Abstract
Background
Hypertensive disorders account for 14% of global maternal deaths. Magnesium sulphate (MgSO4) is recommended for prevention and treatment of pre‐eclampsia/eclampsia. However, MgSO4 remains underused, particularly in low‐ and middle‐income countries (LMICs).
Objective
This qualitative evidence synthesis explores perceptions and experiences of healthcare providers, administrators and policy‐makers regarding factors affecting use of MgSO4 to prevent or treat pre‐eclampsia/eclampsia.
Search strategy
We searched MEDLINE, EMBASE, Emcare, CINAHL, Global Health and Global Index Medicus, and grey literature for studies published between January 1995 and June 2021.
Selection criteria
Primary qualitative and mixed‐methods studies on factors affecting use of MgSO4 in healthcare settings, from the perspectives of healthcare providers, administrators and policy‐makers, were eligible for inclusion.
Data collection and analysis
We applied a thematic synthesis approach to analysis, using COM‐B behaviour change theory to map factors affecting appropriate use of MgSO4.
Main results
We included 22 studies, predominantly from LMICs. Key themes included provider competence and confidence administering MgSO4 (attitudes and beliefs, complexities of administering, knowledge and experience), capability of health systems to ensure MgSO4 availability at point of use (availability, resourcing and pathways to care) and knowledge translation (dissemination of research and recommendations). Within each COM‐B domain, we mapped facilitators and barriers to physical and psychological capability, physical and social opportunity, and how the interplay between these domains influences motivation.
Conclusions
These findings can inform policy and guideline development and improve implementation of MgSO4 in clinical care. Such action is needed to ensure this life‐saving treatment is widely available and appropriately used.
Tweetable abstract
Global qualitative review identifies factors affecting underutilisation of MgSO4 for pre‐eclampsia and eclampsia.
Keywords: Eclampsia, health systems, hypertension, magnesium sulphate, pre‐eclampsia, pregnancy, qualitative evidence synthesis, systematic review
Tweetable abstract
Global qualitative review identifies factors affecting underutilisation of MgSO4 for pre‐eclampsia and eclampsia.
 This article includes Author Insights, a video abstract available at https://vimeo.com/manage/videos/623192027
This article includes Author Insights, a video abstract available at https://vimeo.com/manage/videos/623192027
Linked article This article is commented on by LA Magee, p. 392 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16971.
Introduction
Pre‐eclampsia and eclampsia are hypertensive disorders of pregnancy experienced by 4.6% and 1.4% of women, respectively, during pregnancy and childbirth. 1 They are characterised by high blood pressure, proteinuria and (once progressed to eclampsia) seizures, which can be fatal to both woman and baby. 2 , 3 Hypertensive disorders are the second leading direct cause of maternal mortality, accounting for 14% of maternal deaths. 4 However, the precise contribution is unknown for many low‐ and middle‐income countries (LMICs) because of suboptimal detection and reporting of these conditions.
Magnesium sulphate (MgSO4) is recommended by the World Health Organization (WHO) for women with severe pre‐eclampsia/eclampsia, 5 and has been proven to be effective in major trials 6 , 7 and meta‐analyses. 8 , 9 , 10 , 11 Administering MgSO4 to women who have pre‐eclampsia halves their risk of developing eclampsia, 10 and more than halves their risk of death. 4 MgSO4 is cost‐effective 12 and safe, 13 , 14 , 15 , 16 , 17 and has been on the WHO Essential Medicines List since 1996. 18 , 19 The current WHO recommendation for severe pre‐eclampsia/eclampsia is the administration of a full intravenous/intramuscular regimen, including an initial loading dose and maintenance doses over 24 hours. 5 Women must be monitored for signs of magnesium toxicity between doses, therefore it is recommended that MgSO4 be administered in health facilities with adequate staffing and clinical resources for monitoring. 20 Alternative dosage regimens of MgSO4 are also being evaluated. 21 , 22
Despite strong evidence of effectiveness and cost‐effectiveness, MgSO4 is often underused for these indications, 13 , 23 , 24 , 25 particularly in LMICs. 26 Previous research has explored factors affecting MgSO4 guideline implementation, identifying system and market failures that restrict drug availability, absence of clinical protocols and staff reluctance. 18 , 27 , 28 , 29 However, to our knowledge, no systematic review has synthesised evidence on factors affecting use of MgSO4 for women with pre‐eclampsia/eclampsia. A qualitative evidence synthesis (QES) may help to understand why MgSO4 is not more widely adopted in practice. The aim of this QES is to explore the perceptions and experiences of healthcare providers, administrators and policy‐makers regarding factors affecting use of MgSO4 to prevent or treat pre‐eclampsia/eclampsia administered globally, and to develop a conceptual understanding of how these factors influence MgSO4 use.
Methods
We followed the Cochrane Effective Practice and Organisation of Care (EPOC) QES guidance 30 and report our findings according to the ‘Enhancing transparency in reporting the synthesis of qualitative research’ (ENTREQ) statement (Appendix [Link], [Link]); the review protocol is registered with PROSPERO (CRD42020167185). 31 There was no patient or public involvement, and a core outcome set was not applicable in this review.
Inclusion criteria
Eligible studies considered factors affecting use of MgSO4 (any dosage regimen) for pre‐eclampsia/eclampsia prevention or treatment during the antenatal, intrapartum and postpartum periods. Eligible studies included participants who were health system stakeholders (policy‐makers, administrators, health workers and lay health workers). Eligible studies were conducted in healthcare facilities of any level (e.g. hospitals, clinics and primary health care) in any country. Home or community settings were excluded as MgSO4 is not recommended for use outside healthcare facilities. Eligible studies were primary studies using qualitative methods for data collection and analysis. 30 Conference abstracts, posters and clinical case studies were excluded. Mixed‐method studies were eligible if they used qualitative data collection and analysis methods.
Search methods
We searched MEDLINE, EMBASE, Emcare, CINAHL, Global Health and Global Index Medicus for studies from 1 January 1995 (when the Eclampsia Collaborative study definitively established MgSO4 as an effective treatment for eclampsia 6 ) to 28 June 2021. Search strategies were developed using MgSO4, anticonvulsant, pre‐eclampsia/eclampsia and hypertension terms (Table [Link], [Link]). We did not use a methodological filter. 30 We searched OpenGrey, AHRQ, NICE, Jhpiego, Population Council, WHO international and Google Scholar for grey literature, and reviewed reference lists of included studies. 8 , 9 , 10 , 11 , 21
Study selection and data extraction
Two reviewers (from KEE, RIZ, MAB, JPV) independently reviewed each title and abstract for eligibility. We retrieved full texts for potentially relevant studies and two reviewers independently assessed eligibility (from KEE, RIZ, MAB). Disagreements at either stage were resolved by discussion, involving a third reviewer if necessary. We used Google Translate to translate titles and abstracts published in languages other than English; where translation indicated possible inclusion, we planned to list as ‘studies awaiting classification’ (no articles met these criteria). Where more than one paper reported the same study (using the same sample and methods), the papers were collated to ensure the study was the unit of interest. 30 A data extraction form was developed and used to extract data on context and design, and qualitative data (author themes, interpretation and participant quotes). 30
Methodological limitations of included studies
Critical appraisal of included studies was conducted by two reviewers independently (KEE, RIZ) using an adaptation of the Critical Skills Appraisal Programme (CASP) tool. 32 Consensus was reached through discussion. We assessed study aims, methodology, design, recruitment, data collection, data analysis, reflexivity, ethical considerations, findings and research contribution. 33 We did not exclude studies based on this assessment, but used the assessments in GRADE‐CERQual (Confidence in the Evidence from Reviews of Qualitative Research) assessments. 34
Data management, analysis and synthesis
We used an inductive ‘thematic synthesis’ approach 35 to synthesise themes emerging from the data (KEE, RIZ, MAB). We began by free line‐by‐line coding results of five highly relevant studies covering different health system levels and stakeholder types. Codes were organised into a hierarchy, grouping related codes under descriptive themes. Results from included studies were coded line‐by‐line in nvivo 36 using the codebook, which developed iteratively throughout analysis. Text assigned to each code was checked for consistency and further division into sub‐codes. We developed higher‐order analytical themes to identify health system factors affecting use of MgSO4. We then used COM‐B behaviour change theory 37 to explore and categorise health system stakeholders’ Capability, Opportunity, Motivation to appropriately use MgSO4.
