Abstract
Objective
This study aims to determine the relationship between the presence of urinary nitrite and bacterial resistance to antimicrobial therapy in patients with uncomplicated urinary tract infections.
Methods
During a six-month time period (April-October, 2020), we reviewed the urine samples of 59 adult outpatients from the Urology Department of Tbilisi State Medical University the First University Clinic with the diagnosis of urinary tract infection. The infecting microorganisms and the presence of urine nitrite were recorded. Resistance rates to the antibiotics were compared between the positive and negative nitrite groups. Chi-squared test was used to perform the statistical analysis using Prism software version 9.3.1 (GraphPad Software, Inc., San Diego, California).
Results
We examined the correlation between the nitrite-positive and -negative groups with the resistance pattern to ceftriaxone, trimethoprim/sulfamethoxazole (TMP-SMX), ampicillin-sulbactam, fosfomycin, amikacin, doxycycline, cefuroxime, cefotaxime, ceftazidime, and nitrofurantoin.
A total of 59 outpatients with a mean age of 37 years met the inclusion criteria between April and October 2020. In the positive and negative nitrite groups, there were 23 and 36 patients, respectively. Three (17.6%) of the 17 gram-positive organisms and 20 (62.5%) of the 42 gram-negative organisms yielded positive nitrite results.
In nitrite-positive group, resistance rates to ceftriaxone, TMP-SMX, ampicillin-sulbactam, fosfomycin, amikacin, doxycycline, cefuroxime, cefotaxime, ceftazidime, and nitrofurantoin were 52.2%, 70.8%, 63.5%, 67.7%, 25.8%, 31.9%, 29.6%, 32.5%, 22.5% and 83.8%, respectively. These values in the nitrite-negative group were 6.5%, 41.3%, 60.7%, 72.9%, 49%, 3%, 2.3%, 3.3%, 4.3% and 81.9%, respectively.
Highest relative resistance rate was recorded against cefuroxime (12.9), followed by doxycycline (10.6), cefotaxime (9.8), ceftriaxone (8.03), ceftazidime (5.2), TMP-SMX (1.71), ampicillin-sulbactam (1.05), nitrofurantoin (1.02), fosfomycin (0.93), and amikacin (0.53).
The most commonly isolated pathogen was Escherichia coli, which was detected in 35 (71%) isolates. Other bacteria commonly found were Proteus spp in five (12%) isolates, Klebsiella spp in two (5%) isolates, and Enterococcus in five (12%) isolates.
Conclusion
The findings revealed that out of 10 antibiotics, nitrite-positive groups demonstrated higher resistance only against ceftriaxone, cefuroxime, cefotaxime, and doxycycline. Other antibiotics showed no statistically significant differences in resistance. Furthermore, the highest relative resistance rate was recorded against cefuroxime, whereas amikacin revealed the lowest. Therefore, we suggest physicians to not adjust antibiotic therapy for urinary tract infections (UTIs) based on the presence of nitrite. Urine bacteriology should be ordered.
Keywords: antibiotic resistance, anti-bacterial agents, escherichia coli, nitrite, dysuria, urinary tract infection
Introduction
Urinary tract infection (UTI) is a common disease. It is caused by pathogenic microorganisms (bacteria, viruses, or fungi) invading the urinary tract system [1,2]. Cystitis, pyelonephritis, and asymptomatic bacteriuria are the three manifestations of the condition [1,2]. In 70%-90% of cases, Escherichia coli is the etiologic agent [1,2]. Early detection and timely and adequate treatment of the disease are critical to preventing major complications such as renal scarring, hypertension, and chronic renal failure [3,4]. Regarding the rising treatment resistance of microorganisms causing UTIs, appropriate selection of the initial antibiotic before collecting urine culture and antibiogram results are critical [1,2,5-9]. The question is whether it is possible to predict microorganism resistance using markers, such as the nitrite test, prior to preparing urine cultures and antibiogram results. According to Weisz et al., urine nitrite results can be used to identify cephalosporin resistance [10]. On the other hand, other studies contradict this viewpoint [11,12].
Antimicrobial resistance is increasing globally, making infections more difficult to treat, and is related to increased mortality, morbidity, and cost [13,14]. A growing proportion of community-acquired uncomplicated UTIs is caused by multidrug-resistant gram-negative bacilli. As a result, empirical therapy is more likely to fail. Because there are no oral treatment alternatives, an increasing number of individuals with uncomplicated UTIs require hospitalization for intravenous antibiotics.
