ABSTRACT
Controlled trials assessing quadratus lumborum block (QLB) for post-operative analgesia in hip surgery are scarce. This study aimed to compare ultrasound-guided QLB and lumbar plexus block (LPB) for clinical efficacy in hip arthroscopy. Patients undergoing hip arthroscopy in Beijing Jishuitan Hospital in January–June 2019 were randomized to the lumbar plexus (L) and quadratus lumborum (Q) groups (n = 25/group). After either ultrasound-guided block for 30 min, both groups were prepared for surgery after muscle strength measurement in the affected limbs. Opioid doses for patient-controlled analgesia (PCA), visual analog scale (VAS) scores in the resting and active states, upon leaving the post-anesthesia care unit (PACU), and at 2–48 h post-surgery were recorded, and post-operative complications were also recorded. Muscle strength in the affected limbs was significantly higher in the Q group compared with the L group (4.0 versus 2.0, P < 0.001). VAS scores were similar in both groups post-surgery (P > 0.05). One patient had epidural spread in the L group, with no other complications. Compared with ultrasound-guided LPB, ultrasound-guided QLB provides similar and good post-operative analgesia after hip arthroscopy, with less impact on muscle strength and fewer complications. These results should be confirmed in larger trials.
INTRODUCTION
Arthroscopy has become the gold standard for the early diagnosis and treatment of hip joint diseases [1]. Indeed, hip arthroscopy is an increasingly common procedure thanks to advanced surgical tools and method refinement [2], which helps address pathology in and around the hip joint. Compared with open hip surgery, hip arthroscopy has the advantages of a small surgical incision and reduced damage to the joint cavity and surrounding soft tissues [3]. Hip arthroscopy is a comprehensive therapeutic process, and post-operative rehabilitation is crucial for its therapeutic effect; however, obvious post-operative pain limits its application [4].
Lumbar plexus block (LPB) affects the main branches of hip joint capsule nerves, including the femoral, lateral femoral cutaneous and obturator nerves, and was first introduced in 1973 [5]. LPB is an analgesic regimen after surgery involving the hip’s anterior capsule, reducing post-operative pain and opioid dose [6]. On the other hand, quadratus lumborum block (QLB), a variant of transversus abdominis plane (TAP) block, was proposed in 2007 [7]. Currently, four QLB types are available, i.e. QLB1-4 as lateral, posterior, anterior/transmuscular and intramuscular types, respectively [8]. Because L1 and L2 also run between the thoracolumbar and intra-abdominal fasciae before leaving the intervertebral foramen, injection at this site can partially block the lateral femoral cutaneous, femoral and obturator nerves branched from L2 [9]. Consequently, as a trunk block, QLB can be used for multimodal analgesia after hip surgery.
Currently, two case reports [10, 11] and some recent studies [12–14] have described QLB for hip surgery with good post-operative analgesia. Trials are also currently ongoing (e.g. ClinicalTrials.gov NCT03408483 and [15]). In addition, a recent randomized, double-blind, placebo-controlled trial demonstrated that ultrasound-guided QL3 block represents an effective pain management tool following Total Hip Arthroplasty [13]. However, the latter clinical controlled trial was a single-center study.
The hypothesis of this pilot study was that QLB is similar to LPB and can provide good post-operative analgesia for hip arthroscopy. Therefore, this pilot study aimed to assess comparatively the clinical efficacies of ultrasound-guided QLB and LPB in hip arthroscopy. The results could help find a safer and more convenient method for perioperative analgesia for hip arthroscopy.
MATERIALS AND METHODS
Patients
Patients admitted to the XXX Hospital for hip arthroscopy due to glenoid ligament injury in January–June 2019 were enrolled. Inclusion criteria were: planned unilateral hip arthroscopy; grade I–III American Society of Anesthesiologists (ASA) classification of physical condition [16]; 18–60 years old and body mass index (BMI) <35 kg/m2. Exclusion criteria were: infection at the puncture site; anatomical variation; use of anticoagulants; uncooperative position; abnormal nerve function of the affected limb; declining surgery or continuous post-operative patient-controlled analgesia (PCA).
This study was approved by the Institutional Review Board of XXX Hospital (number 201805-19) and registered in the Clinical Trial Registry (clinical trials.gov identifier: ChiCTR1900020612). Written informed consent was provided by each participant before enrollment.