Confidence in the review findings
Two review authors (KEE, RIZ) used the GRADE‐CERQual approach to assess confidence in each review finding, 38 based on methodological limitations, 34 coherence, 39 adequacy 40 and relevance. 41 We assessed each component by levels of concern (no or very minor/minor/moderate/serious), then made a judgement about the overall confidence in the review finding (high/moderate/low/very low). 42 All findings started as high and were downgraded where concerns about GRADE‐CERQual components were identified. We present summaries of findings and GRADE‐CERQual assessments in a summary of qualitative findings table (Table [Link], [Link]) and evidence profile (Table [Link], [Link]).
Reflexivity
We maintained a reflexive stance throughout the review process and regularly discussed and critically reflected on our positionality. 30 , 43 At the outset of this review, the review team considered that MgSO4 is an effective intervention that should be used for prevention and treatment of pre‐eclampsia/eclampsia, and that health system barriers probably limit the implementation in practice. The review team has expertise in public health (KEE, RIZ, JPV, MAB), women's health (KEE, RIZ, JPV, MAB), health economics (KEE), social science (MAB), medicine (JPV) and epidemiology (KEE, RIZ, JPV, MAB). We remained mindful of our presuppositions to minimise the risk of these skewing our analysis or interpretation. 30 , 43 Specifically, we used refutational analysis techniques, such as exploring and explaining any contradictory findings between studies. 33
Results
We included 25 papers from 22 studies (Figure 1, Table 1), published between 2005 and 2021. One global study was conducted in 24 countries, 44 the remaining 21 studies were conducted in 12 countries: Bangladesh, Brazil, Ethiopia, India, Kenya, Malawi, Mexico, Mozambique, Pakistan, Nigeria, South Africa and Zimbabwe. All but the global study were from LMICs. Studies included perspectives of facility administrators, 13 , 14 , 27 , 28 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 health researchers, 29 , 44 , 50 , 55 policy‐makers, 29 , 44 , 45 , 46 , 47 , 48 , 49 , 51 , 52 , 55 doctors, 13 , 27 , 29 , 44 , 48 , 49 , 50 , 56 , 57 , 58 midwives/nurses, 29 , 44 , 47 , 48 , 49 , 52 , 56 , 59 , 60 , 61 and community health workers. 48 , 58 , 60
Figure 1.
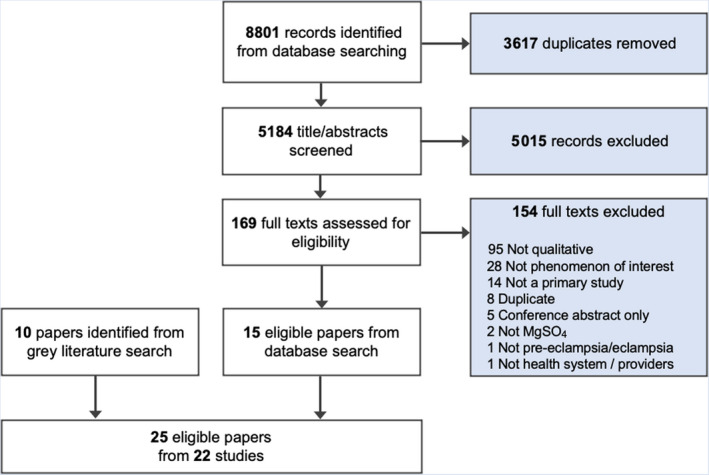
PRISMA diagram.
Table 1.
Characteristics of included studies
| Author and year | Country | Type and number of participants | Data collection | Data analysis |
|---|---|---|---|---|
| Aaserud 2005 | Multiple | Doctors, midwives, researchers, health managers | Observations, 5 group discussions | Thematic and policy framework analysis |
| Alabintei 2021a,b | Nigeria | Health‐facility managers | 29 key informant interviews | Thematic analysis |
| Barua 2011 | India | Nurses, clinicians, obstetricians and gynaecologists | 7 FGDs | Grounded theory |
| Bigdeli 2013 | Pakistan | Healthcare providers, policy‐makers, academics | Document review, 48 IDIs, unknown number of FGDs, observation | Deductive content analysis |
| Charanthimath 2018 | India | Community leaders, doctors, administrators | 14 FGDs and 12 IDIs | Thematic analysis |
| Chaturvedi 2013 | India | Doctors, administrators, district health officials, and programme managers | 39 IDIs, observation, record analysis | Thematic analysis |
| Chikalipo 2020 | Malawi | Nurses, midwives, technicians | 1 FGD and 10 IDIs | Thematic analysis |
| Danmusa 2014 & 2016 | Nigeria | Global experts, local key informants and stakeholders | 23 IDIs | Not specified |
| Hossain 2019 | Bangladesh | Policy‐makers and programme managers | 37 IDIs | Content and thematic analysis |
| Ishaku 2019 | Nigeria | Policy‐makers and programme stakeholders | 64 IDIs | Content and thematic analysis |
| Lotufo 2016 | Brazil | Healthcare managers | Document analysis, observation, interviews | Deductive content analysis |
| Lotufo 2017 | Brazil | Obstetricians | 30 IDIs | Content analysis |
| Ndwiga 2018 | Kenya | Policy‐makers, midwives, doctors, community health workers, and traditional birth attendants | 98 IDIs | Thematic analysis |
| Oguntunde 2015 | Nigeria | Healthcare facility manager | 30 IDIs, survey, observational checklists | Thematic analysis |
| Ramadurg 2016 | India | Nurses, Auxiliary Nurse Midwives, accredited social health activist (ASHAs) | 8 FGDs, surveys | Thematic analysis |
| Raney 2019 | India | Nurse mentors | 12 IDIs | Thematic content analysis |
| Sheikh 2016 | Pakistan | Lady health workers, lady health supervisors, traditional birth attendants, doctors | 7 FGDs and 26 IDIs | Thematic analysis |
| Sripad 2018 | Ethiopia | Policy‐makers, health officers, representatives from MoH and professional associations | 62 IDIs | Thematic analysis |
| Van Dijk 2013 | Mexico | Maternal health researchers, doctors | 13 IDIs | Content analysis |
| Warren 2015a | Nigeria | Policy‐makers and programme stakeholders | 72 IDIs | Content analysis |
| Warren 2015b | Bangladesh | Policy‐makers and programme manager stakeholders | 50 IDIs | Content and thematic analysis |
| Woelk 2009 and Sevene 2005 | Mozambique, South Africa, Zimbabwe | Policy‐makers | Document review, 49 IDIs | Thematic analysis |
Danmusa 2014 and Danmusa 2014 are from a single study; Woelk 2009 and Sevene 2005 are from a single study; Alabintei 2021 and Alabintei 2021 are from a single study.
FGD, focus group discussion; GD, group discussion; IDI, in‐depth interview; MoH, Ministry of Health.
Studies primarily used in‐depth interviews and/or focus group discussions. Detailed critical appraisals are provided (Table [Link], [Link]); the primary reasons for downgrading were limitations in recruitment/sampling strategies, reflexivity, informed consent and ethics approval, data analysis, and insufficient evidence to support findings. Explanations for GRADE‐CERQual assessments are in Table [Link], [Link]: of 28 review findings, we graded ten as high confidence, 17 as moderate confidence and one as very low confidence. The primary reason for downgrading was for relevance – this was a global review with almost all evidence from LMICs.
