Abstract
The exercise pressor reflex (EPR), a neurocirculatory control mechanism, is exaggerated in hypertensive humans and rats. Disease-related abnormalities within the afferent arm of the reflex loop, including mechano- and metabosensitive receptors located at the terminal end of group III/IV muscle afferents, may contribute to the dysfunctional EPR in hypertension. Using control (WKY) and spontaneous hypertensive (SHR) rats, we examined dorsal root ganglion (DRG) gene and protein expression of molecular receptors recognized as significant determinants of the EPR. Twelve lumbar DRGs (6 left, 6 right) were harvested from each of 10 WKY [arterial blood pressure (MAP): 96 ± 9 mmHg] and 10 SHR (MAP: 144 ± 9 mmHg). DRGs from the left side were used for protein expression (Western blotting; normalized to GAPDH), whereas right-side DRGs (i.e., parallel structure) were used to determine mRNA levels (RNA-sequencing, normalized to TPM). Analyses focused on metabosensitive (ASIC3, Bradykinin receptor B2, EP4, P2X3, TRPv1) and mechanosensitive (Piezo1/2) receptors. Although Piezo1 was similar in both groups (P = 0.75), protein expression for all other receptors was significantly higher in SHR compared with WKY. With the exception of a greater Bradykinin-receptor B2 in SHR (P < 0.05), mRNA expression of all other receptors was not different between groups (P > 0.18). The higher protein content of these sensory receptors in SHR indirectly supports the previously proposed hypothesis that the exaggerated EPR in hypertension is, in part, due to disease-related abnormalities within the afferent arm of the reflex loop. The upregulated receptor content, combined with normal mRNA levels, insinuates that posttranscriptional regulation of sensory receptor protein expression might be impaired in hypertension.
Keywords: blood pressure, dorsal root ganglion, exercise pressor reflex, hypertension, mRNA
INTRODUCTION
With the onset of exercise, contraction-induced mechanical and chemical stimuli begin to activate molecular receptors on the terminal end of both group III (mainly mechanosensitive) and group IV (mainly metabosensitive) muscle afferents. This increases the projection of these sensory neurons to cardiovascular control centers in the brainstem and, subsequently, raises the sympathetic outflow to the heart and blood vessels. This neurocirculatory control mechanism is known as the exercise pressor reflex (EPR), which, in health, optimizes the hemodynamic response to physical activities (1–6).
However, in hypertensive humans (7, 8) and rats (9, 10), the EPR is dysfunctional and results in excessive sympathoexcitation, impaired peripheral hemodynamics, and an exaggerated blood pressure response to exercise. Importantly, these abnormal cardiovascular responses occur regardless of medication status (11) and reflect the inability of current pharmacological approaches to address the underlying causes of the malfunctioning EPR in hypertensive humans. As an elevated blood pressure response to exercise amplifies the risk of cardiovascular events (e.g., stroke) (12, 13), it is imperative to understand the mechanisms behind EPR dysfunction and identify potential therapeutic targets to improve clinical care for patients with hypertension.
EPR dysfunction may result from maladaptive alterations at any stage of the reflex arc, including intramuscular metabolic perturbations during exercise, central nervous system processing of afferent signals, end-organ responsiveness to sympathetic activity, and structural changes within the afferent arm of the reflex loop (including receptors on group III/IV fibers). By focusing on the function of individual receptors, previous studies have identified the metabosensitive acid-sensing ion channel 3 (ASIC3) (14), bradykinin receptor 2 (BDKRB2) (15), prostaglandin E2 receptor 4 (EP4) (16), purinergic receptor 2X3 (P2X3), (17), transient receptor potential cation channel 1 (TRPv1), (18), and the mechanosensitive Piezo1 and 2 receptors (19) as major determinants of the EPR in health. Importantly, a limited number of studies in hypertension used receptor antagonists and identified the activation of TRPv1 (20), Gadolinium-sensitive mechanoreceptors (10), or purinergic (P2) receptors (21) as key determinants of the exaggerated EPR in this population. As mentioned earlier, one reason for this unfavorable functional transformation in hypertension could be due to a structural change within the afferent arm of the EPR, more specifically, an augmented expression of molecular receptors on group III/IV muscle afferents (21).
