Keywords: autonomic, heart rate variability, heat therapy, stress, sympathetic
Abstract
Acute whole body heat stress evokes sympathetic activation. However, the chronic effects of repeated moderate heat exposure (RMHE) on muscle sympathetic nerve activity (MSNA) in healthy individuals remain unclear. We performed RMHE with 4 wk (5 days/wk) of warm baths (∼40°C, for 30 min) in nine healthy older (59 ± 2 yr) volunteers. Hemodynamic variables and MSNA were examined before, 1 day after, and 1 wk following 4 wk of RMHE in a laboratory at ∼23°C. Cold pressor test (CPT) and handgrip (HG) exercise were performed during the tests. Under normothermic condition, the resting MSNA burst rate (prior, post, post 1-wk: 31.6 ± 2.0, 25.2 ± 2.0, and 27.7 ± 1.7 bursts/min; P < 0.001) and burst incidence (P < 0.001) significantly decreased after RMHE. Moreover, the resting heart rate significantly decreased after RMHE (62 ± 2, 60 ± 2, and 58 ± 2 beats/min, P = 0.031). The sensitivity of baroreflex control of MSNA and heart rate were not altered by RMHE, although the operating points were reset. The MSNA and hemodynamic responses (i.e., changes) to handgrip exercise or cold pressor test were not significantly altered. These data suggest that the RMHE evoked by warm baths decreases resting sympathetic activity and heart rate, which can be considered beneficial effects. The mechanism(s) should be examined in future studies.
NEW & NOTEWORTHY To our knowledge, this is the first study to observe the effects of repeated warm baths on sympathetic nerve activity during rest and stress in healthy middle age and older individuals. The data suggest that the repeated warm baths decreased resting sympathetic activity and heart rate, which can be considered beneficial effects. This study also provides the first evidence that the repeated warm baths did not alter the baroreflex sensitivity and the sympathetic responses to stress.
INTRODUCTION
The sympathetic nervous system plays a pivotal role in mediating acute cardiovascular responses to stress. Prior studies suggest that acute whole body heat stress (i.e., increases in core temperature Tcore > 0.5°C) evokes significant increases in muscle sympathetic nerve activity (MSNA) in young (1, 2) and older healthy (3, 4) individuals. A prior study (4) showed that MSNA in patients with chronic heart failure decreased significantly from baseline values in the early period of whole body heating when the increase in Tcore was less than Δ0.3°C.
Our prior report showed that resting MSNA in healthy subjects in summer is lower than in winter (5). Although other factors (e.g., diet and daily physical activity, etc.) could contribute to seasonal effects, we speculate that environmental temperature could play a key role in causing the decrease in resting MSNA. Prior studies have suggested beneficial effects of one-time acute heat exposure (6–8) and repeated heat exposures (9–11) in healthy individuals and in patients with cardiovascular diseases. Moreover, a recent study showed that “heat therapy” of 30 1-h hot tub sessions over 8–10 wk decreased resting MSNA in women with polycystic ovary syndrome (12). However, it is unknown if repeated heat exposure can decrease resting sympathetic activity in healthy middle age and older individuals. Both, acute exercise (13) and acute whole body heat exposure induce increases in heart rate (HR) (14), although the mechanisms for the HR increase in the two interventions may differ. It is well known that exercise training reduces resting HR (15). Whether repeated increases in HR induced by repeated whole body heat exposure also result in a reduction in resting HR in healthy middle age and older individuals, has not been examined.
Maintenance of homeostasis is a central function of the autonomic nervous system. The baroreflex plays a key role in maintaining blood pressure (BP). Under resting conditions, there is a close inverse relationship between MSNA bursts and diastolic BP (16). A recent study (12) showed that repeated heat exposure decreased MSNA as well as BP in women with polycystic ovary syndrome. Whether baroreflex function is altered in healthy middle age and older individuals by repeated heat exposure is unclear. To address this issue, it is necessary to examine the effects of repeated heat exposure on baroreflex control of MSNA and HR. Sympathetic activation in response to stress (e.g., exercise) serves to support appropriate blood flow delivery to the relevant end organs. However, altered sympathetic reactivity in response to stress may differ from changes in basal sympathetic activity. To our knowledge, the effects of repeated heat exposure on the sympathetic responses to various stressors have not been examined.
In this study, we hypothesized that repeated moderate heat exposure (RMHE) by repeated warm water baths would decrease resting MSNA and HR in middle age and older subjects. We further hypothesized that such an effect might be due to altered baroreflex sensitivity. We therefore examined the effects of RMHE on baroreflex function and sympathetic responses to stressors such as handgrip (HG) exercise and the cold pressor test (CPT).
METHODS
Subjects
Nineteen healthy subjects (15 males, 4 females) participated in this study. The average age was 59 ± 2 yr with a height of 176 ± 2 cm and a weight of 86 ± 4 kg [body mass index (BMI) 28 ± 1 kg/m2]. Volunteers were recruited from local communities in central Pennsylvania. Inclusion criteria for the studies in this report were as follows: 1) healthy women and men aged 45–75 yr; 2) any race or ethnicity; 3) unremarkable history and physical examination, which were performed during a screening procedure; 4) absence of acute medical conditions; and 5) not currently taking any medications. Subjects with any chronic medical conditions were excluded. The experimental protocol was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. Each subject had the purposes and risks of the protocol explained to them before written informed consent was obtained.
Measurements
As described in our previous reports (4), in the tests in the laboratory, systolic (SBP), diastolic (DBP), and mean arterial pressure (MAP) during resting conditions were measured with an automated sphygmomanometer from the brachial artery (SureSigns VS3, Philips, Philip Medical System). Beat-by-beat BP was recorded from a finger of the nonexercising arm (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands) with resting values verified with the upper arm cuff pressure. HR was monitored from the electrocardiogram (Cardicap/5, Datex-Ohmeda, GE Healthcare, NJ). Respiratory trace was monitored using piezoelectric pneumography.
Multifiber recordings of MSNA were obtained with a tungsten microelectrode inserted in the peroneal nerve. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The signal was amplified, filtered with a bandwidth of 500–5,000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA). The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (17). The nerve signal was routed to a computer screen and a loudspeaker for monitoring throughout the study.
RMHE Paradigm
The RMHE studies were performed in nine subjects (8 males, 1 female, 59 ± 2 yr, 176 ± 3 cm, 89 ± 7 kg) during spring (subject number n = 3), summer (n = 2), fall (n = 2), and winter (n = 2), respectively. Subjects took a warm bath for ∼30 min a day, 5 days a week, for 4 wk (20 sessions total). This paradigm was a modification of the approach used by Hooper (18). Subjects were in the recumbent position in a bathtub with water up to their mid chest while their arms were positioned outside of the bathtub. Water temperature was monitored with a temperature data logger (Supco, SL500XT, Temperature Data Logger, MicroDAQ, Ltd.). The initial water temperature was ∼41°C (<42°C). When water temperature dropped close to 39°C, warm water was added to the bathtub to raise the temperature back to 41°C. This procedure was repeated as necessary two to three times during the 30-min bath. Our pilot study showed that the increase in core temperature with this bathing protocol was Δ0.9 ± 0.1°C. For the subjects’ safety, BP and HR were monitored by an automated sphygmomanometer BP monitor (Omron BP791IT) (19) every 2 min during the baths.