Nitrite tests look for the metabolites of nitrite reductase, an enzyme generated by a variety of microorganisms. Unless there is a UTI, these compounds are not generally present. This test has a sensitivity and specificity of 25% and 94%-100%, respectively. The limited sensitivity has been linked to infection caused by enzyme-deficient bacteria or low-grade bacteriuria. A positive nitrite test result is fairly specific for UTI, mainly due to urease-positive organisms such as Proteus species and, on rare occasions, E. coli; nevertheless, it is very insensitive as a screening tool, with only 25% of UTI patients having a positive nitrite test result.
The link between urinary nitrite and UTIs was initially documented in 1914 and has since been the subject of extensive attention [2]. The benefits of using urinary nitrites include the low cost, the speed with which the results are provided, and the ability to categorize patients into two distinct groups - nitrite positive or negative [14].
Knowing the pathogen spectrum and resistance patterns in the community allows the physician to choose an effective empiric agent. There has been little research on whether the absence of urine nitrites reflects resistance to popular antibiotics used to treat uncomplicated UTIs. Furthermore, the findings of the few studies that have looked into this correlation are contradictory [15]. This study aimed to investigate if nitrite findings in urinalysis could be utilized to guide bacterial isolate and resistance when treating UTIs.
Materials and methods
The urine samples of 59 adult outpatients with a mean age of 37 years and a diagnosis of UTI were reviewed. The Urology Department at Tbilisi State Medical University (TSMU) the First University Clinic provided samples throughout a six-month period (April-October 2020). Patients who were below the age of 18 years, who did not have a urine culture sent to the laboratory, or those with the diagnosis of UTI but a negative culture were excluded from the study.
The tests for bacterial identification and antibiotic sensitivity were carried out at the clinical microbiology laboratory of TSMU the First University Clinic. Samples were promptly transported to the laboratory and processed within 30 minutes of being collected. If a delay of more than one to two hours was expected, the specimen was refrigerated. All specimens were cultured on blood agar and MacConkey agar for 24 hours at 37°C.
All samples were subjected to the following tests: (a) gram staining of the colonies, (b) biochemical reactions using the Analytical Profile Index (API) identification system (API20E, API20 NE, APIstaph, APIstrep; bioMérieux, France), and (c) identification and antimicrobial sensitivity testing using the Kirby-Bauer disk diffusion method.
Ceftriaxone, trimethoprim-sulfamethoxazole (TMP-SMX), ampicillin-sulbactam, fosfomycin, amikacin, doxycycline, cefuroxime, cefotaxime, ceftazidime, and nitrofurantoin were all tested on isolated bacteria. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) standard was used to assess antibiotic susceptibility/resistance.
Statistical analysis was performed using Prism software version 9.3.1 (GraphPad Software, Inc., San Diego, California). Chi-squared test was used to compare the variables. P-value ≤ 0.05 was considered statistically significant.
Results
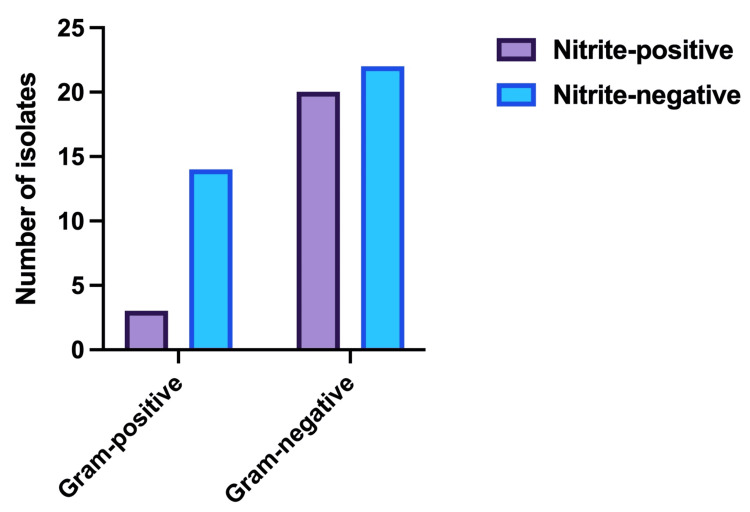
We examined the correlation between the nitrite-positive and -negative groups with the resistance pattern to ceftriaxone, TMP-SMX, ampicillin-sulbactam, fosfomycin, amikacin, doxycycline, cefuroxime, cefotaxime, ceftazidime, and nitrofurantoin. A total of 59 outpatients with a mean age of 37 years met the inclusion criteria between April and October 2020. Table 1 reveals that in the positive and negative nitrite groups, there were 23 and 36 patients, respectively. Three (17.6%) of the 17 gram-positive organisms and 20 (62.5%) of the 42 gram-negative organisms yielded positive nitrite results.
