Abstract
Background and purpose
The diagnosis of late‐onset (age ≥50 years old) relapsing remitting multiple sclerosis (LORRMS) has been increasingly described in clinical practice, whereas data focusing on the specific therapeutic management of LORRMS are scarce. Our objective was to compare the effectiveness of injectable and oral first‐line disease‐modifying therapies (DMTs) in a cohort of LORRMS patients with time to first relapse, time to confirmed disability progression (CDP), and time to discontinuation.
Methods
This is a multicenter, observational, retrospectively acquired cohort study on LORRMS‐naïve patients from the Italian MS Register who started either injectable or oral first‐line DMTs between January 1, 2013 and December 31, 2017. LORRMS patients were divided into two groups, namely the injectable group (IG) and oral group (OG). Cox models adjusted with inverse probability‐weighted propensity score were built for the investigated outcomes.
Results
Of a cohort of 3989 patients, 302 were enrolled (203 in the IG and 99 in the OG). The two cohorts did not differ in baseline characteristics. Time to first relapse did not show any difference between the two groups (hazard ratio [HR]: 1.10; 95% confidence interval [CI]: 0.50–2.46, p = 0.797). Furthermore, no differences were found between the two groups with respect to the risk of CDP (HR: 1.04; 95% CI: 0.35–3.06, p = 0.939), nor for the risk of DMT discontinuation (HR: 0.90; 95% CI: 0.17–2.08, p = 0.425).
Conclusions
Real‐world data from the Italian MS Register suggested that both injectables and oral first‐line DMTs similarly controlled the investigated outcomes in LORRMS.
Keywords: first choice, injectable disease‐modifying therapies, late onset, multiple sclerosis, oral disease‐modifying therapies

INTRODUCTION
Multiple sclerosis (MS) is among the most common causes of neurological disability in young adults when the onset of disease happens at about 30 years of age [1]. However, about 10% of all patients with MS have late‐onset MS (LOMS), defined as onset of the disease at ≥50 years of age [2].
The diagnosis of LOMS in the relapsing remitting multiple sclerosis (RRMS) population (LORRMS) has been increasingly described in clinical practice, due to the improvement of the diagnostic sensitivity of the new diagnostic criteria [3].
It is described that LORRMS is associated with an adverse prognosis in terms of disability accrual, but established marker(s) of such poor prognosis are still lacking in clinical practice [4, 5, 6, 7].
At the same time, data focusing on the specific therapeutic management of LORRMS are scarce; and all of the licensed disease‐modifying therapies (DMTs) have been studied in clinical trials that usually recruit patients up to 55 years of age [8]. However, DMTs should be studied in all age groups, and trial participants should be representative of the patient population receiving the therapy daily in medical practices.
The aim of the current study was to evaluate long‐term outcomes of first‐line DMTs in terms of time to first relapse, disability progression, and time to discontinuation in LORRMS‐naïve patients by focusing on the direct comparison between injectable (interferons and glatiramer acetate) and oral (dimethyl fumarate and teriflunomide) first‐line DMTs [9].
METHODS
Study design and setting
A multicenter, observational, retrospectively acquired cohort was utilized for the current study. Anonymized clinical data of patients with RRMS were extracted from the Italian MS Register from their first treatment prescription with injectable and oral DMTs (between January 1, 2013 and December 31, 2017) to their last follow‐up with the same treatment [10].
Participants
Key eligibility criteria included: (i) a diagnosis of RRMS according to the 2010 revised McDonald criteria [11] with an age of onset ≥50 years, here defined as LORRMS, (ii) start of first‐line DMTs (injectable or oral) between January 1, 2013 and December 31, 2017, (iii) continuous exposure to the investigated DMTs for ≥6 months, and (iv) patients with at least three visits (including baseline) with the Expanded Disability Status Scale (EDSS) evaluation.
LORRMS‐naïve patients who matched the required criteria were divided into two groups for the analyses, the injectables group (IG) and oral group (OG). The IG included LORRMS patients who were treated with either glatiramer acetate (40 mg/ml, three times per week subcutaneously and at least 48 h apart) or interferons (interferon β‐1a and interferon β‐1b, 30 µg/0.5 ml, once weekly, intramuscularly or interferon β‐1a, either 22 µg or 44 µg, three times per week subcutaneously) [12, 13, 14, 15]. The OG included LORRMS patients who were treated with either dimethyl fumarate (120 mg twice per day for the first 7 days, then 240 mg twice per day) or teriflunomide (14 mg once per day) [16, 17].
