Abstract
Fungal multicopper oxidases have many potential industrial applications, since they perform reactions under mild conditions. We isolated a phenol oxidase from the fungus Acremonium murorum var. murorum that was capable of decolorizing plant chromophores (such as anthocyanins). This enzyme is of interest in laundry-cleaning products because of its broad specificity for chromophores. We expressed an A. murorum cDNA library in Saccharomyces cerevisiae and subsequently identified enzyme-producing yeast colonies based on their ability to decolor a plant chromophore. The cDNA sequence contained an open reading frame of 1,806 bp encoding an enzyme of 602 amino acids. The phenol oxidase was overproduced by Aspergillus awamori as a fusion protein with glucoamylase, cleaved in vivo, and purified from the culture broth by hydrophobic-interaction chromatography. The phenol oxidase is active at alkaline pH (the optimum for syringaldazine is pH 9) and high temperature (optimum, 60°C) and is fully stable for at least 1 h at 60°C under alkaline conditions. These characteristics and the high production level of 0.6 g of phenol oxidase per liter in shake flasks, which is equimolar with the glucoamylase protein levels, make this enzyme suitable for use in processes that occur under alkaline conditions, such as laundry cleaning.
Blue oxidases are a subfamily of multicopper enzymes, including laccases, ascorbate oxidases, and vertebrate ceruloplasmin, that are produced by a large number of plants and fungi (20). These enzymes catalyze the four-electron reduction of molecular oxygen to water with the concurrent one-electron oxidation of a substrate, usually a polyphenolic compound (16). Relatively little is known about the physiological role of these enzymes in nature. Laccases, for example, are implicated in a number of processes such as conidial pigmentation, lignin degradation, pathogenicity, and fruiting-body formation (reviewed in reference 22).
Fungal multicopper oxidases are receiving increasing interest as potential industrial enzymes in applications such as detoxification of toxic phenolic compounds and azo dyes (reviewed in reference 12), enzymatic bleaching of kraft pulp (2), and delignification (30) because these oxidases catalyze the oxidation of phenols. Also, it is often desirable to convert compounds under mild conditions to create new product properties or to maintain other properties of a beverage or food product in other processes, e.g., food processing. In the area of laundry cleaning, enzymatic bleach might be a good alternative to current chemical bleaches.
Blue oxidase genes have been cloned from a number of species, mainly plants, white-rot basidiomycetes, and some plant pathogens (for a review, see references 4 [and references therein] and 20). However, in most fungi, oxidases (mainly laccases) are produced at levels that are too low for commercial purposes, even when cloned genes are expressed in heterologous hosts (14, 17). For any of these potential applications to become reality, an inexpensive oxidase source must be available. Consequently, applications to produce consumer goods need redox enzymes, especially those that can be produced easily by recombinant strains.
We identified a fungus, Acremonium murorum, which secretes an unknown phenol oxidase capable of decolorizing chromophores such as cyanidin and pelargonidin. Our objectives in this study were (i) to clone the corresponding phenol oxidase gene, (ii) to express the gene at high levels in Aspergillus awamori, a fungus which is used in industry for the production of proteins (8, 23), and (iii) to characterize the enzyme with respect to its suitability for laundry cleaning.
MATERIALS AND METHODS
Bacterial and fungal strains.
For standard bacterial cloning, Escherichia coli DH5α (9) was used. For cloning of a cDNA library, E. coli XL1-Blue MRF′ {(mcrA)183 (mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac (F′ proAB lacIqZ M15 Tn10 [Tetr])} (Invitrogen, Carlsbad, Calif.) was used. Saccharomyces cerevisiae strain VW-K1 (MATa leu2) was used for expression of the cDNA library. Acremonium murorum var. murorum CBS 157.72 was obtained from the Centraal Bureau voor Schimmelcultures (CBS), Baarn, The Netherlands. A. awamori AWC4.20 is a pyrG mutant strain derived from A. awamori 40 (described in World Patent 91/19782, p.13) and a derivative of A. awamori CBS 115.52.
Cultivation of A. murorum
A shake flask containing 100 ml of potato dextrose broth (Difco Laboratories, Detroit, Mich.) was inoculated with spores of A. murorum obtained from a culture growing on a potato dextrose agar (Oxoid, Ogdensburg, N.Y.) plate that had been incubated for 1 week at 25°C. The culture was grown for 3 days at 25°C in a rotary shaker (250 rpm), and then it was transferred to 100 ml of minimal medium (1), enriched with 0.5% yeast extract, and grown for another 3 days at 25°C.
Extraction of total RNA and isolation of poly(A)+ RNA.
Total RNA was prepared by extraction with Trizol (Life Technologies, Inc., Rockville, Md.). The RNA concentration was determined by measuring absorbance at optical densities of 260 and 280 nm (OD260/280). Purification of poly(A)+ mRNA from total RNA was carried out with the Oligotex mRNA kit (Qiagen, Valencia, Calif.) according to the protocol provided by the supplier.
cDNA synthesis.
cDNA synthesis was carried out by using a cDNA synthesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's protocol, except that reverse transcriptase Superscript II (Life Technologies, Inc.) was used instead of Moloney murine leukemia virus reverse transcriptase.