Dorsal root ganglia (DRG) are located bilaterally at each vertebral segment and contain the cell bodies of all sensory neurons including muscle, joint, and cutaneous afferents. The protein expression of molecular receptors within the DRG is considered a surrogate marker reflecting the content of these receptors in the periphery (22). Importantly, based on the observation that alterations in DRG gene and protein expression of receptors are related, although not exclusively, to the EPR mirror changes in EPR function in various animal models, earlier studies suggested a link between structural changes in the afferent arm of the EPR and alterations in EPR function (23–25). Although protein expression of one of the receptors previously identified as key determinants of the EPR, namely TRPv1, has been shown to be upregulated in spontaneously hypertensive rats (SHR) (20), a comprehensive investigation focusing on gene and protein expression of other EPR-related receptors is currently missing. Addressing this knowledge gap is important as disease-related alterations in the expression of these molecular receptors may, as mentioned earlier, represent a mechanism underpinning the well-documented EPR abnormalities in hypertension (7–10).
It was therefore the purpose of the current study to characterize DRG transcription factors (mRNA sequencing) and protein expression (Western blotting) of EPR-related molecular receptors in normotensive Wistar-Kyoto (WKY) and SHR rats. It was hypothesized that SHR would exhibit greater mRNA levels and an increased protein expression of afferent receptors known to be involved in the EPR.
METHODS
Animal Model
All animal experiments conformed to the Guide and Use of Laboratory Animals and were approved by the University of Utah and Salt Lake City Veterans Affairs Medical Center Animal Care and Use Committees. Ten SHR (age: 91 ± 3 days, weight: 285 ± 10 g, mean arterial pressure: 144 ± 9 mmHg) and 10 WKY (age: 90 ± 4 days, weight: 325 ± 48 g, mean arterial pressure: 96 ± 9 mmHg) adult male rats (Charles River) were studied. Given the known sex differences in the EPR (26, 27) and in the prevalence and pathophysiology of hypertension (28–30), this investigation was limited to male animals. Upon arrival, animals were acclimated for 1 wk in their housing cages on a 12:12-h light-dark cycle (lights on 0800–2000) at 20°C, and fed with standard laboratory rodent chow (Catalog No. 2920X, Envigo). At the end of this acclimatization period, resting blood pressure data were acquired at rest via tail photoplethysmography (BP-2000, Visitech System, Inc, Apex, NC) (31). Euthanasia was performed by enclosing the animals in an air-tight container saturated with isoflurane until respiratory arrest and then followed by decapitation. Twelve lumbar DRGs (6 left, 6 right) were harvested from each animal and quickly frozen and stored at −80°C for later analysis. The six DRGs from the left side were pooled and used for immunoblotting (i.e., protein expression), whereas and the six right DRGs were pooled for RNA sequencing (i.e., mRNA levels expression).
Immunoblotting
The proteins of interest were measured in WKY and SHR animals by Western blot analysis. Protein concentration was determined using the Bradford technique (32). One microgram of homogenate was separated by polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and incubated with primary and secondary antibodies directed against the proteins of interest. Membranes were imaged on a ChemiDoc XRS (Bio-Rad) and quantified with Image Lab software (Bio-Rad). The specific antibodies used to detect proteins included: anti-Piezo1 rabbit antibody (1:100; APC-087, Alomone laboratories, Jerusalem, Israel), anti-Piezo2 rabbit antibody (1:400; a kind gift from Dr. Ardem Patapoutian, The Scripps Research Institute, La Jolla, CA), ASIC3 antibody (1:500; LS C11723, LifeSpan BioScience, Inc., Nottingham, UK), TRPv1 (1:500; GT 15129, Neuromics), P2X3 (1:500; RA 14139, Neuromics), EP4 antibody (1:500; sc-55596, Santa Cruz Biotechnology, Inc., Dallas, TX), and Bradykinin B2 R antibody (1:500; sc-25671, Santa Cruz Biotechnology, Inc., Dallas, TX). Protein abundance was normalized to GAPDH (1:500; ab9485, Abcam, Cambridge, MA), which served as a loading control.