Study design
According to Randomizer Study Version 4.0 (http://www.randomizer.org/), the patients were randomly allocated in a 1:1 ratio to the lumbar plexus (L) and quadratus lumborum (Q) groups using a random number table. The random numbers were placed in an opaque envelope, which was opened by the anesthesiologist before surgery. The anesthesiologists who performed the blocks were not the same as those who monitored the patients. The anesthesiologists did not participate in patient evaluation. The surgeon and the patients didn’t know about grouping.
Surgical procedures
The patients underwent routine fasting for 6 h before surgery. The venous access was opened in the anesthesia-preparation room, and continued oxygen inhalation with a mask (3 l/min) was conducted. Electrocardiographic features, heart rate (HR), noninvasive blood pressure (NiBP) and pulse oxygen saturation (SpO2) were monitored. Then, intravenous anesthesia with fentanyl (1–1.5 μg/kg) and midazolam (0.03–0.04 mg/kg) was performed by an anesthesiologist with >3 years of experience in ultrasound-guided peripheral nerve block. An M-Turbo ultrasound system (SonoSite, USA) in the neuroimaging mode and transducers of the L group were used to connect the C60x/5-2 MHz convex array probe to perform the ‘shamrock’ method of LPB as previously described [17]. Briefly, after the abdominal muscles were identified, the probe was moved slowly to the back and tilted to the caudal side. Typical shamrock-shaped images composed of the L4 transverse process, psoas major, erector spinae and quadratus lumborum were obtained. At this time, the hyperechoic structure in the 1/4 quadrant of the posterior psoas major was the lumbar plexus nerve (Fig. 1). The probe was fixed, and the area 4 cm away from the spinous process of the L4 was located as the puncture point. A 22G, 120-mm nerve stimulation needle tip Stimuplex D Plus (B. Braun, Germany), was guided to the lumbar plexus area and a local anesthetic was injected.
Fig. 1.
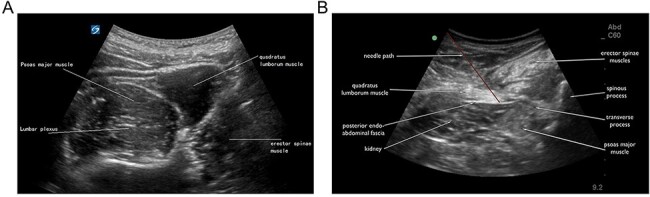
Ultrasound-guided approaches by the ‘shamrock’ method. A. Lumbar plexus block (LPB). B. Quadratus lumborum block (QLB).
Transducers in the Q group were connected to the C60x/5-2 MHz convex array probe, and the QLB method [7] described by Blanco was performed. In the lateral position, a low-frequency convex array probe was placed vertically above the iliac crest. Then, a 22G, 120-mm nerve stimulation needle tip Stimuplex D Plus (B. Braun, Germany) was guided to pass through the quadratus lumborum via the anteromedial direction from the back of the probe until the needle tip was located between the quadratus lumborum and the psoas major, and a local anesthetic was injected into the fascia (Fig. 2).
Fig. 2.

Study flow diagram. All enrolled patients completed the study.
In both groups, the local anesthetic was 0.4% ropivacaine at 0.4 ml/kg. The blocking procedure time was recorded from the beginning of ultrasonic scanning to the end of local anesthetic injection. The block was performed for 30 min, and muscle strength of the quadriceps femoris in the affected limbs was measured. Two senior anesthesiologists performed all blocks.
Subsequently, general anesthesia was induced by target control infusion (TCI) of propofol (4.0–4.5 μg/ml), sufentanil (0.2 μg/kg) and cisatracurium (0.2 mg/kg). Then, a laryngeal mask was placed for mechanical ventilation, and TCI propofol maintained a bispectral index (BIS) of 40–55. Then, surgery was performed, and propofol infusion was discontinued immediately post-surgically. Both groups were next transferred to the PACU and underwent PCA immediately. The electronic analgesia pump (Auto Med 2000, ROK) comprised 2 μg/kg sufentanil, 10 mg tropisetron and 100 ml normal saline; the background and single doses were 2 ml/h and 0.5 ml, respectively. The lock time was 15 min.
All arthroscopies were performed by the same senior arthroscopic surgeon.
