Abstract
Aim
To evaluate the effects of empagliflozin versus placebo on subsequent insulin initiation or dosing changes in a large cardiovascular outcomes trial.
Materials and Methods
In EMPA‐REG OUTCOME, 7020 patients with type 2 diabetes and cardiovascular disease received empagliflozin 10 mg, 25 mg, or placebo. Median follow‐up was 3.1 years. After 12 weeks of treatment, changes in background antihyperglycaemic therapy were permitted. Among insulin‐naïve patients, we assessed the effects of pooled empagliflozin arms versus placebo on time to initiation of insulin. Among insulin‐treated patients, we assessed effects on time to an increase or decrease in insulin dose of more than 20%.
Results
In 3633 (52%) participants not treated with insulin at baseline, empagliflozin reduced new use of insulin versus placebo by 60% (7.1% vs. 16.4%; adjusted HR 0.40 [95% CI 0.32‐0.49]; P < .0001). In 3387 (48%) patients using insulin at baseline, empagliflozin reduced the need for a greater than 20% insulin dose increase by 58% (14.4% vs. 29.3%; adjusted HR 0.42 [95% CI 0.36‐0.49]; P < .0001) and increased the proportion achieving sustained greater than 20% insulin dose reductions without subsequent increases in HbA1c compared with placebo (9.2% vs. 4.9%; adjusted HR 1.87 [95% CI: 1.39‐2.51]; P < .0001). Sensitivity analyses confirmed consistent findings when insulin dose changes of more than 10% or more than 30% were considered.
Conclusions
In patients with type 2 diabetes and cardiovascular disease, empagliflozin markedly and durably delays insulin initiation and substantial increases in insulin dose, while facilitating sustained reductions in insulin requirements over time.
Keywords: diabetes, insulin, sodium‐glucose co‐transporter‐2 inhibitors
1. INTRODUCTION
Insulin is a core antihyperglycaemic therapy and is often needed in the longitudinal management of type 2 diabetes to control blood glucose concentrations. Global insulin requirements are projected to increase by more than 20% by 2030, driven by forecasted increases in the prevalence of type 2 diabetes, in large part as a result of the longer survival of people living with type 2 diabetes. 1 Although insulin use does not appear to directly adversely influence cardiovascular risk or overall mortality, 2 , 3 , 4 the need for insulin intensification in practice represents a marker of clinical progression and tracks with the accumulation of cardiometabolic risk factors. 5 Indeed, insulin use identifies a particularly high‐risk cohort of patients with type 2 diabetes for adverse cardiovascular events and premature death. 6 , 7 , 8 Depending on the health system, insulin can be expensive and requires time, training, and resource expenditure to support its use. 9 Treatment‐attendant adverse effects of insulin such as hypoglycaemia and weight gain 2 , 3 , 4 may be particularly problematic for patients with concomitant cardiovascular disease. 10 Especially in the context of availability of newer antihyperglycaemic options, delaying, preventing, or reducing the need for insulin intensification represents an attractive adjunctive management goal for both patients and practitioners.
The sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors have modest glycaemic effects 11 and may improve β‐cell function and insulin sensitivity. 12 , 13 In the EMPA‐REG OUTCOME trial involving participants with type 2 diabetes and established atherosclerotic cardiovascular disease (ASCVD), empagliflozin reduced cardiovascular and kidney outcomes, 14 , 15 independent of glycaemic control 11 , 16 and irrespective of background antihyperglycaemic therapy, including the use of insulin. 17 Limited data were available regarding the effects of SGLT‐2 inhibitor initiation on subsequent need for insulin commencement or intensification.
We determined the treatment effects of empagliflozin relative to placebo and in addition to standard of care on the time to initiation of insulin treatment among insulin‐naïve participants, and time to substantial increases or decreases in insulin dose among insulin‐treated participants. In exploratory analyses, assuming stable treatment effects over time, we estimated the lifetime benefits of empagliflozin in delaying the need for insulin initiation using validated actuarial methods.
2. MATERIALS AND METHODS
2.1. The EMPA‐REG OUTCOME trial
This was a post hoc analysis of the EMPA‐REG OUTCOME randomized, double‐blind, placebo‐controlled trial. The trial design 18 and primary results 14 , 15 have been previously published. In brief, patients with type 2 diabetes with an HbA1c of 7.0%‐9.0% for drug‐naïve patients, and 7.0%‐10.0% for those on stable antihyperglycaemic therapy, established ASCVD, and estimated glomerular filtration rate (eGFR) of 30 mL/min/1.73 m2 or higher, were randomly assigned to empagliflozin 10 mg, 25 mg, or placebo administered once daily in addition to standard of care. Among insulin‐treated participants, total daily prescribed dose could not be changed within the 12 weeks prior to randomization by more than 10% from the dose at randomization. During the initial 12 weeks of treatment, antihyperglycaemic therapies were to be kept unchanged to assess the early glycaemic and metabolic effects of empagliflozin. However, changes were permitted if the patient had a confirmed fasting glucose level of more than 240 mg/dL (>13.3 mmol/L) or for symptomatic hypoglycaemia. After 12 weeks, investigators were encouraged to follow local guidelines for achieving glycaemic control by adjusting background antihyperglycaemic therapy as needed, and for treating other cardiovascular risk factors. Median follow‐up was 3.1 years.