Study endpoints
The primary study outcome was the evaluation of time to first relapse and time to confirmed disability progression (CDP). The time interval from baseline to the first event (for patients with an event) or to the last evaluation at follow‐up (for patients without an event) was examined. Additionally, the time to discontinuation of the first prescribed DMT was evaluated.
Procedures and outcomes
Patients were included in the study at the initiation of treatment (baseline) and were monitored over their full time on the medication, with data collection performed at baseline and approximately every 6 months during the time of exposure. Patients were censored at treatment discontinuation or at their last recorded clinical visit.
A relapse was defined as new symptoms or an exacerbation of existing symptoms persisting for ≥24 h in the absence of concurrent illness/fever and occurring ≥30 days after a previous relapse.
CDP events were defined as ≥6‐month confirmed increases of either ≥0.5 points for patients with a baseline EDSS score >5.5, ≥1.0 point for those with a baseline EDSS score of 1 and 5.5, and ≥1.5 points for those with a baseline EDSS score of 0.
A minimum of three visits, including the baseline visit, with an EDSS score evaluation, was required. EDSS scores recorded within 30 days after the onset of a relapse were excluded.
Discontinuation of investigated drugs was defined as a gap of treatment for 60 or more days. Time to discontinuation (in months) was measured as the time between the index date and the end of the supply of the prescriptions dispensed.
Statistical analyses
Data are presented as counts (proportions) for categorical variables and mean (SD) or median (interquartile range [IQR]) for continuous variables. Unpaired t tests and Mann‐Whitney tests were used to compare continuous variables according to their distribution. χ2 tests were used to compare categorical variables. Univariate nonparametric Kaplan‐Meier curves and log‐rank tests were used to evaluate the events under investigation in the entire sample.
A Schoenfeld global test was used to verify the proportional hazards assumption for the time on treatments. Once the proportionality assumption was verified, a Cox proportional model was built for each investigated outcome in the entire sample. A Cox proportional hazard univariate regression model was used to estimate the hazard ratio (HR) and its 95% confidence interval (CI).
To take into account the imbalance of the two groups, a propensity score (PS) was calculated.
Additionally, logistic regression was conducted to evaluate all patients using treatment (injectable vs. oral) as the independent variable and baseline levels of sex, age, type of MS onset (monofocal or multifocal), EDSS score, number of relapses in the year prior to onset, and disease duration as covariates. Inverse probability of treatment weight (IPTW) and the stabilized inverse probability of treatment weight were also calculated.
Standardized differences calculated in weighted (using the stabilized weights) and unweighted samples were used to assess the balance of baseline covariates between treated and control.
Multivariable Cox proportional hazard regression models weighted for IPTW were performed to evaluate the relationship between outcomes and treatment groups. HRs and 95% CIs were calculated to evaluate the relationship between outcomes and the treatment group.
For the analysis of relapse outcomes, a negative binomial model and weighted negative binomial model were conducted, using the annual relapse rate as the dependent variable and group as the independent variable.
To better examine the differences between the two treatment strategies in mild‐to‐moderate patients, subgroup univariate analyses were conducted, stratifying patients on baseline EDSS scores (≤2 and >2) and for number of relapses in the prebaseline year (one or more than one), and a Cox proportional hazard univariate regression model was applied to each subgroup. HRs and their 95% CIs were reported.
Missing data were handled through multiple imputation. The analysis used normalized weights to approximate the inferences in the data with data missing not at random [18]. The associations between missingness of the baseline data and other demographical and clinical characteristics were calculated with a multivariate logistic regression analysis, as previously published [19, 20].
A sensitivity analysis was conducted on patients with at least 30 months of follow‐up. All results were considered significant at 0.05. SPSS 21.0 (IBM) was used for all analyses.
Protocol approvals standard, registrations, and patient consents
The ethics committee of the University of Bari (Italy) approved the study as the coordinator center (reference nos. 0055587 and 0052885) and the local ethics committees of all participant centers with the final approval of the Scientific Committee of the Italian MS Register. All studies were undertaken with the understanding and written consent of each subject, and that the study conforms with World Medical Association Declaration of Helsinki.