Construction of a cDNA library.
cDNA was cloned as EcoRI/XhoI fragments into plasmid pYES2.0 (Invitrogen). For large-scale ligations, approximately 200 ng of cDNA was ligated to 1.5 μg of EcoRI/XhoI-digested pYES2.0, in a total volume of 7.5 μl with 1 U of T4 DNA ligase for 5 h at room temperature. Aliquots of 2.5 μl were used to transform 50 μl of electrocompetent E. coli XL1-Blue MRF′ cells (Stratagene) (conditions, 1,700 V, 200 ω, and 25 μF). After addition of 1 ml of SOC (per liter: 20 g of Bacto-tryptone [Difco Laboratories], 5 g of Bacto-yeast extract [Difco], 0.58 g of NaCl, 0.18 g of KCl, 2.0 g of MgCl2 · 6 H2O, 2.46 g of MgSO4 · 7 H2O, 3.6 g of glucose) to each mix, the cells were regenerated for 1 h at 37°C, plated on Luria-Bertani medium (LB) (1% Bacto-tryptone [Difco], 0.5% Bacto-yeast extract [Difco], 10 g of NaCl liter−1, pH 7.0) with ampicillin (100 μg/ml), and grown at 37°C for another 16 h. Dilutions were plated to calculate the titer of the library. To each plate was added 3 ml of capable of decolorizing plant chromophores (such as anthocyanins), and bacteria were scraped off, pooled, and stored in small aliquots. Large-scale DNA was prepared from 200- to 500-ml cultures of LB inoculated with an aliquot of transformants and propagated overnight.
Transformations.
Lithium acetate-mediated transformations of S. cerevisiae VW-K1 were carried out by the method of Gietz and Woods (5). Cells were washed with 1 M sorbitol and finally plated onto selective medium [0.67% yeast nitrogen base minimal medium (without amino acids and with ammonium sulfate)] and 2% glucose and incubated for 3 to 5 days at 30°C. Transformation of A. awamori was carried out as described previously (6).
Screening of an A. murorum cDNA library in S. cerevisiae for decolorization of cyanidin.
Approximately 50,000 colonies of an A. murorum cDNA expression library in S. cerevisiae VW-K1 were plated on medium containing 4% (wt/vol) galactose, 0.5% (wt/vol) glucose, 0.67% (wt/vol) yeast nitrogen base minimal medium, 0.1 M sodium phosphate (pH 7.2), and 120 mg of cyanidin liter−1 to yield approximately 3,000 colonies per plate, and incubated at 30°C. The plates were screened daily for halo-producing transformants.
Isolation of plasmid DNA from yeast.
Plasmid DNA was isolated as described by Hoffman and Winston (11), using a mixture of lysis buffer, phenol, and glass beads followed by centrifugation, transformation of E. coli with a small sample of the supernatant, and isolation of the plasmid from E. coli.
DNA sequence analysis.
DNA sequence analysis was carried out on an LKB automated laser fluorescent DNA sequencer (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.).
Plasmid construction.
For production of A. murorum oxidase by A. awamori, the gene encoding the A. murorum oxidase (AMO) was inserted into expression vector pAWGLA2 (7). The oxidase gene was fused to the 3′ end of the Aspergillus niger glucoamylase gene. The genes are separated by a DNA sequence encoding a KEX2-type recognition site (Asn-Val-Ile-Ser-Lys-Arg). The expression signals (promoter and transcription terminator) are derived from the A. awamori β-1,4-endoxylanase A gene. Since the N-terminal amino acid sequence of wild-type AMO could not be determined due to the low production levels by Acremonium, the fusion was based on cleavage of AMO by the rules of Von Heijne (25, 26) (cleavage between signal peptide and protein). Based on this hypothesis, the glucoamylase was fused to amino acid 22 (Met) of AMO.
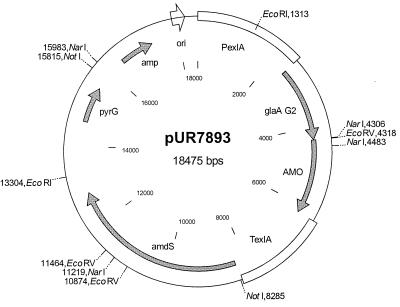
For a correct fusion at the 3′ end of the phenol oxidase gene, pUR7876 was digested with XhoI and XbaI and ligated with two annealed oligonucleotides (5′-TCGAGCTTAAGT-3′ and 5′-CTAGACTTAAGC-3′), thereby introducing an AflII site, resulting in pUR7880. For a correct fusion at the 5′ end, pUR7880 was modified by replacing an EcoRV/NarI fragment with a 170-bp EcoRV/NarI PCR-derived DNA fragment, giving plasmid pUR7890. This vector contains part of the KEX2 recognition site starting at an EcoRV site and the 5′ part of the A. murorum gene up to the NarI site. The PCR fragment was obtained with the primers Acr06 (5′-GAGAGAGATATCCAAGCGCATGCCCAAGTTCGAGCTGGACATTCCTGAGG-3′) and Acr02 (5′-GCTTGATCTCGATCTCATAGTAGT-3′) on plasmid pUR7876 as a template. From pUR7890, pUR7891 was constructed by inserting the A. murorum gene, present on a 2-kb EcoRV/AflII fragment, into the Aspergillus expression vector pAWGLA2, which was also digested with EcoRV and AflII. Finally, pUR7891 was digested with NotI and ligated with the Aspergillus nidulans amdS and the A. awamori pyrG double-selection marker from pAW10S-4 (24), resulting in pUR7893 (Fig. 1).