Outcome measures
Baseline patient data were recorded, including gender, age, BMI, ASA class, complications, surgery time, block procedure time and muscle strength after block. The main outcome was the total dose of sufentanil for PCA 24 h post-surgery. Secondary outcomes were a total dose of sufentanil for PCA upon leaving the PACU, and at 2, 4, 8, 12 and 48 h post-surgery, respectively; VAS scores in the active (flexion, intorsion or extorsion) state at 4 and 24 h post-surgery, respectively; VAS scores in the resting state upon leaving the PACU, and at 2, 4, 8, 24 and 48 h post-surgery, respectively [7]; HR, SpO2, NiBP and complications within 48 h post-surgery such as epidural spread, bilateral block, post-operative nausea and vomiting, respiratory depression, itching, urinary retention, kidney damage, bleeding and hematoma at the puncture site.
The quadriceps muscle strength was recorded after 30 min of the block by the anesthesiologist, who did not perform the block but administered the anesthesia. The quadriceps muscle strength was evaluated by freehand muscle strength examination. The patient was told to take the lateral decubitus position with the affected limb on top. If the affected limb could not be kept straight after hip flexion and knee bending, the muscle strength was grade 0 (without muscle contraction) or grade 1. If the affected limb could be straightened, muscle strength was grade 2. Then the patient took the supine position with hip flexion. If the patient could straighten himself after knee bending, muscle strength was grade 3. If the ankle joint of the affected limb gave a certain resistance, muscle strength was grade 4 (mild resistance) or grade 5 (maximum resistance).
The VAS was marked according to the pain degree, between 0 (no pain) and 10 (severe pain). The VAS was conducted by a PCA group member.
Definitions and follow up
Data collection lasted 48 h, and the patients and researchers involved in post-operative data collection were blinded to the specific anesthesia received by the patients.
Sample size
Sample size calculation was performed with Power and sample size calculation version 3.1.2 (Vanderbilt University, Nashville, TN, USA), based on VAS scores after QLB [18] and LPB [19] for hip arthroscopy. We assumed VAS scores after QLB and LPB are similar and LPB groups, significance levels of α = 0.05 and β = 0.10, the sample size for each group was estimated as 22 cases. Considering a potential dropout rate of 10%, about 25 cases were needed for each group.
Statistical analysis
SPSS version 21.0 (SPSS, USA) was used for statistical analysis. Normally distributed measurements are mean ± standard deviation (SD) and were compared by independent samples t-test. For repeated measurement data, repeated measures analysis of variance (ANOVA) was used to compare within groups at different time points; meanwhile, multivariate analysis of variance was used for between-group comparisons. Measurement data with skewed distribution were presented as median (interquartile range, IQR), and the rank-sum test was performed for between-group comparisons. Count data were compared by the chi-square test and ranked data by the rank-sum test. P < 0.05 was considered statistically significant.
RESULTS
Patient general information
Fifty patients were included in this trial, with 25 cases in each group. All patients completed the study. The mean patient ages in the L and Q groups were 41.4 ± 11.3 and 37.5 ± 9.5 years, respectively. Demographic characteristics, block procedure time, surgery time and other baseline features were similar in both groups (Table I).
Table I.
General patient data
| L group (n = 25) | Q group (n = 25) | P value | |
|---|---|---|---|
| Age (y) | 41.4 ± 11.3 | 37.5 ± 9.5 | 0.183 |
| Gender (Male/Female) | 13/12 | 12/13 | 0.777 |
| BMI (kg/m2) | 23.9 ± 3.0 | 22.9 ± 2.8 | 0.306 |
| ASA classification I (n) | 20 | 19 | 0.735 |
| ASA classification II (n) | 5 | 6 | 0.735 |
| Hypertension (n) | 2 | 4 | 0.389 |
| Diabetes (n) | 4 | 3 | 0.687 |
| Surgery time (min) | 54.6 ± 14.9 | 56.8 ± 20.1 | 0.306 |
| Blocking procedure time (min) | 4.0 ± 2.2 | 4.4 ± 2.2 | 0.885 |
| Muscle strength after block | 2.0 (1.0–3.0) | 4.0 (4.0–4.0) | P < 0.001 |
BMI: Body mass index; ASA: American Society of Anesthesiologists; L group: lumbar plexus group; Q group: quadratus lumborum group.
Muscle strength after block
After 30 min of the block, quadriceps muscle strength was significantly increased in the Q group [2.0 (1.0–3.0)] compared with the L group [4.0 (4.0–4.0)] (P < 0.001; Table I).