Data availability statement
Anonymized data will be shared by request from a qualified investigator for the sole purpose of replicating procedures and results presented in the report, provided that the data transfer is in agreement with European Union legislation on the general data protection regulation.
RESULTS
Participants
Out of 3989 RRMS patients in the Italian MS Register who had started their first DMT during the index window, 302 (203 in the IG and 99 in the OG) matched the required criteria and have been considered eligible for the analyses (Figure 1).
FIGURE 1.
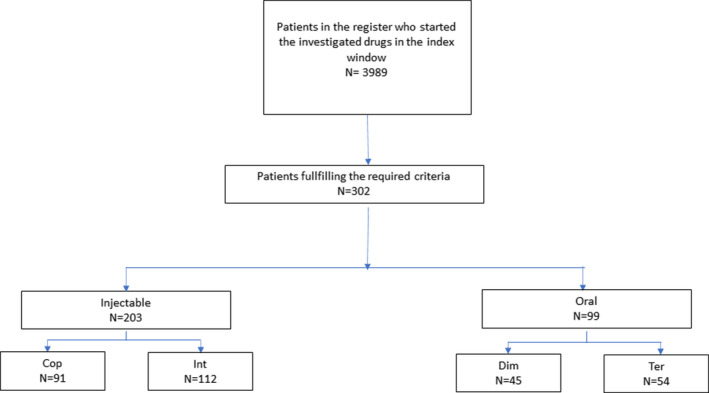
Patients' selection flow chart. Cop, Copaxone; Int, interferons; Dim, dimethyl fumarate; Ter, teriflunomide
Baseline characteristics by group are shown in Table 1. Patients in the two groups were similar for the baseline characteristics (Table 1). The median follow‐up of the total cohort was 25.8 months (IQR: 14.5–38.2 months), the median follow‐up in the IG was higher than the OG (31.1 months, IQR: 17.8–43.6 months vs. 17, IQR: 12.4–24.5 months, p < 0.0001).
TABLE 1.
Baseline characteristics of the two groups
| No. | IG | OG | p | Unweighted standardized mean difference | Weighted standardized mean difference | ||
|---|---|---|---|---|---|---|---|
| 203 | 99 | ||||||
| Female, n (%) | 130 | (64.0) | 72 | (72.7) | 0.132 | −0.087 | −0.184 |
| Age, years, mean (SD) | 55.1 | (4.8) | 55.0 | (5.0) | 0.896 | 0.082 | 0.16 |
| Monofocal onset, n (%) | 195 | (96) | 96 | (97) | 0.692 | −0.009 | −0.048 |
| EDSS at baseline, median (q1–q3) | 2.0 | (1.5–3.0) | 2.5 | (1.5–3.0) | 0.492 | −0.11 | −0.084 |
| Relapses in the year before treatment start, n (%) | 179 | (88.2) | 89 | (89.9) | 0.657 | −0.017 | 0.174 |
| DD, median (q1–q3) | 58 | (18–167) | 70.5 | (22–195) | 0.982 | 2.12 | 0.0002 |
Abbreviations: DD, disease duration; EDSS, Expanded Disability Status Scale; IG, injectable group; OG, oral group, q1‐q3, first and third quartiles.
*Only in patients with relapses in the last year.
Survival analyses
During the follow‐up, 52 patients relapsed (n = 41 [20.2%] in the IG, n = 11 [11.1%] in the OG).
A log‐rank test revealed that the risk of experiencing the first relapse was similar between the two groups (p = 0.395) (Figure 2), which was also confirmed by the IPTW‐adjusted Cox model (HR: 1.10; 95% CI: 0.50–2.46, p = 0.797) (Figure 3).
FIGURE 2.
