Keywords: arterial stiffness, COVID-19 recovery, daytime blood pressure, nighttime blood pressure, SARS-CoV-2
Abstract
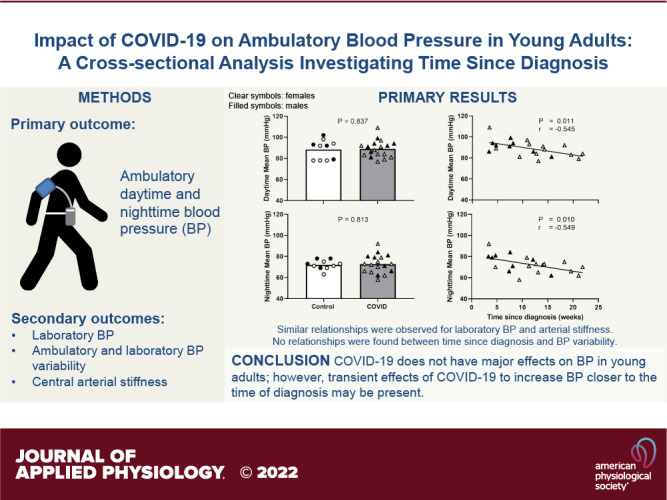
Previous studies have reported detrimental effects of COVID-19 on the peripheral vasculature. However, reports on blood pressure (BP) are inconsistent, and measurements are made only in the laboratory setting. To date, no studies have measured ambulatory BP. In addition, in previous studies, time since COVID-19 diagnosis among participants varied across a wide range, potentially contributing to the inconsistent BP results. Thus, we aimed to perform a comprehensive assessment of BP and BP variability using ambulatory and laboratory (brachial and central) measurements in young adults who had COVID-19. We hypothesized that ambulatory BP would be elevated post-COVID-19 and that measures of BP would be inversely related with time since diagnosis. Twenty-eight young adults who had COVID-19 [11 ± 6 (range 3–22) wk since diagnosis] and 10 controls were studied. Ambulatory daytime, nighttime, and 24-h systolic BP, diastolic BP, and mean BP were not different between the control and COVID groups (e.g., daytime systolic BP: control, 122 ± 12 mmHg; COVID, 122 ± 10 mmHg; P = 0.937). Similar results were observed for laboratory BPs (all P > 0.05). However, ambulatory daytime, nighttime, and 24-h BPs as well as laboratory brachial BPs were inversely correlated with time since COVID-19 diagnosis (e.g., daytime systolic BP: r = −0.444; P = 0.044, nighttime systolic BP: r = −0.518; P = 0.016). Ambulatory and laboratory-measured BP variability were not different between groups nor correlated with time since diagnosis. Collectively, these data suggest that adverse effects of COVID-19 on BP in young adults are minimal and likely transient.
NEW & NOTEWORTHY We report for the first time that ambulatory daytime, nighttime, and 24-h blood pressure (BP), as well as laboratory BP, were not different between control and COVID participants. However, a significant inverse relationship with time since COVID-19 diagnosis was found (i.e., greater BP with more recent infection). Ambulatory and laboratory BP variability were unaffected and not related with diagnosis time. These findings suggest that COVID-19 may exert only short-lasting effects on BP in young adults.
INTRODUCTION
The number of COVID-19 cases reported to date globally has exceeded 400 million with over 6 million deaths since the first case was detected in December 2019 (1), and it is predicted that COVID-19 will remain an endemic disease for the foreseeable future (2). Notably, the incidence of future cardiovascular disease has been reported to be substantially higher in individuals who had COVID-19, including in those without preexisting risk factors and those who had only mild-moderate acute illness (3). However, the underlying factors that contribute to a greater cardiovascular disease burden in COVID-19 are not fully understood. Although high blood pressure (BP) is a major risk factor for the development of cardiovascular disease, the short- and long-term effects of COVID-19 on BP remains unclear.
There is a growing interest in investigating the effects of COVID-19 on cardiovascular health in young adults given that this age group accounts for almost one-fourth of the reported cases in the United States (4). Studies have reported greater central arterial stiffness (5, 6), blunted peripheral vascular function (5, 7, 8), and elevated resting sympathetic nerve activity (9) in previously healthy young adults who had mild-to-moderate COVID-19 compared with those who never had COVID-19. In contrast to these findings of the negative effects of COVID-19 on the vasculature in young adults, it is notable that reports on BP have been inconsistent. Indeed, while some studies report normal BP (5, 7) others have reported elevated BP (6) following COVID-19. Although the reason for this discrepancy is unclear, one important consideration is that all these studies assessed BP only in the laboratory environment.
Although laboratory BP measurements provide valuable information on cardiovascular health, it does not capture the circadian variation and short-term fluctuations in BP that occur throughout the day (10). Moreover, laboratory BP measurements may be confounded by phenomena such as “white coat hypertension” (i.e., elevated BP only in an office/clinic setting) and “masked hypertension” (i.e., normal office/clinic BP despite elevated ambulatory/home BP; 11, 12). Thus, a more comprehensive assessment of BP using 24-h ambulatory BP is warranted to fully capture the potential impact of COVID-19 on BP and BP variability.
Another consideration is that the duration since the onset of SARS-CoV-2 infection at the time participants were studied has been variable between the studies that reported BP. Indeed, some studied individuals within 4 wk from diagnosis (5, 6), whereas others were beyond 4 wk at the time of testing (7, 9), including up to 1 year after diagnosis (13). Notably, data from previous studies indicate that central arterial stiffness, which is well-known to affect BP (14), is elevated in young adults within 4 wk from COVID-19 diagnosis (5, 6), but not when tested after 4 wk (7). Whether time from COVID-19 diagnosis similarly impacts BP is yet to be determined.
