Keywords: autophagy, chlorophyllin, green plant pigment, inflammation, inflammatory bowel diseases
Abstract
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are intestinal complications characterized by chronic inflammation, autophagy abnormality, and lysosomal stress, which are derived from genetic predisposition and environmental risk factors. It is generally precepted that dietary green vegetable is beneficial for physiological homeostasis. In this study, we found that dextran sulfate sodium (DSS)-induced colitis and altered intestinal epithelia in mice were attenuated by oral administration of chlorophyllin (CHL), a water-soluble derivate of chlorophyll. In DSS-treated mice, autophagy was persistently activated in intestinal tissues and associated with bowel disorders. Conversely, supplement of CHL in diet or gavage suppressed intestinal inflammation, downregulated autophagy flux in intestinal tissue, and relieved endoplasmic reticulum stress. In vitro studies show that CHL could activate Akt and mTOR pathways, leading to downregulation of autophagic and lysosomal flux. Thus, consumption of green vegetables and chlorophyllin may be beneficial for IBD recovery in part through alleviation of inflammation and autolysosomal flux.
NEW & NOTEWORTHY Inflammatory bowel disease (IBD) is a chronic and recurrent gastrointestinal disease, while the etiology remains poorly understood. Dietary composition and lifestyle are crucial for pathogenesis and progression of IBD. In this study, we observed that autophagy in the intestinal tissue was persistently activated in IBD mice. Chlorophyllin (CHL), a water-soluble derivate of chlorophyll, can attenuate colitis by regulating autophagy and inflammation. Thus, consumption of green vegetables and chlorophyllin may be beneficial for IBD recovery.
INTRODUCTION
Inflammatory bowel diseases (IBDs) are chronic and relapsing gastrointestinal disorders, featured as ulcerative colitis (UC) and Crohn’s disease (CD) (1). Although the etiology of IBD remains poorly understood, accumulating evidence suggests that dietary, environmental, genetic, epigenetic, and immunological factors may promote initiation and progression of the disease (2–5). Two forms of IBD syndromes share certain common mechanisms but are distinct in their pathophysiological characteristics. In Crohn’s disease (CD), inflammation is typically segmental, asymmetrical, and transmural. CD impacts the whole gastrointestinal tract and pathological findings are mostly found in the terminal ileum and colon (6). Conversely, UC exhibits a continuous pattern of illness, involving superficial mucosal and submucosal, and is limited to the colon (7). The prevalence of IBD is high in Europe and North America, while the rate is rising in Asia, Africa, and South America (8). The rising costs for health care for IBD have become a global health burden (9). Current therapeutic strategies for IBD are often based on anti-inflammatory drugs, immunosuppressive agents, biological therapy, and surgery (10, 11). However, these treatments have shortcomings such as serious side effects and surgical risk. Moreover, many patients do not respond well to these treatments. Therefore, alternative therapeutic approaches and even diets or dietary supplements should be explored.
Imbalanced diet composition is well known for the pathogenesis of IBD. Diets rich in high sugar and high fat are closely related to the incidence of IBD (12, 13), whereas fruit and vegetable diets are negatively correlated (12, 14). Consistent with this notion, incidences of IBD are high in Western Europe and North America in association with diet and lifestyle. Conversely, low incidences of IBD in Asia and other regions are related to a diet rich in green vegetables. Various vitamins and fiber in green vegetables are beneficial for human health and can prevent many illnesses including IBD. However, the green pigment in green vegetables, namely chlorophyll, has yet to be examined for improvement of IBD. Chlorophyllin (CHL, C34H31CuN4Na3O6) is a water-soluble and semisynthetic food-grade derivative of chlorophyll, which is widely used in food industry (15). Numerous studies have demonstrated the roles of CHL in antigenotoxicity, antioxidation, and anticarcinogenesis (16–20), however, the potential therapeutical effects of CHL on IBD have yet to be investigated.
On the other hand, autophagy in physiological dynamics is essential for maintaining intestinal homeostasis. Autophagy is a basic biological process for all eukaryotes and it is vital for environmental response and adaptation machinery for nutrient and calorie availability (21). A major function of autophagy is turnover of long-lived or misfolded proteins and damaged organelles for metabolic recycling (22). Autophagic flux is concerted with lysosomal digestion and plays an important role in maintaining metabolic homeostasis in programmed cell death, cell differentiation, intracellular pathogen clearance, and immunomodulation (23, 24). Previous studies showed that genetic variation of autophagy genes, including autophagy-related 16-like 1 (ATG16L1), immunity-related GTPase family M (IRGM), and Unc-51-like kinase 1 (ULK1), is associated with IBD susceptibility (25–27). Mice with Atg16l1 deletion are susceptible to dextran sulfate sodium (DSS)-induced colitis partly due to Paneth cell abnormalities or enhanced IL-1β production (28, 29). Conversely, abnormal activation of intestinal autophagy can aggravate IBD progression (30–32). Given the fundamental roles of nutrients in regulation of mTOR and autophagy, it is thus interesting to know if chlorophyllin, a dietary green pigment, can regulate intestinal autophagy in the context of IBD. In this study, we found persistent activation of autophagy in experimental colitis. Furthermore, we uncovered that CHL could alleviate experimental colitis partly due to activation of Akt/mTOR pathway, leading to suppression of autophagic flux in the IBD model. Thus, this study indicates a potential beneficial role of CHL and green vegetables in mitigation of IBD syndromes.
MATERIALS AND METHODS
Animal Care and Use
Male C57BL/B6 mice at age of 5–6 wk were purchased from HFK Bioscience (Beijing, China). The mice were kept in a 12-h light/dark cycle environment with free access to food and water. All experimental animal protocols followed The Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH). The animal protocol was approved by Animal Ethics Committee in the College of Life Sciences, Sichuan University.
Induction of IBD in Mice
Chronic colitis model was generated by repeatedly treating mice with 3% DSS (molecular mass at 40,000 Da, from Aladdin China, Cat. No. D122354) in drinking water. After 6 days of treatment with DSS, the mice were given a recovery period of 2 wk, followed by another round of DSS treatment for 6 days, and finally, the mice were euthanized in 35 days (Fig. 1A). For CHL group, chlorophyllin (Chengdu Tongde Pharmaceuticals, China) was added to AIN93 diet at 200 μg/g. Food consumption of mice was in the range of 4–6 g/day. Accordingly, the mice consumed 0.8–1.2 mg CHL daily. For comparison, spinach contains ∼1 mg/g of chlorophyllin, and thus it was estimated that ∼0.8–1.2 g of spinach per day was consumed by the mice in this study. For estimation, this amount of CHL is equivalent to 200–400 g of spinach per day for human consumption. Acute colitis in mice was generated by treating animals with DSS at 3% in drinking water for 6 days. For one experiment, CHL (40 mg/kg body wt) was given through gavage from day 1 to day 6. In another experiment to induce autophagic stress, chloroquine (60 mg/kg body wt) or control saline was intraperitoneally injected into the mice for consecutive 6 days.
Figure 1.