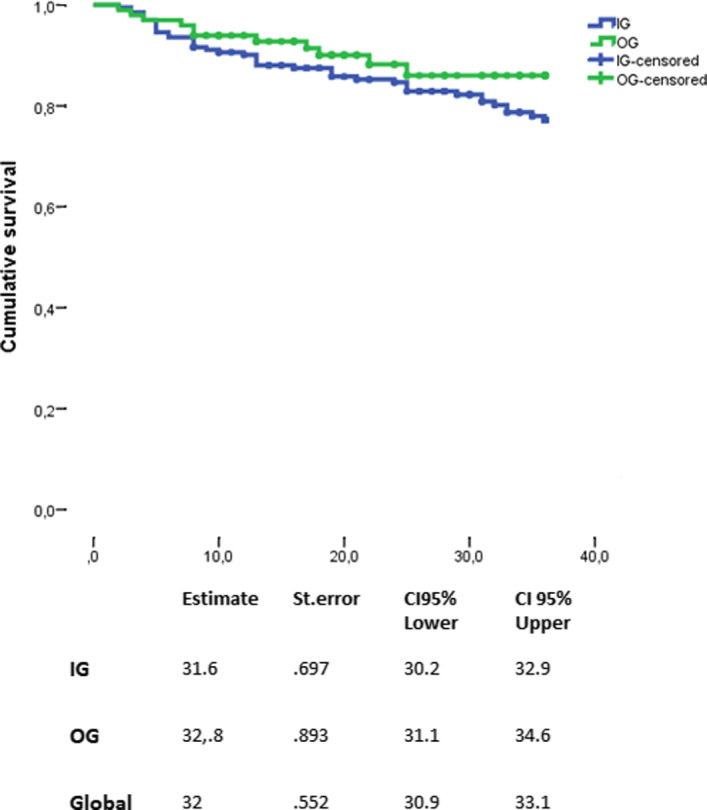
Time to first relapse between the two groups. CI, confidence interval; IG, injectable group; OG, oral group; St. error, standard error
FIGURE 3.
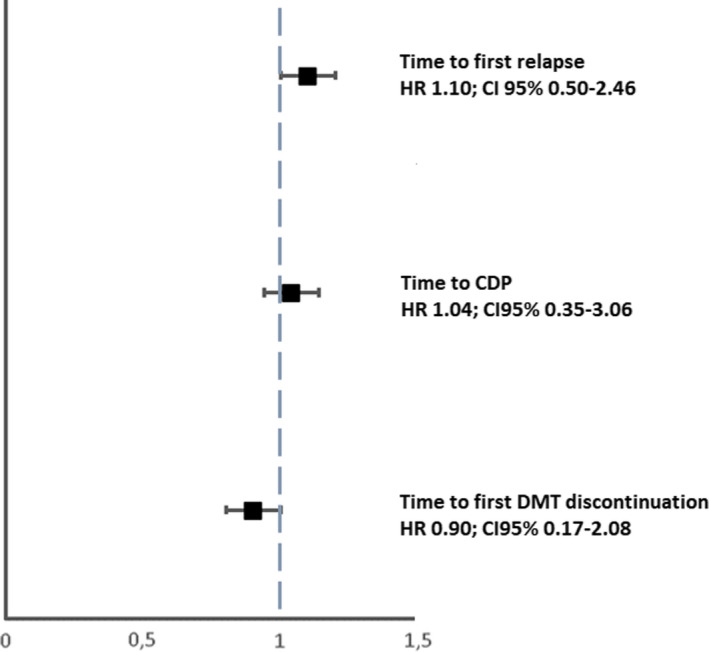
Analysis of treatment effects in time to first relapse, time to CDP, and time to DMT discontinuation. The treatment effects were explored by a propensity‐score adjustment in quintiles for age, duration of disease from onset, Expanded Disability Status Scale at baseline, relapses in the previous year, sex, and clinical onset. CDP, confirmed disability progression; CI, confidence interval; DMT, disease‐modifying therapies; HR, hazard ratio
A negative binomial model and weighted negative binomial model were applied using the annual relapse rate as the dependent variable and group (IG vs. OG) as the independent variable. The incidence rate ratio (OG vs. IG) was 0.80 (95% CI: 0.54–1.19, p = 0.281).
After an inverse probability weighting, the incidence rate ratio was 0.79 (95% CI: 0.52–1.23, p = 0.305). Taking this into consideration with the total count of relapses, the two groups did not show any differences in the risk to occurrence of relapses.
The event CDP was observed in 76 patients (n = 59 [31.6%] in the IG, n = 17 [18.7%] in the OG). The risk to reach CDP did not differ between the two groups using a log‐rank test (p = 0.790) (Figure 4). This was confirmed by the IPTW‐adjusted Cox model (HR: 1.04; 95% CI: 0.35–3.06, p = 0.939) (Figure 3).