Herein, we sought to perform a comprehensive assessment of BP and BP variability using ambulatory as well as laboratory measurements in young adults who had COVID-19. In this cross-sectional investigation, we hypothesized that ambulatory BP would be elevated in those who had COVID-19 compared with those with no prior history of COVID-19 and that measures of BP would be inversely correlated with time since COVID-19 diagnosis (i.e., greater BP in those closer to their diagnosis).
METHODS
Study Population
Twenty-eight young otherwise healthy adults (11 males) who had a laboratory-confirmed diagnosis (SARS-CoV-2 RT-PCR or antigen test) of COVID-19 (COVID group) and 10 adults (4 males) without a prior history of COVID-19 (control group) were recruited and studied between March 20th and November 1st, 2021. COVID participants were studied between 2 wk and 6 mo from their diagnosis. Sixteen of the COVID participants were unvaccinated against COVID-19 mainly due to unavailability at the time, 10 participants were fully vaccinated, and two others had received their 1st dose. All except one of our control participants were vaccinated at the time of assessment. All participants were nonsmokers, were not on any prescription medications, and were free from any known cardiovascular, cerebrovascular, metabolic, or neurological diseases based on a health history questionnaire. After receiving a detailed verbal and written explanation of the experimental protocol, participants provided informed written consent. All experimental procedures conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at the University of Texas at Arlington (No. 2021-0197). Participants were instructed to abstain from caffeine and any over-the-counter medication for at least 12 h and alcohol and exercise for at least 24 h before the study visit. All participants completed the long form of the self-administered International Physical Activity Questionnaire to estimate average physical activity (15). Laboratory assessments were carried out following an overnight fast in a temperature-controlled (20°C–22°C) dimly lit room.
Experimental Protocols
Laboratory BP.
Participants were instrumented with a standard lead II electrocardiogram (model Q710, Quinton, Bothell, WA) to continuously measure heart rate (HR). Resting brachial artery BP was obtained using an automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY). Beat-to-beat arterial BP was measured via finger photoplethysmography (Finometer PRO, Finapres Medical Systems, Amsterdam, The Netherlands). Respiratory movements were monitored using a strain-gauge pneumobelt (Pneumotrace II 1132, UFI, Morro Bay, CA) around the abdomen to monitor for respiratory-driven fluctuations in BP and HR (e.g., deep breath, sigh etc.). After instrumentation, participants rested supine for at least 20 min before data collection. Then, HR, beat-to-beat BP, and respiration were recorded continuously during a 5-min resting baseline. Automated brachial artery BPs were obtained every minute. Participants were instructed to remain quiet and awake during this period.
Central BP and arterial stiffness.
For assessment of central BP, a brachial BP measurement was obtained using the Sphygmocor device and software (XCEL 1.3, Atcor Medical, Sydney, Australia), which analyzes the brachial waveform and provides an estimate of central BP (16). In addition, we also measured central arterial stiffness as carotid-femoral pulse wave velocity (PWV), as previously described (7). Briefly, a cuff was placed on the thigh, and carotid and femoral pulses were palpated at the strongest points. Measurements were made between three sites (carotid artery to sternal notch, sternal notch to thigh cuff, and femoral artery to thigh cuff). An arterial BP waveform was detected using a handheld tonometer placed over the carotid artery while the thigh cuff was inflated. PWV was calculated (XCEL 1.3, Atcor Medical, Sydney, Australia) as the carotid-femoral artery distance divided by the pulse transit time. Some of the PWV data used for this study have been previously published (7); however, the hypothesis tested and relationships examined are novel and independent from the previous study.
Ambulatory BP.
Following laboratory measurements, participants were fitted with an appropriately sized brachial cuff and Oscar 2 oscillometric ambulatory BP monitor (model 250, Sun Tech Medical, Morrisville) to wear for a continuous 24-h period. Participants were instructed to perform normal daily activities but not to perform any moderate-vigorous physical activity during the 24-h period. Measurements were obtained every 20 min during daytime and every 30 min during nighttime. Day and night periods were preprogrammed based on each individual’s expected sleep time and wake-up time for the day of the assessment (17–19). Sleep and wake-up times were also confirmed postassessment and adjusted if needed via the software, before downloading the report. Ambulatory BP was measured in all controls and 24 COVID participants.
Data Analysis
All continuous data were recorded at 1,000 Hz using PowerLab (ADInstruments, Bella Vista, Australia) and stored offline for later analysis. HR was averaged over the 5-min resting period. Brachial artery BP in the laboratory setting was measured and reported as the average of three readings. The first measurement during the 5 min baseline period was discarded to avoid potential erroneous readings at the start of data collection. Central BP was calculated as the average of two readings. Central arterial stiffness was quantified using the average of two measures of PWV that were within 0.5 m/s of each other (20). For ambulatory BP, following criteria were used to identify a satisfactory assessment per published guidelines: 1) 24-h recording with ≥70% of expected measurements, 2) ≥ 20 valid awake readings, and 3) ≥ 7 valid asleep readings (17, 19). For quantification of daytime BP, 2 h immediately after waking and immediately before bedtime were discarded from the analysis to avoid measurement artifacts during the transition period. Likewise, for quantification of nighttime BP, 1 h immediately after bedtime and immediately before waking up were removed (17, 19). Adequate number of readings for daytime and nighttime were obtained in all controls and 21 COVID participants. Average systolic BP, diastolic BP, and mean BP were quantified separately for daytime, nighttime, and 24 h. Nocturnal dip was calculated as percentage difference between average daytime and nighttime systolic BP (21). Ambulatory BP variability was quantified as standard deviation of daytime BP (SDday), nighttime BP (SDnight), 24 h SD weighted for daytime and nighttime BP variability [SDdn = ] (22), and average real variability (ARV = − |, where N denotes the number of BPs and k denotes the chronological order of the measurements; 23). Beat-to-beat BP variability was also quantified from the 5-min Finometer-derived BP measures, which were calibrated to the average of three automated sphygmomanometer readings for systolic BP, diastolic BP, and mean BP to ensure absolute values were matched. Following parameters of BP variability were then calculated: SD, coefficient of variation [CV% = ], and ARV (23).