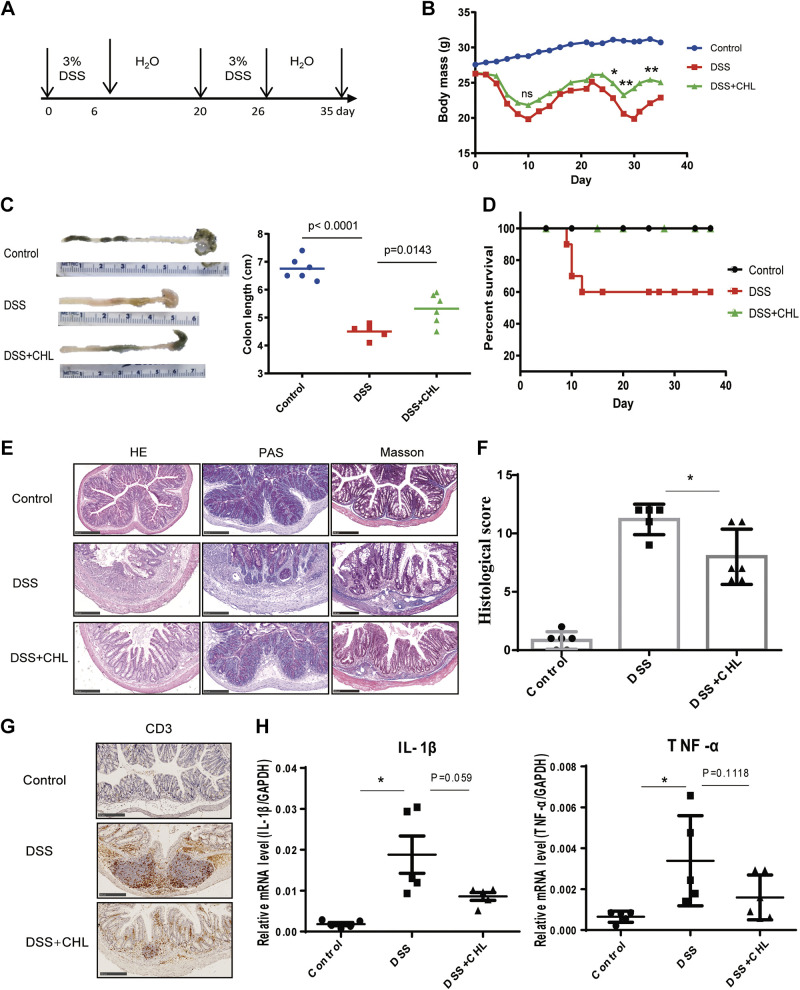
Chlorophyllin (CHL) attenuates dextran sulfate sodium (DSS)-induced biogenesis of chronic colitis. A: to simulate human chronic and recurrent colitis model, mice were treated with 3% DSS for 6 days. After being switched to pure water for 2 wk, the mice were treated with DSS for another 6 days and euthanized until 35 days. For CHL treatment group, CHL was added to the AIN93 diet at 200 μg/g (n = 6 animals/group). B: changes in body weight during the experiment. C and D: as key measurements for IBD, colon length and survival rate are recorded. E: hematoxylin-eosin (H&E), periodic acid-Schiff staining for mucopolysaccharides, and Masson’s trichrome staining for colon fibrosis. Scale bar = 250 μm. F: colon histology score. G: CD3+ lymphocytes were measured via immunohistochemical staining. Scale bar = 250 μm. H: colonic inflammatory cytokines IL-1β and TNF-α were detected by RT-qPCR analysis. The results shown are means ± SE. Statistical significance was determined by one-way ANOVA. *P < 0.05; **P < 0.01. IBD, inflammatory bowel disease.
Histochemical Examination
For histology examination, freshly collected colon tissues (0.5 cm from the distal rectum) were fixed in 4% paraformaldehyde followed by embedding in paraffin. Sections were stained with a hematoxylin-eosin (H&E) kit for histological assessment. To measure intestinal mucins, tissue sections were fixed by Carnoy’s solution and stained with a Periodic Acid Schiff (PAS) kit. Colon fibrosis was determined by Masson’s trichrome staining. Histology scoring was performed using a modified protocol from Lee et al. (33). The details of the histological scoring were described previously. Briefly, colonic epithelial damage was scored blindly as follows: grade 0 for normal; grade 1 for hyperferation, irregular crypts, and loss of Goblet cells; grade 2 for mild-to-moderate crypt loss (10%–50%); grade 3 for severe crypt loss (50%–90%); grade 4 for complete crypt loss, surface epithelium intact; grade 5 for small to medium-sized ulcer (<10 crypt widths); and grade 6 for large ulcer (>10 crypt widths). Infiltration with inflammatory cells was scored separately for mucosa (0 for normal; 1 for mild; 2 for modest; 3 for severe), submucosa (0 for normal; 1 for mild to modest; 2 for severe), and muscle/serosa (0 for normal and 1 for moderate to severe). The slides were scanned and images were captured by NanoZoomer-SQ instrument.
Measurement of Intestinal Permeability
Measurement of intestinal permeability was described previously (34). Briefly, after 6 days of DSS treatment, the mice were fasted for 6 h, followed by oral gavage administration with fluorescein isothiocyanate (FITC)-dextran (500 µg/kg body wt, molecular mass: 40 kDa, Sigma, Cat. No. FD40S). Serum was harvested for 2 h. The FITC-dextran concentration in the serum was measured by fluorescence with Multiskan Spectrum (Thermo Varioskan Flash) with an excitation wavelength of 485 nm and emission wavelength of 535 nm.
Immunohistochemistry Staining
The tissue slides were deparaffinized and rehydrated, and antigens were retrieved by boiling in citrate buffer (50 mM sodium citrate at pH 6.8). The slides were sequentially treated with 3% H2O2, followed by 0.1% TritonX-100, and blocked with 5% bovine albumin. The slides were incubated with primary antibodies against CD3 (Abcam, Cat. No. ab16669), p62 (Zen BioScience, Cat. No. 380672, China), LC3 (Zen BioScience, Cat. No. 310133, China), Atg7 (Cell Signaling Technology, Cat. No. 8558), LAMP1 (R&D, Cat. No. 8558), GRP78 (Assay Designs, Cat. No. SPA-826), CHOP (Cell Signaling Technology, Cat. No. 2895), F4/80 (Novus Biologicals, Cat. No. NBP1-60140), Occludin (ABclonal, Cat. No. A12621, China), ZO-1(Santa Cruz Biotechnology, Cat. No. sc-33752), and E-cad (Abways technology, Cat. No. CY1155, China) followed by corresponding secondary antibody labeled with horseradish peroxidase (HRP) (ZSJQ Biotech, PV9003/PV6000, China). The dilution ratio of primary antibody was 1:200. For the negative control, the slides were incubated with rabbit or mouse IgG Isotype Control (Cell Signaling Technology, Cat. No. 3900S, 5415S). Finally, the slides were developed with a DAB kit. The slides were scanned and the images were captured by NanoZoomer-SQ instrument.