FIGURE 4.
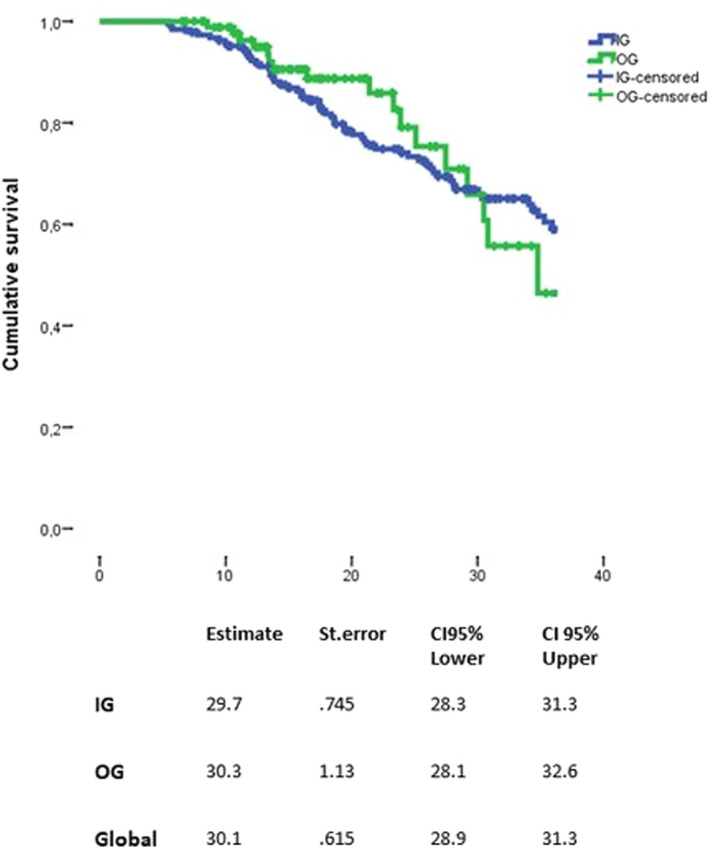
Time to confirmed disability progression between the two groups. CI, confidence interval; IG, injectable group; OG, oral group; St. error, standard error
Finally, DMT discontinuation was observed in 69 patients, 56 patients (27.6%) in the IG, 13 patients (18.8%) in the OG). The risk to discontinue DMT did not differ between the two groups using a log‐rank test (p = 0.079) (Figure 5). This was confirmed by the IPTW‐adjusted Cox model (HR: 0.90; 95% CI: 0.17–2.08, p = 0.425) (Figure 3).
FIGURE 5.
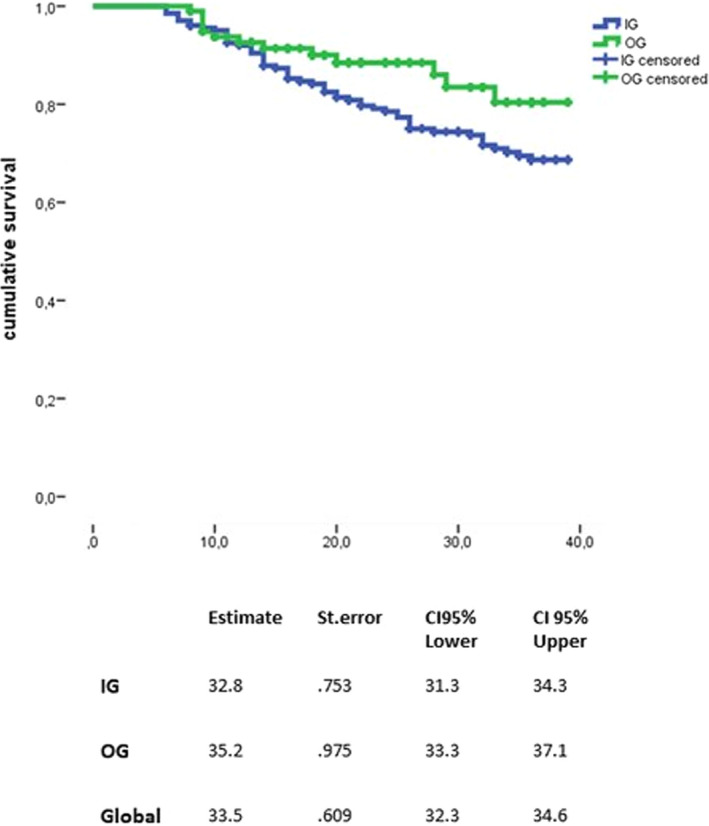
Time to first disease‐modifying treatment discontinuation between the two groups. CI, confidence interval; IG, injectable group; OG, oral group; St. error, standard error
Subgroup analyses
Subgroup analyses were also performed for each investigated outcome to examine any difference in the two groups in mild‐to‐moderate patients. IG and OG subjects were stratified based on their baseline EDSS score (≤2 or >2) and the number of relapses during the year prior to their baseline visit (1 or >1) (Supplementary Table S1).