Table 1. Nitrite test results in gram-positive and gram-negative isolates.
| Gram stain/Nitrite test | Nitrite-positive | Nitrite-negative | Total |
| Gram-positive | 3 | 14 | 17 |
| Gram-negative | 20 | 22 | 42 |
| Total | 23 | 36 | 59 |
The gram stain and nitrite test patterns for all isolated pathogens are shown in Figure 1.
Figure 1. Gram stain and nitrite test pattern for isolated pathogens.
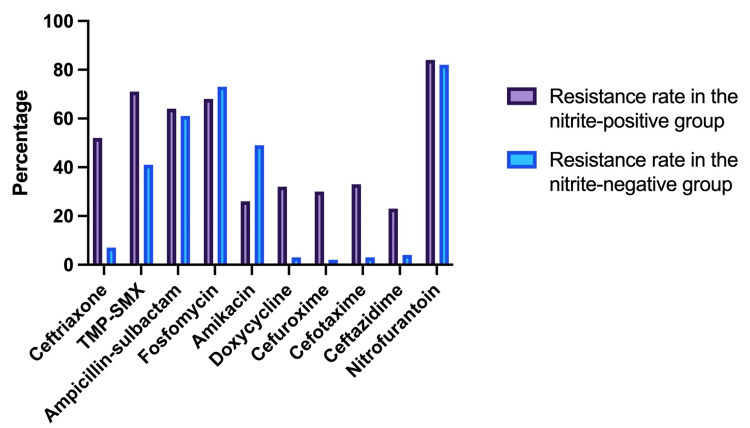
Table 2 shows that in nitrite-positive group, resistance rates to ceftriaxone, TMP-SMX, ampicillin-sulbactam, fosfomycin, amikacin, doxycycline, cefuroxime, cefotaxime, ceftazidime, and nitrofurantoin were 52.2%, 70.8%, 63.5%, 67.7%, 25.8%, 31.9%, 29.6%, 32.5%, 22.5%, and 83.8%, respectively. These values in the nitrite-negative group were 6.5%, 41.3%, 60.7%, 72.9%, 49%, 3%, 2.3%, 3.3%, 4.3%, and 81.9%, respectively. Only ceftriaxone, cefuroxime, cefotaxime, and doxycycline showed a statistically significant difference (p < 0.05). Highest relative resistance rate was recorded against cefuroxime (12.9), followed by doxycycline (10.6), cefotaxime (9.8), ceftriaxone (8.03), ceftazidime (5.2), TMP-SMX (1.71), ampicillin-sulbactam (1.05), nitrofurantoin (1.02), fosfomycin (0.93), and amikacin (0.53).
Table 2. Antibiotic resistance rates in nitrite-negative and nitrite-positive groups.
TMP-SMX: Trimethoprim-sulfamethoxazole.
| Antibiotics | Resistance rate in the nitrite-positive group (%) | Resistance rate in the nitrite-negative group (%) | P-value | Relative resistance rate in nitrite-positive versus nitrite-negative group |
| Ceftriaxone | 52.2 | 6.5 | 0.00005 | 8.03 |
| TMP-SMX | 70.8 | 41.3 | 0.56 | 1.71 |
| Ampicillin-sulbactam | 63.5 | 60.7 | 1 | 1.05 |
| Fosfomycin | 67.7 | 72.9 | 1 | 0.93 |
| Amikacin | 25.8 | 49 | 0.62 | 0.53 |
| Doxycycline | 31.9 | 3 | 0.004 | 10.6 |
| Cefuroxime | 29.6 | 2.3 | 0.005 | 12.9 |
| Cefotaxime | 32.5 | 3.3 | 0.005 | 9.8 |
| Ceftazidime | 22.5 | 4.3 | 0.19 | 5.2 |
| Nitrofurantoin | 83.8 | 81.9 | 1 | 1.02 |
Figure 2 shows the correlation between nitrite findings and resistance patterns to various antibiotics that are being used to treat UTIs.
Figure 2. Comparison of resistance rates between the nitrite-positive and nitrite-negative groups.
TMP-SMX: Trimethoprim-sulfamethoxazole.