RT-qPCR Analysis
Total RNA was extracted with TRIzol and reversely transcribed into cDNA pool with the Transcriptor First Strand cDNA Synthesis Kit (Roche, Cat. No. 04897030001). Real-time qPCR (RT-qPCR) reactions contained 2 µL of cDNA, 0.2 µL of primers (200 nM), and 5 µL of MIX in a final volume of 10 µL. Amplification was performed using Bio-Rad cfx96. Primer sequences are listed in Table 1. The relative mRNA expression was normalized to the expression of GAPDH mRNA expression.
Table 1.
List of mouse primers for qPCR analysis
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| TNF-α | TGGGACAGTGACCTGGACTGT | TTCGGAAAGCCCATTTGAGT |
| IL-1β | TCGCTCAGGGTCACAAGAAA | CATCAGAGGCAAGGAGGAAAAC |
| GAPDH | GCACAGTCAAGGCCGAGAAT | GCCTTCTCCATGGTGGTGAA |
Cell Culture and Treatment
Caco-2 and HCT116 cells were maintained at 37°C in a 5% CO2 atmosphere. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum with penicillin (100 U/mL) and streptomycin (100 mg/mL). A plasmid for the expression of tandem monomeric RFP-GFP-tagged LC3 was provided by Dr. Ying Tong. Plasmid transfection was carried out by Lipofectamine 3000 (Life Technologies) according to the manufacturer’s instructions. For inhibition of autophagy by CHL, cells were cultured with DMEM including 10% FBS together with CHL at the concentration mentioned in figure legends. For inhibition of mTOR or Akt, cells were cultured with DMEM including 10% FBS together with rapamycin (MedChemExpress, Cat. No. HY-10219) or MK-2206 (MedChemExpress, Cat. No. HY-108232). For lysosome staining, cells were cultured in dish with or without CHL for 24 h, then treated with 75 nM LysoTracker (Thermo Fisher, Waltham, MA) for 1 h followed by confocal microscopy or FACS analysis.
Western Blot Analysis
Proteins were extracted from the colonic tissues or cells using reducing RIPA buffer containing protease inhibitors. Total protein concentration was determined by a Pierce BCA Protein Assay Kit (Thermo). Equal quality of protein samples was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane. The membranes were blocked with 5% skim milk, followed by incubation with primary antibodies overnight at 4°C. The primary antibodies for AKT (Cell Signaling Technology, Cat. No. 8596), p-AKT (Cell Signaling Technology, Cat. No. 4685), mTOR (Cell Signaling Technology, Cat. No. 2983), p-mTOR (Cell Signaling Technology, Cat. No. 5536), p70s6k (Cell Signaling Technology, Cat. No. 2708), p-p70s6k (Cell Signaling Technology, Cat. No. 9209), GAPDH (Zen BioScience, Cat. No. 250133, China), and β-actin (Zen BioScience, Cat. No. 250132, China) were applied in the study. The dilution ratio of primary antibody was 1:1,000. After incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies with dilution at 1:5,000, the blots were developed with Clarity TM Western ECL Substrate.
Statistical Analysis
Data were recorded, processed, and operated using Microsoft Excel 2007 and GraphPad Prism 6.0 (GraphPad Software, Inc.). Animal survival rates were analyzed by the Kaplan–Meier log-rank analysis. Student’s t test was used to analyze the difference between two groups. One-way ANOVA was used to analyze the difference between ≥3 groups, followed by Tukey multiple comparison testing. Statistical significance was designated as *P < 0.05, **P < 0.01, or ***P < 0.001.
RESULTS
Chlorophyllin Mitigates Dextran Sulfate Sodium-Induced Biogenesis of Chronic Colitis in Mice
As shown in Fig. 1A, chronic colitis was induced by repeating DSS treatments, according to a modified protocol adapted from Wirtz et al. (35). Briefly, mice were given 3% DSS in drinking water for 6 days, followed by a recovery period with pure water for 2 wk, and then the mice were given another round of treatment and recovery until day 35. To determine impact of chlorophyllin on colitis, CHL was added to diet at 200 μg/g. As shown, CHL treatment significantly prevented the DSS-mediated body weight loss (Fig. 1B) and colon length shortening (Fig. 1C). Likewise, stool consistency and blood in the stool were significantly reduced. Consistently, DSS treatment induced 40% mortality in mice at day 35, and chlorophyllin substantially reduced the mortality rate in agreement with relieved symptoms of colitis (Fig. 1D). Indeed, histochemical examination showed that CHL could 1) reduce damage to intestinal mucosa, 2) suppress infiltration of inflammatory cells, 3) restore the number of Goblet cells, and 4) suppress the intestinal fibrosis and improve histological scores (Fig. 1, E and F). Moreover, the mRNA levels of proinflammatory cytokines TNF-α and IL1-β were also reduced in the colon by CHL treatment compared with the DSS group (Fig. 1H). Infiltration of CD3+ lymphocytes in the DSS mice, a major feature of the intestinal inflammation, was markedly relieved by CHL treatment (Fig. 1G). Taken together, these results demonstrate that oral administration of chlorophyllin can significantly impede the biogenesis of experimental colitis.
Chlorophyllin Relieves Established IBD in Mice
We further determined if the administration of CHL can relieve established colitis in mice. Severe colitis was induced by treating the animals with 3% DSS for 7 days, accompanied by weight loss. After 7 days of treatment with DSS, the mice were given a recovery period with water. For the CHL treatment group, chlorophyllin was added to drinking water at 40 mg/L on day 7. Finally, the mice were euthanized in 15 days (Fig. 2A). Expected, CHL treatment significantly accelerated the weight recovery (Fig. 2B) and reversed colon length shortening (Fig. 2C). Likewise, stool consistency was significantly reduced. Strikingly, the DSS-induced mortality at 62.5% rate in mice at day 15 was substantially reduced by chlorophyllin treatment, showing a reduced mortality rate at 12.5%, which agreed with relieving symptoms of colitis (Fig. 2D). Histochemical examination showed reduction of damaged intestinal mucosa and less inflammatory cells infiltration (Fig. 2E). These results demonstrated that CHL can improve established colitis in mice.
Figure 2.
Chlorophyllin (CHL) mitigates the established IBD in mice. A: to determine if CHL could reverse established colitis, mice were treated with 3% DSS for 7 days. Then, the mice were given a recovery period with water. For CHL group, chlorophyllin was added into water at 40 mg/L on day 7. Finally, the mice were euthanized in 15 days (control n = 3 animals, DSS/DSS+CHL group n = 8 animals). B: changes in body weight during the experiment. C and D: colon length and survival rate are recorded. E: hematoxylin-eosin staining (H&E). Scale bar = 250 μm. The results shown are means ± SE. *P < 0.05; **P < 0.01. DSS, dextran sulfate sodium; IBD, inflammatory bowel disease.