RNA Extraction and RNA Sequencing
RNA was extracted using RNeasy-mini kits (Qiagen), and treated with RNase-free DNase-I (Qiagen) (33). Immediately after extraction, RNA eluted in water was flash frozen and stored at −80°C until ready for library building. Library construction was performed using the Illumina TruSeq Stranded Total RNA Sample Preparation Kit with Ribo-Zero Human/Mouse/Rat (RS-122-2201, RS-122-2202). NovoSeq 150 Cycle paired-read sequencing was used with 25 million read-pairs per sample. Values for mRNA were then normalized as transcripts per million (TPM).
Statistical Analyses
All data are expressed as means ± SD. Unpaired Student’s t tests were used to conduct a priori planned comparisons between WKY and SHR animals for DRG protein and mRNA expression.
RESULTS
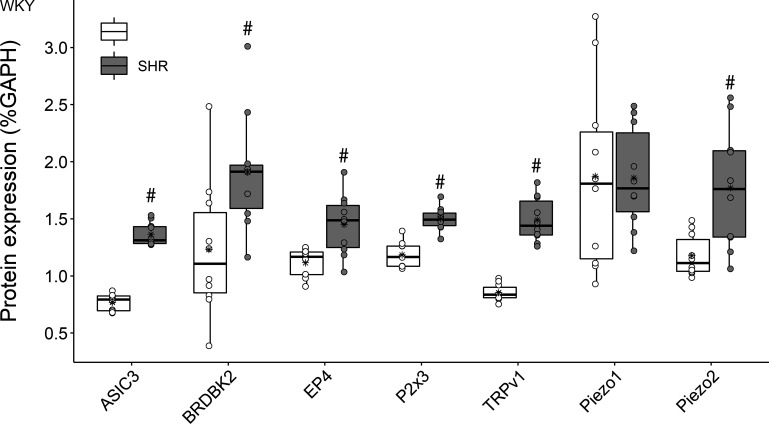
Representative Western blots for the receptors of interest are presented in Fig. 1. Quantification of DRG protein expression is presented in Fig. 2. With the exception of Piezo1 (Piezo1: 1.86 ± 0.47 vs. 1.87 ± 0.87%, P = 0.75), SHR animals demonstrated significantly (P < 0.01) greater protein expression for ASIC3 (+78%; 1.36 ± 0.09 vs. 0.77 ± 0.07%), BDKRB2 (+55%; 1.91 ± 0.55 vs. 1.23 vs. 0.63%), EP4 (+30%; 1.45 ± 0.21 vs. 1.11 ± 0.13%), P2X3 (+27%; 1.49 ± 0.11 vs. 1.18 ± 0.11%), TRPv1 (+75%; 1.47 ± 0.20 vs. 0.86 ± 0.06%), and Piezo2 (+50%; 1.77 ± 0.53 vs. 1.18 ± 0.19%) compared with WKY animals.
Figure 1.