Qualitative synthesis findings
We developed nine overarching themes under three domains: provider competence and confidence, health system capability and knowledge translation (Figure 2). Table [Link], [Link] presents the summary of qualitative findings and CERQual assessments.
Figure 2.
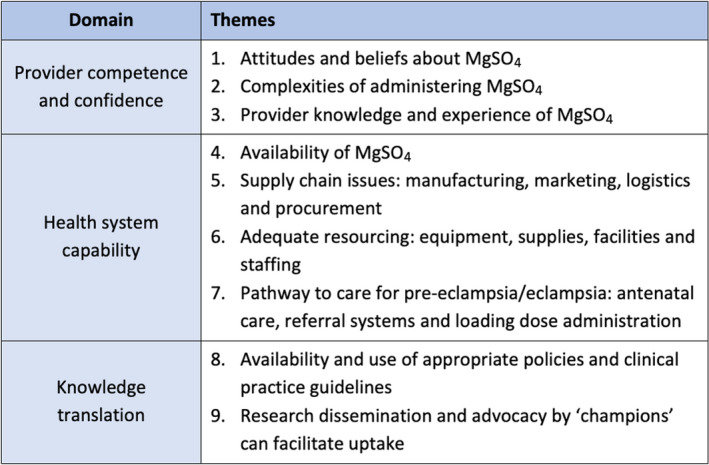
Domains and themes.
Provider competence and confidence
-
1.
Attitudes and beliefs about MgSO4
Finding 1.1: MgSO4 is often perceived by providers, administrators and policy‐makers as useful and effective, and acknowledged as the preferred treatment for pre‐eclampsia/eclampsia. In Latin America, some believe that MgSO4 is overused for women with mild pre‐eclampsia (moderate confidence). 13 , 27 , 29 , 44 , 50 , 52 , 53 , 55 , 56 , 60 Providers in two studies were more aware of MgSO4’s effectiveness for eclampsia than severe pre‐eclampsia, 13 , 29 potentially because effectiveness was first demonstrated for eclampsia.
Finding 1.2: Some providers preferred alternative drugs (particularly diazepam and phenytoin) because of past training, less cumbersome protocols for use, perceived safety or familiarity. Providers’ decisions to use alternatives may be based on positive attitudes towards the alternative, or negative perceptions of MgSO4 (moderate confidence). 29 , 50 , 55 , 56 , 58 , 60 , 62 Many providers were trained and comfortable in using diazepam; for some, familiarity outweighed ‘newer’ and less familiar MgSO4 regimens. 29 , 55 , 62 Compared with diazepam, MgSO4 was considered more difficult to administer and less safe. 29 , 62 Phenytoin was considered an alternative treatment in India and Mexico, where some believed it to be superior. 50 , 56
Finding 1.3: Fear of complications or adverse events from MgSO4 can undermine beliefs about its safety and can create barriers to its use. Some providers felt fearful, cautious or concerned when using MgSO4 based on a perceived likelihood of adverse events (e.g. maternal respiratory arrest, death). This fear was exacerbated when providers were at risk of being blamed for adverse events (high confidence). 13 , 18 , 29 , 48 , 49 , 50 , 56 , 57 , 58 , 59 , 61 Providers’ fear, caution and concern were most commonly due to their perceived risk that complications may cause harm or death. 13 , 18 , 29 , 48 , 49 , 50 , 56 , 57 , 58 , 59 , 61 Some providers held persisting beliefs about the “toxicity” of MgSO4. 29 , 48 , 57 , 58 For some, fears were based on lived experiences. 29 , 55 Fears can lead providers to feel inadequate or stressed using MgSO4, 13 , 29 , 57 resulting in hesitation or avoidance. 13 , 56
-
2.
Complexities of administering MgSO4
Finding 2.1: Providers may be less likely to administer MgSO4, or may administer it incorrectly, because of confusion about when and how to administer, perceived difficulty of administering and a fear of incorrect use. However, some providers think the difficulty of administering MgSO4 is overstated (moderate confidence). 29 , 48 , 49 , 50 , 56 , 57 , 59 , 60 , 61 Some providers were unsure about recognising disease severity (mild versus severe pre‐eclampsia) and indications for MgSO4 use. 49 , 56 , 61 Providers also experienced confusion about methods for administration, 49 , 56 , 60 , 61 and believe that administration is difficult. 48 , 50 , 59 However, some felt those not using it are ‘lazy’. 59
Finding 2.2: Difficulties identifying and preparing the correct dose undermine providers’ willingness, confidence and competence to administer MgSO4 (high confidence). 29 , 48 , 49 , 50 , 57 , 59 , 61 Training may be inadequate or incorrect, 29 and requirements are harder to remember when pre‐eclampsia/eclampsia is encountered relatively infrequently. 57 Variability in local dosage regimens causes confusion, 29 , 59 and MgSO4 packaging sizes differ from recommended dose amounts. 59 , 61 Difficulties with dosing lead to providers delaying or avoiding MgSO4 use. 48 , 59
Finding 2.3: The need to monitor women after administering MgSO4 increases the perceived burden and riskiness of using it, which can contribute to underutilisation (moderate confidence). 29 , 44 , 48 , 50 , 56 , 57 , 59 Monitoring is considered resource and labour‐intensive, contributing to a perception that MgSO4 can only be used in higher‐level facilities. 44 , 48 , 56 , 57 , 59
Finding 2.4: Determining the appropriate cadre of healthcare provider to administer MgSO4 (e.g. through task‐shifting or task‐sharing) can be a barrier or facilitator to appropriate use, especially in resource‐constrained settings where higher cadre providers are unavailable or have limited capacity (moderate confidence). 28 , 46 , 47 , 48 , 51 , 53 , 58 , 59 , 60 There were diverse views about what cadres of provider should administer MgSO4. 28 , 46 , 47 , 48 , 58 , 59 , 60 Some considered that it can only be administered by doctors, 28 , 48 , 53 , 58 , 60 whereas others felt that trained nurses and midwives can administer it. 47 , 48 , 51 , 60 Concerns included that lower cadres may not have sufficient skills or motivation to monitor women post‐administration, 59 and allowing them to administer could result in abuse or harm. 51
-
3.
Provider knowledge and experience of MgSO4
Finding 3.1: Knowledge about the benefits and risks of MgSO4, its indications for use and how it should be administered (including appropriate regimens) is a facilitator for appropriate use, whereas insufficient knowledge can reduce providers' confidence and competence (moderate confidence). 29 , 48 , 49 , 50 , 53 , 56 , 57 , 58 , 60 , 62 Limited knowledge can lead to inappropriate practice, such as sending a woman home after administering the loading dose. 62
Finding 3.2: Effective and practical training can increase providers' knowledge and (simulated) experience of MgSO4, thereby improving their capability and motivation to use it. Training does not guarantee use, but is seen by some as a pre‐requisite to use (high confidence). 14 , 29 , 44 , 46 , 48 , 49 , 52 , 53 , 55 , 56 , 57 , 58 , 59 , 61 , 62 Training increases perceptions that providers can administer MgSO4 correctly. 27 , 44 , 52 , 56 , 57 Training is more likely to be effective if it is practical 48 , 49 , 59 , 61 and recent. 29 , 49 , 59
Finding 3.3: Practical, first‐hand experience administering MgSO4 (including clinical experience and simulation or practical skills training) encourages providers to use MgSO4 appropriately. Conversely, the absence of recent, frequent experience can undermine providers' confidence, competence and willingness to administer MgSO4 (moderate confidence). 29 , 48 , 50 , 55 , 57 , 58 , 59 , 60 , 61 , 62 Observing the positive effects of MgSO4 influenced providers' attitudes, becoming ‘convinced’ and advocating for its use. 55 , 62
Health system capability
-
4.