The 1st, 11th, and the last baths for each subject were performed in the laboratory. Subjects were instructed to operate the BP and temperature monitors and how to properly control the water temperature within the target range during the baths in the laboratory. The other baths were performed in the subjects’ homes. The time and date of the 5 baths/wk during the 4 wk depended on subjects’ schedule. However, consecutive nonbath days were limited to less than 3 days (i.e., 1 or 2 days). The temperature data logger recorded the date and time when each of the sessions began and ended and the curve of water temperature during the baths performed in the subjects’ homes. The last bath was performed in the laboratory in the morning that was ∼23 h before the post-RMHE tests in all subjects. Subjects were asked to not take any warm bath between “post-RMHE” and “post-1wk.” Investigators verified the conditions (e.g., water temperature, date and time, BP and HR, etc.) of all baths at the subjects’ home via checking the data logger. During the return visits “post-RMHE” and “post-1wk,” subjects were questioned regarding any significant changes in their health (e.g., sick days, etc.) and daily life (e.g., appetite, diet, exercise routine, etc.) during the study period.
Nonheating Bath Trial
The paradigm was the same as the warm bath trial except that the water temperature was maintained at ∼34°C–35°C (20). In our pilot study, a 30-min water bath at ∼34°C–35°C did not increase core temperature. The 4-wk nonheating bath trial was performed on two of the nine subjects who performed the warm bath trial. The interval between the nonheating bath trial and the warm bath trial was >3 mo.
Nonheating Time Control
Time control studies were performed in 13 subjects (10 males, 3 females) during spring (subject number n = 4), summer (n = 3), fall (n = 3), and winter (n = 3), respectively. The control subjects were matched for age (59 ± 2 vs. 61 ± 3 yr, P = 0.588) and BMI (28 ± 2 vs. 27 ± 1 kg/m2, P = 0.568) with the subjects in RMHE group. The period between the first and the second visit was 28–36 days (32 ± 1 days). Subjects in the nonheating time control trial did not perform RMHE between visits 1 and 2. Recordings from eight of 13 subjects in the time control group were included in the data analysis in our previous reports. However, the hemodynamic variables in the two visits in these eight subjects were from two separate studies and were not used and reported together in any prior report. Moreover, the data on baroreflex sensitivity from all subjects in the control group were not analyzed and reported previously. Time control studies (no intervention) in a control group is an established model in prior reports with similar interventions (12, 21).
Experimental Protocols
For the RMHE trial and the nonheating bath trial, all protocols in this study were performed in three laboratory visits: 1) within 1 wk before the start of RMHE (pre-RMHE), 2) 1 day after 4 wk of RMHE (post-RMHE), and 3) 1 wk after the end of RMHE (post-1wk). For personal reasons, one of the nine subjects did not complete a post-1wk visit.
Subjects refrained from caffeine and alcohol for 24 h before the testing. To reduce any potential diurnal variations in the measurements, all testing was performed in the morning. All procedures were conducted in a room with an ambient temperature of ∼23°C. In each of the three visits, maximal force generated from a voluntary handgrip contraction (MVC) was determined upon repeated (i.e., ∼3) isometric contractions from the nondominant arm using a handgrip dynamometer.
Resting BP, Cardiac Output, HR, and MSNA
At first, automated sphygmomanometer BPs were obtained two or three times in the supine position. Prior to microneurography, transthoracic echocardiography/Doppler was performed using a Vivid 7 system (General Electric Healthcare) with an M4S probe using well-established procedures (22). Left ventricular (LV) stroke volume was determined from measurement of the LV outflow tract diameter (parasternal long-axis view) to provide cross-sectional area below the aortic annulus (½ diameter2 × π; in cm2), and the time-velocity-integral within the LV outflow tract (TVI, apical 5-chamber view; in cm) to calculate stroke volume as cross-sectional area × TVI (in cm3 or mL). Cardiac output (L/min) was then calculated as LV stroke volume × the corresponding HR. All echocardiographic measurements were averaged over three to five cardiac cycles. Total peripheral resistance (TPR) was estimated as MAP/cardiac output.
After a brief break, microneurography was performed. Then, subjects were asked to keep quiet for an acclimation period (5–10 min) until the hemodynamic variables and MSNA were stable. Subsequently, all variables were continuously recorded for 6 min with the subjects at rest. Automated sphygmomanometer BPs were obtained two or three times during this period. Recordings of the electrocardiogram, beat-by-beat BP, and MSNA were used to assess spontaneous baroreflex function.
Baroreflex Function with Modified Oxford Technique
After recording for baseline, baroreflex modulation of MSNA and HR were assessed via the modified Oxford technique as previously described (23). In brief, 100 µg of sodium nitroprusside was administered intravenously via a catheter, followed ∼60 s later by 150 µg of phenylephrine HCl. These drugs induce a decrease, followed by an increase, in BP. This protocol was repeated three times during each visit, separated by >10-min intervals. This duration was sufficient for arterial BP, HR, and MSNA to return to the baseline levels. The gain of baroreflex control of MSNA from these trials was averaged. The Oxford procedure was performed during the three visits of the RMHE trial on seven subjects.
Responses to Stress
After a resting period, 3 min of baseline data was collected. Then, each subject performed static isometric handgrip at 30% MVC to fatigue followed by 2-min postexercise circulatory occlusion (PECO). A visual force indicator was used so that subjects could maintain the force necessary for 30% MVC. When fatigue (i.e., extremely hard) was reported, before the subject stopped gripping a cuff on the upper arm was inflated up to 250 mmHg for 2 min.
After another resting period (>20 min), 3 min of baseline data was collected. Then, a cold pressor test (CPT) was performed by immersing one hand in ice water for 2 min. The order for the handgrip and CPT was random between the subjects, but the same order was maintained for the three visits within each subject. Between handgrip and CPT, subjects rested for at least 20 min and until HR and BP returned to baseline.
Vascular function was also assessed with ultrasound in this project. Because of space limitations in one single report, these data will be presented separately. The vascular function tests were performed before performing microneurography. After the test, subjects were released from the testing table and had a brief break. We believe these tests should have minimal effects on the other testing protocols described in this report.
For the nonheating time control studies, MSNA and hemodynamic variables were recorded under resting conditions for 6 min. Thereafter, handgrip exercise with PECO was performed as described earlier. These procedures were performed before and after a period of 28–36 days (32 ± 1 days).
Data Analysis
Data were sampled at 200 Hz via a data acquisition system (MacLab, ADInstruments, Castle Hill, Australia). MSNA bursts were evaluated by a computer program that identified bursts based upon fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (4). Integrated MSNA was normalized by assigning a value of 100 to the mean amplitude of the largest 10% of the bursts during the 6-min baseline period (24). Normalization of the MSNA signal was performed to reduce variability between subjects attributed to factors including needle placement. Total MSNA was identified from burst area of the integrated neurogram. The beat-by-beat HR, RR interval (RRI), SBP, DBP, MAP, and MSNA were calculated simultaneously using a computer program (4). Mean values of MSNA data were reported as burst rate (bursts/min), burst incidence (bursts/100 heart beats), and total activity (total burst area/min).