FIG. 1.
Plasmid pUR7893. Open bars, A. awamori 5′ and 3′ regulatory sequences; filled arrows, coding sequences. Abbreviations and designations: glaA, glucoamylase gene; amdS, acetamidase gene; pyrG, orotidine 5′-monophosphate decarboxylase gene; amp, β-lactamase gene, exlA, β-1,4-endoxylanase gene; ori, origin of replication. Only relevant restriction sites are indicated.
Construction of recombinant A. awamori strains.
Strain A. awamori AWC4.20 was transformed with pUR7893, and transformants were selected in two ways, either by restored growth on minimal medium (1) due to the integration of the wild-type pyrG gene or by growth on minimal medium with 10 mM acetamide as a sole nitrogen source, due to the integration of the amdS gene. The latter selection method usually results in transformants containing multiple copies of the plasmid, since these transformants grow better and faster on acetamide-containing medium. Transformants were purified twice on Aspergillus minimal medium (1). Conidia were obtained by growing mycelium on potato dextrose agar plates for 5 to 7 days at 30°C.
Shake flask induction experiments with A. awamori
Production in shake flasks of the AMO by A. awamori under control of the β-1,4-endoxylanase A transcription control sequences was carried out according to the method of Gouka et al. (6). The induction medium was supplemented with 0.5 mM CuCl2.
Protein analysis.
For analysis of secreted proteins, the medium was separated from the mycelium by filtration through Miracloth (Calbiochem-Behring, La Jolla, Calif.). Concentration of the proteins in the medium was carried out by ammonium sulfate precipitation (80% saturation). The precipitate was kept at 4°C for 16 h and pelleted by centrifugation for 45 min at 25,000 × g The protein pellet was dissolved in 2.5 ml of 30 mM sodium phosphate (pH 8.5) and subsequently desalted using a Sephadex G25 column (Amersham Pharmacia Biotech). Proteins were eluted with 3.5 ml of 30 mM sodium phosphate (pH 8.5). Enzyme analysis was carried out by polyacrylamide gel electrophoresis (PAGE), plate assays, and enzyme activity assays. For PAGE, samples were boiled in 1% sodium dodecyl sulfate (SDS) without reducing agent. Glucoamylase was detected as previously described (7).
Plate assays.
Fungal conidia were inoculated onto agar plates, containing minimal medium (1) with 1.5% agar and a substrate, either an anthocyanidin (120 mg of cyanidin liter−1 [Fluka; Sigma-Aldrich Corp., St. Louis, Mo.] or 240 mg of pelargonidin liter−1 [Roth, Karlsruhe, Germany]) or 2 mM ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)]. The plates were incubated at 25°C and screened daily for the presence of clearing zones (anthocyanidins) or green halos (ABTS) as a result of extracellular enzyme activity. To detect enzyme production by recombinant strains containing the phenol oxidase gene fused to the exlA promoter, d-xylose was added to the plates at a 5% concentration.
Enzyme activity assays.
Decolorization of anthocyanidins was measured in 1 ml of solution containing 100 mM sodium phosphate (pH 7.5), 0.1 mM cyanidinchloride or pelargonidinchloride, and 20 to 200 μl of enzyme sample. The change in absorbance (per minute) was measured during 5 min in a UV-VIS spectrum (wavelength, 200 to 700 nm; absorbance peak, 579 nm). Standard ABTS oxidation assays were carried out by adding the appropriate amount of enzyme to 50 mM sodium phosphate (pH 6.0)–2 mM ABTS solution (final volume, 1 ml) and monitoring the absorbance increase at 414 nm (extinction coefficient, 35 mM cm−1). One unit of enzyme activity was defined as the amount of enzyme that oxidizes 1 μmol of ABTS per min per ml at 20°C.
For determination of the AMO activity as a function of pH, standard amounts of AMO (1,500 U) were added to 1 ml of Britton and Robinson buffer (B&R buffer) (3) (pH range, 3.0 to 11.0)–2 mM ABTS solution, and the activity was measured at 414 nm. Similarly, syringaldazine (SGZ) oxidation activity was determined in B&R buffer–100 μM SGZ by monitoring the absorbance change at 530 nm. B&R buffer was prepared by mixing a 100-ml solution of 28.6 mM citric acid, 28.6 mM KH2PO4, 28.6 mM boric acid, and 28.6 mM diethylbarbituric acid, which was set at the appropriate pH with 0.2 M sodium hydroxide and then diluted with water to 200 ml. AMO activity as a function of temperature was determined by measuring the activity of standard amounts of AMO (1,500 U) in B&R buffer (pH 4.5)–2 mM ABTS at various temperatures (without preincubation of AMO). Stability as a function of pH was measured by incubating standard amounts of AMO in B&R buffer (pH range 3.0 to 9.6) at 4°C and determining the residual activity at different times using the standard ABTS oxidation assay described above. Stability as a function of temperature was measured by incubating standard amounts of AMO in B&R buffer (pH 8.5) at different temperatures and determining the residual activity at different times using the standard ABTS oxidation assay.