Availability of MgSO4
Finding 4.1: MgSO4 is often unavailable in some facilities (particularly rural or lower‐level facilities in LMICs), and stock‐outs are an obstacle to its use. MgSO4 is also not consistently available in many LMICs at country, regional and district levels, suggesting that facility‐level stock‐outs may reflect system‐level unavailability (high confidence). 13 , 14 , 18 , 28 , 29 , 44 , 45 , 46 , 47 , 48 , 49 , 51 , 53 , 56 , 57 , 62 Stock‐outs occur across different facility levels, including primary care settings, secondary and tertiary hospitals, and rural facilities. 13 , 29 , 48 , 56 , 57 Even in settings where MgSO4 is available there can be a disconnect between perceptions of system‐level and facility‐level availability, with those in administrative or policy roles unaware that stock‐outs are occurring, or those at facility‐level unaware that it is available to procure. 18 , 46 , 47 , 48 , 51 Access is less likely to be a problem in high‐income countries. 44
-
5.
Supply chain issues: manufacturing, marketing, logistics and procurement
Finding 5.1: Registration, licensing and inclusion of MgSO4 on national Essential Medicines Lists are acknowledged to be important pre‐requisites, although they will not alone guarantee MgSO4 use (high confidence). 18 , 28 , 29 , 44 , 48 , 52 MgSO4 may not be registered for use in all settings. 18 , 28 , 44 Reasons include that policy‐makers may be unconvinced it is necessary, with some believing diazepam is equally effective. 18
Finding 5.2: MgSO4 is generally perceived as cheap and affordable. However, in some settings, affordability is undermined in practice by higher retail prices and families’ low incomes (moderate confidence). 13, 14, 18, 29, 44, 51, 52
Finding 5.3: Limited local production, a relatively small market for MgSO4 compared with other drugs with multiple indications and problems with distribution and storage all contribute to unavailability of MgSO4 in some LMIC settings. The low price of MgSO4 price can contribute to these availability issues, as the financial returns available to pharmaceutical companies are low and consequently the incentive to produce and market MgSO4 is limited (moderate confidence). 18 , 29 , 44 , 45 , 46 , 49 , 51 , 52 , 55 , 62 Several studies note the importance of distribution and logistics for MgSO4 access, 18 , 46 , 49 , 62 and the presence of bottlenecks and problems in distribution. 18 , 49 , 55 Providers described expiration of MgSO4 because of relatively infrequent use, particularly at lower‐level facilities. 49 , 51
Finding 5.4: MgSO4 availability at health facilities relies on effective national and local procurement systems; even where MgSO4 is available, failures in complex and fragmented procurement systems in some settings contribute to facility‐level stock‐outs. Facility‐level managers may seek to bypass official procurement channels that are perceived as ineffective, which can increase fragmentation and undermine centralised systems (moderate confidence). 13 , 18 , 29 , 46 , 47 , 48 , 49 , 53 , 62 Problems with procurement included: mismatched demand from hospital departments and national medicines lists, 29 clinicians failing to request MgSO4, 18 , 29 exclusion of MgSO4 from central procurement, 18 , 52 and complex, unreliable requisition processes. 13 , 49 , 52 Failures can occur at central or local government levels, 46 , 48 or facility‐level. 18 , 53 Primary and community health facilities are more likely to experience problems obtaining MgSO4. 46 , 47 If pre‐eclampsia/eclampsia is not encountered often or is considered uncommon at a facility, administrators may choose not to procure MgSO4. 53 Failures in national procurement systems contribute to stock‐outs. 13 , 18 , 29 , 46 , 47 , 48 , 49 , 62 Procurement could be more effective with improved supply monitoring, 62 transparency regarding quantities required, 46 and facility‐level coordination to match supply and demand. 49
Finding 5.5: Women or their relatives may be required to privately purchase MgSO4 from hospital pharmacies or retail outlets in some settings where medicines cannot be sourced from the facility directly. For some families, this may cause financial hardship or they may not have money readily available to purchase it, which can cause delays or barriers to use (high confidence). 14 , 29 , 47 , 48 , 49 , 56 , 62 Private purchase may be required because of facility stock‐outs, 14 , 48 , 49 , 56 or as standard practice to manage demand. 29 , 47 , 49 , 62 Retail outlets generally charge higher prices. 14 , 48 , 49
-
6.
Adequate resourcing: equipment, supplies, facilities and staffing
Finding 6.1: Providers' capability and confidence to safely administer MgSO4 is undermined if the necessary equipment and supplies are missing and facilities are inadequate. MgSO4 is considered safer to use in well‐equipped facilities, with access to sphygmomanometers, urine dipstick tests, laboratory and diagnostic equipment, medications such as calcium gluconate, and sufficient rooms and beds for monitoring (high confidence). 28, 29, 48, 49, 53, 56, 57, 59, 61, 62
Finding 6.2: Insufficient staffing, competing clinical duties, workload pressure and inflexible work schedules restrict the use of MgSO4, especially given that administration requires ongoing monitoring for toxicity. Staffing constraints are especially problematic for women experiencing severe pre‐eclampsia or eclampsia outside usual hours (high confidence). 14 , 28 , 48 , 49 , 53 , 55 , 56 , 57 , 58 , 59 , 61 Staff shortages occurred across multiple levels, involving nurses, 49 , 56 , 61 midwives, 14 , 49 , 59 doctors 18 , 28 , 53 , 56 , 57 , 58 , 61 and paramedics, 56 at primary and community health centres 28 , 48 , 49 , 57 , 58 , 61 or hospitals. 18 , 29 , 48 , 49 , 56 , 59
Finding 6.3: Perspectives varied on appropriate facility level(s) for administration of MgSO4. Its use in hospitals is generally accepted, but some consider that MgSO4 should not be administered at lower‐level facilities. As a result, women seeking care at lower‐level facilities may not receive treatment. Reasons for preferring higher‐level facilities include the availability of equipment, a particular cadre of provider and access to intensive care facilities (high confidence). 28 , 29 , 44 , 46 , 49 , 52 , 53 , 57 , 58 , 60 , 61 Some suggested that MgSO4 could be used at primary health facilities or emergency mobile health services with training and resourcing; 28 , 29 , 52 others felt use in primary health settings was inappropriate. 52 , 57 , 58 , 60 Use of MgSO4 may not always occur at lower‐level hospitals. 28 , 29 , 46
-
7.
Pathway to care for pre‐eclampsia/eclampsia: antenatal care, referral systems and loading dose administration
Finding 7.1: MgSO4 use relies on identifying women with symptoms of pre‐eclampsia or eclampsia. Timely identification relies on women accessing quality antenatal care that includes regular blood pressure checks, preventive treatment of hypertension, detection and classification of pre‐eclampsia/eclampsia symptoms, and prompt treatment or referral (moderate confidence). 46, 51, 53, 58, 60
Finding 7.2: Prompt, successful referral to higher‐level facilities enables timely and effective MgSO4 treatment, especially for women in remote or rural areas. Challenges to effective referrals in some settings include lack of capability at referral and referring sites, problems with transport, costs and complex inter‐facility communication processes. Timeliness of treatment can be improved where a loading dose is given at a lower‐level healthcare facility before referral (moderate confidence). 46, 48, 52, 53, 58, 61, 62
Finding 7.3: Administration of a loading dose of MgSO4 by lower‐cadre providers before referral is considered beneficial, safer and less complicated to administer than the full regimen; attitudes toward task shifting its administration to lower‐cadre healthcare workers (including paramedics, community health workers, skilled birth attendants, midwives and nurses) were generally positive. However, some were concerned about the knowledge and capability of these providers (moderate confidence). 13 , 27 , 29 , 46 , 47 , 48 , 49 , 58 , 60 , 61 , 62 Loading dose administration is considered less complicated than the full regimen, described by some as ‘easy’ and ‘safe’. 27 , 46 Administration of a loading dose was considered necessary because of barriers for women from rural areas accessing higher‐level care 27 , 46 , 47 , 49 and skilled staff shortages. 46 , 47 However, a loading dose may not be given even where permitted because of perceived safety issues, 29 , 49 inadequate resourcing 13 , 29 , 46 , 58 , 61 or providers being unaware of its importance. 46 , 58
Knowledge translation
-
8.