Baroreflex Sensitivity
As described in prior reports (2, 4, 25), spontaneous baroreflex control of MSNA (burst incidence, burst area, and total activity) was evaluated by analyzing the relationship between beat-by-beat spontaneous variations in DBP and MSNA. In brief, DBP for all of the cardiac cycles were grouped into 3 mmHg intervals (bins). For a given 3 mmHg DBP bin, “burst incidence” (i.e., 100 × the MSNA bursts number/the cardiac cycle number), “burst area” (i.e., total burst area/the MSNA bursts number), and “total activity” (i.e., total burst area/the cardiac cycle number) were calculated, respectively. With all binned data, the slopes of the relationship between mean DBP and “burst incidence,” “burst area,” and “total MSNA,” respectively, were identified using linear regression analyses. The regression data were accepted when the correlation coefficient (R) between DBP and MSNA burst incidence was higher than 0.7. For all linear regression analyses, the data were weighted for the number of cardiac cycles for each DBP bin. These slopes were used as indices of MSNA baroreflex sensitivity (BRS). The MSNA BRS during the Oxford procedure was calculated with the same approach.
As described in prior reports (4, 26–28), cardiac baroreflex control was estimated with the sequence technique. The beat-by-beat time series of SBP and RRI during the resting baseline were analyzed using CardioSeries software v2.7 (29). Briefly, sequences of three or more consecutive beats where SBP and RRI changed in the same direction were identified as arterial baroreflex sequences (29). A linear regression was applied to each individual sequence, and only those sequences in which R2 > 0.80 were accepted. The slopes of these sequences were calculated as a measure of spontaneous cardiac BRS.
Responses to Stress
The mean values over the 3-min resting baseline before handgrip, the last minute of handgrip, and the last 1.5 min of PECO were calculated for statistical analyses. Similarly, the mean values over the 3-min resting baseline and the 2-min CPT were calculated for statistical analyses.
Statistical analyses were performed using commercially available software (SigmaPlot 14.0, Systat Software Inc.). The effects of the time of RMHE (i.e., pre-RMHE, post-RMHE, post-1wk) on the resting hemodynamic variables, MSNA, and BRS were evaluated via a one-way repeated-measures ANOVA, followed by Dunnett’s post hoc analyses for multiple comparisons where appropriate. The effects of the RMHE (factor 1: pre-RMHE, post-RMHE, post-1wk) on the variables during the stages of the intervention (factor 2: e.g., resting baseline, handgrip, PECO) were evaluated via a two-way repeated-measures ANOVA, followed by Dunnett’s post hoc analyses for multiple comparison where appropriate. The responses to the stressors (i.e., the changes) during the three visits were also evaluated via a one-way repeated-measures ANOVA. Data distribution in the aforementioned analysis was examined via Shapiro–Wilk test. Most of the variables were normally distributed. When the normality test failed (e.g., HR data in handgrip trial and CPT trial), a two-way repeated-measures ANOVA was performed. In the time control group, the variables before and after the time control were compared with paired t tests. All values are reported as means ± SE. P values <0.05 were considered statistically significant.
RESULTS
Effects of RMHE on Resting Hemodynamic Variables and MSNA
All subjects performed the 20 bath sessions within 4 wk. The bath water temperature ranged from 39°C–42°C (mean 40.2 ± 0.3°C) in all of the 20 bath sessions. The warm baths raised HR significantly (74 ± 2 to 87 ± 2 beats/min, P < 0.001, paired t test) within the first and the last 3 min of the 30 min baths with the subjects sitting in the laboratory’s bathtub.
All subjects reported no significant changes in their health and activities of daily life during the study period. Body weight, BMI, and supine BPs and HR during the three visits are shown in Table 1. After 4 wk of RMHE, body weight decreased in eight of nine subjects. Supine SBP, DBP, and MAP before and during microneurography (Table 1 and Fig. 1) did not change significantly during the three visits (one-way repeated-measures ANOVA). The mean HR from the 6-min baseline ECG (Fig. 1) significantly decreased during the three visits (P = 0.031, one-way repeated-measures ANOVA). Moreover, the post-RMHE HR was significantly lower than pre-RMHE (post hoc). MSNA (Fig. 1) expressed as both burst rate (P < 0.001) and burst incidence (P < 0.001) significantly decreased by RMHE (one-way repeated-measures ANOVA). Post-RMHE MSNA burst rate and burst incidence were significantly lower than pre-RMHE values.
Table 1.
Resting hemodynamic variables during the three visits
| Pre-RMHE | Post-RMHE | Post-1wk | P | |
|---|---|---|---|---|
| Weight, kg | 88.7 ± 6.9 | 87.5 ± 6.6 | 86.4 ± 7.3 | 0.090 |
| BMI | 28.3 ± 1.7 | 27.9 ± 1.5 | 27.5 ± 1.7 | 0.135 |
| Before MSNA recording | ||||
| SBP, mmHg | 118 ± 4 | 116 ± 1 | 119 ± 4 | 0.554 |
| DBP, mmHg | 78 ± 2 | 77 ± 2 | 78 ± 2 | 0.696 |
| MAP, mmHg | 91 ± 3 | 90 ± 2 | 91 ± 3 | 0.674 |
| SV, mL | 103.9 ± 10.9 | 108.0 ± 11.8 | 105.9 ± 11.5 | 0.573 |
| CO, L/min | 6.74 ± 0.64 | 6.65 ± 0.68 | 6.56 ± 0.73 | 0.951 |
| TPR, mmHg·min/L | 14.4 ± 1.3 | 14.6 ± 1.5 | 15.1 ± 1.7 | 0.924 |
| During MSNA recording | ||||
| SBP, mmHg | 123 ± 3 | 120 ± 4 | 121 ± 3 | 0.660 |
| DBP, mmHg | 83 ± 2 | 82 ± 2 | 81 ± 3 | 0.675 |
CO, cardiac output; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity; RMHE, repeated moderate heat exposure; SBP, systolic mean arterial pressure; SV, stroke volume; TPR, total peripheral resistance. P, P value of one-way repeated-measures ANOVA. Subject number: n = 9 for Pre-RMHE and Post-RMHE, n = 8 for Post-1wk.
Figure 1.
Mean and individual muscle sympathetic nerve activity (MSNA), mean arterial pressure (MAP), and heart rate (HR) under resting condition during pre-repeated moderate heat exposure (RMHE) (Pre), post-RMHE (Post), and post-1wk (Post-1wk) visits. The small symbols represent the individual data. P: P value of one-way repeated-measures ANOVA. Post hoc vs. Pre, aP = 0.040; bP = 0.045; cP < 0.001; dP = 0.001; eP < 0.001; and fP = 0.018.
In the three visits (pre, post, and post-1 wk) of the nonheating bath trial (subject number n = 2), the resting MAP (subject A: 89, 92, and 97 mmHg; subject B: 87, 88, and 87 mmHg), resting HR (subject A: 61, 63, and 67 beats/min; subject B: 68, 71, and 59 beats/min), and resting MSNA burst rate (subject A: 42.5, 43.0, and 44.7 bursts/min; subject B: 34.8, 36.0, and 31.8 bursts/min) did not clearly differ.
In the nonheating time control group (subject number n = 13), body weight (83.7 ± 4.2 vs. 83.5 ± 4.0 kg, P = 0.372, paired t test) did not change over time. Furthermore, HR, MAP, and MSNA burst rate and incidence did not change over time in these subjects (Fig. 2).
Figure 2.