Statistical Analysis
Normality was assessed using the Shapiro–Wilk test. All comparisons between control and COVID were made using Student’s t test for independent samples, or Mann–Whitney U test when data were not normally distributed (SPSS, version 25). To examine the relationship between time since diagnosis and the outcome variables, we performed curve-fitting analysis to determine the nature of the relationship between the independent and dependent variables and observed that the data fit both a linear model and an exponential model. However, the difference between these models were not significant and did not change the interpretation of the data. Therefore, we performed a linear regression analysis to determine the relationship between time since COVID-19 diagnosis and measures of BP, BP variability, and PWV. All data are presented as means ± SD, and the significance level was set a priori at α < 0.05.
RESULTS
Participant Characteristics
The control and COVID groups were matched for age (control, 23 ± 3 yr; COVID, 23 ± 4 yr; P = 0.757), body mass index (control, 23.0 ± 2.6 kg/m2; COVID, 24.5 ± 3.1 kg/m2; P = 0.172), and physical activity levels (control, 4,177 ± 3,624 METmin/wk; COVID, 5,080 ± 4,912 METmin/wk; P = 0.909). Resting HR was also not different between the groups (control, 58 ± 8 beats/min; COVID, 62 ± 11 beats/min; P = 0.244). For the COVID group, the mean time since diagnosis was 11 ± 6 (range: 3–22) wk. All COVID participants had mild illness (24) and none had required hospitalization. Twelve participants reported having 1–3 persistent symptoms (loss of smell and/or taste, fatigue, and muscle pain after exertion), whereas 16 reported having no symptoms at the time of testing. However, there were no differences in any of the reported experimental measures between those with and without symptoms (data not shown, P > 0.05 for all measures).
Ambulatory and Laboratory BP and Arterial Stiffness
There was no difference in ambulatory daytime or nighttime systolic BP, diastolic BP, or mean BP between control and COVID groups (Fig. 1). Similar results were obtained for the overall 24-h period (P > 0.05 for all). There was also no difference in nocturnal dipping between the two groups (control, 14 ± 5%; COVID, 14 ± 4%; P = 0.844). Similarly, there were no differences in laboratory measured brachial systolic BP, diastolic BP, or mean BP between control and COVID groups (Table 1). Last, central systolic BP (control, 100 ± 7 mmHg; COVID, 103 ± 9 mmHg; P = 0.334), diastolic BP (control, 68 ± 6 mmHg; COVID, 72 ± 7 mmHg; P = 0.168), mean BP (control, 79 ± 6 mmHg; COVID, 82 ± 8 mmHg; P = 0.207), and PWV (control, 5.5 ± 0.7 m/s; COVID, 5.4 ± 0.9 m/s; P = 0.552) were not different between groups.