Opioid dose
Upon leaving the PACU, and at 2–48 h after surgery, opioid doses for PCA in the L group were 1.1 (1.9–1.6), 4.4 (4.1–4.6), 8.6 (8.1–9.1), 16.6 (16.1–17.1), 24.6 (24.1–25.1), 48.6 (48.2–49.1) and 96.6 (96.1–97.1) ml, respectively, versus 1.1 (1.0–1.3), 4.4 (4.1–6.6), 8.6 (8.2–8.7), 16.5 (16.1–16.8), 24.6 (24.1–24.7), 48.6 (48.1–48.9) and 96.6 (96.2–96.7) ml for the Q group, respectively, indicating no significant differences (Table II).
Table II.
Cumulative doses of PCA at different time points after surgery (ml)
| L group (n = 25) | Q group (n = 25) | P value | |
|---|---|---|---|
| Leaving the PACU | 1.2 (1.0–1.6) | 1.1 (1.0–1.3) | 0.372 |
| 2 h after surgery | 4.4 (4.1–4.6) | 4.4 (4.1–4.6) | 0.674 |
| 4 h after surgery | 8.6 (8.1–9.1) | 8.6 (8.2–8.7) | 0.761 |
| 8 h after surgery | 16.6 (16.1–17.1) | 16.5 (16.1–16.8) | 0.430 |
| 12 h after surgery | 24.6 (24.1–25.1) | 24.6 (24.1–24.7) | 0.538 |
| 24 h after surgery | 48.6 (48.2–49.1) | 48.6 (48.1–48.9) | 0.442 |
| 48 h after surgery | 96.6 (96.1–97.1) | 96.6 (96.2–96.7) | 0.552 |
PCA: Patient-controlled anesthesia; L group: lumbar plexus group; Q group: quadratus lumborum group.
QLB and LPB cause similar levels of pain in hip arthroscopy
Resting VAS scores in the Q group were 4.0 (3.0–6.0) immediately after surgery, and 3.0 (2.0–4.0), 2.0 (1.5–3.0), 2.0 (0.5–2.5), 0 (0–1.0) and 0 (0–0) at 2–48 h after surgery, respectively, with no significant differences compared with the L group at various time point after hip arthroscopy [4.0 (0.5–5.0), 2.0 (1.0–4.0), 2.0 (0–3.5), 1.0 (0–2.5), 0 (0–0.5) and 0 (0–0), respectively; (Table III)].
Table III.
VAS scores at different time points after surgery
| L group (n = 25) | Q group (n = 25) | P value | ||
|---|---|---|---|---|
| Resting state VAS | Leaving the PACU | 4.0 (0.5–5.0) | 4.0 (3.0–6.0) | 0.066 |
| 2 h after surgery | 2.0 (1.0–4.0) | 3.0 (2.0–4.0) | 0.07 | |
| 4 h after surgery | 2.0 (0–3.5) | 2.0 (1.5–3.0) | 0.081 | |
| 8 h after surgery | 1.0 (0–2.5) | 2.0 (0.5–2.5) | 0.233 | |
| 24 h after surgery | 0 (0–0.5) | 0 (0–1.0) | 0.347 | |
| 48 h after surgery | 0 (0–0) | 0 (0–0) | 0.615 | |
| Active state VAS | 4 h after surgery | |||
| Flexion | 3.0 (1.0–5.0) | 3.0 (3.0–4.0) | 0.322 | |
| Intorsion | 2.0 (0.5–4.0) | 2.0 (2.0–4.0) | 0.106 | |
| Extorsion | 2.0 (0.5–4.5) | 2.0 (1.5–3.5) | 0.478 | |
| 24 h after surgery | ||||
| Flexion | 1.0 (0.5–2.5) | 1.0 (1.0–2.0) | >0.999 | |
| Intorsion | 1.0 (0–2.0) | 1.0 (0–1.0) | 0.496 | |
| Extorsion | 1.0 (0–2.0) | 0 (0–1.0) | 0.388 |
VAS: Visual analog scale; PACU: post-anesthesia care unit; L group: lumbar plexus group; Q group: quadratus lumborum group.