Mean and individual mean arterial pressure (MAP), heart rate (HR), muscle sympathetic nerve activity (MSNA) burst rate and incidence before and after the time control. The small symbols represent the individual data. P: P value of paired t test.
Effects of RMHE on Baroreflex Function
Bolus infusion of sodium nitroprusside followed by phenylephrine HCl significantly altered MAP, HR, and MSNA in all visits (Table 2). The cardiac BRS and the sensitivity of baroreflex control of MSNA during the modified Oxford procedure did not change during the three visits (Table 3). The spontaneous MSNA BRS during the 6-min resting baseline did not change significantly either (Fig. 3). Moreover, spontaneous cardiac BRS by sequence technique did not change after RMHE (Fig. 3).
Table 2.
Hemodynamic variables and MSNA during Oxford procedure
| Pre-RMHE | Post-RMHE | Post-1wk | P | |
|---|---|---|---|---|
| MAP, mmHg | ||||
| Base | 94 ± 2 | 94 ± 2 | 93 ± 3 | 0.995 |
| SNP | 84 ± 3* | 81 ± 3* | 83 ± 3* | <0.001 |
| PE | 98 ± 2^ | 98 ± 1^* | 97 ± 3^ | 0.321 |
| HR, beats/min | ||||
| Base | 61 ± 2 | 59 ± 3 | 58 ± 2 | 0.296 |
| SNP | 70 ± 2* | 65 ± 2* | 65 ± 3* | <0.001 |
| PE | 59 ± 3^* | 56 ± 3^ | 54 ± 2^* | 0.651 |
| MSNA BR, bursts/min | ||||
| Base | 31.5 ± 2.4 | 25.2 ± 1.8 | 26.2 ± 1.9 | 0.001 |
| SNP | 40.3 ± 3.3* | 35.0 ± 1.9* | 35.9 ± 4.3* | <0.001 |
| PE | 28.0 ± 2.5^ | 21.0 ± 2.4^# | 21.4 ± 4.2^ | 0.932 |
BR, burst rate; HR, heart rate; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity; PE, mean values from 60 to 120 s after phenylephrine HCl; RMHE, repeated moderate heat exposure; SNP, mean values from 30 to 90 s after sodium nitroprusside infusion. P: P values for the factors in two-way repeated-measures ANOVA (up to down): visit (i.e., effects of RMHE), drug, interaction. Subject number: n = 7 for Pre and Post, n = 6 for Post-1wk.
Post hoc: *P < 0.05 vs. base within visit; ^P < 0.05 vs. SNP within visit; #P = 0.042 vs. Pre-RMHE between visits.
Table 3.
Baroreflex sensitivity with modified Oxford technique
| Pre-RMHE | Post-RMHE | Post-1wk | P | |
|---|---|---|---|---|
| CBRS, ms/mmHg | 11.8 ± 1.2 | 13.6 ± 1.5 | 13.5 ± 2.0 | 0.545 |
| Sequence number | 34 ± 4 | 32 ± 7 | 34 ± 5 | 0.846 |
| MBRS BI, bursts/100 beats/mmHg | −2.3 ± 0.5 | −2.1 ± 0.5 | −2.0 ± 0.4 | 0.944 |
| MBRS area, U/burst/mmHg | −0.45 ± 0.10 | −0.55 ± 0.19 | −0.45 ± 0.16 | 0.836 |
| MBRS total, U/beat/mmHg | −0.69 ± 0.13 | −0.70 ± 0.13 | −0.56 ± 0.12 | 0.881 |
CBRS, cardiac baroreflex sensitivity, which was calculated via sequence technique with the data during the Oxford procedure; MBRS, sensitivity of baroreflex control of muscle sympathetic nerve activity (MSNA); MBRS BI, area, total, the sensitivity of baroreflex control of MSNA burst incidence, burst area, and total activity, respectively; RMHE, repeated moderate heat exposure; sequence number, number of detected sequences for CBRS calculation. P: P value of one-way repeated-measures ANOVA. Subject number: n = 7 for Pre-RMHE and Post-RMHE, n = 6 for Post-1wk.
Figure 3.
Mean and individual spontaneous baroreflex sensitivity under resting conditions during the three visits. The small symbols represent the individual data. CBRS, cardiac baroreflex sensitivity. MBRS BI (bursts/100 beats/mmHg), MBRS area (U/mmHg), MBRS total (units/beats/mmHg): the sensitivity of baroreflex control of muscle sympathetic nerve activity (MSNA) burst incidence, burst area, and total activity. The sequence number detected for CBRS [Pre-repeated moderate heat exposure (RMHE), Post-RMHE, Post-1wk: 113 ± 23, 110 ± 17, and 119 ± 21, P = 0.730]. P: P value of one-way repeated-measures ANOVA. There were no significant differences among the three visits.
In the three visits of the nonheating bath trial (subject number n = 2), the spontaneous MSNA BRS (subject A: −0.87, −0.76, −0.68 U/beat/mmHg; subject B: −0.57, −0.89, −0.53 U/beat/mmHg) and the spontaneous cardiac BRS (subject A: 14.6, 13.0, 16.3 ms/mmHg; subject B: 8.7, 8.2, 10.2 ms/mmHg) did not clearly differ.
In the nonheating time control group (subject number n = 13), the spontaneous cardiac BRS (13.5 ± 1.8 vs. 12.9 ± 1.7 ms/mmHg, P = 0.531) did not change. The detected sequence numbers for cardiac baroreflex sensitivity (CBRS) were not different in the two visits (112 ± 17 vs. 121 ± 17, P = 0.625). The spontaneous BRS control of MSNA burst incidence (−2.45 ± 0.27 vs. −2.16 ± 0.44 bursts/100 beats/mmHg, P = 0.503), burst area (−0.38 ± 0.11 vs. −0.49 ± 0.16 U/mmHg, P = 0.520), and total activity (−0.67 ± 0.05 vs. −0.65 ± 0.06 U/beat/mmHg, P = 0.703) did not change.