Persistent Activation of Autophagy and Endoplasmic Reticulum Stress in Experimental Chronic Colitis Are Suppressed by Chlorophyllin Treatment
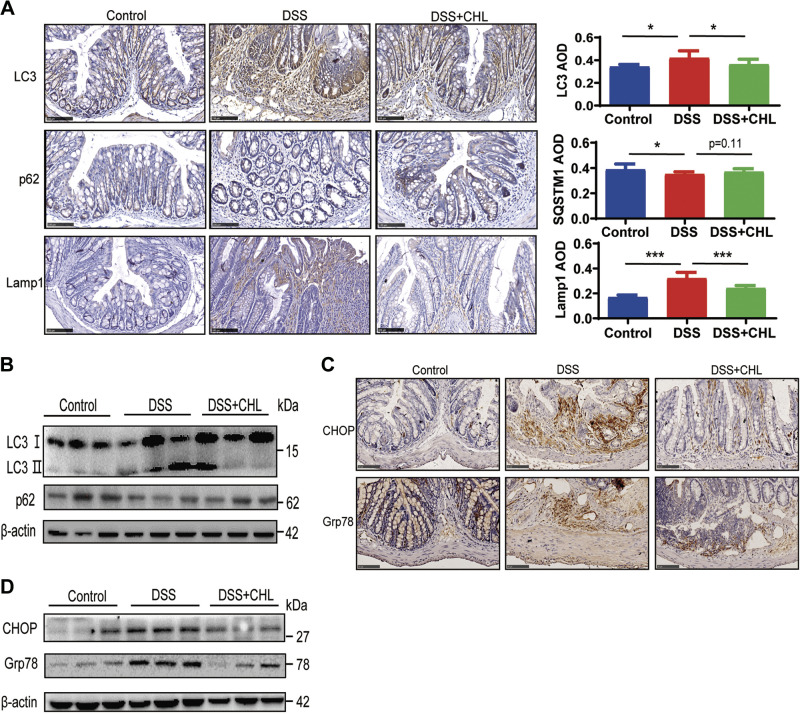
Previous studies through genetic analysis demonstrated that abnormal autophagy activation plays an important role in the pathogenesis and development of IBD (29, 30). We reasoned that green pigment might relieve IBD in part through modulation of autophagy in the intestine. As shown in Fig. 3, DSS treatment significantly accelerated autophagic flux in colon tissue, as indicated by accumulation phospholipid conjugation of LC3 (LC3-II), and increased turnover of autophagic substrate such as SQSTM1/p62, an autophagosome cargo protein. Lysosomal biogenesis, as indicated by lysosomal membrane protein LAMP1, was significantly increased in intestinal cells of the colitis mice, compared with that in control group. Notably, chlorophyllin (CHL) treatment largely reduced excessive autophagic activation, showing attenuated turnover of LC3-II and LAMP1, as well as restoration of p62 (Fig. 3, A and B). Patients with Crohn's disease and ulcerative colitis showed endoplasmic reticulum (ER) stress (36). We then analyzed the unfolded protein response (UPR) by measurement of the markers, chaperone BiP (GRP78) and C/EBP homologous protein (CHOP) in the colonic epithelium. As shown, ER stress, which was induced in the DSS-mediated colitis model, was significantly relieved by the green pigment (Fig. 3, C and D).
Figure 3.
Persistent activation of autophagy and endoplasmic reticulum stress in experimental chronic colitis are suppressed by chlorophyllin (CHL) treatment. A: representative images of p62/Sqstm1, LC3, LAMP1 expressed in the colon were determined by immunohistochemistry and positive signals were analyzed by ImageJ software. Three visual fields were counted for each mouse. Control n = 3 animals; DSS n = 4 animals; DSS+CHL n = 4 animals. The results shown are means ± SE. Statistical significance was determined by one-way ANOVA. *P < 0.05; ***P < 0.001. Scale bar = 100 μm. B: Western blot analysis of LC3 and p62 protein extracts of colon. C and D: expression of CHOP and Grp78 in the colon were determined by immunohistochemistry analysis and Western blot. DSS, dextran sulfate sodium.
Inhibition of Provoked Autophagy Attenuates DSS-Induced Acute Colitis in Mice
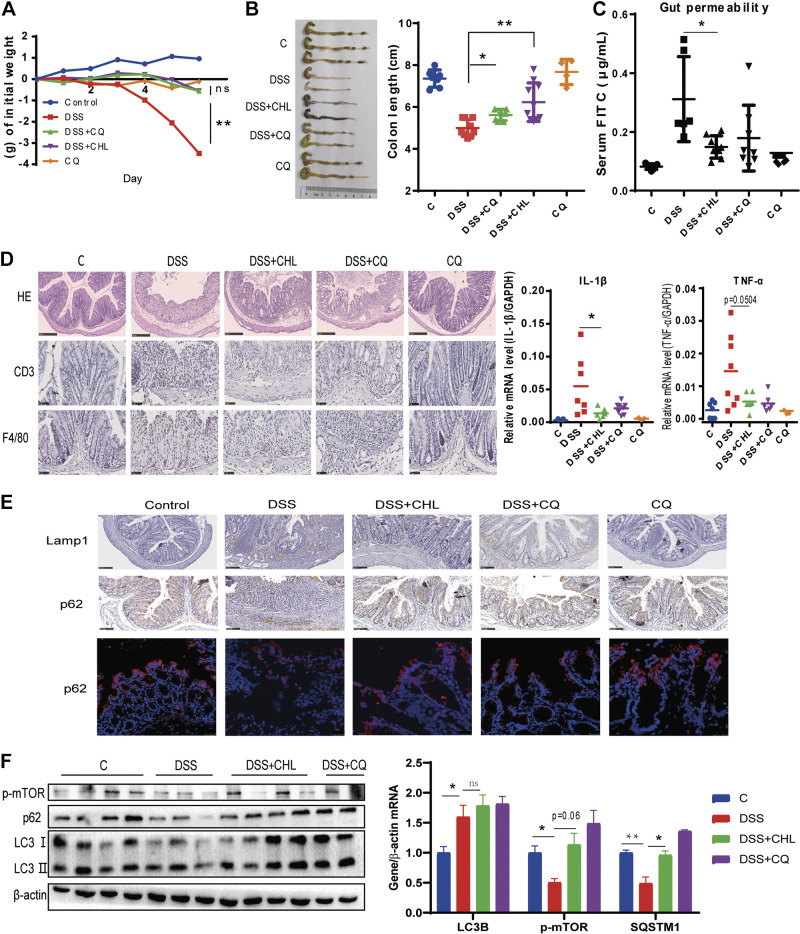
To determine whether provoked autophagy plays the key role in DSS-induced colitis, we examined the impacts of autophagy inhibitor chloroquine on a murine model of colitis. Briefly, severe colitis was induced by treating the animals with 3% DSS for 6 days, characterized by sustained weight loss (Fig. 4A) and shortening of colon length (Fig. 4B). These phenomena were attenuated by chloroquine treatment in parallel with CHL treatment. One of the most typical characteristics of IBD in its pathogenesis is epithelial barrier disruption (37, 38). Here, chloroquine and CHL significantly rescued the DSS-induced impairment of gut permeability, respectively (Fig. 4C). Dysfunction of intestinal barrier may lead to penetration of gut bacterial components and food antigens into the body, which can consequently initiate and accelerate intestinal inflammation. As expected, the mRNA levels of TNF-α and Il1-β in the colon tissue were suppressed in the colitis mice treated by CHL or chloroquine accordingly. Likewise, infiltration of CD3+ lymphocytes and F4/80+ macrophages in the submucosa of the DSS-induced colitis was also alleviated by chloroquine or CHL, respectively (Fig. 4D). Consistently, histological analysis by H&E staining revealed improvement of the ulcers and crypt loss by chloroquine and CHL individually. As shown, chloroquine or CHL treatment significantly attenuated DSS-induced autophagic flux, showing downregulation of Lamp1, a major lysosomal membrane protein, and accumulation of p62/Sqstm1, a cargo protein for autolysosomal flux (Fig. 4E). Western blot analysis also demonstrated accumulation of p62 and LC3-II, in agreement with the phosphorylation and activation of mTOR, exerted by chloroquine or CHL treatment (Fig. 4F). Taken together, these results indicated that persistent and provocative activation of autophagy is involved in the pathogenesis of IBD, which may serve as potential therapeutic targets.