No statistically significant differences were found between the two groups for all the investigated outcomes (Supplementary Table S1).
Sensitivity analysis
A sensitivity analysis was conducted on 163 (136 IG and 27 OG) subjects with at least 30 months (out of 36) of follow‐up. The IQR around the median at follow up visits became much smaller, with median follow up of 36.7 months (IQR: 29.7–47.9 months). In the IG, the median was also 39.3 months (IQR: 30.7–50.5 months), whereas in the OG it was 31.5 months (IQR: 26.7–36.6 months).
Time to first relapse (p = 0.512), time to CDP (p = 0.631), and time to DMT discontinuation (p = 0.190) did not show differences when analyzed with a log‐rank test. The IPTW PS‐adjusted Cox regression model revealed no differences in hazard ratios for investigated outcomes: time to first relapse (HR: 1.01; 95% CI: 0.81–1.23, p = 0.992), time to CDP (HR: 0.79; 95% CI: 0.42–1.47, p = 0.460), and time to first DMT discontinuation (HR: 0.46; 95% CI: 0.95–2.26, p = 0.342).
DISCUSSION
In this multicenter, observational, retrospectively acquired cohort study on LORRMS, we found no differences between the two investigated groups in terms of risk of relapse occurrence, CDP, and treatment discontinuation.
In our cohort, the rates of the three investigated events were higher in patients in the IG group, but when these data were analyzed over time (first with univariate survival analysis and then with a multivariate IPTW PS‐adjusted Cox model), we did not find any difference.
In clinical practice, LORRMS has been investigated in terms of clinical characteristics and prognostic factors, but difference in effectiveness of first line DMTs in such a cohort has been considered lightly, simply as the rate of patients exposed or not [6]. No specific treatment guidelines exist for LORRMS that have been considered a cohort of MS patients at major risk of worst clinical prognosis [2].
A recent work by Thakolwiboon et al. [21] showed preliminary results on the effectiveness and tolerability of DMTs in a cohort of 44 LORRMS also considering first‐line DMTs. The effectiveness was assessed by no evidence of disease activity (NEDA) score after 12 months on therapy, and tolerability was reported as rates of discontinuation for safety alerts [21]. After 12 months, the NEDA status was obtained by 18 of 27 patients on glatiramer acetate, 6 of 13 patients on interferons, 4 of 6 patients on dimethyl fumarate, and 10 of 19 on teriflunomide [21]. However, efficacy of each DMT in that study should not be compared due to small sample size and possible selection bias.
LORRMS represents a very frail cohort of people with RRMS, because they are more prone to the worst prognosis in term of disability accrual, as previously described [5, 6, 7, 22, 23].
Immunosenescence is a matter of debate in the neuroimmunology field for the qualitative and quantitative changes occurring to adaptive and innate immunity compartments during aging [24].
These changes may be additive or synergistic with the effects produced by immunomodulatory and immunosuppressive medications, which could lead to a lower efficacy and tolerance of drugs with a higher rate of therapeutic failure and side effects [24].
To date, more studies are needed for a better understanding of the benefits of DMTs in LORRMS, both in clinical trials and in the real‐world setting.
Big data registries offer the opportunity to study real‐world clinical outcomes in large cohorts of patients, and the strengths of our work include the generalizability, the representation of daily clinical MS practice, and the large cohort of patients collected in the Italian MS Register, which is the largest national clinical database with about 140 connected Italian MS centers [10, 25].