Effects of RMHE on MSNA and Hemodynamic Responses to Stress
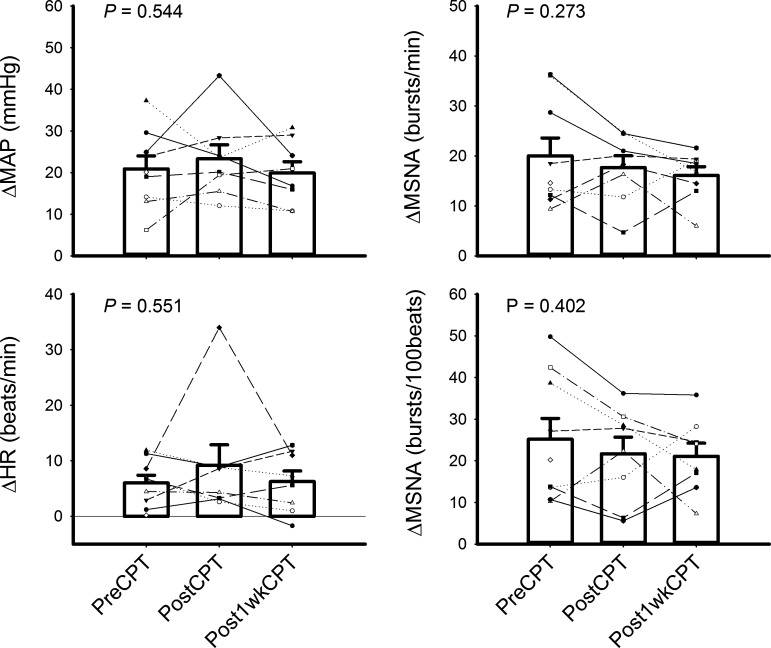
There was no significant difference in BPs and MSNA between the initial 6-min resting baseline and the 3-min baseline before handgrip or CPT. During each of the three visits, CPT evoked significant increases in MAP and MSNA burst rate and incidence (all P < 0.001) (Table 4). CPT also evoked significant increases in MSNA total activity in pre-RMHE (661 ± 59 vs. 1,132 ± 97, U/min, P < 0.001, paired t test), post-RMHE (554 ± 69 vs. 1,077 ± 110 U/min, P < 0.001), and post-1wk visits (615 ± 48 vs. 1,195 ± 141 U/min, P < 0.001). Compared with the pre-RMHE visit, HR during the whole CPT trial (i.e., base + CPT) in post-RMHE visits (P < 0.001) and post-1wk visits (P = 0.018) was significantly lower. MSNA during the whole CPT trial in post-RMHE visits was significantly lower (P = 0.021) than that in the pre-RMHE visit. Moreover, MSNA burst rates during the CPT period in the post-RMHE visits (P = 0.015) and post-1wk (P = 0.018) visits were lower than those during the pre-RMHE visits. However, the responses (i.e., the changes between the last min of CPT and the prior CPT baseline) of MAP, HR, and MSNA burst rate and incidence of CPT did not differ significantly between the three visits (Fig. 4, all P > 0.05, one-way repeated-measures ANOVA).
Table 4.
Hemodynamic variables and MSNA during cold pressor test
| Pre-RMHE | Post-RMHE | Post-1wk | P | |
|---|---|---|---|---|
| MAP, mmHg | ||||
| Base | 96 ± 2 | 96 ± 2 | 97 ± 2 | 0.501 |
| CPT | 117 ± 4* | 120 ± 4* | 117 ± 3* | <0.001 |
| 0.544 | ||||
| HR, beats/min | ||||
| Base | 64 ± 2 | 57 ± 2a | 60 ± 2 | <0.001 |
| CPT | 70 ± 2* | 66 ± 3 | 67 ± 2* | 0.006 |
| 0.551 | ||||
| MSNA BR, bursts/min | ||||
| Base | 29.0 ± 2.1 | 23.9 ± 3.1 | 25.6 ± 2.8 | 0.026 |
| CPT | 49.0 ± 4.2* | 41.6 ± 4.4*b | 41.7 ± 3.2*c | <0.001 |
| 0.273 | ||||
| MSNA BI, bursts/100 beats | ||||
| Base | 45.2 ± 2.9 | 42.4 ± 5.6 | 42.6 ± 4.8 | 0.449 |
| CPT | 70.3 ± 6.0* | 64.1 ± 7.9* | 63.6 ± 6.2* | <0.001 |
| 0.402 |
BI, burst incidence; BR, burst rate; CPT, cold pressor test; HR, heart rate; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity; RMHE, repeated moderate heat exposure. P: P values for the factors in two-way repeated measures ANOVA (up to down): Visit (i.e., effects of RMHE), stress (i.e., CPT), Interaction. Subject number: n = 9 for Pre, n = 8 for Post and Post-1wk.
Post hoc: within visit *P < 0.05 vs. Base. Between visits: vs. Pre-RMHE, aP = 0.003; bP = 0.015; cP = 0.018.
Figure 4.
Mean and individual responses of mean arterial pressure (MAP), heart rate (HR), and muscle sympathetic nerve activity (MSNA) burst rate and incidence to cold pressor test (CPT). The small symbols represent the individual data. PreCPT, PostCPT, Post-1wkCPT: the response by CPT during pre-repeated moderate heat exposure (RMHE), post-RMHE, and post-1wk visits. There were no significant differences among the three visits. P: P value of one-way repeated-measures ANOVA.
MVC (28.7 ± 2.7, 29.0 ± 3.0, and 28.9 ± 3.3 kg, P = 0.922) was not different in the three visits. The mean handgrip force (8.5 ± 0.9, 8.7 ± 0.9, and 8.7 ± 1.0 kg, P = 0.764) and duration (184 ± 15, 186 ± 18, and 164 ± 19 s, P = 0.512) were not different in the three visits. Handgrip exercise induced significant increases in HR, MAP, and MSNA burst rate and incidence in all three visits (all P < 0.001, Table 5). Handgrip and PECO evoked significant increases in MSNA total activity in pre-RMHE (Base, HG, PECO: 721 ± 44, 1,349 ± 69*, 1,206 ± 69* U/min, P < 0.001, one-way repeated-measures ANOVA, *P < 0.05 vs. base), post-RMHE (580 ± 60, 1,169 ± 163*, 992 ± 116 U/min, P < 0.001), and post-1wk visits (593 ± 72, 1,085 ± 141*, 912 ± 116*, P < 0.001). During PECO, MSNA and BP (all P < 0.001) were significantly greater than the pre-exercise baseline, whereas HR was not significantly higher than the baseline during the three visits. Compared with the pre-RMHE visit, HR during the whole handgrip trial (i.e., base + HG + PECO) in post-RMHE visits (P = 0.008) and post-1wk visits (P = 0.026) was significantly lower. Compared with pre-RMHE visit, MSNA burst rate during the whole handgrip trial in post-RMHE (P = 0.001) and post-1wk (P < 0.001) visits was significantly lower. MSNA burst incidence throughout the handgrip trial post-RMHE was also lower than pre-RMHE (P = 0.032). The responses (i.e., the changes) in MAP, HR, and MSNA burst rate and incidence to handgrip or PECO were not different in the three visits (Fig. 5).
Table 5.
Hemodynamic variables and MSNA during handgrip trail
| Pre-RMHE | Post-RMHE | Post-1wk | P | |
|---|---|---|---|---|
| MAP, mmHg | ||||
| Base | 98 ± 3 | 95 ± 2 | 98 ± 2 | 0.933 |
| HG | 117 ± 4* | 116 ± 5* | 116 ± 4* | <0.001 |
| PECO | 110 ± 4* | 110 ± 4* | 109 ± 3* | 0.904 |
| HR, beats/min | ||||
| Base | 65 ± 2 | 60 ± 2c | 59 ± 2d | 0.009 |
| HG | 75 ± 2* | 69 ± 2*b | 70 ± 1*d | <0.001 |
| PECO | 65 ± 2^ | 61 ± 2^d | 61 ± 2^ | 0.929 |
| MSNA BR, bursts/min | ||||
| Base | 31.0 ± 2.3 | 24.8 ± 3.1 | 24.1 ± 3.7 | <0.001 |
| HG | 47.4 ± 3.1* | 39.1 ± 4.6*a | 35.6 ± 4.2*b | <0.001 |
| PECO | 44.0 ± 3.1* | 34.5 ± 3.9*a | 32.7 ± 4.0*a | 0.267 |
| MSNA BI, bursts/100 beats | ||||
| Base | 48.0 ± 3.2 | 42.3 ± 5.8 | 41.0 ± 6.3 | 0.045 |
| HG | 63.6 ± 3.8* | 56.5 ± 6.8* | 51.2 ± 6.2*d | <0.001 |
| PECO | 68.3 ± 4.7* | 57.3 ± 6.6*d | 54.6 ± 7.2*c | 0.447 |
BI, burst incidence; BR, burst rate; HG, handgrip; MSNA, muscle sympathetic nerve activity PECO, postexercise circulatory occlusion; RMHE, repeated moderate heat exposure. P: 3 P values for the factors in two-way ANOVA (up to down): Visit (i.e., effects of RMHE), Stress (i.e., handgrip), Interaction. Subject number: n = 9 for Pre and Post, n = 8 for Post-1wk.