Availability and use of appropriate policies and clinical practice guidelines
Finding 8.1: Policies and guidelines should clearly recommend MgSO4 for eclampsia and severe pre‐eclampsia and describe indications and administration methods in detail appropriate to the intended audience and context. Policy should specify those settings in which administration is recommended. Translating higher‐level policy into local protocols including algorithms and job aids can be useful. Inconsistent and ambiguous policy or guidance creates confusion for healthcare workers and may undermine MgSO4 use (high confidence). 18 , 28 , 29 , 44 , 48 , 50 Guidelines should specify indications for MgSO4 use, 44 , 48 and how to administer it, including dosage and administration methods. 29 , 48 , 53 , 54 , 62
Finding 8.2: The process for developing policies, guidelines and protocols varies. Processes that are consultative and include diverse stakeholders are perceived by some to increase ownership and uptake of guidelines. Stakeholders may include clinicians, governments and non‐governmental organisation representatives (very low confidence). 44 , 50 , 53 , 55 , 62 It is unclear whether including local clinicians or administrators to create ownership and increase uptake is effective. 18 , 50 Governments' receptiveness to input from clinicians is considered an important facilitator in policy development. 55
Finding 8.3: For policy and guidelines to influence clinical practice, guidance must be disseminated and available to administrators and healthcare providers at all facility levels where MgSO4 is to be used. There is confusion in some settings about whether national policy exists, and concern that national and regional policies are not properly disseminated to intended audiences (moderate confidence). 18 , 29 , 46 , 47 , 48 , 50 , 51 , 53 , 54 , 56 , 58 , 59 , 62 Policies should be translated into clinical practice guidelines and made available at facility‐level 18 , 29 , 46 , 48 , 50 , 56 in wards. 48 , 59 , 62
Finding 8.4: In practice, guidelines are sometimes not followed. Although some providers are interested in guidelines and believe that they are useful, others are resistant to change, unaware or disinterested. At facility‐level, the protocols used are sometimes inconsistent with national and international guidelines. Increased monitoring and obligations are perceived to improve compliance (moderate confidence). 18 , 29 , 44 , 46 , 47 , 48 , 50 , 55 , 58 , 59 , 62 A lack of interest or awareness about guidelines and updates is noted particularly among older physicians. 50 , 55 , 58 Facility administrators and providers are not obligated to follow policies or guidelines. 18 , 44 , 50 , 55 Variable protocols at facility‐level can leave staff confused and result in practice that is not consistent with national or international recommendations. 44 , 50 , 56 , 59
-
9.
Research dissemination and advocacy by ‘champions’ can facilitate uptake
Finding 9.1: Evidence from research, such as clinical trials of effectiveness, can influence clinical practice and improve MgSO4 use if key results are properly disseminated and translated for the local setting. Local experience may be more persuasive for some than international research. Accordingly, MgSO4 uptake may be more likely where local researchers have participated in trials, because they advocate its use and enhance credibility of findings for the local setting (moderate confidence). 29 , 44 , 50 , 55 , 62 Local participation in international trials may increase the uptake of MgSO4 by developing local champions 29 and increasing the local credibility of findings or providing local complementary evidence. 55 , 62 Dissemination of research findings may be via journal publication 44 and participation by researchers in policy formulation. 55
Finding 9.2: Stakeholders can act as ‘champions’ of MgSO4 use by raising awareness of its effectiveness, issuing guidelines or policies for use, and encouraging uptake at both policy and implementation levels. This role can be performed by individuals (including policy‐makers and clinicians) and organisations (including medical associations, non‐profit organisations and international agencies) (moderate confidence). 29 , 44 , 49 , 55 , 62 Champions' influence may increase ‘political will’ and put the issue ‘on the policy agenda’. 44 , 49 , 55 , 62
Figure 3 depicts abbreviated findings using the COM‐B model to understand how addressing factors affecting implementation may influence behaviour change toward appropriate use of MgSO4. Within each COM‐B domain, we mapped facilitators and barriers to physical and psychological capability, and physical and social opportunity, and how the interplay between these domains influences motivation (reflective and automatic). When these facilitators are reinforced, and barriers are addressed, we expect behaviour (appropriate use of MgSO4 for pre‐eclampsia/eclampsia) to improve. In turn, positive experience using MgSO4 reinforces providers' capability, opportunity and motivation to continue its use.
Figure 3.
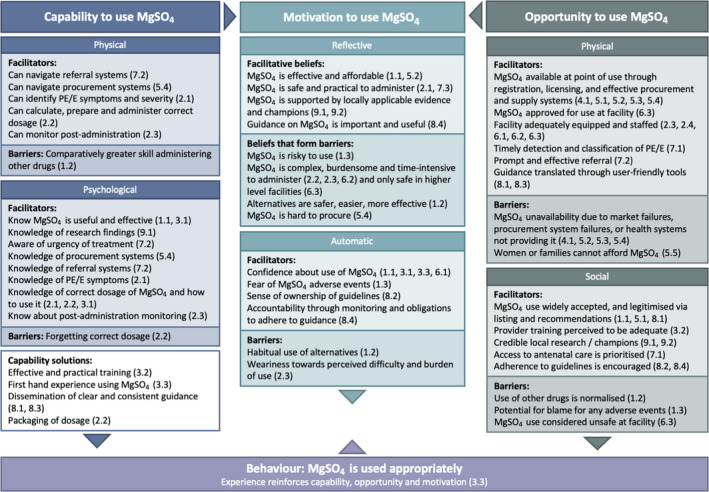
COM‐B model of appropriate use of MgSO4.
Discussion
Main findings
This QES revealed a broad range of factors affecting MgSO4 use operating across multiple health system levels. First, whether providers will use MgSO4 appropriately depends on their competence and confidence, which may be undermined by MgSO4's unfamiliarity and perceived riskiness, and strengthened through training and experience. Second, appropriate MgSO4 use depends on the capability of health systems to ensure that it is consistently available at facilities that are adequately resourced and accessible to women. Finally, appropriate MgSO4 use depends on effective knowledge translation through research dissemination, clear guidelines and protocols, and local champions.
The COM‐B model shows how factors may be addressed to improve the implementation of MgSO4. Appropriate MgSO4 use depends on the collective behaviour of policy‐makers promoting MgSO4 and ensuring its availability, administrators translating policy into practice by creating an enabling environment 63 for MgSO4 use, and providers making appropriate clinical decisions to administer MgSO4 for individual women. The model can be used to inform development of interventions for particular settings by providing a framework to consider the full range of options and select the best option to target specific barriers.