Age at onset should be associated with a personalized treatment algorithm, considering the disease heterogeneity and the increasing number of DMTs available. Additionally, the long‐term data on the DMTs’ efficacy and safety are mandatory to inform decision making.
The primary limitation of our study is the observational nature of the data. To mitigate the imbalance due to heterogeneity of data, we have employed a IPTW PS adjustment. Furthermore, to overcome biases related to data collection, we have conducted a sensitivity analysis limited to patients with at least 30 months of follow‐up [26, 27, 28, 29].
Another limit is due to the lack of magnetic resonance imaging data, because the current criteria for defining the efficacy of a treatment of MS must consider the presence of new or active (enhancing) lesions on magnetic resonance imaging scans [30]. These data are missing in many real‐world studies. The level of evidence of a descriptive study without MRI data is limited and cannot replace a non‐inferiority trial.
Furthermore, we have no detailed data to adequately compare the safety profile of the investigated drugs, because data were not sufficiently complete to enable such an analysis.
Further research is needed to more accurately identify the DMTs that could fit better in a LORRMS cohort, both for efficacy and for safety issues in a real‐world setting.
CONFLICT OF INTEREST
All authors have nothing to disclose related to the submitted article.
AUTHOR CONTRIBUTIONS
Aurora Zanghì: Conceptualization (equal), data curation (equal), formal analysis (equal), methodology (equal), writing–original draft (equal). Carlo Avolio: Data curation (equal), investigation (equal), methodology (equal), supervision (equal), validation (equal). Maria Pia Amato: Data curation (equal), methodology (equal), supervision (equal), validation (equal). Massimo Filippi: Conceptualization (equal), data curation (equal), validation (equal), visualization (equal). Maria Trojano: Conceptualization (equal), data curation (equal), supervision (equal), validation (equal). Francesco Patti: Conceptualization (equal), data curation (equal), validation (equal), visualization (equal). Emanuele D'Amico: Conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), supervision (equal), validation (equal), visualization (equal), writing–original draft (equal).
Supporting information
Table S1
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Catania within the CRUI‐CARE Agreement. [Correction added on 23 May 2022, after first online publication: CRUI funding statement has been added.]
Zanghì A, Avolio C, Amato MP, et al. First‐line therapies in late‐onset multiple sclerosis: An Italian registry study. Eur J Neurol. 2021;28:4117–4123. 10.1111/ene.15006
Aurora Zanghì and Carlo Avolio contributed equally to this article.
Funding information
The researchers were independent from funders and sponsors. All researchers could access all of the data.
DATA AVAILABILITY STATEMENT
Anonymized data will be shared by request from a qualified investigator for the sole purpose of replicating procedures and results presented in the report, provided that the data transfer is in agreement with European Union legislation on the general data protection regulation.
REFERENCES
- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinelli V, Rodegher M, Moiola L, Comi G. Late onset multiple sclerosis: clinical characteristics, prognostic factors and differential diagnosis. Neurol Sci. 2004;25(Suppl 4):S350‐S355. [DOI] [PubMed] [Google Scholar]
- 3. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162‐173. [DOI] [PubMed] [Google Scholar]
- 4. Mirmosayyeb O, Brand S, Barzegar M, et al. Clinical characteristics and disability progression of early‐ and late‐onset multiple sclerosis compared to adult‐onset multiple sclerosis. J Clin Med. 2020;9(5):1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Amico E, Patti F, Zanghì A, Chisari CG, Lo Fermo S, Zappia M. Late‐onset and young‐onset relapsing‐remitting multiple sclerosis: evidence from a retrospective long‐term follow‐up study. Eur J Neurol. 2018;25(12):1425‐1431. [DOI] [PubMed] [Google Scholar]
- 6. Jakimovski D, Dujmic D, Hagemeier J, et al. Late onset multiple sclerosis is associated with more severe ventricle expansion. Mult Scler Relat Disord. 2020;46:102588. [DOI] [PubMed] [Google Scholar]
- 7. Alroughani R, Akhtar S, Ahmed S, Behbehani R, Al‐Hashel J. Is time to reach EDSS 6.0 faster in patients with late‐onset versus young‐onset multiple sclerosis? PLoS One. 2016;11(11):e0165846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Salter A, Wallström E, Cutter G, Stüve O. Evolution of clinical trials in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419826547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Amico E, Leone C, Caserta C, Patti F. Oral drugs in multiple sclerosis therapy: an overview and a critical appraisal. Expert Rev Neurother. 2015;15(7):803‐824. [DOI] [PubMed] [Google Scholar]
- 10. Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci. 2019;40(1):155‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDonald WI, Compston A, Edan G, et al. multiple Rdcf, Panel sgftI. Neurol otdomsA, 121–127.