Post hoc: within visit: *P < 0.05 vs. Base; ^P < 0.05 PECO vs. HG. Between visits: vs. Pre-RMHE aP < 0.001; b0.001 < P < 0.01; c0.01 < P < 0.02; d0.02 < P < 0.05.
Figure 5.
Mean and individual responses of mean arterial pressure (MAP), heart rate (HR), and muscle sympathetic nerve activity (MSNA) burst rate and incidence to handgrip exercise and postexercise muscle ischemia (PECO) in the three visits. The small symbols represent the individual data. PreHG, PostHG, Post-1wkHG: the response by handgrip exercise during pre-repeated moderate heat exposure (RMHE), post-RMHE, and post-1wk visits. P: P value of one-way repeated-measures ANOVA. There were no significant differences during the three visits.
In the three visits of the nonheating bath trial (n = 2), the responses (i.e., the changes) to CPT in MAP (subject A: 14, 12, and 11 mmHg; subject B: 24, 28, and 29 mmHg), HR (subject A: 7, 3, 1 beats/min; subject B: 3, 9, and 12 beats/min), and MSNA burst rate (subject A: 13.3, 11.8, and 8.2 bursts/min; subject B: 18.5, 16.3, and 19.4 bursts/min) did not clearly differ. Similarly, the responses to handgrip exercise or PECO did not clearly differ in these two subjects.
In nonheating time control group (n = 13), the responses (i.e., the changes) to handgrip exercise in MAP (17 ± 1 vs. 21 ± 2 mmHg, P = 0.101, paired t test), HR (11 ± 1 vs. 12 ± 2 beats/min, P = 0.254), and MSNA burst rate (14.4 ± 2.9 vs. 15.5 ± 1.9 bursts/min, P = 0.741) and burst incidence (11.6 ± 3.6 vs. 10.8 ± 1.8 bursts/100 beats, P = 0.816) did not differ (n = 13). Similarly, the responses (i.e., the changes) to PECO in MAP (15 ± 1 vs. 17 ± 3 mmHg, P = 0.573), HR (2 ± 1 vs. 2 ± 1 beats/min, P = 0.534), and MSNA burst rate (14.7 ± 2.5 vs. 12.9 ± 1.9 bursts/100 beats, P = 0.563) and incidence (18.7 ± 3.6 vs. 14.1 ± 1.7 bursts/100 beats, P = 0.148) did not differ.
DISCUSSION
The main findings of this study are that 4 wk of repeated warm baths induces a decrease in resting MSNA and a decrease in resting HR. The sensitivity of baroreflex control of MSNA and the cardiac baroreflex sensitivity are not altered by the repeated warm baths, although the operating points are reset. Moreover, the MSNA and hemodynamic responses (i.e., changes) to a variety of stressors are not altered.
Resting MSNA, Hemodynamic Variables, and Baroreflex Function
The beneficial effects of heat exposure with traditional approaches (e.g., sauna bathing, Wagon therapy, etc.) have been reported for years (6, 8, 30–33). Recently, the effects of acute or chronic heat exposure with hot water immersion on cardiovascular function in healthy individuals and patients have been examined by several groups (7, 11, 12, 21, 34–37). Many of these prior studies focused on the effects of heat exposure on peripheral vascular function (8, 21, 30, 31, 36, 37). For example, repeated 1-h hot water immersion over 8 wk increased flow-mediated dilatation in healthy subjects (21).
Although in prior reports hemodynamic variables were examined after repeated heat exposure (11, 21), only one recent study by Ely et al. (12) examined the effects of “heat therapy” on MSNA. In that study, 30 1-h hot tub sessions over 8–10 wk reduced resting MSNA burst frequency and BP in women with obesity with polycystic ovary syndrome (12). In our study, the resting MSNA burst rate and incidence in healthy individuals decreased after 4 wk of warm baths. The decreases in MSNA suggest that sympathetic activity decreased after 4 wk of RMHE. These effects were not observed in the time control studies or in the nonheating bath trial.
Importantly, our data showed that 4 wk of warm baths also decreased resting HR while resting HR did not change in control studies. In the prior report, HR after repeated hot water immersion decreased from that before the intervention in women with obesity with polycystic ovary syndrome (12). The mechanism of the reduction in resting HR is unknown. We speculate that since the warm baths induced increases in HR, the subsequent decrease in resting HR might be related to a “training effect.” It is known that resting HR is inversely related to physical fitness and highly associated with mortality (38). Thus, a decrease in resting HR can indicate a beneficial effect of repeated heat exposure.
Although the final warm bath itself could contribute to the effects seen during the post-RMHE tests, our data suggest that the repeated heat exposures over 4 wk cumulatively contribute to the effects we observed. A recent report (39) showed that HR variability increased during the cooling down period following a single session of 30 min of sauna bathing. However, both HR variability and HR returned to near baseline values after 30 min of recovery (39). These observations (39) suggest that one session of whole body heating treatment can have important immediate postintervention effects. However, the duration of these effects is limited. We would also like to note that a 30-min exposure to sauna bathing could represent a stronger stimulus than that of a single warm water bath used in the current study. For these reasons, we conclude that the repeated heat exposures by RMHE over 4 wk contribute significantly to the effects observed in our study.
In contrast to prior reports that examined the effects promptly following “heat therapy,” we also studied its effects 1 wk after completing RMHE. One week after RMHE, the MSNA burst rates in all subjects were still lower than pre-RMHE. Moreover, 1 wk after RMHE, the MSNA burst rate over the whole handgrip trial was still significantly lower than during the pre-RMHE visit, and the HR during the whole CPT trial and whole handgrip trials was significantly lower than during the pre-RMHE visit, respectively. These data suggest that the effects of RMHE on the autonomic sytem last for at least several days.
After 4 wk of RMHE, the resting BPs were not significantly different from the pre-RMHE values, although MAP decreased in six of nine subjects (see Fig. 1). Several prior reports (12, 21) suggested that repeated heat exposure significantly decreased resting BP while other studies (33) did not. This could be due to the differences in the population of subjects and/or the paradigm he heat exposure. For example, in the prior report (12) seven of 18 subjects were classified as “stage one hypertensive,” whereas none of the subjects in our studies were hypertensive. In the present study, BP in the subjects with relatively higher values pre-RMHE decreased after RMHE (see Fig. 1).