Purification of A. murorum phenol oxidase.
Hydrophobic-interaction chromatography was used to purify AMO from the fermentation broth. A phenyl-Sepharose 6 fast-flow column (Amersham Pharmacia Biotech) was equilibrated with 50 mM sodium phosphate–30% (1.3 M) ammonium sulfate (pH 6.0). Ammonium sulfate was added to the enzyme sample to a final concentration of 1.3 M. Proteins were eluted with a linear decreasing-salt gradient. Enzyme activity in the different fractions was measured at pH 6.0 using 2 mM ABTS as substrate. Those samples containing phenol oxidase were pooled and dialyzed against 20 mM sodium phosphate (pH 8.5) and stored at −20°C.
Determination of the amino-terminal sequence of AMO.
Protein samples were analyzed using an apparatus consisting of a Porton LF3000 sequencer and online phenylthiohydantoine analysis (Beckman Instruments Inc., Fullerton, Calif.) with a Beckman high-performance liquid chromatograph type 125S and a Beckman detector type 168. The phenylthiohydantoine derivatives were analyzed online using a C18 Microbore RP column (Beckman). Separation was achieved by using a gradient of 5% tetrahydrofuran in water and acetonitrile. Detection was done at 268 nm.
Nucleotide sequence accession number.
The sequence data for AMO have been submitted to the EMBL database under accession number no. AJ271104.
RESULTS
Isolation, cloning and characterization of AMO.
The fungus A. murorum var. murorum CBS 157.72 produced a clearing zone when cultured on solidified media containing either cyanidin or pelargonidin, and it secreted a phenol oxidase into the medium when grown in shake flask cultures.
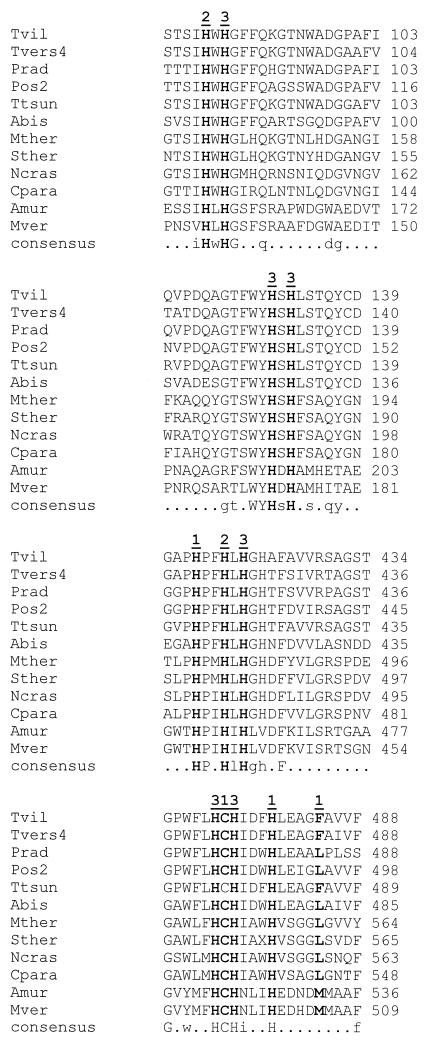
From an A. murorum cDNA expression library in S. cerevisiae VW-K1, we identified a transformant that produced a clearing zone (halo) around the colony on cyanidin-containing plates. This transformant was purified, and plasmid DNA, designated pUR7876, was isolated. Retransformation of S. cerevisiae with pUR7876 DNA again resulted in halo-forming colonies. The DNA sequence of the EcoRI/XhoI cDNA insert in pUR7876 was determined by subcloning fragments in pUC19 (29). The insert consisted of 2,120 nucleotides, including 5′ and 3′ nontranslated sequences and a poly(A) tail. The DNA sequence, with the ATG codon at position 135, comprised an open reading frame of 1,806 nucleotides, encoding an enzyme of 602 amino acids. The 3′ nontranslated region was 165 bases and was followed by a poly(A) tail. An in-frame ATG codon located 63 bp downstream of the first one also could be used to start translation, resulting in a protein of 581 amino acids. Comparison of the deduced amino acid sequence with the sequences of proteins in the databases (Table 1) showed an identity of 66% with a bilirubin oxidase isolated from the fungus Myrothecium verrucaria (13) (see Table 1). Furthermore, the A. murorum phenol oxidase was similar to the consensus sequences of the four copper-binding sites present in laccases (4) (Fig. 2). The homology with laccases was restricted to those consensus areas, and the overall identity of AMO with laccases was <15% (Table 1).
TABLE 1.