Flexion, intorsion and extorsion VAS scores (0 to 10 points) at 4 h after surgery in the Q group were 3.0 (3.0–4.0), 2.0 (2.0–4.0) and 2.0 (1.5–3.5), respectively, with no significant differences compared with the L group [3.0 (1.0–5.0), 2.0 (0.5–4.0) and 2.0 (0.5–4.5), respectively; (Table III)]. Flexion, intorsion and extorsion VAS scores (0 to 10 points) at 24 h after surgery in the Q group were 1.0 (1.0–2.0), 1.0 (0–1.0) and 0 (0–1.0), respectively, with no significant differences compared with the L group [1.0 (0.5–2.5), 1.0 (0–2.0) and 1.0 (0–2.0), respectively); (Table III)].
Secondary outcomes
NiBP, HR and SpO2 during surgery were similar in both groups (Table IV). For post-operative complications, one patient had epidural spread (4%) in the L group; there were no other complications such as nausea and vomiting in the whole study population (Table V).
Table IV.
Noninvasive blood pressure, heart rate and blood oxygen saturation at different points during surgery
| L group (n = 25) | Q group (n = 25) | P value | ||
|---|---|---|---|---|
| Systolic pressure | Entering the room | 129.0 ± 7.6 | 127.2 ± 7.2 | 0.852 |
| Laryngeal mask insertion | 113.0 ± 9.7 | 110.6 ± 9.4 | 0.915 | |
| Starting the surgery | 106.9 ± 7.6 | 105.3 ± 6.7 | 0.539 | |
| Completing the surgery | 105.7 ± 8.7 | 104.5 ± 6.6 | 0.232 | |
| Diastolic pressure | Entering the room | 73.7 ± 9.3 | 72.44 ± 9.4 | 0.91 |
| Laryngeal mask insertion | 64.4 ± 8.1 | 63.0 ± 8.4 | 0.806 | |
| Starting the surgery | 60.0 ± 6.6 | 59.5 ± 6.9 | 0.64 | |
| Completing the surgery | 58.8 ± 8.7 | 60.2 ± 7.0 | 0.511 | |
| Heart rate | Entering the room | 79.8 ± 5.6 | 78.6 ± 6.3 | 0.284 |
| Laryngeal mask insertion | 65.5 ± 4.7 | 66.0 ± 6.3 | 0.06 | |
| Starting the surgery | 60.8 ± 4.8 | 63.6 ± 6.2 | 0.177 | |
| Completing the surgery | 60.2 ± 6.0 | 63.3 ± 6.7 | 0.297 | |
| Blood oxygen saturation | Entering the room | 99.0 ± 0.8 | 98.8 ± 0.9 | 0.329 |
| Laryngeal mask insertion | 100.0 ± 0.0 | 100.0 ± 0.0 | – | |
| Starting the surgery | 100.0 ± 0.0 | 100.0 ± 0.0 | – | |
| Completing the surgery | 100.0 ± 0.0 | 100.0 ± 0.0 | – |
L group: lumbar plexus group; Q group: quadratus lumborum group.
Table V.
Post-operative complications
| Q group (n = 25) | L group (n = 25) | P value | |
|---|---|---|---|
| Epidural spread | 0 | 1 | >0.999 |
| Bilateral block | 0 | 0 | – |
| Post-operative nausea and vomiting | 0 | 0 | – |
| Respiratory depression | 0 | 0 | – |
| Itching | 0 | 0 | – |
| Urinary retention | 0 | 0 | – |
| Kidney damage | 0 | 0 | – |
| Bleeding and hematoma at the puncture site | 0 | 0 | – |
L group: lumbar plexus group; Q group: quadratus lumborum group.
DISCUSSION
This study demonstrated that patients undergoing QLB had significantly higher muscle strength in the affected limbs compared with those administered LPB, with no significant differences in sufentanil dose at various time points after surgery, as well as VAS scores in the resting and active states.
Although hip arthroscopy is a minimally invasive procedure, like other open surgeries, post-operative pain remains an important issue [20]. Therefore, it was proposed that an effective analgesia method must include the basic contents for post-operative rehabilitation in hip arthroscopy, which can directly affect the surgical effects and the long-term function of the joint [21].
Previous studies have shown that LPB can reduce post-operative pain and opioid dose after total hip arthroplasty [22]. For example, a randomized trial of patients undergoing hip fracture repair showed that LPB reduces pain scores and improves patient satisfaction [23]. In addition, ultrasound-guided LPB by the ‘shamrock’ method [17] could effectively avoid the interference of bony structure acoustic shadow. Studies by Gürkan et al. showed that the shamrock approach could prolong the analgesic time after hip surgery [24].