Figure 1.
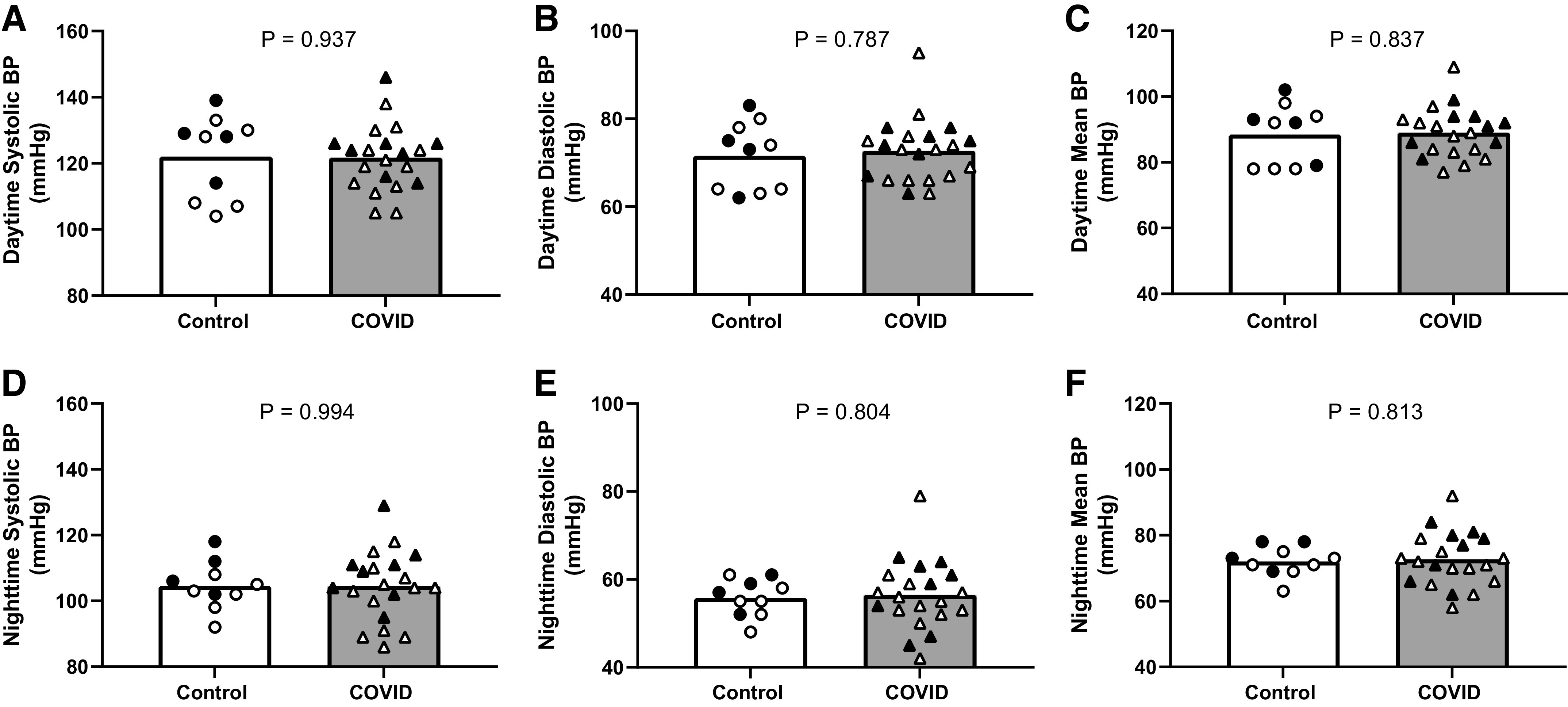
Group and individual data for ambulatory daytime (A, B, and C) and nighttime (D, E, and F) systolic blood pressure (BP), diastolic BP, and mean BP between control (white bars and circles; n = 10; 4 males) and COVID (gray bars and triangles; n = 21; 8 males). Black symbols represent males and white symbols represent females. Comparisons between groups were made using Student’s t test for independent samples.
Table 1.
Laboratory brachial blood pressure and blood pressure variability
| Parameter | Control | COVID | P Value |
|---|---|---|---|
| Systolic, mmHg | |||
| BP | 110 ± 7 | 111 ± 8 | 0.722 |
| SD | 5.0 ± 1.0 | 4.4 ± 0.9 | 0.134 |
| CV% | 4.5 ± 1.1 | 4.0 ± 0.8 | 0.116 |
| ARV | 2.1 ± 0.6 | 2.3 ± 0.7 | 0.471 |
| Diastolic, mmHg | |||
| BP | 66 ± 4 | 69 ± 6 | 0.135 |
| SD | 3.5 ± 0.8 | 3.5 ± 0.8 | 0.900 |
| CV% | 5.2 ± 1.2 | 5.1 ± 1.2 | 0.683 |
| ARVa | 1.8 ± 0.9 | 1.8 ± 0.7 | 0.590 |
| Mean, mmHg | |||
| BP | 81 ± 5 | 83 ± 6 | 0.334 |
| SDa | 3.7 ± 0.6 | 3.7 ± 0.9 | 0.732 |
| CV% | 4.6 ± 0.8 | 4.5 ± 1.1 | 0.809 |
| ARV | 1.3 ± 0.5 | 1.3 ± 0.3 | 0.977 |
Values are means ± SD. ARV, average real variability; BP, blood pressure; CV, coefficient of variation; SD, standard deviation. Independent sample t tests were used to compare between control (n = 10; 4 males) and COVID (n = 28; 11 males) groups. aNon-normalized data were analyzed using Mann-Whitney U test.
Relationships with Time since Diagnosis
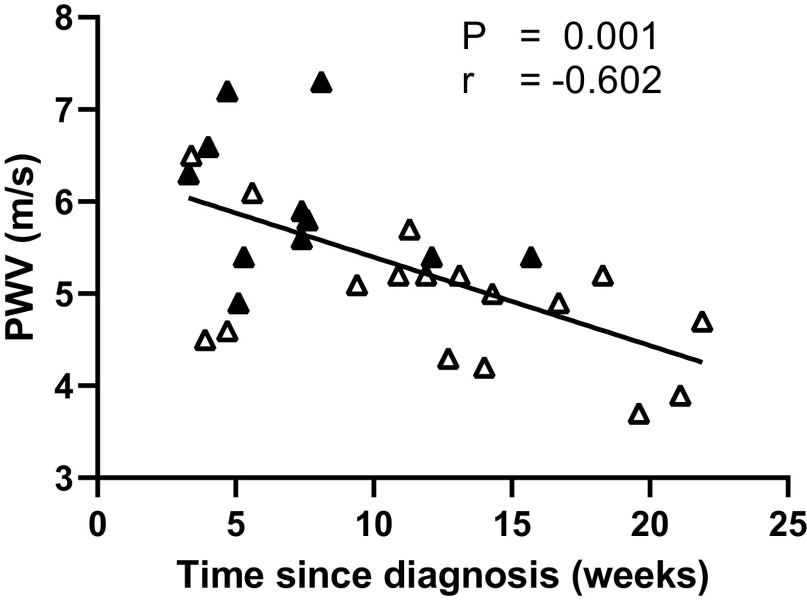
Ambulatory daytime and nighttime systolic BP, diastolic BP, and mean BP were inversely related with time since COVID-19 diagnosis (Fig. 2). Similar results were observed for 24-h systolic BP (r = −0.516; P = 0.017), diastolic BP (r = −0.574; P = 0.006), and mean BP (r = −0.592; P = 0.005). Likewise, laboratory brachial systolic BP (r = −0.474; P = 0.011), diastolic BP (r = −0.449; P = 0.017), and mean BP (r = −0.462; P = 0.013), were inversely correlated with time since diagnosis, whereas no relationships were found for central systolic, diastolic, or mean BP (all P > 0.05). PWV was also inversely related with time since diagnosis (Fig. 3). This relationship remained after accounting for the potential influence of laboratory measured BP on PWV (r = 0.699, P < 0.001). Both time since diagnosis (P = 0.016) and mean BP (P = 0.020) were significant determinants of PWV.