Percent identity (calculated by the ClustalW method) of A. murorum polyphenol oxidase to other blue copper oxidasesa
| Enzyme | Identity (%) with polyphenol oxidase fromb:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amur | Mver | Tvil | Tvers4 | Pos2 | Prad | Abis | Ncras | Mther | Sther | Bcin | Cpara | Anid | |
| A. murorum polyphenol oxidase | 66 | 15 | 15 | 14 | 15 | 14 | 12 | 11 | 14 | 14 | 13 | 11 | |
| M. verrucaria bilirubin oxidase | 15 | 16 | 12 | 14 | 13 | 12 | 15 | 12 | 11 | 17 | 6 | ||
| T. villosa laccase (lcc1) | 68 | 62 | 63 | 47 | 25 | 28 | 27 | 30 | 31 | 16 | |||
| T. versicolor laccase (lcc4) | 64 | 64 | 47 | 27 | 27 | 26 | 29 | 26 | 17 | ||||
| P. ostreatus laccase (pox2) | 38 | 48 | 27 | 25 | 25 | 26 | 24 | 19 | |||||
| P. radiata laccase | 43 | 23 | 24 | 25 | 26 | 24 | 17 | ||||||
| A. bisporus laccase | 26 | 25 | 27 | 22 | 23 | 17 | |||||||
| N. crassa laccase | 60 | 56 | 35 | 57 | 18 | ||||||||
| M. thermophila laccase | 63 | 32 | 56 | 18 | |||||||||
| S. thermophilum laccase | 29 | 51 | 18 | ||||||||||
| B. cinereus laccase | 37 | 20 | |||||||||||
| C. parasitica laccase | 19 | ||||||||||||
| A. nidulans laccase | |||||||||||||
Enzyme amino acid sequences (numbers in parentheses below are accession numbers) are the polyphenol oxidase from A. murorum (this paper), bilirubin oxidase from M. verrucaria (Q12737), and laccases from T. villosa (lcc1) (Q99044), Trametes versicolor (lcc4) (Q12719), P. ostreatus (pox2) (Q12739), Phlebia radiata (Q01679), A. bisporus (Q12542), N. crassa (P10574), M. thermophila (AR023901), S. thermophilum (AR007280), Botrytis cinerea (Q12570), Cryphonectria parasitica (Q03966), and A. nidulans (P17489). When neccessary, DNA sequences were translated into amino acid sequences.
Abbreviations: Amur, A. murorum; Mver, M. verrucaria; Tvil, T. villosa; Tvers4, T. versicolor (lcc4); Pos2, P. ostreatus (pox2); Prad, P. radiata; Abis, A. bisporus; Ncras, N. crassa; Mther, M. thermophila; Sther, S. thermophilum; Bcin, B. cinereus; Cpara, C. parasitica; Anid, A. nidulans.
FIG. 2.
Amino acid sequence alignment of AMO with other blue copper enzymes. Only those areas that contain the types I, II, and III copper ligands (marked in bold as 1, 2, and 3) are shown. Amino acid sequence data were obtained as described in Table 1. For abbreviations on left, see Table 1, footnote b. T. tsunodae bilirubin oxidase has GenBank accession number AB006824. A consensus sequence is given below each 13-row set. An amino acid identity of 100% among all enzymes is shown in uppercase, and an identity between 80% and 100% is in lowercase.
Heterologous production of AMO by A. awamori
We transformed A. awamori AWC4.20 with pUR7893 to obtain transformants that overproduced AMO. In a cyanidin plate assay, all transformants produced large halos around the fungal colony, indicating that cyanidin was converted into a colorless compound by the secreted enzyme. Similar plates, in which cyanidin was substituted for the oxidase substrate ABTS, showed that ABTS also was oxidized by this enzyme. Four A. awamori AWC4.20-pUR7893 transformants were analyzed in submerged cultivation and had similar activities on cyanidin. These activities corresponded to a decrease in the OD579 (absorbance peak of cyanidin at pH 7.5) of approximately 0.06 to 0.07/μl of medium sample in 1 min, which is 2 to 3 orders of magnitude more than the production levels obtained with culture medium of A. murorum.
The amounts of extracellular AMO activity, as determined with an ABTS activity assay, reached up to 25 U/ml after 30 h of induction. Based on specific activity of 40 U/mg (see below), the level of recombinant enzyme secreted in these shake flask cultures was approximately 600 mg per liter.
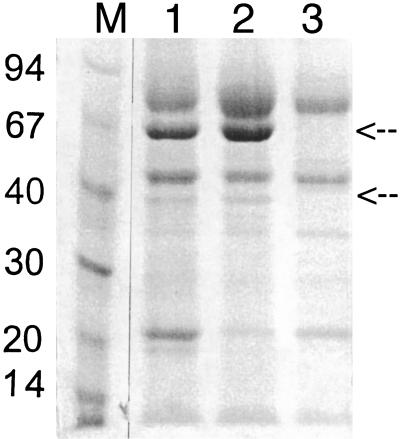
Medium samples of two transformants, AWC-pUR7893-5p and -10A, also were analyzed on SDS-PAGE (8 to 18% gradient gel), stained with Coomassie brilliant blue (Fig. 3). Both samples contained high levels of recombinant enzyme, visible as a main band of about 67 kDa. In addition, a second, minor band of about 40 kDa was visible. Apparently, this smaller band represented a faster-migration form of denatured AMO, since both proteins have the same amino-terminal sequence (SPLSPAYTLF) and, under nondenaturing conditions, only a single band is visible (see below). The amounts of AMO were almost equimolar with the amounts of glucoamylase (visible as a band at approximately 80 kDa), indicating that degradation was minimal. The identity of glucoamylase also was confirmed by Western blot analysis (data not shown).
FIG. 3.