Case reports have suggested that local injection of anesthetics into the quadratus lumborum can effectively relieve pain in patients after various hip and lower limb surgeries [25–28]. As shown in the present study, the dose and requirements of sufentanil in patients administered QLB at different time intervals after surgery were significantly lower than those in the control (L) group. Meanwhile, there were no significant differences between the two groups in sufentanil dose and resting and active state VAS scores at various time points after surgery (all lower than 3/10, P > 0.05), suggesting that compared with ultrasound-guided LPB, ultrasound-guided QLB provides similar post-operative analgesia after hip arthroscopy. These findings corroborated a recent placebo-controlled trial demonstrating that ultrasound-guided QL3 block represents an effective pain management method following THA [13]. However, it should be noted that the present study is the first to compare QLB and the widely used LPB with a prospective controlled design.
Although LPB usually has few complications [29], it may be associated with serious adverse events, with epidural spread being the most common complication [30]. In a recent study, 5 of 17 volunteers administered LPB had MRI-confirmed distribution of the drug in the epidural space [31]. In this trial, a 52-year-old female patient in the L group developed epidural spread with stable hemodynamics during surgery and no other discomfort postoperatively. After 6 h of the block, the epidural effect disappeared and the patient reported no specific discomfort, suggesting a good post-operative analgesic effect. However, after 24 h, this individual showed a slightly higher VAS score compared with the remaining patients of the same group. The reason may be that after the anesthetic spread to the epidural area, the drug concentration around the lumbar plexus was reduced, not effectively providing prolonged analgesia. QLB is a typical intramuscular drug injection approach, and its effects can spread through the thoracolumbar fascia to the paravertebral space or directly affect the transverse abdominis level [32]. The needle approach and the location of local anesthesia are not far from the abdominal cavity, internal abdominal organs and large blood vessels, and local anesthetics are not directly injected into the adjacent areas of the large nerve [32]. Therefore, the likelihood of various complications is much lower than that of other nerve blocks. So far, serious complications have not been reported, including in the present study, indicating that QLB might be safe, but a comparison with LPB will have to be made.
An unnecessary femoral nerve block is considered a possible complication of QLB3. A reasonable theoretical explanation lies in the direct anatomical continuity of Thoracolumbar fascia and iliac fascia and the possibility of anesthetic spreading downwards along the iliac fascia, resulting in quadriceps weakness [33–36]. QLB3 without puncturing the psoas major muscle fascia (PMM) was performed, and the contrast agent could not spread to the tail end [27]. This finding indicates that avoiding PMM perforation results in no extra quadriceps weakness. In this trial, muscle strength was 4.0 (4.0–4.0) at 30 min after QLB, demonstrating that it was unaltered. It may be that anesthesiologists in this trial were all experienced in ultrasound-guided nerve block and may not puncture the PMM during the block, so the local anesthetic did not spread to the tail end, thus avoiding quadriceps weakness. As shown above, the muscle strength after surgery in the L group was 2.0 (1.0–3.0), which was significantly lower than that of the Q group (P < 0.05). These data suggest that QLB has little to no impact on specific muscle strength, which is a major advantage of this approach over LPB. As a result, the risk of falling after surgery can be reduced, hip surgery patients could get out of bed as early as possible, and complications could be prevented after QLB.
The limitations of this study should be mentioned. First, it only assessed the clinical efficacies of QLB and LPB in hip arthroscopy. Whether QLB and LPB provide similar analgesic effects in other types of surgery needs further investigation. Secondly, although this was a randomized prospective trial, all patients were treated in the same hospital. Thirdly, we did not correct for multiple comparisons during the analysis of secondary endpoints because of the small sample size of this exploratory study. In addition, the study might have been underpowered for the secondary endpoints. Therefore, multicenter randomized prospective trials should be conducted to confirm our findings. Finally, the mechanism of action of QLB remains unclear, and more trials are needed to explore its analgesic mechanism for promoting the clinical application of this approach.
In conclusion, compared with ultrasound-guided LPB, ultrasound-guided QLB provides similar, good post-operative analgesia after hip arthroscopy, with less impact on muscle strength. Complications will have to be examined in future trials. However, further research is needed to explore whether it could replace LPB to provide perioperative analgesia in other hip surgeries.