Interpretation
The factors identified in this QES are consistent with those found in other studies, such as Ridge et al. who used a case study to develop a ‘fishbone diagram’ of requirements for rational use of MgSO4 at a health facility in Zambia. 64 Our QES furthers this understanding, by combining evidence from a global synthesis of qualitative evidence with the COM‐B model to explore how such factors affect behaviour. Many of our findings align with evidence from quantitative studies, including providers' preference for diazepam 65 , 66 , 67 and desire for simplified dosing regimens, 25 , 26 , 68 concerns about availability of MgSO4, 25 , 64 , 65 , 69 , 70 and the impact of inadequate resourcing. 67 , 71 , 72 , 73 Task‐shifting has been proposed as a solution to address staffing shortages and barriers to accessing higher‐level facilities; WHO guidelines currently allow for nurses and midwives to administer MgSO4 under certain circumstances (e.g. as a loading dose, or where higher‐cadre staff are unavailable). 74
Implications for practice
We developed the following questions based on our findings to assist health system administrators, policy‐makers and other stakeholders. When developing interventions and policies to identify and address barriers to appropriate use of MgSO4 for pre‐eclampsia/eclampsia in a given context, these individuals could consider the following:
Increasing provider competence and confidence
Are providers aware of the benefits of MgSO4 compared with alternatives?
Do providers have pre‐existing fears regarding adverse events that need to be addressed?
Do providers receive regular and refresher in‐service training that is sufficiently practical (including clinical simulations)?
Can MgSO4 administration processes be simplified, including through simpler, lower‐dose regimens and improving calibration of how MgSO4 is packaged and administered (e.g. through ready‐to‐use doses)?
Are resources and training adapted for the local context?
Improving health system capability
Is MgSO4 consistently available at all health facilities in which it is recommended for use? If not, how can bottlenecks in supply chains or procurement systems be removed?
Is MgSO4 supplied free‐of‐charge or at an affordable price for women and families?
Is MgSO4 supplied in a form that is appropriate for the recommended dosage regimen?
Is the health facility sufficiently well‐equipped to facilitate MgSO4 administration?
In lower‐resource settings with staffing shortages, can MgSO4 be safely administered by lower‐cadre healthcare workers?
Ensuring effective knowledge translation
Are national policies consistent with international guidelines, translated into local guidelines and tools (including algorithms or job aids), and disseminated effectively?
Are local stakeholders equipped to ‘champion’ use of MgSO4?
Strengths and limitations
A limitation of this QES is that data regarding system‐level issues (e.g. procurement and supply) were often specific to particular local contexts – only high‐level, generalised conclusions could be drawn. System‐level constraints and bottlenecks are likely to vary between countries and regions, and should be assessed for each implementation context. All relevant qualitative evidence since effectiveness of MgSO4 for pre‐eclampsia/eclampsia was demonstrated (2002 and 1995, respectively) was included, and factors affecting use may have changed over time. However, over 90% of included studies were published after 2010, so available evidence probably reflects current practices. Finally, six grey literature reports were linked to work by the Population Council’s Ending Eclampsia projects in four countries, 46 , 47 , 48 , 49 , 51 , 52 and the issues explored in these projects (specifically, policy awareness and task‐shifting of loading dose) may be somewhat overrepresented. This was mitigated during analysis by exploring these issues within themes identified across all included studies, and considering evidence from pre‐ and post‐intervention reports concurrently. Although a comparison of views of different cadres of health workers would provide interesting insight into factors affecting MgSO4 use from different perspectives, we were unable to explore this in our analysis, as most included studies did not report this level of detail.
Despite these limitations, this QES presents the first global review of factors affecting implementation of MgSO4 to treat pre‐eclampsia/eclampsia from the perspective of health system stakeholders. Taking a health systems approach encompasses supply‐side barriers at all levels, enabling a full picture of things that can ‘go wrong’ in the chain of events required to get MgSO4 to all women who need it.
Conclusion
This QES identified a range of factors affecting use of MgSO4 for pre‐eclampsia/eclampsia relating to provider competence and confidence, health system capability and knowledge translation. Policy‐makers and researchers should consider these findings when designing and implementing policies and interventions to increase appropriate use. Contrary to some providers' beliefs about the riskiness of MgSO4, in practice adverse events from MgSO4 are uncommon and translation of this knowledge could improve uptake. 75 , 76 Practical training for providers, translating knowledge into clear clinical practice guidelines adapted for local contexts, and addressing systematic problems in supply chains and procurement mechanisms may improve MgSO4 uptake. Studies are also exploring the efficacy of simpler, lower‐dose regimens 21 , 22 , 77 that may be easier and safer to administer. As this review did not find evidence regarding specific dosage regimens, further primary research is needed to understand provider perspectives on specific regimens and whether some are easier to use than others.
Further research is also needed to explore the experiences, attitudes and beliefs of women and their partners or families regarding use of MgSO4 for pre‐eclampsia/eclampsia – including, for example, experience of pain or adverse effects. Context‐specific research on facility‐level stock audits or surveys of providers' knowledge and preferences could complement insights from this QES to inform local implementation strategies. Ultimately, action by policy‐makers, administrators and providers across multiple health system levels will be crucial to improve uptake of this life‐saving treatment.
Disclosure of interests
None disclosed.
Contribution to authorship
KEE designed this study with input from JPV and MAB. KEE, RIZ, MAB and JPV conducted study screening, data extraction, critical appraisal and GRADE‐CERQual assessments. KEE led the analysis and manuscript writing, with input from RIZ, JPV and MAB. All authors reviewed and approved the final manuscript.
Details of ethics approval
Not applicable, this is a systematic review of published qualitative studies.
Funding
The authors received no funding for this work, which was completed as part of KEE's MPH at University of Melbourne School of Population and Global Health.
Acknowledgements
We would like to acknowledge the support from our institutions in facilitating the completion of this work. RIZ is sponsored by an Australian Awards Scholarship (ST000SSF2) for her master's education, funded by the Australian Department of Foreign Affairs and Trade (DFAT). MAB's time is supported by an Australian Research Council Discovery Early Career Researcher Award (DE200100264) and a Dame Kate Campbell Fellowship (University of Melbourne Faculty of Medicine, Dentistry, and Health Sciences). JPV is supported by a National Health and Medical Research Council Investigator Grant (1194248).
Supporting information
Table S1. Summary of qualitative findings.
Table S2. Evidence profile.
Table S3. CASP assessments of included studies.
Appendix S1. Search strategies.
Appendix S2. ENTREQ statement.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Eddy KE, Vogel JP, Zahroh RI, Bohren MA. Factors affecting use of magnesium sulphate for pre‐eclampsia or eclampsia: a qualitative evidence synthesis. BJOG 2022;129:379–391.
 This article includes Author Insights, a video abstract available at https://vimeo.com/manage/videos/623192027
This article includes Author Insights, a video abstract available at https://vimeo.com/manage/videos/623192027
Contributor Information
KE Eddy, Email: katherine.eddy@burnet.edu.au.
MA Bohren, Email: meghan.bohren@unimelb.edu.au.
Data availability
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.