Figure 2.
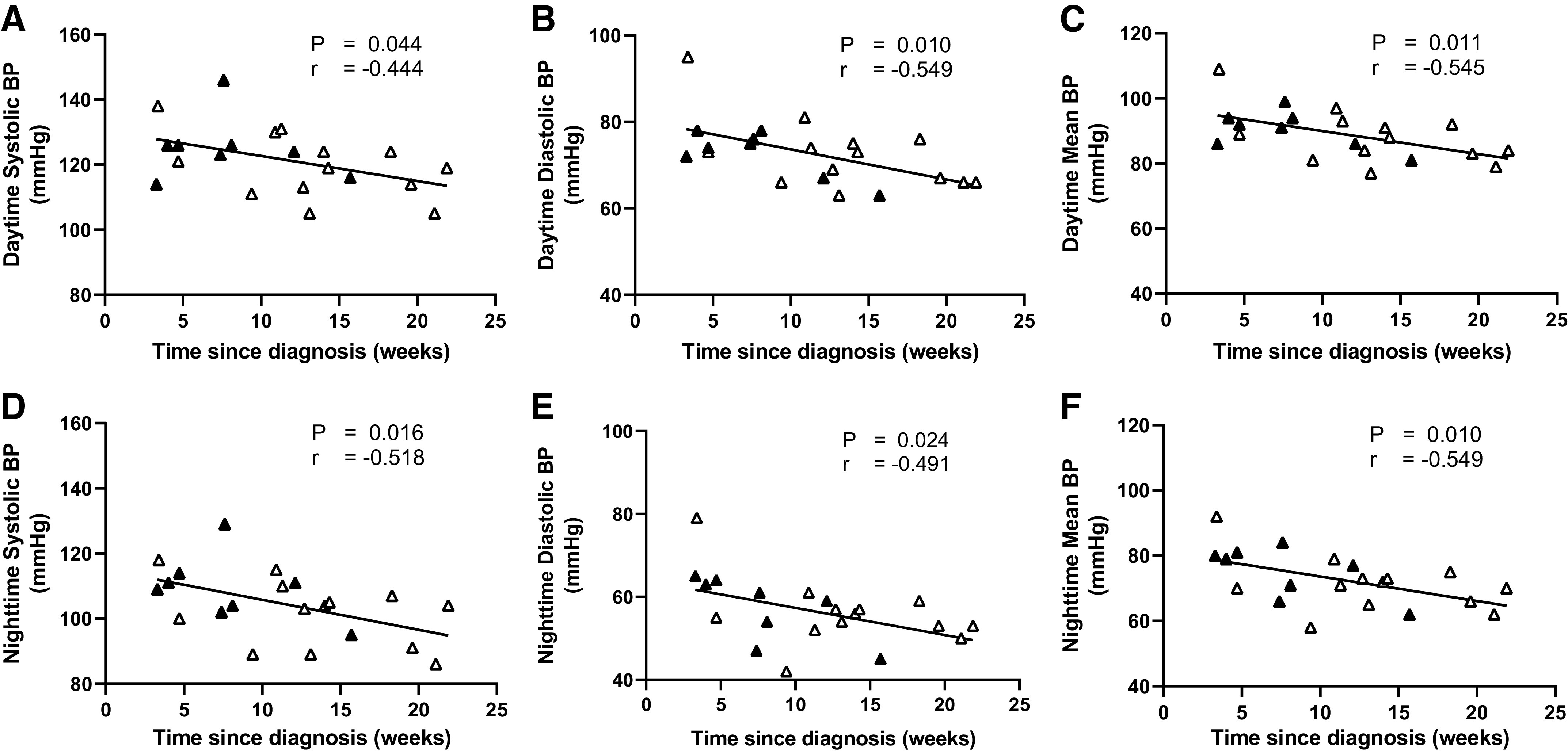
Relationship between time since diagnosis and ambulatory daytime (A, B, and C) and nighttime (D, E, and F) systolic blood pressure (BP), diastolic BP, and mean BP in the COVID group (n = 21; 8 males). Black symbols represent males and white symbols represent females. r = correlation coefficient.
Figure 3.
Relationship between time since diagnosis and carotid-femoral pulse wave velocity (PWV) in the COVID group (n = 28; 11 males). Black symbols represent males and white symbols represent females. r = correlation coefficient.
Ambulatory and Beat-to-Beat BP Variability
There was no difference in ambulatory BP variability for systolic BP, diastolic BP, or mean BP (i.e., SDday, SDnight, SDdn, and ARV) between control and COVID groups (Table 2). Likewise, beat-to-beat BP variability measurements were not different between the control and COVID groups (Table 1). There were also no significant relationships between time since COVID-19 diagnosis and ambulatory or beat-to-beat BP variability with the exception of CV% for beat-to-beat diastolic BP variability (Table 3).