ACKNOWLEDGEMENTS
The authors would like to thank all study participants who were enrolled in this study.
Contributor Information
Liangjing Yuan, Department of Anesthesiology, Beijing Jishuitan Hospital, Beijing 100000, China.
Chengshi Xu, Department of Anesthesiology, Beijing Jishuitan Hospital, Beijing 100000, China.
Ye Zhang, Department of Anesthesiology, Beijing Jishuitan Hospital, Beijing 100000, China.
Geng Wang, Department of Anesthesiology, Beijing Jishuitan Hospital, Beijing 100000, China.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None declared.
ETHICS APPROVAL
The study protocol was approved by the Ethics Committees of the Beijing Jishuitan Hospital (201 805–19).
INFORMED CONSENT
Written informed consent was provided by each participant before enrollment.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. de Amorim Cabrita HA, de Castro Trindade CA, de Campos Gurgel HM et al. Hip arthroscopy. Rev Bras Ortop 2015; 50: 245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cvetanovich GL, Chalmers PN, Levy DM et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy 2016; 32: 1286–92. [DOI] [PubMed] [Google Scholar]
- 3. Kirsch JM, Khan M, Bedi A. Does hip arthroscopy have a role in the treatment of developmental hip dysplasia? J Arthroplasty 2017; 32: S28–31. [DOI] [PubMed] [Google Scholar]
- 4. Ramos L, Kraeutler MJ, Marty E et al. Pain scores and activity tolerance in the early postoperative period after hip arthroscopy. Orthop J Sports Med 2020; 8: 2325967120960689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amiri HR, Zamani MM, Safari S. Lumbar plexus block for management of hip surgeries. Anesth Pain Med 2014; 4: e19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bech NH, Hulst AH, Spuijbroek JA et al. Perioperative pain management in hip arthroscopy; what options are there? J Hip Preserv Surg 2016; 3: 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanco R. Tap block under ultrasound guidance: the description of a “no pops” technique. Reg Anesth Pain Med 2007; 32: 130. [Google Scholar]
- 8. Akerman M, Pejčić N, Veličković I. A review of the quadratus lumborum block and ERAS. Front Med (Lausanne) 2018; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sá M, Cardoso JM, Reis H et al. Quadratus lumborum block: are we aware of its side effects? A report of 2 cases. Rev Bras Anestesiol 2018; 68: 396–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueshima H, Yoshiyama S, Otake H. The ultrasound-guided continuous transmuscular quadratus lumborum block is an effective analgesia for total hip arthroplasty. J Clin Anesth 2016; 31: 35. [DOI] [PubMed] [Google Scholar]
- 11. La Colla L, Uskova A, Ben-David B. Single-shot quadratus lumborum block for postoperative analgesia after minimally invasive hip arthroplasty: a new alternative to continuous lumbar plexus block? Reg Anesth Pain Med 2017; 42: 125–6. [DOI] [PubMed] [Google Scholar]
- 12. Brixel SM, Biboulet P, Swisser F et al. Posterior quadratus lumborum block in total hip arthroplasty: a randomized controlled trial. Anesthesiology 2021; 134: 722–33. [DOI] [PubMed] [Google Scholar]
- 13. He J, Zhang L, He WY et al. Ultrasound-guided transmuscular quadratus lumborum block reduces postoperative pain intensity in patients undergoing total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Pain Res Manag 2020; 2020: 1035182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Dai F, Ona Ayala KE et al. Transmuscular quadratus lumborum and lateral femoral cutaneous nerve block in total hip arthroplasty. Clin J Pain 2021; 37: 366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kikuchi M, Mihara T, Mizuno Y et al. Anterior quadratus lumborum block for postoperative recovery after total hip arthroplasty: a study protocol for a single-center, double-blind, randomized controlled trial. Trials 2020; 21: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doyle DJ, Goyal A, Bansal P et al. American Society of Anesthesiologists Classification. Treasure Island, FL: StatPearls Publishing, 2021. Copyright ©. [PubMed] [Google Scholar]
- 17. Lin JA, Lu HT, Chen TL. Ultrasound standard for lumbar plexus block. Br J Anaesth 2014; 113: 188–9. [DOI] [PubMed] [Google Scholar]