References
- 1. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre‐eclampsia/eclampsia and its adverse outcomes in low‐ and middle‐income countries: a WHO secondary analysis. PLoS One 2014;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . The World Health Report 2005: Make Every Mother and Child Count. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 4. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A‐B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . WHO Recommendations for Prevention and Treatment of Pre‐eclampsia and Eclampsia. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 6. Duley L, The Eclampsia Trial Collaborative Group . Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet 1995;345:1455–63. [PubMed] [Google Scholar]
- 7. Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre‐eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo‐controlled trial. Lancet 2002;359:1877–90. [DOI] [PubMed] [Google Scholar]
- 8. Duley L, Henderson‐Smart DJ, Walker GJ, Chou D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev 2010;(12):CD000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duley L, Henderson‐Smart DJ, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev 2010;(10):CD000128. [DOI] [PubMed] [Google Scholar]
- 10. Duley L, Gulmezoglu AM, Henderson‐Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database Syst Rev 2010;(11):CD000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duley L, Gülmezoglu AM, Chou D. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database Syst Rev 2010;(9):CD002960. 10.1002/14651858.CD002960.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon J, Gray A, Duley L. Cost‐effectiveness of prophylactic magnesium sulphate for 9996 women with pre‐eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG: Int J Obstet Gynaecol 2006;113:144–51. [DOI] [PubMed] [Google Scholar]
- 13. Chaturvedi S, Randive B, Mistry N. Availability of treatment for eclampsia in public health institutions in Maharashtra, India. J Health Popul Nutr 2013;31:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oguntunde O, Charyeva Z, Cannon M, Sambisa W, Orobaton N, Kabo IA, et al. Factors influencing the use of magnesium sulphate in pre‐eclampsia/eclampsia management in health facilities in Northern Nigeria: a mixed methods study. BMC Pregnancy Childbirth 2015;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magpie Trial Follow up Collaborative group . The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre‐eclampsia. Outcome for women at 2 years. BJOG: Int J Obstet Gynaecol 2007;114:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magpie Trial Follow up Collaborative group . The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre‐eclampsia. Outcome for children at 18 months. BJOG: Int J Obstet Gynaecol 2007;114:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shepherd E, Salam RA, Manhas D, Synnes A, Middleton P, Makrides M, et al. Antenatal magnesium sulphate and adverse neonatal outcomes: a systematic review and meta‐analysis. PLoS Med 2019;16:e1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sevene E, Lewin S, Mariano A, Woelk G, Oxman AD, Matinhure S, et al. System and market failures: the unavailability of magnesium sulphate for the treatment of eclampsia and pre‐eclampsia in Mozambique and Zimbabwe. BMJ (Clin Res Ed) 2005;331:765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . World Health Organization: Model List of Essential Medicines. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 20. World Health Organization . Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 21. Duley L, Matar HE, Almerie MQ, Hall DR. Alternative magnesium sulphate regimens for women with pre‐eclampsia and eclampsia. Cochrane Database Syst Rev 2010;(8):CD007388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pratt JJ, Niedle PS, Vogel JP, Oladapo OT, Bohren M, Tunçalp Ö, et al. Alternative regimens of magnesium sulfate for treatment of preeclampsia and eclampsia: a systematic review of non‐randomized studies. Acta Obstet Gynecol Scand 2016;95:144–56. [DOI] [PubMed] [Google Scholar]
- 23. Lumbiganon P, Metin Gulmezoglu A, Piaggio G, Langerc A, Grimshawd J. Magnesium sulfate is not used for pre‐eclampsia and eclampsia in Mexico and Thailand as much as it should be. Bull World Health Organ 2007;85:763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Silva DA, Proctor L, von Dadelszen P, McCoach M, Lee T, Magee LA. Determinants of magnesium sulphate use in women hospitalized at <29 weeks with severe or non‐severe pre‐eclampsia. PLoS One 2017;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katageri G, Charantimath U, Joshi A, Vidler M, Ramadurg U, Sharma S, et al. Availability and use of magnesium sulphate at health care facilities in two selected districts of North Karnataka, India. Reprod Health 2018;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long Q, Oladapo OT, Leathersich S, Vogel JP, Carroli G, Lumbiganon P, et al. Clinical practice patterns on the use of magnesium sulphate for treatment of pre‐eclampsia and eclampsia: a multi‐country survey. BJOG: Int J Obstet Gynaecol 2017;124:1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charanthimath U, Vidler M, Katageri G, Ramadurg U, Karadiguddi C, Kavi A, et al. The feasibility of task‐sharing the identification, emergency treatment, and referral for women with pre‐eclampsia by community health workers in India. Reprod Health 2018;15:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lotufo FA, Parpinelli MA, Osis MJ, Surita FG, Costa ML, Cecatti JG. Situational analysis of facilitators and barriers to availability and utilization of magnesium sulfate for eclampsia and severe preeclampsia in the public health system in Brazil. BMC Pregnancy Childbirth 2016;16:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bigdeli M, Zafar S, Assad H, Ghaffar A. Health system barriers to access and use of magnesium sulfate for women with severe pre‐eclampsia and eclampsia in Pakistan: evidence for policy and practice. PLoS One 2013;8:e59158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glenton C, Bohren MA, Downe S, Paulsen EJ, Lewin S, et al. EPOC Qualitative Evidence Syntheses: Protocol and Review Template (version 1.1). Oslo, Norway: Norwegian Institute of Public Health; 2020. [Google Scholar]
- 31. National Institute for Health Research . PROSPERO [Factors Affecting use of Magnesium Sulphate for Pre‐eclampsia or Eclampsia: A Qualitative Evidence Synthesis]. York, UK: National Institute for Health Research; [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=167185]. Accessed 28 April 2020. [Google Scholar]
- 32. CASP UK . Critical Appraisal Skills Programme. Oxford, UK: CASP UK; 2020. [https://casp‐uk.net/]. Accessed 31 March 2020. [Google Scholar]
- 33. Bohren M, Munthe‐Kaas H, Berger B, Allanson E, Tunçalp Ö. Perceptions and experiences of labour companionship: a qualitative evidence synthesis (Protocol). Cochrane Database Syst Rev 2016;(12). 10.1002/14651858.CD012449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munthe‐Kaas H, Bohren MA, Glenton C, Lewin S, Noyes J, Tunçalp Ö, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings‐paper 3: how to assess methodological limitations. Implement Sci 2018;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. QSR International Pty Ltd . NVivo. Doncaster, VIC; QSR International Pty Ltd; 2020. [https://www.QSR.international.com/nvivo‐qualitative‐data‐analysis‐software/home]. Accessed 1 March 2020. [Google Scholar]
- 37. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lewin S, Booth A, Glenton C, Munthe‐Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci 2018;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colvin CJ, Garside R, Wainwright M, Munthe‐Kaas H, Glenton C, Bohren MA, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings‐paper 4: how to assess coherence. Implement Sci 2018;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glenton C, Carlsen B, Lewin S, Munthe‐Kaas H, Colvin CJ, Tunçalp Ö, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings‐paper 5: how to assess adequacy of data. Implement Sci 2018;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noyes J, Booth A, Lewin S, Carlsen B, Glenton C, Colvin CJ, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings‐paper 6: how to assess relevance of the data. Implement Sci 2018;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lewin S, Bohren M, Rashidian A, Munthe‐Kaas H, Glenton C, Colvin CJ, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings—paper 2: how to make an overall CERQual assessment of confidence and create a Summary of Qualitative Findings table. Implement Sci 2018;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xyrichis A, Mackintosh N, Terblanche M, Bench S, Philippou J, Sandall J. Healthcare stakeholders' perceptions and experiences of factors affecting the implementation of critical care telemedicine (CCT): qualitative evidence synthesis. Cochrane Database Syst Rev 2017;(2). 10.1002/14651858.CD012876.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aaserud M, Lewin S, Innvaer S, Paulsen EJ, Dahlgren AT, Trommald M, et al. Translating research into policy and practice in developing countries: a case study of magnesium sulphate for pre‐eclampsia. BMC Health Serv Res 2005;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Danmusa S, Coeytaux F, Potts J, Wells E. Scale‐up of magnesium sulfate for treatment of pre‐eclampsia and eclampsia in Nigeria. Int J Gynaecol Obstet: Off Organ Int Federation Gynaecol Obstet 2016;134(3):233–6. [DOI] [PubMed] [Google Scholar]