Table 2.
Ambulatory blood pressure variability
| Parameter | Control | COVID | P Value |
|---|---|---|---|
| Systolic BP, mmHg | |||
| SDday | 9.3 ± 2.5 | 10.4 ± 2.0 | 0.175 |
| SDnight | 8.3 ± 2.9 | 8.8 ± 3.0 | 0.615 |
| SDdn | 9.0 ± 2.1 | 9.8 ± 1.7 | 0.228 |
| ARVa | 9.0 ± 1.6 | 9.6 ± 1.6 | 0.201 |
| Diastolic BP, mmHg | |||
| SDdaya | 8.1 ± 1.5 | 9.0 ± 2.5 | 0.441 |
| SDnight | 6.7 ± 1.9 | 6.6 ± 2.2 | 0.905 |
| SDdn | 7.6 ± 1.2 | 8.2 ± 2.0 | 0.414 |
| ARV | 7.4 ± 1.0 | 7.8 ± 1.5 | 0.428 |
| Mean BP, mmHg | |||
| SDday | 7.7 ± 1.8 | 8.7 ± 2.3 | 0.244 |
| SDnight | 6.8 ± 1.9 | 6.6 ± 2.4 | 0.803 |
| SDdn | 7.4 ± 1.4 | 7.9 ± 1.9 | 0.456 |
| ARV | 7.2 ± 0.9 | 7.6 ± 1.4 | 0.363 |
Values are means ± SD. ARV, average real variability; BP, blood pressure; SDday, daytime standard deviation; SDdn, 24-h standard deviation weighted for daytime and nighttime variability; SDnight, nighttime standard deviation. Independent sample t tests were used to compare between control (n = 10; 4 males) and COVID (n = 21; 8 males) groups. aNon-normalized data were analyzed using Mann-Whitney U test.
Table 3.
Relationship between time since COVID-19 diagnosis and blood pressure variability
| Parameter | Ambulatory BP Variability |
Beat-to-Beat BP Variability |
||||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | |||
| Systolic BP, mmHg | SDday | −0.212 | 0.357 | SD | 0.025 | 0.901 |
| SDnight | −0.143 | 0.537 | CV% | 0.205 | 0.295 | |
| SDdn | −0.240 | 0.295 | ARV | 0.058 | 0.771 | |
| ARV | −0.109 | 0.639 | ||||
| Diastolic BP, mmHg | SDday | 0.020 | 0.932 | SD | 0.298 | 0.124 |
| SDnight | −0.265 | 0.246 | CV% | 0.460 | 0.014 | |
| SDdn | −0.095 | 0.681 | ARV | 0.343 | 0.074 | |
| ARV | 0.050 | 0.828 | ||||
| Mean BP, mmHg | SDday | 0.020 | 0.931 | SD | 0.119 | 0.548 |
| SDnight | −0.181 | 0.433 | CV% | 0.262 | 0.178 | |
| SDdn | −0.094 | 0.686 | ARV | 0.095 | 0.632 | |
| ARV | 0.004 | 0.986 | ||||
n = 21 (8 males) for ambulatory BP variability and n = 28 (11 males) for beat-to-beat BP variability. ARV, average real variability; BP, blood pressure; CV, coefficient of variation; r, correlation coefficient; SD, standard deviation; SDday, daytime standard deviation; SDdn, 24 h standard deviation weighted for daytime and nighttime variability; SDnight, nighttime standard deviation.
DISCUSSION
To our knowledge, this was the first study to comprehensively investigate the effects of COVID-19 on ambulatory BP and BP variability in young adults. Contrary to our hypothesis, ambulatory BP was not different between young adults who had COVID-19 and controls who never had COVID-19. However, we found that ambulatory daytime, nighttime, and 24-h BP as well as laboratory brachial BP were inversely correlated with time since COVID-19 diagnosis, with higher BP presenting closer to the onset of infection. Interestingly, a similar inverse relationship was observed between time since diagnosis and central arterial stiffness. In addition, we show that COVID-19 does not adversely impact ambulatory or laboratory BP variability in young adults, and no relationship to time since COVID-19 diagnosis was found when studying individuals within 6 mo from diagnosis. Collectively, these data suggest that COVID-19 does not have major effects on BP in young adults; however, transient effects of COVID-19 to increase BP and central arterial stiffness closer to diagnosis may be present.
We (7) and others (5, 13) have previously reported that brachial artery BP measured in the laboratory setting is not different between young adults who had COVID-19 and those who never had COVID-19. Although measuring BP in the laboratory setting is conventional and provides important information, it does not allow for the measurement of BP over an extended time period during regular daily activities, nor does it consider nocturnal dipping, the presence of whitecoat hypertension or masked hypertension. Indeed, elevated daytime, nighttime, and 24-h ambulatory BP as well as having either reduced or exaggerated nocturnal dipping are strong independent predictors of adverse cardiovascular outcomes (25, 26). In the current study, we found that ambulatory daytime, nighttime, 24-h BP, and nocturnal dipping were not different between young adults who are within 6 mo from diagnosis and those without a history of COVID-19. Ambulatory and beat-to-beat BP variability, which are known to offer independent prognostic information on cardiovascular outcomes, (23, 27) were also unaffected by COVID-19. For our study, we recruited control participants during the pandemic since that would ensure better matching of participants with regards to any lifestyle changes that may have been unavoidable during this period. One caveat to this is that it is impossible to know whether some of the control participants may have had asymptomatic infection. Nevertheless, given the growing research indicating an elevated cardiovascular disease risk associated with COVID-19 (3, 28), these negative findings are promising in that we found no major effects on BP or BP variability in young adults who had COVID-19. Nevertheless, comprehensive studies of BP in those who are older, have more severe acute illness, and those with persistent sequalae from COVID-19 are warranted.