SDS–8 to 18% gradient PAGE from supernatants of AWC-7893 transformants cultivated in shake flasks. The samples were boiled without reducing agent. The gel was stained with Coomassie brilliant blue. The arrows indicate the position of the phenol oxidase. Lane 1, AWC-7893-5p; lane 2, AWC-7893-10A; lane 3, AWGLA, which contains a single copy of the A. niger glucoamylase gene (7). M, molecular size marker (kDa).
Determination of the amino-terminal sequence gave the sequence SPLSPAYTLF, indicating that the enzyme was processed after amino acid 61. Thus, although the fusion was actually based on the theoretical cleavage site of a signal peptide between amino acids 21 and 22 (the fusion of glucoamylase was made with amino acid 22 of AMO), AMO is cleaved after amino acid 61. This means that the glucoamylase-AMO fusion molecule contained two consecutive propeptides: the prosequence of glucoamylase (NVISKR, containing the KEX2 cleavage site) and the prosequence of AMO (theoretically 40 amino acids). A zymogram containing cyanidin as a substrate showed that only AMO was responsible for the decolorization of cyanidin.
Characterization of recombinant AMO.
We purified the enzyme from the culture broth by hydrophobic-interaction chromatography. The major peak in enzyme activity, which eluted at approximately 45% salt, was clearly visible as a blue band on the column during elution. The specific activity of the purified enzyme was about 40 U/mg of protein.
From SDS-PAGE, the molecular size of AMO was estimated to be 67 kDa, whereas the calculated molecular size was 60 kDa. The 7-kDa difference could be explained by N-glycosylation at two putative sites (Asn-X-Thr). Furthermore, the 40-kDa band that was observed in the medium samples of the transformants also was present in purified AMO. When AMO was not boiled, a single band of approximately 32 kDa was visible on SDS-PAGE (data not shown). Apparently, the presence of 1% SDS is not sufficient to fully denature the protein. This was confirmed by analysis of the activity after incubation of AMO in 1% SDS, which showed no decrease in AMO activity. Boiling completely destroyed the activity.
On an isoelectric focusing gel, a band with a pI that was near 3.5 was enzymatically active when the gel was incubated with ABTS. This pI was lower than the calculated pI of 4.3.
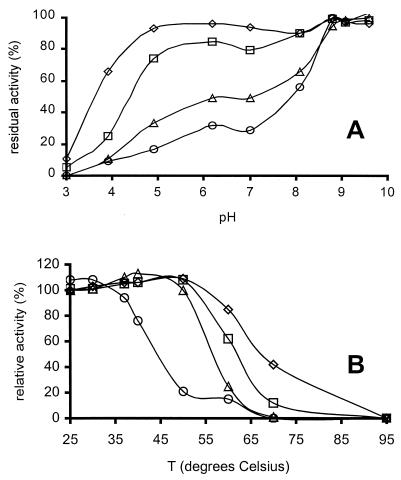
Recombinant AMO had an optimal pH of 4 to 4.5 with ABTS as substrate (Fig. 4A). With SGZ as a substrate, the maximum activity was observed at pH 8.5 to 9. At 60°C, the highest AMO activity was measured, and it was approximately 2.5-fold higher than the activity observed at 20°C (Fig. 4B).
FIG. 4.
Dependence of AMO activity on pH and temperature. (A) AMO activity as a function of pH (normalized to the optimum activity) with ABTS (2 mM) as substrate (□) and SGZ (100 μM) as substrate (⋄). Assays were performed in B&R buffer at the indicated pH at 30°C. (B) AMO activity as a function of temperature (normalized to the activity at 20°C) with ABTS (2 mM) as substrate in B&R buffer, pH 4.5. Both experiments were carried out in duplicate; standard errors were <10%.
The stability of the purified enzyme was measured as a function of pH and temperature. The enzyme was highly unstable at low pH, whereas at alkaline pH the enzyme retained full activity for at least 280 h when incubated at 4°C (Fig. 5A). Thermostability analysis showed that the enzyme was stable at 50°C for 3 h and almost fully stable for 20 min at 60°C. After prolonged incubation or incubation at higher temperatures, the activity decreased (Fig. 5B).
FIG. 5.
The effect of pH and temperature on AMO stability. (A) AMO stability as a function of pH. AMO was incubated at different pHs (3–10) in B&R buffer at 4°C, and the residual activity (normalized to t = 0 h) was analyzed after 3 (⋄), 20 (□), 168 (▵), and 280 (○) h in B&R buffer (pH 6) with 2 mM ABTS. (B) AMO stability as a function of temperature and time. AMO was incubated at different temperatures in B&R buffer (pH 8.5), and the residual activity (normalized to t = 0 h) was analyzed after 0.3 (⋄), 1 (□), 3 (▵), and 21 (○) h in B&R buffer (pH 6) with 2 mM ABTS. Both experiments were carried out in duplicate; standard errors were <10%.