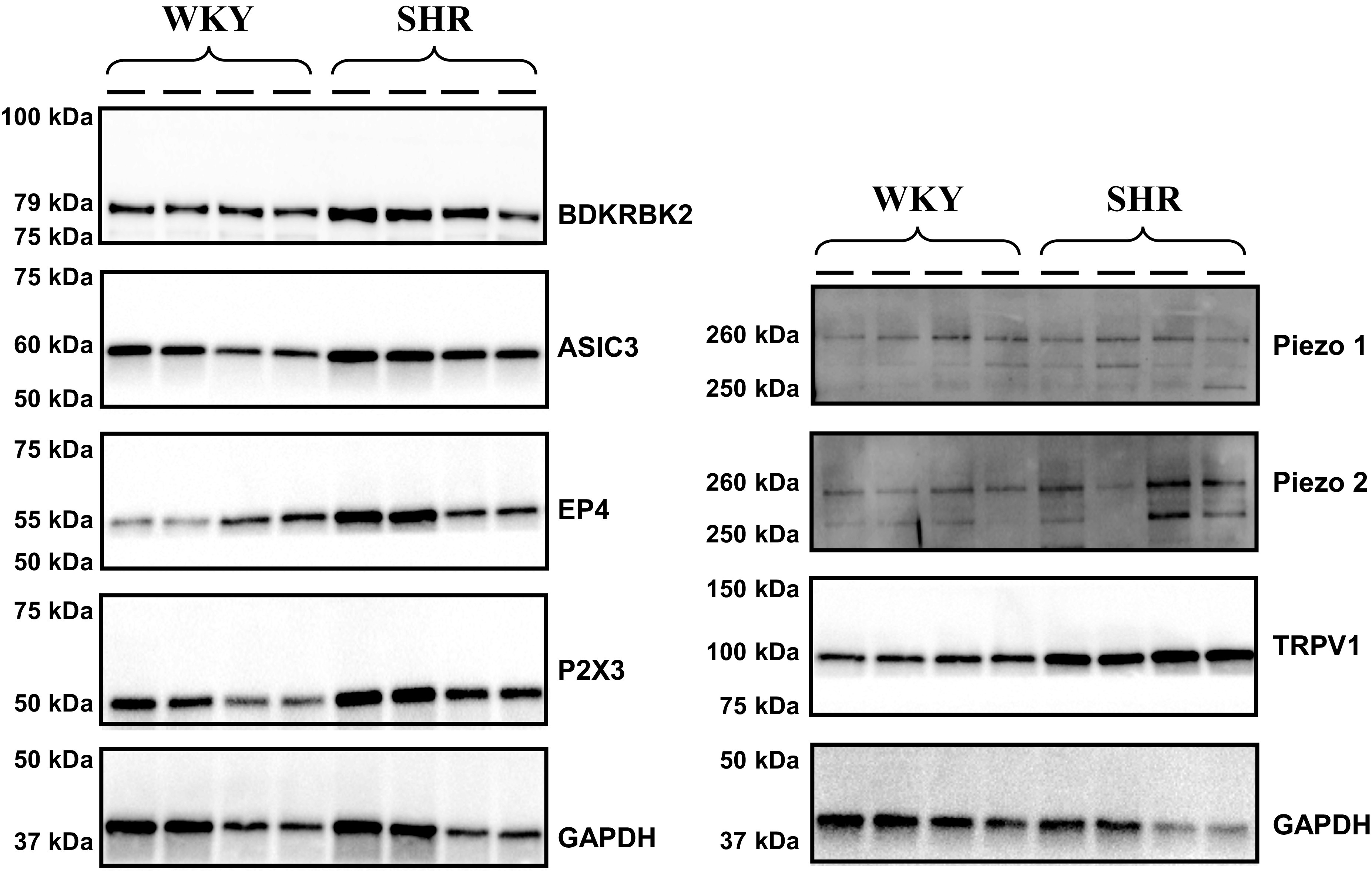
Representative Western Blots of molecular dorsal root ganglion receptors in normotensive (Wistar-Kyoto, WKY, n = 4) and hypertensive (spontaneously hypertensive rats, SHR, n = 4) male rats.
Figure 2.
Protein expression of molecular dorsal root ganglion (DRG) receptors in normotensive (Wistar-Kyoto, WKY, n = 10) and hypertensive (spontaneously hypertensive rat, SHR, n = 10) male rats. In the box plots, the boundary of the box closest to zero indicates the 1st quartile, a black line within the box marks the median, and the boundary of the box farthest from zero indicates the 3rd quartile. Asterisk represents group mean. Whiskers represent the lowest and highest values which are not outliers. Points above and below the whiskers indicate outliers identified as 1.5 times interquartile range above the 3rd quartile or below the 1st quartile. A priori, unpaired Student’s t tests were used to test group differences. Data are means ± SD. #P < 0.05 vs. WKY.
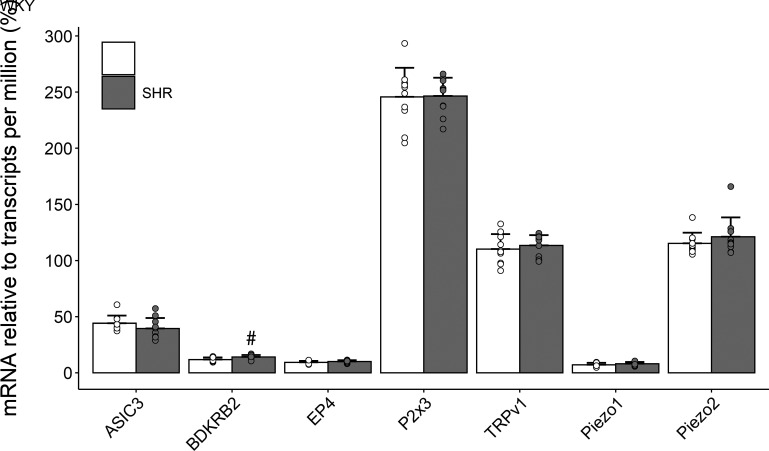
The DRG mRNA levels of all tested molecular receptors are presented in Fig. 3. With the exception of BDKRB2 (14 ± 2 vs. 12 ± 2 TPM, P < 0.05), mRNA content was similar (P > 0.18) between SHR and WKY animals for ASIC3 (39 ± 9 vs. 44 ± 7 TPM), EP4 (10 ± 1 vs. 9 ± 1 TPM), P2X3 (246 ± 16 vs. 246 ± 26 TPM), TRPv1 (113 ± 9 vs. 110 ± 13 TPM), Piezo1 (7 ± 2 vs. 8 ± 1 TPM), and Piezo2 (121 ± 17 vs. 115 ± 9 TPM).