Before the study, we postulated that RMHE might affect sympathetic outflow by altering baroreflex sensitivity. However, whereas our data showed that RMHE resets the operating point to lower MSNA and HR, the sensitivity of baroreflex control of MSNA and HR did not change after RMHE. This result is in contrast to a prior report (12), in which sensitivity of baroreflex control of MSNA increased after 30 sessions of hot immersions. We speculate that the differences in the subject populations (e.g., healthy subjects vs. patients) and the heating protocols could contribute to the observed differences in the results. Moreover, the approaches used to assess baroreflex sensitivity differed between studies. In that study (12), baroreflex sensitivity was calculated from the ratio of MSNA burst changes over DBP changes during the Valsalva maneuver. In our study, the MSNA BRS was assessed by the modified Oxford procedure and during spontaneous BP changes at rest. In both assessments in our study, the MSNA BRS was calculated on a beat-by-beat basis over several minutes. Based on these data, we speculate that the decrease in resting MSNA after RMHE is not the result of a change in MSNA BRS. We would like to note that all subjects in our studies were healthy. The unchanged BRS at least suggests that RMHE does not decrease the capacity of BP control in healthy individuals.
Whereas there was no significant change in BMI, after RMHE, body weight was decreased in eight of nine subjects. The reason for the decrease in body weight is unclear and should be examined in future studies. Although diet and exercise were not controlled during the study period, the subjects did not report any significant changes in their health or activities of daily life (e.g., appetite, diet, exercise). We cannot exclude the possibility that a decrease in body weight contributed to the decrease in resting MSNA.
Responses to Stressors
Although resting MSNA decreased after RMHE, the responses (i.e., changes) in MSNA and hemodynamic variables to CPT or handgrip did not change. To our knowledge, there is no prior report on the effects of repeated heat exposure on sympathetic responses to CPT and handgrip. In the studies during acute heating, the MSNA responses to exercise were accentuated by local muscle heating (40–42) or whole body heat stress (43), whereas in other studies these responses were not changed during whole body heating (44). Prior reports speculated that acutely elevated muscle temperature might alter the sensitivity of muscle afferents (40–42). Thus, we speculate that RMHE may not alter the sensitivity of muscle afferents under normothermic conditions in these healthy individuals. Prior studies showed that the MSNA response to CPT during acute whole body heating was not different from the normothermic condition in young (1) and older (3) healthy subjects, whereas the BP response was attenuated during acute body heating. In the present study, both the MSNA and BP responses to CPT were maintained after RMHE. These results suggest that both autonomic neural outflow and the end-organ responsiveness to these stressors are maintained after RMHE in these healthy aged individuals. Of note, these functional responses in sympathetic activity and BP to stressors are necessary and important to the system. Thus, RMHE does not impair the autonomic and cardiovascular responses to stressors in healthy aged individuals.
A number of chronic disease processes are associated with chronically high levels of resting sympathetic nerve activity. These conditions include obesity (45), diabetes (46), hypertension (47), metabolic syndrome (48), heart disease (49), and the aging process itself (50). In chronic heart failure, increased sympathetic nerve activity and the associated rise in vascular resistance eventuallyleads to worsening cardiac performance (51), and is associated with reduced survival (52). Thus, interventions such as RMHE in the present study may have beneficial effects on these patients. Extreme heat exposure may have a higher risk in patients with cardiovascular disease (53, 54). Thus, in future studies, the effects of RMHE should be examined in a systematic fashion in various cardiovascular disease states.
Study Limitations
The nonheating water bath trial was only performed in two subjects. This was because the control of water temperature for “neutral temperature” (i.e., ∼34°C–35°C) was more challenging than for warm baths. A slightly higher or lower temperature could cause warm or cold stimulation and thereby affect the parameters of interest in an unpredictable manner. Thus, the study was performed at first on two subjects who were highly skilled at maintaining the water temperature in the required range. Importantly, prior reports also showed that a thermoneutral water immersion sham treatment did not result in any health improvements in sedentary control subjects (21). Thus, we believe that the effects seen after 4 wk of warm baths were not the effects of hydrostatic pressure or other factors (e.g., a period of rest) during the water immersion. To further confirm that the decreases in resting MSNA and HR were not time effects, we also included time control studies from a group of subjects (n = 13) that represent data from repeat studies over time (i.e., no intervention) rather than repeat studies following a series of nonheating baths. Although we acknowledge this limitation, time control studies as used in our report represent an established model in previous studies that examined similar interventions (12, 21). Thus, we believe that the effects seen after 4 wk of warm baths were not time effects.
In conclusion, the present study suggests that repeated heat exposure can induce decreases in resting sympathetic activity and resting HR under normothermic conditions in older healthy individuals, which could potentially translate into sustained beneficial effects on the cardiovascular system. Repeated heat exposure does not alter baroreflex function or the sympathetic responses to stressors under normothermic condition.
GRANTS
This work was supported by the National Institutes of Health Grants MPI R01 HL141198 and UL1 TR002014.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. and L.I.S. conceived and designed research; J.C., Z.G., U.A.L., C.B., J.C.L., and M.D.H. performed experiments; J.C., Z.G., U.A.L., and J.C.L. analyzed data; J.C. and L.I.S. interpreted results of experiments; J.C. prepared figures; J.C. and L.I.S. drafted manuscript; J.C. and L.I.S. edited and revised manuscript; J.C., U.A.L., C.B., J.C.L., M.D.H., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We express appreciation to the subjects for willingness to participate in this protocol.
REFERENCES
- 1.Cui J, Shibasaki M, Low DA, Keller DM, Davis SL, Crandall CG. Heat stress attenuates the increase in arterial blood pressure during the cold pressor test. J Appl Physiol (1985) 109: 1354–1359, 2010. doi: 10.1152/japplphysiol.00292.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol 593: 2225–2235, 2015. doi: 10.1113/JP270162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J, Boehmer J, Blaha C, Sinoway LI. Muscle sympathetic nerve activity response to heat stress is attenuated in chronic heart failure patients. Am J Physiol Regul Integr Comp Physiol 312: R873–R882, 2017. doi: 10.1152/ajpregu.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Muller MD, Blaha C, Kunselman AR, Sinoway LI. Seasonal variation in muscle sympathetic nerve activity. Physiol Rep 3: e12492, 2015. doi: 10.14814/phy2.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91: 2582–2590, 1995. doi: 10.1161/01.CIR.91.10.2582. [DOI] [PubMed] [Google Scholar]
- 7.Thomas KN, van Rij AM, Lucas SJ, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol 312: R281–R291, 2017. doi: 10.1152/ajpregu.00404.2016. [DOI] [PubMed] [Google Scholar]
- 8.Lee E, Laukkanen T, Kunutsor SK, Khan H, Willeit P, Zaccardi F, Laukkanen JA. Sauna exposure leads to improved arterial compliance: findings from a non-randomised experimental study. Eur J Prev Cardiol 25: 130–138, 2018. doi: 10.1177/2047487317737629. [DOI] [PubMed] [Google Scholar]