DISCUSSION
We isolated and characterized a phenol oxidase from the fungus A. murorum. Amino acid sequence comparison shows a high degree of identity (66%) with a bilirubin oxidase (BOX) isolated from the fungus Myrothecium verrucaria (13). As with other blue copper enzymes, e.g., laccases and ascorbate oxidase, four consensus domains for all types (I, II, and III) of copper ligands are present in AMO (Fig. 2). Three other fungal bilirubin oxidases have been reported, from Trachyderma tsunodae (10), Penicillium janthinellum (18), and Pleurotus ostreatus (15). However, these enzymes differ from both AMO and Myrothecium bilirubin oxidase. The T. tsunodae bilirubin oxidase amino acid sequence is very similar to the sequences of laccases (Fig. 2) with, for example, 74% identity with the amino acid sequence of T. villosa laccase encoded by the lcc4 gene. The identity of T. tsunodae bilirubin oxidase with AMO and M. verrucaria bilirubin oxidase is only 12 and 14%, respectively. Similarly, the P. ostreatus bilirubin oxidase appears to be identical to P. ostreatus laccase POX2 (15) and has only 13% identity with the amino acid sequence of AMO and M. verrucaria bilirubin oxidase. The P. janthinellum enzyme, which contains copper, zinc, and iron atoms, also is very different from AMO and M. verrucaria bilirubin oxidase, since these proteins contain only copper.
The amino-terminal part of the protein shows the characteristics of a signal sequence. The predicted (26) signal peptide cleavage site for AMO is between amino acids 21 (Ala) and 22 (Met). The protein also contains two dibasic amino acid sequences, residues 51/52 and 60/61 (both Arg-Arg), which might be cleaved by a KEX2-like protease and which could indicate that AMO is initially produced as a proenzyme (21). The first residue of the mature recombinant enzyme is Ser-62 (although the N-terminal sequence of AMO produced by A. murorum could not be determined exactly, this sequence was not in contradiction with the sequence obtained from the recombinant form). Based on these results, residues 22 through 61 probably comprise a propeptide whose proteolytic removal occurs during maturation of AMO. Consequently, the recombinant fusion molecule probably contains two consecutive propeptides: the glucoamylase prosequence NVISKR and the prosequence of AMO. Based on the amino-terminal-sequence data, processing occurs correctly after the AMO prosequence. If translation begins at the second in-frame ATG codon, the resulting protein does not contain a theoretical site for cleavage of a signal peptide, suggesting that the first ATG is the translation initiation codon that is used in vivo.
To make the application of multicopper enzymes feasible in industrial processes or products, the production levels in shake flasks should be at least approximately 1 g per liter. However, the amounts that have been reported are usually low. In a homologous system the amounts can range from a few milligrams per liter up to 80 mg per liter for Botrytis cinereus laccase (19). However, these levels are still low for commercial purposes, and cultivation of these fungi is often difficult. Although laccases have been isolated from a large number of ascomycetes (e.g., A. nidulans, Neurospora crassa, and Podospora anserina), deuteromycetes (Botrytis cinereus), and basidiomycetes (e.g., Coriolus hirsutus, Trametes villosa [or Polyporus pinsitus], Agaricus bisporus, Polyporus versicolor, and Pleurotus ostreatus), their production levels in heterologous hosts are also usually <100 mg per liter (14, 17). In contrast, the yield of the recombinant phenol oxidase from A. awamori transformants grown in shake flasks was high (600 mg per liter). As normally the production yield is improved when shake flask experiments are replaced by fed-batch fermentation processes, AMO has potential commercial utility for industrial purposes or consumer products.
AMO had an optimal activity for ABTS and SGZ as substrates at pH from 4 to 4.5 and 8.5 to 9, respectively. These optima are at least equal and often higher than described for other polyphenol oxidases such as laccases (27, 28) and indicate that AMO has potential to be used under alkaline conditions. Furthermore, under alkaline conditions the enzyme is fully stable for at least 3 h at 50°C and loses only 15% activity after 20 min at 60°C. This is close to the stability observed for the thermophilic fungi Myceliophthora thermophila and Scytalidium thermophilum (28) and higher than for laccases isolated from Polyporus pinsitus and Rhizoctonia solani (28).
In conclusion, we isolated a new phenol oxidase derived from the fungus A. murorum var. murorum CBS 157.72. The enzyme converted anthocyanidins to colorless compounds and could be applied as a mild alternative for chemical bleaching, such as in laundry cleaning. This enzyme forms an attractive alternative to other polyphenol oxidases due to (i) its potential to be produced at high levels (at least 0.6 g/liter) by cultivation of a recombinant strain of A. awamori, (ii) its high stability under alkaline conditions and high temperatures, (iii) its high activity at 50 to 60°C, and (iv) its activity under even extreme alkaline conditions (pH 9 to 10).
ACKNOWLEDGMENTS
We thank John Chapman and Maarten Egmond for critical reading of the manuscript and Han van Brouwershaven for amino-terminal sequence analysis.