Figure 3.
Quantity of mRNA in dorsal root ganglion (DRG) of normotensive (Wistar-Kyoto, WKY, n = 10) and hypertensive (spontaneously hypertensive rat, SHR, n = 10) male rats. A priori, unpaired Student’s t tests were used to test group differences. Data are means ± SD. #P < 0.05 vs. WKY.
DISCUSSION
With the purpose of further investigating the mechanisms determining the well-documented EPR abnormalities characterizing humans and animals with hypertension, this study sought to evaluate gene and protein expression of EPR receptors in the DRG of WKY and SHR. Despite mostly similar levels of mRNA, the protein content of these sensory receptors was determined to be higher in SHR. Although the receptors investigated in this study are not limited to mediating the EPR, these findings indirectly support the idea that the exaggerated EPR in hypertension may, at least in part, result from disease-related abnormalities within the afferent arm of the reflex loop and that posttranscriptional regulation of EPR receptor protein expression could account for this abnormality.
The current study is the first to demonstrate that protein expression of multiple metabosensitive receptors (i.e., ASIC3, BDKRB2, EP4, P2X3, and TRPv1) is upregulated by 25%–80% in the DRG of SHR (Fig. 2). These findings confirm, and extend, a previous investigation reporting a ∼90% higher TRPv1 content in the DRG of SHR compared with WKY (20). This earlier study also quantified the functional consequence associated with the upregulated protein content and found that when Capazepine was used to block TRPv1 receptors, both blood pressure and renal sympathetic nerve activity decreased significantly more in SHR compared with WKY (20). These observations strengthen the rationale for the hypothesis that a higher metaboreceptor content in SHR could, in addition to the documented abnormal central processing of afferent signals (20, 34), impaired functional sympatholysis (35), and increased receptor sensitivity (36), be considered a structural alteration of the reflex loop that might contribute to the abnormal EPR in hypertension. However, although earlier findings from animal studies suggest this concept (23–25), direct evidence for the existence of this relationship in hypertension remains to be established. Due to the relatively high number of receptors included in this investigation, quantifying the functional relevance of each receptor via agonist/antagonist experiments would not have been feasible. Thus, it should be emphasized that the observed increase in metabosensitive receptor protein expression in SHR may not necessarily translate into an augmented EPR response. Additional studies are warranted to experimentally test the hypothesis that increases in the content of EPR-receptor within DRGs contribute to the abnormal EPR in hypertension.
Although metabosensitive receptors of group III/IV muscle afferents have been shown to independently augment the EPR in many different animal models of cardiovascular disease (20, 37–40), the activation of a single receptor using a metabolite concentration within the physiological range may not increase group III/IV muscle afferent firing (41, 42). Indeed, as the EPR is only triggered when a combination of receptors are activated in concert (41, 43), the finding that numerous group III/IV afferent receptors are substantially upregulated in SHR is of critical importance and indicates that therapies targeting one receptor family may not be sufficient. Finally, as inhibition of mechano-gated channels has been shown to attenuate the EPR in healthy animals (19, 44), the current study also quantified the content of these two mechanosensitive receptors. Interestingly, while the expression of Piezo1, which primarily mediates the function of non-neuronal cells (45), was not different between the groups, protein content of Piezo2, which is predominately expressed in sensory neurons (46, 47), was significantly higher in SHR compared with WKY (Fig. 2). Although the exact mechanisms determining the increased DRG protein content in the SHR remain unknown, abnormalities in pre- or posttranscriptional protein regulation could play a role.