Our findings also indicated that central BP was not elevated following COVID-19. This is in contrast to findings by Szeghy et al. (6) who reported that central systolic BP and mean BP were elevated in young adults who had COVID-19 compared with controls. Notably, a key difference is that all participants (n = 15) in the study by Szeghy et al. (6) were within 4 wk from diagnosis, whereas only four participants in the current study were within that time frame at the time of testing. Hence, the difference in the timing since infection may have contributed to the divergent findings between studies. Indeed, in line with this hypothesis, in the current study, we found that ambulatory and laboratory brachial BP were inversely correlated with time since diagnosis, with individuals who were closer to COVID-19 diagnosis presenting with greater BP values compared with those further away from diagnosis up to 6 mo. Likewise, arterial stiffness was also shown to be inversely related to time since diagnosis. These findings are in agreement with the findings of a recent follow up study by Szeghy et al. (29), which showed improvement in carotid-femoral PWV over the first 6 mo from diagnosis in young otherwise healthy adults who had COVID-19. Although no significant relationship with time since diagnosis was found for central BP in the current study, Szeghy et al. (29) reported an improvement in central systolic BP and mean BP at 6 mo compared with 1 mo after diagnosis. Collectively, these findings provide evidence for a potential transient impact of COVID-19 on indices of cardiovascular health in young adults.
Data from previous studies lend some insight into factors that may contribute to a transient elevation in BP. For example, studies have demonstrated that during the early phase of the illness, young adults who had COVID-19 exhibit impaired vasodilation (5) and the potential for increased vasoconstriction with elevated sympathetic nervous system activity (9). Central artery stiffness and central BP have also been shown to be higher within 3–4 wk from the COVID-19 diagnosis compared with controls (5). Findings from studies that included individuals further out from diagnosis suggests no elevation in arterial stiffness (7), and no impairment in vascular function (13) or impairment in only those with persistent symptoms (7). Although the temporal relationship between vascular dysfunction, elevated arterial stiffness, and elevated BP is a topic of some debate, it has been suggested that increased peripheral vascular resistance due to alterations in smaller arteries (i.e., impaired vasodilation and increased vasoconstriction) leads to elevated brachial BP, which causes greater large artery stiffness (30, 31), followed by a rise in central BP. Increased central BP is then thought to contribute to structural changes in the smaller arteries, which again leads to a rise in brachial BP (30, 31). Although previous studies suggest that central BP is elevated within 4 wk from COVID-19 diagnosis (6), our data indicates that the effects of COVID-19 on brachial BP and arterial stiffness in young adults are likely not sufficient to cause a significant impact on central BP, nor cause permanent structural alterations on the vasculature.
Perspectives and Significance
Despite previous studies reporting negative effects of COVID-19 on the peripheral vasculature and autonomic function in young adults, it is encouraging to see that the impact of COVID-19 on BP, a primary risk factor for the development of cardiovascular disease, is likely minimal and not persistent in this age group. Indeed, this is important because to date, nearly 15 million young adults (i.e., ∼ 30% of those between 18 and 29 yr) have been affected by COVID-19 in the United States alone (32). Given that SARS-CoV-2 virus is continuously changing and evolving into new variants with varying virulence, investigating the long-term effects of COVID-19 becomes complex and challenging. However, longitudinal studies are necessary to fully understand the risk of future cardiovascular disease with COVID-19. In contrast to our findings in young adults, several studies have reported new-onset hypertension post COVID-19 in older adults and those with preexisting comorbidities (33–35). Whether this is a permanent outcome of COVID-19 in this population is still unknown and warrants further investigation. Moreover, the exact mechanisms that trigger negative vascular alterations following COVID-19 are still unclear. However, two plausible mechanisms are direct vascular inflammation (36, 37) and imbalance in the renin-angiotensin-aldosterone system as a result of viral binding and downregulation of angiotensin-converting enzyme-2 receptors (38). Additional studies investigating the role of these potential mechanisms in causing adverse cardiovascular outcomes are needed to better understand the short and long-term influence of COVID-19 on overall cardiovascular health.
GRANTS
This work was supported by The University of Texas at Arlington College of Nursing and Health Innovation. Both D.N. (827597/2021) and B.Y.S. (20PRE34990010) are supported by American Heart Association predoctoral fellowships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.N. and P.J.F. conceived and designed research; D.N., R.J.S., A.-K.G., B.Y.S., and B.E.Y. performed experiments; D.N. analyzed data; D.N., R.J.S., A.-K.G., B.Y.S., B.E.Y., and P.J.F. interpreted results of experiments; D.N. prepared figures; D.N. drafted manuscript; D.N., R.J.S., A.-K.G., B.Y.S., B.E.Y., and P.J.F. edited and revised manuscript; D.N., R.J.S., A.-K.G., B.Y.S., B.E.Y., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the participants for volunteering their time for the study.