REFERENCES
- 1.Bennett J W, Lasure L L. Growth media. In: Bennett J W, Lasure L L, editors. More gene manipulations in fungi. San Diego, Calif: Academic Press, Inc.; 1991. pp. 441–458. [Google Scholar]
- 2.Bourbonnais R, Paice M G, Reid I D, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and the role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol. 1995;61:1876–1880. doi: 10.1128/aem.61.5.1876-1880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton H T S, Robinson R. Universal buffer solutions and the dissociation constant of veronal. J Chem Soc. 1931;458:1456. [Google Scholar]
- 4.Cullen D. Recent advances on the molecular genetics of lignolytic fungi. J Biotechnol. 1997;53:273–289. doi: 10.1016/s0168-1656(97)01684-2. [DOI] [PubMed] [Google Scholar]
- 5.Gietz R D, Woods R A. Transformation of yeast by the lithium acetate/single stranded carrier DNA/PEG method. Methods Microbiol. 1998;26:53–66. [Google Scholar]
- 6.Gouka R J, Hessing J G M, Punt P J, Stam H, van den Hondel C A M J J. An expression system based on the promoter region of the 1,4-β-endoxylanase A gene of Aspergillus awamori. Appl Microbiol Biotechnol. 1996;46:28–35. doi: 10.1007/s002530050779. [DOI] [PubMed] [Google Scholar]
- 7.Gouka R J, Punt P J, van den Hondel C A M J J. Glucoamylase gene fusions alleviate limitations for protein production in Aspergillus awamori at the transcriptional and (post-)translational level. Appl Environ Microbiol. 1997;63:488–497. doi: 10.1128/aem.63.2.488-497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gouka R J, Punt P J, van den Hondel C A M J J. Efficient production of secreted proteins by Aspergillus: progress, limitations, and prospects. Appl Microbiol Biotechnol. 1997;47:1–11. doi: 10.1007/s002530050880. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan J. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Hiromi K, Yamaguchi Y, Sugiura Y, Iwamoto H, Hirose J. Bilirubin oxidase from Trachyderma tsunodae K-2593, a multi-copper enzyme. Biosci Biotechnol Biochem. 1992;56:1349–1350. [Google Scholar]
- 11.Hoffman C S, Winston F. A ten minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 12.Husain Q, Jan U. Detoxification of phenols and aromatic amines from polluted wastewater by using phenol oxidases. J Sci Ind Res. 2000;59:286–293. [Google Scholar]
- 13.Koikeda S, Ando K, Kaji H, Inoue T, Murao S, Takeuchi K, Samejima T. Molecular cloning of the gene for bilirubin oxidase from Myrothecium verrucaria and its expression in yeast. J Biol Chem. 1993;268:18801–18809. [PubMed] [Google Scholar]
- 14.Kojima Y, Tsukuda Y, Kawai Y, Tsukamoto A, Sugiura J, Sakaino M, Kita Y. Cloning, sequence analysis, and expression of ligninolytic polyphenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem. 1990;265:15224–15230. [PubMed] [Google Scholar]
- 15.Masuda-Nishimura I, Ichikawa K, Hatamoto O, Abe K, Koyama Y. cDNA cloning of bilirubin oxidase from Pleurotus ostreatus strain Shinshu and its expression in Aspergillus sojae: an efficient screening of transformants, using the laccase activity of bilirubin oxidase. J Gen Appl Microbiol. 1999;45:93–97. doi: 10.2323/jgam.45.93. [DOI] [PubMed] [Google Scholar]
- 16.Messerschmidt A, Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Eur J Biochem. 1990;187:341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- 17.Saloheimo M, Niku-Paavola M L. Heterologous production of a lignolytic enzyme: expression of a Phlebia radiata laccase gene in Trichoderma reesei. Bio/Technology. 1991;9:987–990. [Google Scholar]
- 18.Seki Y, Takeguchi M, Okura I. Purification and properties of bilirubin oxidase from Penicillium janthinellum. J Biotechnol. 1996;46:145–151. [Google Scholar]
- 19.Slomczynski D, Nakas J P, Tanenbaum S W. Production and characterization of laccase from Botrytis cinerea 61-34. Appl Environ Microbiol. 1995;61:907–912. doi: 10.1128/aem.61.3.907-912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon E I, Sundaram U M, Machonkin T E. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 21.Steiner D F, Smeekens S P, Ohagi S, Chan S J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992;267:23435–23438. [PubMed] [Google Scholar]
- 22.Thurston C F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 23.van den Hondel C A J J M, Punt P J, van Gorcom R F M. Heterologous gene expression in filamentous fungi. In: Bennett J W, Lasure L L, editors. More gene manipulations in fungi. San Diego, Calif: Academic Press, Inc.; 1991. pp. 396–428. [Google Scholar]
- 24.Van Gemeren I A, Beijersbergen A, Musters W, Gouka R J, van den Hondel C A M J J, Verrips C T. The effect of pre- and pro-sequences and multi-copy integration on heterologous expression of the Fusarium solani pisi cutinase gene in Aspergillus awamori. Appl Microbiol Biotechnol. 1996;45:755–763. doi: 10.1007/s002530050759. [DOI] [PubMed] [Google Scholar]
- 25.Von Heijne G. Signal sequences: the limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 26.Von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4685–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahleithner J A, Xu F, Brown K M, Brown S H, Golightly E J, Halkier T, Kauppinen S, Pederson A, Schneider P. The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr Genet. 1996;29:395–403. doi: 10.1007/BF02208621. [DOI] [PubMed] [Google Scholar]
- 28.Xu F, Shin W, Brown S H, Wahleithner J A, Sundaram U M, Solomon E I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 30.Youn H D, Hah Y C, Kang S O. Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiol Lett. 1995;132:183–188. [Google Scholar]