Figure 4.
Suppression of autophagy by chloroquine attenuates DSS-induced acute colitis in mice. Mice were treated with 3% DSS for 6 days. For several groups, CHL (40 mg/kg) was gavage from day 1 to day 6. Chloroquine (60 mg/kg) was intraperitoneally injected from day 1 to day 6. Saline as vehicle. (CQ group n = 4 animals, others n ≥ 6 animals). A and B: changes of body weight and colon length. C: gut permeability was determined by fluorescein isothiocyanate (FITC)-dextran presented in serum after oral gavage administration. D: representative images of colon tissues. Hematoxylin-eosin (H&E), CD3+ lymphocytes, and macrophages were measured via immunohistochemical staining. RT-qPCR analysis of mRNA levels of TNF-α and IL-1β. E: expression of p62/Sqstm1 and LAMP1 in the colon were determined by immunohistochemistry analysis and immunofluorescence. Scale bar = 100 μm. F: Western blot analysis of p-mTOR, LC3, and p62 of colon. The results shown are means ± SE. Statistical significance was determined by one-way ANOVA. *P < 0.05; **P < 0.01. DSS, dextran sulfate sodium.
DSS-Induced Autophagic Activation in Intestinal Cells Is Attenuated by Chlorophyllin
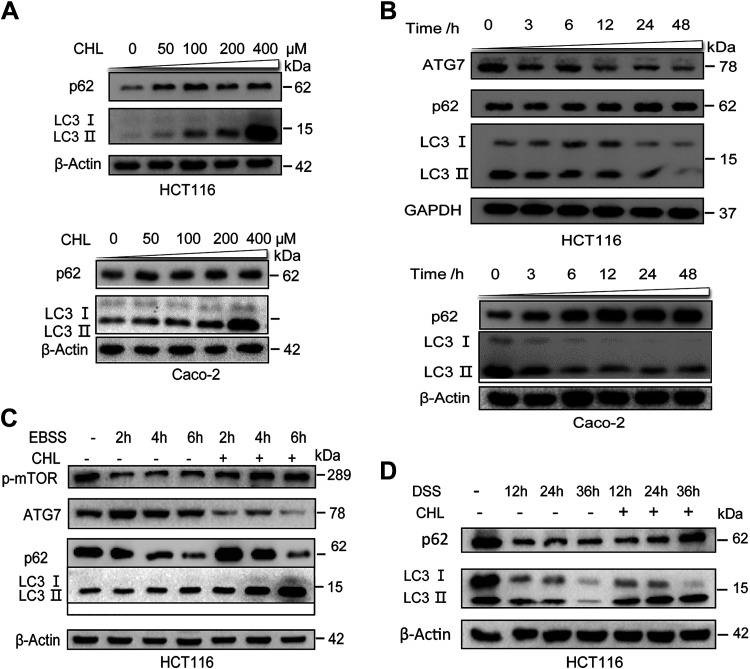
The DSS-induced autophagic activation in vivo is complicated by other factors such as diet composition and intestinal microbiome, and we, therefore, examined the direct impacts of chlorophyllin on the DSS-induced autophagic flux of intestinal cells in vitro. As shown in Fig. 5A, a steady state of autophagic flux in two lines of intestinal cells was attenuated by CHL treatment at 50–400 μM for 24 h, as indicated by accumulation of p62/SQSTM1 and phospholipid conjugation of LC3. Likewise, in a time-course experiment, CHL treatment led to decreased levels of Atg7 in agreement with the accumulation of p62/SQSTM1 and failed phospholipid conjugation of LC3 (Fig. 5B). Amino acid starvation is a major physiological regulation leading to activation of autophagy through suppression of mTOR activation (39). As shown in Fig. 5C, depletion of amino acids quickly promoted autophagic flux, as indicated by reduction of mTOR phosphorylation at Ser2448 and downregulation of p62, while ATG7 and LC3 levels were increased. However, autophagic flux was notably suppressed, showing increased phosphorylation of mTOR and accumulation of LC3 and p62. We also found that DSS could directly induce autophagic flux, as indicated by downregulation of p62 and LC3, whereas CHL treatment suppressed this effect, as indicated by accumulation of p62 and LC3 (Fig. 5D). These results demonstrated that CHL may serve as a feeding signal to attenuate autophagic flux, in part through activation of mTOR.
Figure 5.
DSS-induced activation of autophagy in intestinal cells is inhibited by chlorophyllin (CHL). A: Western blot analysis of SQSTM1/p62, LC3, and β-actin in intestinal cells HCT116 and Caco2 treated by CHL at 0, 50, 100, 200 and 400 μM for 24 h. B: Western blot analysis of Atg7, p62, and LC3 in cells treated with 50 μM CHL for indicated time period. C: representative immunoblots of p-mTOR, Atg7, LC3, and β-actin in amino acid-starved cells for indicated period or treated by 50 μM CHL. D: HCT116 cells were treated with 3% DSS only or together with CHL for indicated time, followed by Western blot analysis. Each experiment was repeated three times. DSS, dextran sulfate sodium.