Gene expression of the sensory receptors of interest was, despite significant differences in protein expression, similar in SHR and WKY (Fig. 3). Although speculative given the limited data available from the current study, this discrepancy indirectly supports the idea of a disease-related impact on posttranscriptional protein regulation. This may result from impairments in mRNA translation mediated via noncoding microRNAs or reduced protein ubiquitination, two alterations that have previously been detected in hypertension (48–50). Future work should examine ribosomal profiling, immunoprecipitation for ubiquitinated proteins, and microRNAs to further evaluate the potential of posttranscriptional dysfunction as a mechanism determining the elevated EPR receptor protein content in hypertension.
Limitations
Since the current study did not experimentally confirm the exaggerated EPR in SHR (9, 10, 20, 44), a causal relationship between EPR-receptor expression and the magnitude of the EPR cannot be determined. In addition, although DRGs have previously been used to determine protein expression of receptors linked with group III/IV muscle afferents (20, 23–25), it is important to emphasize that DRGs also consist of cell bodies from cutaneous and joint sensory afferents. It is therefore possible that the discrepancy in protein expression between the WKY and SHR is, at least to a degree, due to a difference in the content of receptors linked with sensory fibers that do not affect the EPR. Furthermore, it is possible that the higher amount of EPR-receptor proteins produced in the DRG is transported to other tissues (e.g., skin and joint), but not to the nerve endings of group III/IV muscle afferents (20). Finally, given the known sex differences in the EPR (26, 27) and in the prevalence and pathophysiology of hypertension (28–30), this investigation excluded female animals, which limits generalizability of the current findings to male SHR.
Perspectives and Significance
Despite similar levels of mRNA in WKY and SHR, protein expression for EPR-related molecular receptors was increased in the DRG of the hypertensive animals. These findings suggest a dysregulation between gene and protein expression of these molecular receptors as a potential contributor to the abnormal EPR characterizing humans and animals with hypertension. Therapies targeting the translation or degradation of these proteins may therefore be of clinical relevance and ultimately help treating the exaggerated blood pressure response to physical activity frequently observed in hypertensive individuals. However, studies are needed to directly test this hypothesis.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grants HL-116579 and HL-139451 (to M. Amann) and the Veterans Affairs Rehabilitation Research and Development Grant E3343-R (to M. Amann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.W. and M.A. conceived and designed research; J.C.W., O.S.K., R.W.H., and J.Z. performed experiments; J.C.W. and O.S.K. analyzed data; J.C.W. and M.A. interpreted results of experiments; J.C.W. prepared figures; J.C.W. drafted manuscript; J.C.W., O.S.K., R.W.H., J.Z., A.R.L., and M.A. edited and revised manuscript; J.C.W., O.S.K., R.W.H., J.Z., A.R.L., and M.A. approved final version of manuscript.
REFERENCES
- 1.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 5.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O'Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur J, Alvarez A, Hanna HW, Krishnan AC, Senador D, Machado TM, Altamimi YH, Lovelace AT, Dombrowski MD, Spranger MD, O'Leary DS. Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am J Physiol Heart Circ Physiol 311: H1268–H1276, 2016. doi: 10.1152/ajpheart.00501.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidhu SK, Weavil JC, Rossman MJ, Jessop JE, Bledsoe AD, Buys MJ, Supiano MS, Richardson RS, Amann M. Exercise pressor reflex contributes to the cardiovascular abnormalities characterizing hypertensive humans during exercise. Hypertension 74: 1468–1475, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol 590: 1481–1494, 2012. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chant B, Bakali M, Hinton T, Burchell AE, Nightingale AK, Paton JFR, Hart EC. Antihypertensive treatment fails to control blood pressure during exercise. Hypertension 72: 102–109, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11076. [DOI] [PubMed] [Google Scholar]