REFERENCES
- 1.Centers for Disease Control and Prevention. COVID Data Tracker (Online). US Department of Health and Human Services. https://covid.cdc.gov/covid-data-tracker/#datatracker-home[2022 Apr 10]. [Google Scholar]
- 2.Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 371: 741–745, 2021. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 28: 583–590, 2022. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. COVID Data Tracker (Online). US Department of Health and Human Services. https://covid.cdc.gov/covid-data-tracker/#demographics[2022 Apr 10].
- 5.Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/AJPHEART.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szeghy RE, Province VM, Stute NL, Augenreich MA, Koontz LK, Stickford JL, Stickford ASL, Ratchford SM. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp Physiol 1–14, 2021. doi: 10.1113/EP089481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nandadeva D, Young BE, Stephens BY, Grotle A-K, Skow RJ, Middleton AJ, Haseltine FP, Fadel PJ. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am J Physiol Heart Circ Physiol 321: H479–H484, 2021. doi: 10.1152/ajpheart.00368.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jud P, Kessler HH, Brodmann M. Case report: changes of vascular reactivity and arterial stiffness in a patient with Covid-19 infection. Front Cardiovasc Med 8: 671669, 2021. doi: 10.3389/fcvm.2021.671669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stute NL, Stickford JL, Province VM, Augenreich MA, Ratchford SM, Stickford ASL. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol 599: 4269–4285, 2021. doi: 10.1113/JP281888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 53: 96–104, 1983. doi: 10.1161/01.RES.53.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G, Verdecchia P. Clinical value of ambulatory blood pressure: evidence and limits. Circ Res 116: 1034–1045, 2015. doi: 10.1161/CIRCRESAHA.116.303755. [DOI] [PubMed] [Google Scholar]
- 12.Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ 29: 102–111, 2020. doi: 10.1016/j.hlc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Dillon GA, Wolf ST, Alexander LM. Nitric oxide-mediated cutaneous microvascular function is not altered in young adults following mild-to-moderate SARS CoV-2 infection. Am J Physiol Heart Circ Physiol 322: H319–H327, 2022. doi: 10.1152/ajpheart.00602.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 9: 755–762, 2006. doi: 10.1079/PHN2005898. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 16: 2079–2084, 1998. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension 62: 988–994, 2013. doi: 10.1161/HYPERTENSIONAHA.113.02148. [DOI] [PubMed] [Google Scholar]
- 18.JCS Joint Working Group. Guidelines for the clinical use of 24 hour ambulatory blood pressure monitoring (ABPM) (JCS 2010) - digest version. Circ J 76: 508–519, 2012. doi: 10.1253/circj.CJ-88-0020. [DOI] [PubMed] [Google Scholar]
- 19.Parati G, Stergiou G, O'Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion J-M, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32: 1359–1366, 2014. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 20.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomfield D, Park A. Night time blood pressure dip. World J Cardiol 7: 373–376, 2015. doi: 10.4330/WJC.V7.I7.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, Mancia G, Parati G. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens 25: 2058–2066, 2007. doi: 10.1097/HJH.0b013e32829c6a60. [DOI] [PubMed] [Google Scholar]
- 23.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 23: 505–511, 2005. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 24.NIH. Clinical Spectrum|COVID-19 Treatment Guidelines (Online). https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/[2022 Apr 10].
- 25.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 378: 1509–1520, 2018. doi: 10.1056/NEJMoa1712231. [DOI] [PubMed] [Google Scholar]
- 26.Yang W-Y, Melgarejo JD, Thijs L, Zhang Z-Y, Boggia J, Wei F-F, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, Dolan E, Stolarz-Skrzypek K, Malyutina S, Casiglia E, Lind L, Filipovský J, Maestre GE, Li Y, Wang J-G, Imai Y, Kawecka-Jaszcz K, Sandoya E, Narkiewicz K, O'Brien E, Verhamme P, Staessen JA; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 322: 409–420, 2019. doi: 10.1001/jama.2019.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Wang JG, Staessen JA. Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated Chinese. Hypertension 63: 790–796, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02681. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Salazar B, Holwerda M, Stüdle C, Piragyte I, Mercader N, Engelhardt B, Rieben R, Döring Y. COVID-19 and the vasculature: current aspects and long-term consequences. Front Cell Dev Biol 10: 824851, 2022. doi: 10.3389/fcell.2022.824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeghy RE, Stute NL, Province VM, Augenreich MA, Stickford JL, Stickford ASL, Ratchford SM. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J Appl Physiol (1985) 132: 1297–1309, 2022. doi: 10.1152/japplphysiol.00793.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, Regnault V; French Study Group on Arterial Stiffness. Interaction between hypertension and arterial stiffness an expert reappraisal. Hypertension 72: 796–805, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11212. [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res 116: 1007–1021, 2015. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 32.Elflein J. Total Number of COVID-19 Cases by Age U.S. 2022 (Online). Statista. https://www.statista.com/statistics/1254271/us-total-number-of-covid-cases-by-age-group/[2022 Apr 11]. [Google Scholar]
- 33.Chen G, Li X, Gong Z, Xia H, Wang Y, Wang X, Huang Y, Barajas-Martinez H, Hu D. Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS One 16: e0250815, 2021. doi: 10.1371/journal.pone.0250815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akpek M. Does COVID-19 cause hypertension? Angiology, 2021. doi: 10.1177/00033197211053903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasim D, Alme B, Jordal S, Lind Eagan TM, Tadic M, Mancia G, Guttormsen AB, Saeed S. Characteristics of 24-hour ambulatory blood pressure monitoring in a COVID-19 survivor. Future Cardiol 17: 1321–1326, 2021. doi: 10.2217/fca-2020-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells 9: 1652, 2020. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]