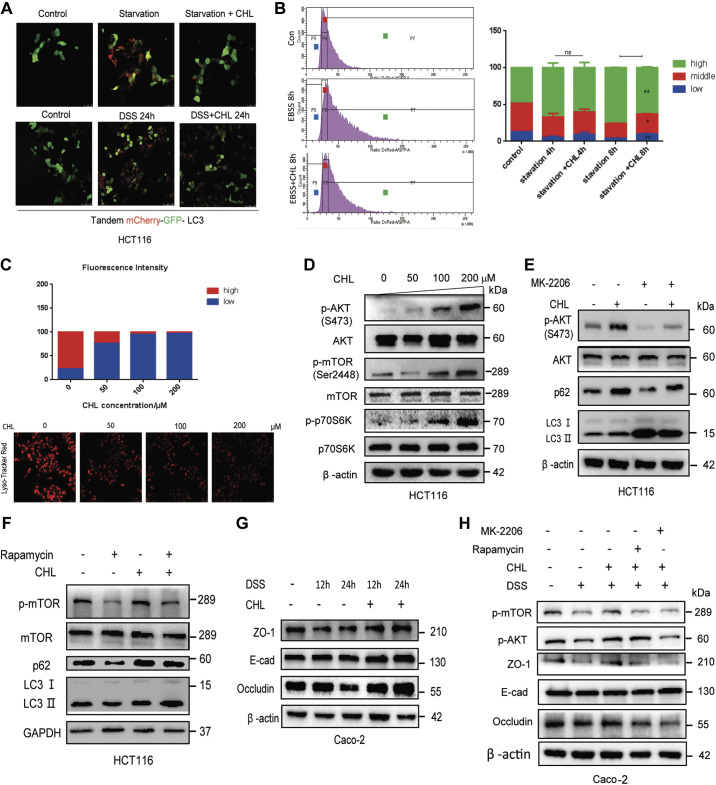
Chlorophyllin Modulates Autophagic Flux and Improves Intestinal Epithelial Barrier Dysfunction through Activation of Akt-mTOR Pathway
Next, we investigated the potential molecular mechanism underlying the suppressive effect of CHL on autophagic flux. We further monitored the dynamics of autophagic flux using a tandem-mCherry-GFP-LC3 probe expressed in HCT116 cells. As shown in Fig. 6A, formation of acidic autophagic-lysosomal puncta, indicated by red fluorescence-LC3 and quench of green fluorescence-LC3, was significantly increased in response to depletion of amino acids (EBSS) or challenge with DSS, indicating autophagic activation. However, the red fluorescent puncta were significantly reduced when the cells were exposed to CHL (Fig. 6A). We also applied FACS analysis to quantitate the autophagic fluxes. As shown in Fig. 6B, depletion of amino acids substantially increased a subset of high mCherry/GFP puncta-containing cells, indicating the increased autophagic flux, while CHL attenuated the autophagic activation. Likewise, we measured the number of lysosomes and lysosomal acidity by LysoTracker staining and found that CHL treatment decreased the number of lysosomes or acidity (Fig. 6C). It is known that Akt-mediated mTOR phosphorylation and activation are essential for the inhibition of autophagic flux (40). Indeed, we found that CHL treatment could increase the phosphorylation of Akt, as well as its downstream targets mTOR and p70S6K (Fig. 6D), in line with suppression of autophagic flux. Taken together, these lines of evidence demonstrated that in response to green pigment, chlorophyllin, cellular Akt-mTOR-p70S6K pathway is activated, leading to inhibition of autophagic flux, which may further counter the DSS-induced excessive activation of autophagy. Furthermore, to determine whether Akt-mTOR was involved in CHL-mediated suppression of autophagic flux and disease progression, we examined the impacts of Akt inhibitor (MK-2206) and mTOR inhibitor (rapamycin) on cellular autophagy and tight junction. As excepted, we found that CHL was able to relieve autophagy activated by MK-2206 or rapamycin, showing partially rescued phosphorylation of Akt and mTOR. Likewise, p62/SQSTM1 showed similar changes (Fig. 6, E and F). One of the important features of IBD is intestinal epithelial damage. In cellular model, we found that tight junction proteins such as ZO-1 and occludin were downregulated under DSS exposure, which could be reversed by CHL treatment (Fig. 6G). However, MK-2206 or rapamycin treatments impaired the protective effect of CHL, showing reduction of tight junction proteins ZO-1 and occludin (Fig. 6H). Our results are in agreement with previous reports indicating that activation of Akt protects the epithelial barrier (41, 42). These data further implied that CHL can attenuate autophagic flux and protect the intestinal epithelial barrier in part through activation of Akt/mTOR.
Figure 6.
Chlorophyllin (CHL) modulates autophagic flux and improves intestinal epithelial barrier dysfunction through activation of Akt-mTOR pathway. A: confocal microscopy analysis of autophagic flux through expression of a probe, LC3-tandem-RFP-GFP, in HCT116 cells challenged with amino acid starvation or DSS, together with or without CHL treatment. Scale bar = 25 μm. The experiment was repeated three times. B: autophagic flux was determined by FACS analysis in cells expressing tandem LC3-RFP-GFP under indicated treatments and presented as histograms for the ratio of RFP/GFP. C: lysosomal biogenesis was determined by Lyso-Tracker staining, followed by confocal microscopy and FACS analysis. Scale bar = 25 μm. D: CHL activated Akt-mTOR pathway in a dose-dependent manner. E and F: HCT116 cells were treated with 500 nM MK-2206 or 200 nM rapamycin only or together with 50 mM CHL for 12 h, followed by Western blot analysis. G: Caco-2 cells were treated with 3% DSS alone or with CHL (50 μΜ) for indicated time, followed by Western blot analysis. H: the proteins levels of tight junction proteins ZO-1 and occludin were determined by Western blot. The experiment was repeated three times. DSS, dextran sulfate sodium.
DISCUSSION
Inflammatory bowel diseases including Crohn’s disease and ulcerative colitis are associated with environmental factors, intestinal infection, and genetic susceptibility/predisposition, which ultimately lead to abnormal mucosal immune response against the intestinal microbiome. Diet habit and composition are critical for human health and well-being. It is known that high-sugar and high-fat diets are strongly correlated with IBD (12, 43, 44), while consumption of high amounts of fiber, particularly fiber from vegetables and fruits, is negatively correlated with IBD (2, 45). However, the role of chlorophyll, a major component in green vegetables, in IBD progression is still unclear.
The exact cause and the pathogenesis of IBD are poorly understood. However, IBD is generally a consequence of defective host immunity. Persistent activation of intestinal immune system caused by viruses, bacteria, and food antigen may exert inflammation and tissue injury in gastrointestinal tract. Autophagy, a basic cellular program, is essential for intestinal homeostasis in part through degradation of damaged cell organelle and maintaining metabolic turnover (28). Previous studies reported that knocking out autophagy-related key genes, including Atg16L1, Atg4B, Atg5, and Atg7 worsens the IBD symptoms in animal models (28, 46–48). These works indicate that basal or physiological levels of autophagy are critical for the maintenance of intestinal integrity. Notably, deletion of Atg16L1, Atg5, and Atg7 in mice displays impairment of Paneth cells, the α-defensin-producing cells in the crypts of small intestine (28, 48). Therefore, loss of physiologically autophagic turnover leads to deterioration of IBD. However, on the other hand, persistent activation of autophagy and its disassociation with the consequent lysosomal degradation may also provoke tissue damage in IBD (49, 50). Indeed, it is known that persistent activation of autophagy can aggravate IBD by inducing autophagic cell death, leading to the destruction of the intestinal barrier and the overproduction of proinflammatory cytokines (30, 31, 51). Likewise, modulation and suppression of autophagy may be used as a potential therapeutical treatment for IBD (31, 32, 51–53). Previous studies showed that persistent activation of autophagy may serve as a potential signal for cell death (54, 55).
In this study, we found persistent autophagy activation in colon tissue of experimental IBD mice. Specifically, DSS as an immunogen or damage signal can provoke autophagic activation and inflammation in the intestinal tissues of the disease models, and such notion is further demonstrated by the experiment with cell culture models. We found that excessive activation of autophagy without subsequent orderly lysosomal degradation may create lysosomal stress, which may release autolysosome contents such as cathepsins for tissue damage and inflammation. Indeed, cathepsin G was found in the supernatants from tissues of patients with IBD compared with healthy controls (49).