- 12.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med 329: 1677–1683, 1993. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 13.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin 14: 263–270, 1996. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- 14.Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1720–H1725, 2008. doi: 10.1152/ajpheart.00623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol (1985) 75: 2061–2068, 1993. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- 16.Stone AJ, Copp SW, Kaufman MP. Role of prostaglandins in spinal transmission of the exercise pressor reflex in decerebrated rats. Neuroscience 277: 26–35, 2014. doi: 10.1016/j.neuroscience.2014.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCord JL, Tsuchimochi H, Kaufman MP. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex. J Appl Physiol (1985) 109: 1416–1423, 2010. doi: 10.1152/japplphysiol.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res 50: 133–139, 1982. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 19.Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594: 641–655, 2016. doi: 10.1113/JP271714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. doi: 10.1152/ajpheart.00575.2013. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs L, Comroe JH Jr.. Reflex apnea, bradycardia, and hypotension produced by serotonin and phenyldiguanide acting on the nodose ganglia of the cat. Circ Res 29: 145–155, 1971. doi: 10.1161/01.res.29.2.145. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Li JD, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X(3) expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011. doi: 10.1152/ajpheart.00188.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA 99: 8360–8365, 2002. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing J, Lu J, Li J. Augmented P2X response and immunolabeling in dorsal root ganglion neurons innervating skeletal muscle following femoral artery occlusion. J Neurophysiol 109: 2161–2168, 2013. doi: 10.1152/jn.01068.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives SJ, McDaniel J, Witman MA, Richardson RS. Passive limb movement: evidence of mechanoreflex sex specificity. Am J Physiol Heart Circ Physiol 304: H154–H161, 2013. doi: 10.1152/ajpheart.00532.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 31: 1247–1254, 2018. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmarakby AA, Sullivan JC. Sex differences in hypertension: lessons from spontaneously hypertensive rats (SHR). Clin Sci (Lond) 135: 1791–1804, 2021. doi: 10.1042/CS20201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension 68: 1322–1327, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharkey LC, Radin MJ, Heller L, Rogers LK, Tobias A, Matise I, Wang Q, Apple FS, McCune SA. Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension-heart failure (SHHF), spontaneously hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol Appl Pharmacol 273: 47–57, 2013. doi: 10.1016/j.taap.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol 32: 9–15, 1994. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 33.Lopes DM, Denk F, McMahon SB. The molecular fingerprint of dorsal root and trigeminal ganglion neurons. Front Mol Neurosci 10: 304, 2017. doi: 10.3389/fnmol.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SA, Leal AK, Murphy MN, Downey RM, Mizuno M. Muscle mechanoreflex overactivity in hypertension: a role for centrally-derived nitric oxide. Auton Neurosci 188: 58–63, 2015. doi: 10.1016/j.autneu.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol 589: 1209–1220, 2011. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brock JA, Van Helden DF. Enhanced excitatory junction potentials in mesenteric arteries from spontaneously hypertensive rats. Pflugers Arch 430: 901–908, 1995. doi: 10.1007/BF01837403. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Xing J, Li J. Bradykinin B2 receptor contributes to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 304: H1166–H1174, 2013. doi: 10.1152/ajpheart.00926.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. doi: 10.1113/jphysiol.2012.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99: 368–380, 2014[Erratum inExp Physiol99: 740, 2014]. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone AJ, Copp SW, Kim JS, Kaufman MP. Combined, but not individual, blockade of ASIC3, P2X, and EP4 receptors attenuates the exercise pressor reflex in rats with freely perfused hindlimb muscles. J Appl Physiol 119: 1330–1336, 2015. doi: 10.1152/japplphysiol.00630.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyman AJ, Tumova S, Beech DJ. Piezo1 channels in vascular development and the sensing of shear stress. Curr Top Membr 79: 37–57, 2017. doi: 10.1016/bs.ctm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18: 1756–1762, 2015. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kuhnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR, Patapoutian A. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med 10: eaat9897, 2018. doi: 10.1126/scitranslmed.aat9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dluzen DF, Noren Hooten N, Zhang Y, Kim Y, Glover FE, Tajuddin SM, Jacob KD, Zonderman AB, Evans MK. Racial differences in microRNA and gene expression in hypertensive women. Sci Rep 6: 35815, 2016. doi: 10.1038/srep35815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep 3: 858–868, 2013. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Abriel H, Loffing J, Rebhun JF, Pratt JH, Schild L, Horisberger JD, Rotin D, Staub O. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle’s syndrome. J Clin Invest 103: 667–673, 1999. doi: 10.1172/JCI5713. [DOI] [PMC free article] [PubMed] [Google Scholar]