcare utilization among adults with type II diabetes mellitus. J Diabetes Complications. 2016;30(8):1488–1493. [DOI] [PubMed] [Google Scholar]
- 15. Berkowitz SA, Kalkhoran S, Edwards ST, Essien UR, Baggett TP. Unstable housing and diabetes-related emergency department visits and hospitalization: A nationally representative study of safety-net clinic patients. Diabetes Care. 2018;41(5):933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davidson KW, McGinn T. Screening for social determinants of health: The known and unknown. JAMA. 2019;322(11):1037–1038. [DOI] [PubMed] [Google Scholar]
- 17. Gottlieb LM, DeSalvo K, Adler NE. Healthcare sector activities to identify and intervene on social risk: An introduction to the American Journal of Preventive Medicine supplement. Am J Prev Med. 2019; 57(6 suppl 1):S1–S5. [DOI] [PubMed] [Google Scholar]
- 18. Alley DE, Asomugha CN, Conway PH, Sanghavi DM. Accountable health communities—Addressing social needs through Medicare and Medicaid. N Engl J Med. 2016;374(1):8–11. [DOI] [PubMed] [Google Scholar]
- 19. Shinn M, Brown SR, Wood M, Gubits D. Housing and service interventions for families experiencing homelessness in the United States: An experimental evaluation. Eur J Homelessness. 2016;10(1):13–30. [PMC free article] [PubMed] [Google Scholar]
- 20. Mabli J OJ, Dragoset L, Castner L, Santos B. Measuring the effect of Supplemental Nutrition Assistance Program (SNAP) participation on food security. Math Policy Res. 2013. [Google Scholar]
- 21. Loskutova NY, Tsai AG, Fisher EB, et al. Patient navigators connecting patients to community resources to improve diabetes outcomes. J Am Board Fam Med. 2016;29(1):78–89. [DOI] [PubMed] [Google Scholar]
- 22. Horny M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: A retrospective cohort study. BMC Health Serv Res. 2017;17(1):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berkowitz SA, Hulberg AC, Standish S, Reznor G, Atlas SJ. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med. 2017;177(2):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kangovi S, Mitra N, Grande D, Huo H, Smith RA, Long JA. Community health worker support for disadvantaged patients with multiple chronic diseases: A randomized clinical trial. Am J Public Health. 2017;107(10):1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hessler D, Bowyer V, Gold R, Shields-Zeeman L, Cottrell E, Gottlieb LM. bringing social context into diabetes care: Intervening on social risks versus providing contextualized care. Curr Diab Rep. 2019;19(6):30. [DOI] [PubMed] [Google Scholar]
- 26. Trump LJ, Mendenhall TJ. Community health workers in diabetes care: A systematic review of randomized controlled trials. Fam Syst Health. 2017;35(3):320–340. [DOI] [PubMed] [Google Scholar]
- 27. Rothschild SK, Martin MA, Swider SM, et al. Mexican American trial of community health workers: A randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. Am J Public Health. 2014;104(8):1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nelson K, Taylor L, Silverman J, et al. randomized controlled trial of a community health worker self-management support intervention among low-income adults with diabetes, Seattle, Washington, 2010-2014. Prev Chronic Dis. 2017;14:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang TS, Funnell M, Sinco B, et al. Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes Care. 2014;37(6):1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carrasquillo O, Lebron C, Alonzo Y, Li H, Chang A, Kenya S. Effect of a community health worker intervention among Latinos with poorly controlled type 2 diabetes: The Miami Healthy Heart Initiative randomized clinical trial. JAMA Intern Med. 2017; 177(7):948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alderwick H, Gottlieb LM. Meanings and misunderstandings: A social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papajorgji-Taylor D, Francisco M, Schneider JL, et al. Bridge to Health/Puente a la Salud: Rationale and design of a pilot feasibility randomized trial to address diabetes self-management and unmet basic needs among racial/ethnic minority and low-income patients. Contemp Clin Trials Commun. 2021;22:100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. AHRQ. Program Uses “Pathways” to Confirm Those At-risk Connect to Community Based Health and Social Services, Leading to Improved Outcomes; 2008. https://innovations.ahrq.gov/node/4433. Accessed February 26, 2019.
- 34. Hill-Briggs F, Lazo M, Peyrot M, et al. Effect of problem-solving-based diabetes self-management training on diabetes control in a low income patient sample. J Gen Intern Med. 2011;26(9):972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fitzpatrick SL, Golden SH, Stewart K, et al. Effect of DECIDE (Decision-making Education for Choices In Diabetes Everyday) program delivery modalities on clinical and behavioral outcomes in urban African Americans with type 2 diabetes: A randomized trial. Diabetes Care. 2016;39(12):2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hill-Briggs F. Problem solving in diabetes self-management: A model of chronic illness self-management behavior. Annals Behav. Med. 2003;25(3):182–193. [DOI] [PubMed] [Google Scholar]
- 37. Sundar KR. Universal screening for social needs in a primary care clinic: A quality improvement approach using the Your Current Life Situation survey. Perm J. 2018; 22:18–089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ADCES. Effects of the COVID-19 Pandemic on People with Diabetes; 2020. https://wwwdiabetesorg/sites/default/files/2020-12/ADA%20Thrivable%20Data%20Deckpdf. Accessed December 1, 2020.
- 39. Bansal M, Shah M, Reilly B, Willman S, Gill M, Kaufman FR. Impact of reducing glycated hemoglobin on healthcare costs among a population with uncontrolled diabetes. Appl Health Econ Health Policy. 2018;16(5):675–684. [DOI] [PubMed] [Google Scholar]
- 40. Juarez D, Goo R, Tokumaru S, Sentell T, Davis J, Mau M. Association between sustained glycated hemoglobin control and healthcare costs. Am J Pharm Benefits. 2013;5(2):59–64. [PMC free article] [PubMed] [Google Scholar]
- 41. Gary TL, Batts-Turner M, Yeh HC, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2009;169(19):1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seligman HK, Smith M, Rosenmoss S, Marshall MB, Waxman E. Comprehensive diabetes self-management support from food banks: A randomized controlled trial. Am J Public Health. 2018;108(9):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thornton PL, Kumanyika SK, Gregg EW, et al. New research directions on disparities in obesity and type 2 diabetes. Ann N Y Acad Sci. 2020;1461(1):5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lynch CP, Egede LE. Optimizing diabetes self-care in low literacy and minority populations—problem-solving, empowerment, peer support and technology-based approaches. J Gen Intern Med. 2011;26(9):953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lohr AM, Ingram M, Nunez AV, Reinschmidt KM, Carvajal SC. Community-clinical linkages with community health workers in the United States: A scoping review. Health Promot Pract. 2018;19(3):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.