- 12. https://www.ema.europa.eu/en/documents/product‐information/rebif‐epar‐product‐information_en.pdf
- 13. https://www.ema.europa.eu/en/documents/psusa/glatiramer‐list‐nationally‐authorised‐medicinal‐products‐psusa/00001529/201411_en.pdf
- 14. https://www.ema.europa.eu/en/medicines/human/EPAR/betaferon
- 15. https://www.emdserono.com/us‐en/pi/rebif‐pi.pdf
- 16. Aubagio (teriflunomide) tablets hp, sanofi.eu/aubagio (accessed April).
- 17. Tecfidera (dimethyl fumarate) hw, ema.europa.eu/docs/it_IT/document_library/, EPAR_‐_Product_Information/human/002601/, WC500162069.pdf (accessed April).
- 18. Carpenter JR, Kenward MG, White IR. Sensitivity analysis after multiple imputation under missing at random: a weighting approach. Stat Methods Med Res. 2007;16(3):259‐275. [DOI] [PubMed] [Google Scholar]
- 19. Ferro MA. Missing data in longitudinal studies: cross‐sectional multiple imputation provides similar estimates to full‐information maximum likelihood. Ann Epidemiol. 2014;24(1):75‐77. [DOI] [PubMed] [Google Scholar]
- 20. Héraud‐Bousquet V, Larsen C, Carpenter J, Desenclos J‐C, Le Strat Y. Practical considerations for sensitivity analysis after multiple imputation applied to epidemiological studies with incomplete data. BMC Med Res Methodol. 2012;12(1):73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thakolwiboon S, Karukote A, Sohn G, Jin D, Avila M. Efficacy and tolerability of disease modifying therapies in late onset multiple sclerosis (2176). Neurology. 2020;94(15 Supplement):2176. [Google Scholar]
- 22. Andersen MA, Buron M, Magyari M. Late‐onset Multiple Sclerosis is associated with an increased rate of reaching Disability Milestones (760). Neurology. 2020;94(15 Supplement):760. [DOI] [PubMed] [Google Scholar]
- 23. Narimatsu K, Freitas L, Mendes N, et al. Late onset multiple sclerosis: an epidemiologic study from the first Brazilian MS center (4625). Neurology. 2020;94(15 Supplement):4625. [Google Scholar]
- 24. Fulop T, Larbi A, Hirokawa K, Cohen AA, Witkowski JM. Immunosenescence is both functional/adaptive and dysfunctional/maladaptive. Semin Immunopathol. 2020;42(5):521‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis ‐ insights from real‐world observational studies. Nat Rev Neurol. 2017;13(2):105‐118. [DOI] [PubMed] [Google Scholar]
- 26. Rosenbaum PR RDCacgumms, 33‐38. mtitpsAS.
- 27. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734‐753. [DOI] [PubMed] [Google Scholar]
- 28. Bergstra SA, Sepriano A, Ramiro S, Landewé R. Three handy tips and a practical guide to improve your propensity score models. RMD open. 2019;5(1):e000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalincik T, Butzkueven H. Observational data: Understanding the real MS world. Mult Scler. 2016;22(13):1642‐1648. [DOI] [PubMed] [Google Scholar]
- 30. Mayssam EN, Eid C, Khoury SJ, Hannoun S. "No evidence of disease activity": Is it an aspirational therapeutic goal in multiple sclerosis? Mult Scler Relat Disord. 2020;40:101935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Anonymized data will be shared by request from a qualified investigator for the sole purpose of replicating procedures and results presented in the report, provided that the data transfer is in agreement with European Union legislation on the general data protection regulation.
Anonymized data will be shared by request from a qualified investigator for the sole purpose of replicating procedures and results presented in the report, provided that the data transfer is in agreement with European Union legislation on the general data protection regulation.