- 9.Tei C, Shinsato T, Miyata M, Kihara T, Hamasaki S. Waon therapy improves peripheral arterial disease. J Am Coll Cardiol 50: 2169–2171, 2007. doi: 10.1016/j.jacc.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol (1985) 121: 716–723, 2016. doi: 10.1152/japplphysiol.00424.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Heart Circ Physiol 316: H1495–H1506, 2019. doi: 10.1152/ajpheart.00151.2019. [DOI] [PubMed] [Google Scholar]
- 12.Ely BR, Francisco MA, Halliwill JR, Bryan SD, Comrada LN, Larson EA, Brunt VE, Minson CT. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 317: R630–R640, 2019. doi: 10.1152/ajpregu.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crandall CG, Gonzalez-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf) 199: 407–423, 2010. doi: 10.1111/j.1748-1716.2010.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation 89: 1648–1655, 1994. doi: 10.1161/01.CIR.89.4.1648. [DOI] [PubMed] [Google Scholar]
- 16.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest: relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallbo AB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 18.Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341: 924–925, 1999. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 19.Ephraim PL, Hill-Briggs F, Roter DL, Bone LR, Wolff JL, Lewis-Boyer L, Levine DM, Aboumatar HJ, Cooper LA, Fitzpatrick SJ, Gudzune KA, Albert MC, Monroe D, Simmons M, Hickman D, Purnell L, Fisher A, Matens R, Noronha GJ, Fagan PJ, Ramamurthi HC, Ameling JM, Charlston J, Sam TS, Carson KA, Wang NY, Crews DC, Greer RC, Sneed V, Flynn SJ, DePasquale N, Boulware LE. Improving urban African Americans’ blood pressure control through multi-level interventions in the Achieving Blood Pressure Control Together (ACT) Study: a randomized clinical trial. Contemp Clin Trials 38: 370–382, 2014. doi: 10.1016/j.cct.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miwa C, Mano T, Saito M, Iwase S, Matsukawa T, Sugiyama Y, Koga K. Ageing reduces sympatho-suppressive response to head-out water immersion in humans. Acta Physiol Scand 158: 15–20, 1996. doi: 10.1046/j.1365-201X.1996.527289000.x. [DOI] [PubMed] [Google Scholar]
- 21.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JF, Kuo LC, Nelson JG, Limacher MC, Quinones MA. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation 70: 425–431, 1984. doi: 10.1161/01.cir.70.3.425. [DOI] [PubMed] [Google Scholar]
- 23.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 24.Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol (1985) 88: 767–773, 2000. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- 25.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291: H482–H483, 2006. doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt U, Vorneweg P, Riedasch M, Hohage H. Acute and persistant effects of smoking on the baroreceptor function. J Auto Pharmacol 19: 105–108, 1999. doi: 10.1046/j.1365-2680.1999.00123.x. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, Groppelli A, Di Rienzo M, Castiglioni P, Parati G. Smoking impairs baroreflex sensitivity in humans. Am J Physiol Heart Circ Physiol 273: H1555–H1560, 1997. doi: 10.1152/ajpheart.1997.273.3.H1555. [DOI] [PubMed] [Google Scholar]
- 29.Dias DP, Silva LE, Katayama PL, Silva CA, Salgado HC, Fazan R. Correlation between RR, inter-systolic and inter-diastolic intervals and their differences for the analysis of spontaneous heart rate variability. Physiol Meas 37: 1120–1128, 2016. doi: 10.1088/0967-3334/37/7/1120. [DOI] [PubMed] [Google Scholar]
- 30.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38: 1083–1088, 2001. doi: 10.1016/S0735-1097(01)01467-X. [DOI] [PubMed] [Google Scholar]
- 31.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/s0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 32.Heinonen I, Laukkanen JA. Effects of heat and cold on health, with special reference to Finnish sauna bathing. Am J Physiol Regul Integr Comp Physiol 314: R629–R638, 2018. doi: 10.1152/ajpregu.00115.2017. [DOI] [PubMed] [Google Scholar]
- 33.Hunter SD, Laosiripisan J, Elmenshawy A, Tanaka H. Effects of yoga interventions practised in heated and thermoneutral conditions on endothelium-dependent vasodilatation: the Bikram yoga heart study. Exp Physiol 103: 391–396, 2018. doi: 10.1113/EP086725. [DOI] [PubMed] [Google Scholar]
- 34.Ely BR, Clayton ZS, McCurdy CE, Pfeiffer J, Needham KW, Comrada LN, Minson CT. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am J Physiol Endocrinol Physiol 317: E172–E182, 2019. doi: 10.1152/ajpendo.00549.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coombs GB, Barak OF, Phillips AA, Mijacika T, Sarafis ZK, Lee AHX, Squair JW, Bammert TD, DeSouza NM, Gagnon D, Krassioukov AV, Dujic Z, DeSouza CA, Ainslie PN. Acute heat stress reduces biomarkers of endothelial activation but not macro- or microvascular dysfunction in cervical spinal cord injury. Am J Physiol Heart Circ Physiol 316: H722–H733, 2019. doi: 10.1152/ajpheart.00693.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 37.Bailey TG, Cable NT, Miller GD, Sprung VS, Low DA, Jones H. Repeated warm water immersion induces similar cerebrovascular adaptations to 8 weeks of moderate-intensity exercise training in females. Int J Sports Med 37: 757–765, 2016. doi: 10.1055/s-0042-106899. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart 99: 882–887, 2013. doi: 10.1136/heartjnl-2012-303375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laukkanen T, Lipponen J, Kunutsor SK, Zaccardi F, Araujo CGS, Makikallio TH, Khan H, Willeit P, Lee E, Poikonen S, Tarvainen M, Laukkanen JA. Recovery from sauna bathing favorably modulates cardiac autonomic nervous system. Complement Ther Med 45: 190–197, 2019. doi: 10.1016/j.ctim.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Ray CA, Gracey KH. Augmentation of exercise-induced muscle sympathetic nerve activity during muscle heating. J Appl Physiol (1985) 82: 1719–1725, 1997. doi: 10.1152/jappl.1997.82.6.1719. [DOI] [PubMed] [Google Scholar]
- 41.Kuipers NT, Sauder CL, Kearney ML, Ray CA. Interactive effect of aging and local muscle heating on renal vasoconstriction during isometric handgrip. Am J Physiol Renal Physiol 297: F327–F332, 2009. doi: 10.1152/ajprenal.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuipers NT, Sauder CL, Kearney ML, Ray CA. Changes in forearm muscle temperature alter renal vascular responses to isometric handgrip. Am J Physiol Heart Circ Physiol 293: H3432–H3439, 2007. doi: 10.1152/ajpheart.00822.2007. [DOI] [PubMed] [Google Scholar]
- 43.Cui J, Shibasaki M, Davis SL, Low DA, Keller DM, Crandall CG. Whole body heat stress attenuates baroreflex control of muscle sympathetic nerve activity during postexercise muscle ischemia. J Appl Physiol (1985) 106: 1125–1131, 2009. doi: 10.1152/japplphysiol.00135.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui J, Blaha C, Sinoway LI. Whole body heat stress attenuates the pressure response to muscle metaboreceptor stimulation in humans. J Appl Physiol (1985) 121: 1178–1186, 2016. doi: 10.1152/japplphysiol.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995. doi: 10.1161/01.HYP.25.4.560. [DOI] [PubMed] [Google Scholar]
- 46.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 108: 3097–3101, 2003. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 47.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 48.Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 48: 1359–1365, 2005. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 49.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 73: 615–621, 1986. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- 50.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993. doi: 10.1161/01.HYP.21.4.498. [DOI] [PubMed] [Google Scholar]
- 51.Zelis R, Parilak L, Huber D, Hogeman C, Leuenberger U. The sympathetic nervous system in heart failure. In: Perspectives in Heart Failure Proceedings of the Congress Italian Society of Cardiology, edited by Dei Cas L, Leier CV.. Milan, Italy, 1995, p. 25–34. [Google Scholar]
- 52.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrao CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 53.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- 54.Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16: 269–277, 1999. doi: 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]