As depicted in Table 3, the most commonly isolated pathogen was E. coli, which was detected in 35 (71%) isolates. Other bacteria included Proteus spp in five (12%) isolates, Klebsiella spp in two (5%) isolates, and Enterococcus in five (12%) isolates.
Table 3. Frequency of isolated uropathogens.
| Pathogen | Number of isolates | Percentage of isolates (%) |
| Escherichia coli | 35 | 71 |
| Proteus spp | 5 | 12 |
| Klebsiella spp | 2 | 5 |
| Enterococcus | 5 | 12 |
Discussion
The purpose of this study was to see if the presence of urine nitrite could help clinicians choose the most appropriate empirical antibiotic before the bacteriological analysis findings. Commonly seen pathogens in complicated UTIs which do not convert nitrates to nitrites include Enterococcus, Pseudomonas, and Acinetobacter [16].
Weisz et al. stated that urine nitrite results are a useful sign of resistance to empiric therapy with first- and third-generation cephalosporins [10]. In our study, we subjected nitrite-positive and nitrite-negative groups to second and third-generation cephalosporins including cefuroxime, cefotaxime, ceftriaxone, and ceftazidime. In our study, only cefuroxime, cefotaxime, and ceftriaxone revealed a statistically significant difference (p ≤ 0.05) among these four antibiotics.
Larson et al. compared the sensitivity of nitrite-positive and nitrite-negative cultures to TMP-SMX. Results revealed that out of 86 nitrite-positive cultures, 22% showed resistance, whereas out of 73 nitrite-negative cultures, only 18% were resistant to the drug. There was no statistically significant difference in the proportion of TMP-SMX-resistant isolates [12]. Our findings revealed a similar pattern as we did not find a statistically significant difference between the two groups. Our results revealed the resistance rates of 70.8% and 41.3% in nitrite-positive and nitrite-negative groups, respectively. The variation in percentages may have been caused by the number of individual microorganisms included in the study since it is known that Enterococci tend to be more susceptible to TMP-SMX therapy [17].
Enterococcus, a less common uropathogen, does not produce nitrite and has a unique treatment resistance pattern [18]. Because of the expression of low-affinity penicillin-binding proteins, all enterococci have decreased sensitivity to penicillin and ampicillin but high-level resistance to most cephalosporins and all semi-synthetic penicillins [19].
A urine pH below 6.0, the amount of bacteriuria, the short time between collection and testing, dilute urine, and the presence of blood, urobilinogen, vitamin C, or medications can all cause a false-negative nitrite result. In addition, although several uropathogens, including E. coli, Klebsiella, and Proteus, can convert nitrate to nitrite, others cannot. Therefore, the nitrite test cannot be used to detect the presence of bacterial infection [10].
The main limitation of our study was the sampling size. Despite the fact that we found a statistically significant difference in resistance to four antibiotics, additional data is needed to draw a more certain conclusion.
Conclusions
The findings revealed that out of 10 antibiotics, nitrite-positive groups demonstrated higher resistance only against ceftriaxone, cefuroxime, cefotaxime, and doxycycline. Other antibiotics showed no statistically significant differences in resistance. The highest relative resistance rate was recorded against cefuroxime, whereas amikacin demonstrated the lowest.