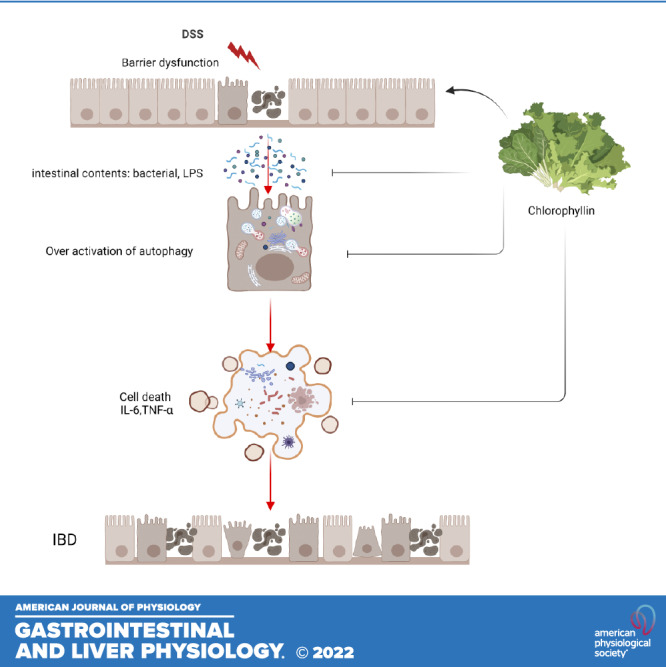
Dietary compositions are crucial for pathogenesis and progression of IBD. Western diet with excessive consumption of sugar and fat is regarded as a risk factor for IBD development (56, 57). Appropriate nutrition may improve the quality of the patients. In this study, we found that a water-soluble form of chlorophyll, namely CHL, can attenuate autophagy in the intestinal cells and alleviate the DSS-induced experimental IBD in mice. In particular, DSS as a negatively charged polysaccharide can activate cellular autophagy. Conversely, attenuation of autophagy by chloroquine improves DSS-mediated IBD. CHL treatment can modulate and suppress autophagy both in vivo and in vitro. Our previous study showed that CHL could directly inhibit LPS-initiated cellular response and downregulate inflammatory cytokines (58). CHL was known for its role in neutralization and sequestration of carcinogens (17–19). In summary, our study revealed a potential application of chlorophyllin for treatment of IBD, and the implication may be further extended to the consumption of green vegetables for mitigating IBD (see graphic summary).
GRANTS
The work was supported by National Natural Science Foundation of China (NSFC) Grants 31571165 and 31771288 (to Y-P.H), 31771559 (to Y.T.), 82070846 (to X.Z.), Science and Technology of Sichuan Province, 2018MM0105, and National Institutes of Health Grant P01DK098108 (to S.J.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.Z. and Y-P.H. conceived and designed research; T.Z. and R.Z. performed experiments; T.Z., G.Z., W.L., L.P., M.J., H.Z., S.J.P., and Y-P.H. analyzed data; T.Z., Y.T., Z.X., S.J.P., and Y-P.H. interpreted results of experiments; T.Z. prepared figures; T.Z. and Y-P.H. drafted manuscript; X.F., Y-P.H., and X.Z. edited and revised manuscript; Y-P.H. and X.Z. approved final version of manuscript.
REFERENCES
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med 347: 417–429, 2002. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 15: 525–535, 2018. doi: 10.1038/s41575-018-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, Wu GD. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 148: 1087–1106, 2015. doi: 10.1053/j.gastro.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8: 458–466, 2008. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 15: 39–49, 2018. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 6.Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn's disease. Lancet 389: 1741–1755, 2017. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 7.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis. Lancet 389: 1756–1770, 2017. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390: 2769–2778, 2017. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 12: 720–727, 2015. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 10.Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 14: 269–278, 2017[Erratum inNat Rev Gastroenterol Hepatol14: 688, 2017]. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 11.Le Berre C, Ananthakrishnan AN, Danese S, Singh S, Peyrin-Biroulet L. Ulcerative Colitis and Crohn's disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol 18: 14–23, 2020. doi: 10.1016/j.cgh.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut 40: 754–760, 1997. doi: 10.1136/gut.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russel MG, Engels LG, Muris JW, Limonard CB, Volovics A, Brummer RJ, Stockbrügger RW. Modern life' in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol 10: 243–249, 1998. doi: 10.1097/00042737-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi Porro G, Panza E. Smoking, sugar, and inflammatory bowel disease. Br Med J (Clin Res Ed) 291: 971–972, 1985. doi: 10.1136/bmj.291.6500.971-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solymosi K, Mysliwa-Kurdziel B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev Med Chem 17: 1194–1222, 2017. doi: 10.2174/1389557516666161004161411. [DOI] [PubMed] [Google Scholar]
- 16.Kamat JP, Boloor KK, Devasagayam TP. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim Biophys Acta 1487: 113–127, 2000. doi: 10.1016/s1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- 17.Kumar M, Verma V, Nagpal R, Kumar A, Gautam SK, Behare PV, Grover CR, Aggarwal PK. Effect of probiotic fermented milk and chlorophyllin on gene expressions and genotoxicity during AFB1-induced hepatocellular carcinoma. Gene 490: 54–59, 2011. doi: 10.1016/j.gene.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Shaughnessy DT, Gangarosa LM, Schliebe B, Umbach DM, Xu Z, MacIntosh B, Knize MG, Matthews PP, Swank AE, Sandler RS, DeMarini DM, Taylor JA. Inhibition of fried meat-induced colorectal DNA damage and altered systemic genotoxicity in humans by crucifera, chlorophyllin, and yogurt. PLoS One 6: e18707, 2011. doi: 10.1371/journal.pone.0018707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Díaz GD, Li Q, Dashwood RH. Caspase-8 and apoptosis-inducing factor mediate a cytochrome c-independent pathway of apoptosis in human colon cancer cells induced by the dietary phytochemical chlorophyllin. Cancer Res 63: 1254–1261, 2003. [PubMed] [Google Scholar]
- 20.Nagini S, Palitti F, Natarajan AT. Chemopreventive potential of chlorophyllin: a review of the mechanisms of action and molecular targets. Nutr Cancer 67: 203–211, 2015. doi: 10.1080/01635581.2015.990573. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocaturk NM, Gozuacik D. Crosstalk between mammalian autophagy and the ubiquitin-proteasome system. Front Cell Dev Biol 6: 128, 2018. doi: 10.3389/fcell.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryter SW, Cloonan SM, Choi AM. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cells 36: 7–16, 2013. doi: 10.1007/s10059-013-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Günther S, Prescott NJ, Onnie CM, Häsler R, Sipos B, Fölsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 39: 207–211, 2007. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 26.Henckaerts L, Cleynen I, Brinar M, John JM, Van Steen K, Rutgeerts P, Vermeire S. Genetic variation in the autophagy gene ULK1 and risk of Crohn's disease. Inflamm Bowel Dis 17: 1392–1397, 2011. doi: 10.1002/ibd.21486. [DOI] [PubMed] [Google Scholar]