- 46. Hossain S, Roy S, Sultana K, Warren C. Assessing the Effect of a Primary Health Care Intervention for Improving Pre‐eclampsia and Eclampsia Knowledge and Practice in Bangladesh. Washington, DC: Population Council and USAID; 2019. [Google Scholar]
- 47. Ishaku S, Nwala E, Adoyi G, Nwigwe C, Kongyamba S, Anaba U, et al. Post‐Intervention Analysis of Pre‐eclampsia and Eclampsia in Three Nigerian States. Washington, DC: Population Council and USAID; 2019. [Google Scholar]
- 48. Ndwiga C, Sripad P, Warren C. Ending Eclampsia Project: Addressing Barriers to Quality of Underutilized Commodities and Services for Prevention and Management of Pre‐eclampsia and Eclampsia in Kenya – Study Report Washington. New York, NY: Population Council; 2018. [Google Scholar]
- 49. Sripad P, Ismail H, Dempsey A, Kirk K, Warren CE. Exploring Barriers and Opportunities for Pre‐eclampsia and Eclampsia Prevention and Management in Ethiopia. Washington, DC: Population Council and USAID; 2018. [Google Scholar]
- 50. Van Dijk MG, Diaz Olavarrieta C, Zuniga PU, Gordillo RL, Gutierrez MER, Garcia SG. Use of magnesium sulfate for treatment of pre‐eclampsia and eclampsia in Mexico. Int J Gynecol Obstet 2013;121:110–4. [DOI] [PubMed] [Google Scholar]
- 51. Warren C, Hossain S, Nur RA, Sultana K, Kirk KR, Dempsey A. Landscape Analysis on Pre‐eclampsia and Eclampsia in Bangladesh. Washington, DC: Population Council and USAID; 2015. [Google Scholar]
- 52. Warren C, Ishaku S, Oginni A, Adoyi G, Kirk KR, Dempsey A. Landscape Analysis of Pre‐eclampsia/Eclampsia in Nigeria. Washington, DC: Population Council and USAID; 2015. [Google Scholar]
- 53. Alabintei O, Abasi I, Alabrah P. The use of magnesium sulphate in the management of severe preeclampsia and eclampsia in Bayelsa State, Nigeria. J Gynecol Womens Health 2021;21. [Google Scholar]
- 54. Alabintei O, Abasi I, Alabrah P. Assessment of compliance with national guidelines on the management of severe preeclampsia and eclampsia in public health facilities in Bayelsa State. Asian Res J Gynaecol Obstet 2021;5:17–28. [Google Scholar]
- 55. Woelk G, Daniels K, Cliff J, Lewin S, Sevene E, Fernandes B, et al. Translating research into policy: lessons learned from eclampsia treatment and malaria control in three southern African countries. Health Res Policy Syst 2009;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barua A, Mundle S, Bracken H, Easterling T, Winikoff B. Facility and personnel factors influencing magnesium sulfate use for eclampsia and pre‐eclampsia in 3 Indian hospitals. Int J Gynaecol Obstet: Off Organ Int Federation Gynaecol Obstet 2011;115:231–4. [DOI] [PubMed] [Google Scholar]
- 57. Lotufo FA, Parpinelli MA, Osis MJ, Surita FG, Costa ML, Cecatti JG. Obstetrician's risk perception on the prescription of magnesium sulfate in severe preeclampsia and eclampsia: a qualitative study in Brazil. PLoS One 2017;12:e0172602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sheikh S, Qureshi RN, Khowaja AR, Salam R, Vidler M, Sawchuck D, et al. Health care provider knowledge and routine management of pre‐eclampsia in Pakistan. Reprod Health 2016;13:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chikalipo MC, Phiri LK, Mndolo N, Mbiza CR, Khisi P, Golombe E, et al. Perception of midwives towards magnesium sulfate use at Chatinkha Maternity Wing in Blantyre, Malawi: a qualitative study. Int J Women's Health 2020;12:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramadurg U, Vidler M, Charanthimath U, Katageri G, Bellad M, Mallapur A, et al. Community health worker knowledge and management of pre‐eclampsia in rural Karnataka State, India. Reprod Health 2016;13:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raney JH, Morgan MC, Christmas A, Sterling M, Spindler H, Ghosh R, et al. Simulation‐enhanced nurse mentoring to improve preeclampsia and eclampsia care: an education intervention study in Bihar, India. BMC Pregnancy Childbirth 2019;19:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Danmusa S, Coeytaux F, Potts J, Wells E. Expanding Use of Magnesium Sulfate for Treatment of Pre‐eclampsia and Eclampsia: Building Towards Scale in Nigeria. New York: Public Health Institute; 2014. [Google Scholar]
- 63. Mathai M. Working with communities, governments and academic institutions to make pregnancy safer. Best Pract Res Clin Obstet Gynaecol 2008;22:465–76. [DOI] [PubMed] [Google Scholar]
- 64. Ridge AL, Bero LA, Hill SR. Identifying barriers to the availability and use of Magnesium Sulphate Injection in resource poor countries: a case study in Zambia. BMC Health Serv Res 2010;10:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith JM, Currie S, Cannon T, Armbruster D, Perri J. Are national policies and programs for prevention and management of postpartum hemorrhage and preeclampsia adequate? A key informant survey in 37 countries. Global Health Sci Pract 2014;2:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Okereke E, Ahonsi B, Tukur J, Ishaku SM, Oginni AB. Benefits of using magnesium sulphate (MgSO4) for eclampsia management and maternal mortality reduction: lessons from Kano State in Northern Nigeria. BMC Res Notes 2012;5:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim YM, Ansari N, Kols A, Tappis H, Currie S, Zainullah P, et al. Prevention and management of severe pre‐eclampsia/eclampsia in Afghanistan. BMC Pregnancy Childbirth 2013;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salinger DH, Mundle S, Regi A, Bracken H, Winikoff B, Vicini P, et al. Magnesium sulphate for prevention of eclampsia: are intramuscular and intravenous regimens equivalent? A population pharmacokinetic study. BJOG: Int J Obstet Gynaecol 2013;120:894–900. [DOI] [PubMed] [Google Scholar]
- 69. Tukur J. The use of magnesium sulphate for the treatment of severe pre‐eclampsia and eclampsia. Ann Afr Med 2009;8:76–80. [DOI] [PubMed] [Google Scholar]
- 70. Duduyemi AO, Okafor IP, Misoprostol OES. Magnesium sulphate and anti‐shock garment: a knowledge, availability and utilization study at the Primary Health Care Level in Western Nigeria. PLoS One 2019;3:e0213491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hofmeyr GJ, Haws RA, Bergström S, Lee ACC, Okong P, Darmstadt GL, et al. Obstetric care in low‐resource settings: what, who, and how to overcome challenges to scale up? Int J Gynecol Obstet 2009;107:S21–45. [DOI] [PubMed] [Google Scholar]
- 72. Munabi‐Babigumira S, Glenton C, Lewin S, Fretheim A, Nabudere H. Factors that influence the provision of intrapartum and postnatal care by skilled birth attendants in low‐ and middle‐income countries: a qualitative evidence synthesis. Cochrane Database Syst Rev 2017;11:CD011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ritchie LMP, Khan S, Moore JE, Timmings C, van Lettow M, Vogel JP, et al. Low‐ and middle‐income countries face many common barriers to implementation of maternal health evidence products. J Clin Epidemiol 2016;76:229–37. [DOI] [PubMed] [Google Scholar]
- 74. World Health Organization . WHO Recommendations: Optimizing Health Worker Roles to Improve Access to Key Maternal and Newborn Health Interventions through Task Shifting. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 75. Smith JM, Lowe RF, Fullerton J, Currie SM, Harris L, Felker‐Kantor E. An integrative review of the side effects related to the use of magnesium sulfate for pre‐eclampsia and eclampsia management. BMC Pregnancy Childbirth 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Duley L, Guelmezoglu AM, Henderson‐Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database Syst Rev 2010;(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gordon R, Magee LA, Payne B, Firoz T, Sawchuck D, Tu D, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J d'obstetrique et gynecologie du Canada: JOGC 2014;36:154–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of qualitative findings.
Table S2. Evidence profile.
Table S3. CASP assessments of included studies.
Appendix S1. Search strategies.
Appendix S2. ENTREQ statement.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.