Based on our findings, we suggest physicians to not adjust antibiotic therapy for UTIs based on the presence of nitrite, and urine bacteriology should be ordered.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Elder JS. Nelson Textbook of Pediatrics. Vol. 18. Philadelphia: Saunders; 2007. Urinary tract infections; p. 222. [Google Scholar]
- 2.Hansson S, Jodal U. Pediatric Nephrology. Vol. 5. Philadelphia: Lippincott, Williams & Wilkins; 2004. Urinary tract infection; p. 10. [Google Scholar]
- 3.The effect of vitamin A on renal damage following acute pyelonephritis in children. Ayazi P, Moshiri SA, Mahyar A, Moradi M. https://doi.org/10.1007/s00431-010-1297-1. Eur J Pediatr. 2011;170:347–350. doi: 10.1007/s00431-010-1297-1. [DOI] [PubMed] [Google Scholar]
- 4.Comparison of procalcitonin and C-reactive protein tests in children with urinary tract infection. [ May; 2022 ];Ayazi P, Mahyar A, Hashemi J, Daneshi MM. https://www.researchgate.net/publication/222107129_Comparison_of_Procalcitonin_and_C-reactive_protein_tests_in_children_with_urinary_tract_infection Iran J Pediatr. 2009 19:381–386. [Google Scholar]
- 5.Antibiotic resistance of urinary tract pathogens and evaluation of empirical treatment in Turkish children with urinary tract infections. Yüksel S, Oztürk B, Kavaz A, et al. https://doi.org/10.1016/j.ijantimicag.2006.08.009. Int J Antimicrob Agents. 2006;28:413–416. doi: 10.1016/j.ijantimicag.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Changing trend in antimicrobial resistance of pediatric uropathogens in Taiwan. Tseng MH, Lo WT, Lin WJ, Teng CS, Chu ML, Wang CC. https://doi.org/10.1111/j.1442-200X.2008.02738.x. Pediatr Int. 2008;50:797–800. doi: 10.1111/j.1442-200X.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- 7.Bacterial resistance to antimicrobials in urinary isolates. Muratani T, Matsumoto T. https://doi.org/10.1016/j.ijantimicag.2004.02.001. Int J Antimicrob Agents. 2004;24:0–31. doi: 10.1016/j.ijantimicag.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Fluoroquinolones and resistance in the treatment of uncomplicated urinary tract infection. Hooton TM. https://doi.org/10.1016/s0924-8579. Int J Antimicrob Agents. 2003;22 Suppl 2:65–72. doi: 10.1016/s0924-8579(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 9.Urinary tract infection caused by fluoroquinolone- and cephem-resistant Enterobacteriaceae. Muratani T, Matsumoto T. https://doi.org/10.1016/j.ijantimicag.2006.05.009. Int J Antimicrob Agents. 2006;28:0–3. doi: 10.1016/j.ijantimicag.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.The presence of urinary nitrites is a significant predictor of pediatric urinary tract infection susceptibility to first- and third-generation cephalosporins. Weisz D, Seabrook JA, Lim RK. https://doi.org/10.1016/j.jemermed.2008.01.010. J Emerg Med. 2010;39:6–12. doi: 10.1016/j.jemermed.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Urine nitrite not correlated with bacterial resistance to cephalosporins. Grant DC, Chan L, Waterbrook A. https://doi.org/10.1016/j.jemermed.2004.09.014. J Emerg Med. 2005;28:321–323. doi: 10.1016/j.jemermed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Can urinary nitrite results be used to guide antimicrobial choice for urinary tract infection? Larson MJ, Brooks CB, Leary WC, Lewis LM. https://doi.org/10.1016/s0736-4679(97)00069-3. J Emerg Med. 1997;15:435–438. doi: 10.1016/s0736-4679(97)00069-3. [DOI] [PubMed] [Google Scholar]
- 13.Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Walker E, Lyman A, Gupta K, Mahoney MV, Snyder GM, Hirsch EB. Clin Infect Dis. 2016;63:960–965. doi: 10.1093/cid/ciw396. [DOI] [PubMed] [Google Scholar]
- 14.Oral and parenteral therapeutic options for outpatient urinary infections caused by enterobacteriaceae producing CTX-M extended-spectrum beta-lactamases. Prakash V, Lewis JS 2nd, Herrera ML, Wickes BL, Jorgensen JH. Antimicrob Agents Chemother. 2009;53:1278–1280. doi: 10.1128/AAC.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Spellberg B, Guidos R, Gilbert D, et al. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 16.Bono MJ, Reygaert WC. StatPearls Publishing. Treasure Island, FL: StatPearls Publishing; [ May; 2022 ]. 2021. Urinary Tract Infection. [Google Scholar]
- 17.Susceptibility of enterococci to trimethoprim and trimethoprim-sulfamethoxazole. Crider SR, Colby SD. https://doi.org/10.1128/AAC.27.1.71. Antimicrob Agents Chemother. 1985;27:71–75. doi: 10.1128/aac.27.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Should the absence of urinary nitrite influence empiric antibiotics for urinary tract infection in young children? Chaudhari PP, Monuteaux MC, Bachur RG. https://doi.org/10.1097/PEC.0000000000001344. Pediatr Emerg Care. 2020;36:481–485. doi: 10.1097/PEC.0000000000001344. [DOI] [PubMed] [Google Scholar]
- 19.Kristich CJR, Rice LB, Arias CA. Boston: Massachusetts Eye and Ear Infirmary. Boston: Massachusetts Eye and Ear Infirmary; [ May; 2022 ]. 2014. Enterococcal Infection—Treatment and Antibiotic Resistance. [Google Scholar]