- 27.Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, McArdle W, Strachan D, Bethel G, Bryan C, Lewis CM, Deloukas P, Forbes A, Sanderson J, Jewell DP, Satsangi J, Mansfield JC, Cardon L; Wellcome Trust Case Control Consortium, Cardon L, Mathew CG. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet 39: 830–832, 2007. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW 4th.. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263, 2008. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456: 264–268, 2008. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 30.Thachil É, Hugot JP, Arbeille B, Paris R, Grodet A, Peuchmaur M, Codogno P, Barreau F, Ogier-Denis É, Berrebi D, Viala J. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn's disease. Gastroenterology 142: 1097–1099.e4, 2012. doi: 10.1053/j.gastro.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Shen T, Li S, Cai LD, Liu JL, Wang CY, Gan WJ, Li XM, Wang JR, Sun LN, Deng M, Liu YH, Li JM. Erbin exerts a protective effect against inflammatory bowel disease by suppressing autophagic cell death. Oncotarget 9: 12035–12049, 2018. doi: 10.18632/oncotarget.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SL, Shao BZ, Zhao SB, Chang X, Wang P, Miao CY, Li ZS, Bai Y. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell Death Dis 10: 391, 2019. doi: 10.1038/s41419-019-1634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8: 1327–1336, 2006. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 34.Wu P, Zhang R, Luo M, Zhang T, Pan L, Xu S, Pan L, Ren F, Ji C, Hu R, Noureddin M, Pandol SJ, Han YP. Liver injury impaired 25-hydroxylation of vitamin D suppresses intestinal Paneth cell defensins, leading to gut dysbiosis and liver fibrogenesis. Am J Physiol Gastrointest Liver Physiol 319: G685–G695, 2020. doi: 10.1152/ajpgi.00021.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12: 1295–1309, 2017. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 36.Tréton X, Pédruzzi E, Cazals-Hatem D, Grodet A, Panis Y, Groyer A, Moreau R, Bouhnik Y, Daniel F, Ogier-Denis E. Altered endoplasmic reticulum stress affects translation in inactive colon tissue from patients with ulcerative colitis. Gastroenterology 141: 1024–1035, 2011. doi: 10.1053/j.gastro.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 37.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 38.John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal 15: 1255–1270, 2011. doi: 10.1089/ars.2011.3892. [DOI] [PubMed] [Google Scholar]
- 39.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344: 427–431, 1999. [PMC free article] [PubMed] [Google Scholar]
- 40.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 41.He S, Guo Y, Zhao J, Xu X, Wang N, Liu Q. Ferulic acid ameliorates lipopolysaccharide-induced barrier dysfunction via microRNA-200c-3p-mediated activation of PI3K/AKT pathway in Caco-2 cells. Front Pharmacol 11: 376, 2020. doi: 10.3389/fphar.2020.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin N, Xu LF, Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem Biophys Res Commun 440: 143–149, 2013. doi: 10.1016/j.bbrc.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 43.Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbrügger RW, Brummer RJ. Diet as a risk factor for the development of ulcerative colitis. Am J Gastroenterology 95: 1008–1013, 2000. doi: 10.1111/j.1572-0241.2000.01942.x. [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K, Kobashi G, Washio M, Yokoyama T, Date C, Tanaka H; Epidemiology Group of the Research Committee on Inflammatory Bowel Disease in Japan. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis 11: 154–163, 2005. doi: 10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology 145: 970–977, 2013. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Zheng L, Chen J, Fukata M, Ichikawa R, Shih DQ, Zhang X. The protection role of Atg16l1 in CD11c+ dendritic cells in murine colitis. Immunobiology 222: 831–841, 2017. doi: 10.1016/j.imbio.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabrera S, Fernández AF, Mariño G, Aguirre A, Suárez MF, Español Y, Vega JA, Laurà R, Fueyo A, Fernández-García MS, Freije JM, Kroemer G, López-Otín C. ATG4B/autophagin-1 regulates intestinal homeostasis and protects mice from experimental colitis. Autophagy 9: 1188–1200, 2013. doi: 10.4161/auto.24797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cadwell K, Patel KK, Komatsu M, Virgin HW 4th, Stappenbeck TS. A common role for Atg16L1, Atg5 and Atg7 in small intestinal Paneth cells and Crohn disease. Autophagy 5: 250–252, 2009. doi: 10.4161/auto.5.2.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denadai-Souza A, Bonnart C, Tapias NS, Marcellin M, Gilmore B, Alric L, Bonnet D, Burlet-Schiltz O, Hollenberg MD, Vergnolle N, Deraison C. Functional proteomic profiling of secreted serine proteases in health and inflammatory bowel disease. Sci Rep 8: 7834, 2018. doi: 10.1038/s41598-018-26282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lügering N, Kucharzik T, Stein H, Winde G, Lügering A, Hasilik A, Domschke W, Stoll R. IL-10 synergizes with IL-4 and IL-13 in inhibiting lysosomal enzyme secretion by human monocytes and lamina propria mononuclear cells from patients with inflammatory bowel disease. Dig Dis Sci 43: 706–714, 1998. doi: 10.1023/a:1018845526434. [DOI] [PubMed] [Google Scholar]
- 51.Yue W, Liu Y, Li X, Lv L, Huang J, Liu J. Curcumin ameliorates dextran sulfate sodium-induced colitis in mice via regulation of autophagy and intestinal immunity. Turk J Gastroenterol 30: 290–298, 2019. doi: 10.5152/tjg.2019.18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagar J, Ranade S, Kamath V, Singh S, Karunanithi P, Subramani S, Venkatesh K, Srivastava R, Dudhgaonkar S, Vikramadithyan RK. Therapeutic potential of chloroquine in a murine model of inflammatory bowel disease. Int Immunopharmacol 21: 328–335, 2014. doi: 10.1016/j.intimp.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Goenka MK, Kochhar R, Tandia B, Mehta SK. Chloroquine for mild to moderately active ulcerative colitis: comparison with sulfasalazine. Am J Gastroenterol 91: 917–921, 1996. [PubMed] [Google Scholar]
- 54.Liu Y, Shoji-Kawata S, Sumpter RM Jr, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, Shaw SY, Clarke PG, Puyal J, Levine B. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA 110: 20364–20371, 2013. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yonekawa T, Thorburn A. Autophagy and cell death. Essays Biochem 55: 105–117, 2013. doi: 10.1042/bse0550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JE, Kim HR, Kim JC, Lee ES, Chung CH, Lee EY, Chung BY. Tetrahydrocurcumin ameliorates skin inflammation by modulating autophagy in high-fat diet-induced obese mice. BioMed Res Int 2021: 6621027, 2021. doi: 10.1155/2021/6621027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding H, Ge G, Tseng Y, Ma Y, Zhang J, Liu J. Hepatic autophagy fluctuates during the development of non-alcoholic fatty liver disease. Ann Hepatol 19: 516–522, 2020. doi: 10.1016/j.aohep.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Zheng H, You Y, Hua M, Wu P, Liu Y, Chen Z, Zhang L, Wei H, Li Y, Luo M, Zeng Y, Liu Y, Luo DX, Zhang J, Feng M, Hu R, Pandol SJ, Han YP. Chlorophyllin modulates gut microbiota and inhibits intestinal inflammation to ameliorate hepatic fibrosis in mice. Front Physiol 9: 1671, 2018. doi: 10.3389/fphys.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]