- 18. Yuan L, Zhang Y, Xu C et al. Postoperative analgesia and opioid use following hip arthroscopy with ultrasound-guided quadratus lumborum block: a randomized controlled double-blind trial. J Int Med Res 2020; 48: 300060520920996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. YaDeau JT, Tedore T, Goytizolo EA et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg 2012; 115: 968–72. [DOI] [PubMed] [Google Scholar]
- 20. Malloy P, Malloy M, Draovitch P. Guidelines and pitfalls for the rehabilitation following hip arthroscopy. Curr Rev Musculoskelet Med 2013; 6: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malloy P, Gray K, Wolff AB. Rehabilitation after hip arthroscopy: a movement control-based perspective. Clin Sports Med 2016; 35: 503–21. [DOI] [PubMed] [Google Scholar]
- 22. de Leeuw MA, Zuurmond WW, Perez RS. The psoas compartment block for hip surgery: the past, present, and future. Anesthesiol Res Pract 2011; 2011: 159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chudinov A, Berkenstadt H, Salai M et al. Continuous psoas compartment block for anesthesia and perioperative analgesia in patients with hip fractures. Reg Anesth Pain Med 1999; 24: 563–8. [DOI] [PubMed] [Google Scholar]
- 24. Gürkan Y, Aksu C, Kuş A et al. One operator’s experience of ultrasound guided lumbar plexus block for paediatric hip surgery. J Clin Monit Comput 2017; 31: 331–6. [DOI] [PubMed] [Google Scholar]
- 25. Kadam VR. Ultrasound-guided quadratus lumborum block as a postoperative analgesic technique for laparotomy. J Anaesthesiol Clin Pharmacol 2013; 29: 550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carvalho R, Segura E, Loureiro MD et al. Quadratus lumborum block in chronic pain after abdominal hernia repair: case report. Rev Bras Anestesiol 2017; 67: 107–9. [DOI] [PubMed] [Google Scholar]
- 27. Visoiu M, Yakovleva N. Continuous postoperative analgesia via quadratus lumborum block - an alternative to transversus abdominis plane block. Paediatr Anaesth 2013; 23: 959–61. [DOI] [PubMed] [Google Scholar]
- 28. Chakraborty A, Goswami J, Patro V. Ultrasound-guided continuous quadratus lumborum block for postoperative analgesia in a pediatric patient. A A Case Rep 2015; 4: 34–6. [DOI] [PubMed] [Google Scholar]
- 29. Njathi CW, Johnson RL, Laughlin RS et al. Complications after continuous posterior lumbar plexus blockade for total hip arthroplasty: a retrospective cohort study. Reg Anesth Pain Med 2017; 42: 446–50. [DOI] [PubMed] [Google Scholar]
- 30. Gadsden JC, Lindenmuth DM, Hadzic A et al. Lumbar plexus block using high-pressure injection leads to contralateral and epidural spread. Anesthesiology 2008; 109: 683–8. [DOI] [PubMed] [Google Scholar]
- 31. Bendtsen TF, Pedersen EM, Haroutounian S et al. The suprasacral parallel shift vs lumbar plexus blockade with ultrasound guidance in healthy volunteers—a randomised controlled trial. Anaesthesia 2014; 69: 1227–40. [DOI] [PubMed] [Google Scholar]
- 32. Blanco R, Ansari T, Riad W et al. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain after cesarean delivery: a randomized controlled trial. Reg Anesth Pain Med 2016; 41: 757–62. [DOI] [PubMed] [Google Scholar]
- 33. Dam M, Moriggl B, Hansen CK et al. The pathway of injectate spread with the transmuscular quadratus lumborum block: a cadaver study. Anesth Analg 2017; 125: 303–12. [DOI] [PubMed] [Google Scholar]
- 34. Carline L, McLeod GA, Lamb C. A cadaver study comparing spread of dye and nerve involvement after three different quadratus lumborum blocks. Br J Anaesth 2016; 117: 387–94. [DOI] [PubMed] [Google Scholar]
- 35. Parras T, Blanco R. Randomised trial comparing the transversus abdominis plane block posterior approach or quadratus lumborum block type I with femoral block for postoperative analgesia in femoral neck fracture, both ultrasound-guided. Rev Esp Anestesiol Reanim 2016; 63: 141–8. [DOI] [PubMed] [Google Scholar]
- 36. Wikner M. Unexpected motor weakness following quadratus lumborum block for gynaecological laparoscopy. Anaesthesia 2017; 72: 230–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