2.2. Insulin‐related endpoints
Total daily insulin dose was captured and estimated at each study visit.
In the current analysis, we explore a broad range of endpoints related to new‐onset insulin use, sustained insulin dose increase, and sustained insulin dose reduction, as well as its discontinuation.
Insulin initiation was defined as time to first initiation of insulin (at any dose) taken for at least two consecutive visits at a minimum of 13 weeks apart. This time interval was selected as the regular long‐term visit schedule frequency in the EMPA‐REG OUTCOME trial, which was 14 ± 1 weeks. This endpoint was analysed in patients not treated with insulin at baseline.
Sustained insulin dose increase was defined as time to first increase in total daily insulin dose by more than 20% from baseline total daily insulin dose, sustained for at least two consecutive visits at a minimum of 13 weeks apart, analysed in patients treated with insulin at baseline. Sensitivity analyses were performed varying this dose escalation threshold by more than 10% and more than 30% increases from baseline. As small absolute dose changes may account for larger relative changes among patients with low baseline insulin requirements, specific definitions for qualifying dose increases were used, in accordance with the EMPA‐REG OUTCOME protocol definitions of rescue therapy (see the Supplemental Methods).
Sustained insulin dose reduction was defined as time to first decrease in total daily insulin dose by more than 20% from baseline with sustained glycaemic control for the remaining time in the study. Sensitivity analyses were performed varying this dose reduction threshold by more than 10% and more than 30% decreases from baseline. Sustained glycaemic control was fulfilled if there was no subsequent change (defined as <0.2% increase) or a subsequent decrease in HbA1c for the remaining time in the study.
Sustained insulin discontinuation was defined as time to discontinuation of insulin with sustained glycaemic control for the remainder of the study analysed among patients treated with insulin at baseline.
We analysed the risk of each insulin‐related endpoint in placebo and pooled empagliflozin groups separately by Kaplan‐Meier estimates. For display purposes, we show annual Kaplan‐Meier estimates through 4 years. Differences in time to first event between the two treatment groups were evaluated by Cox proportional hazards regression models adjusted for treatment arm, geographic region, and baseline factors of body mass index, eGFR, HbA1c, and duration of type 2 diabetes. Effect estimates were summarized as hazard ratios (HRs) and 95% confidence intervals (CIs).
To investigate whether type 2 diabetes duration or baseline glycaemic control modified empagliflozinʼs effect on insulin‐related endpoints, we performed further analyses exploring treatment effects across subgroups of duration of type 2 diabetes (≤5, >5 to 10, >10 years) and of baseline HbA1c (<8, 8 to <9, ≥9%) using Cox regression, including an interaction term between these categorical variables and treatment arm, adjusted for the above covariates.
2.3. Exploratory actuarial analysis of insulin‐free survival
Using validated actuarial methods that have been previously applied to EMPA‐REG OUTCOME, 19 we estimated the long‐term effects on need for insulin initiation if empagliflozin was continued for lifetime use among insulin‐naïve participants. Given the known competing risks of death with long‐term therapeutic assessment, we were unable to evaluate the insulin use endpoints in isolation and instead evaluated time to first new insulin initiation (sustained over ≥2 consecutive study visits) or death (from any cause). As such, we estimated the effect of empagliflozin versus placebo on ‘residual lifespan free of new insulin initiation’ among patients not treated with insulin at baseline. Instead of time from randomization to last follow‐up, this analysis examined age at randomization and the age at the time of the outcome. We constructed Kaplan‐Meier survival curves for every age from 45 to 80 years for each treatment group using age rather than time from randomization as the time scale. Area under the survival curve (up to a maximum of 90 years) reflected average time alive and free from insulin initiation. The difference in these survival time estimates is the time that empagliflozin prolongs lifespan without need for insulin initiation. Representative age‐based Kaplan‐Meier plots are presented for three selected ages (45, 60, and 75 years). The estimated treatment differences in insulin‐free survival and accompanying 95% CIs were smoothed with a locally weighted scatterplot‐smoothing procedure.
All analyses are post hoc and not adjusted for multiplicity. All statistical analyses were performed using SAS software (version 9.4).
3. RESULTS
At baseline, 3387 (48%) patients were treated with insulin. Among insulin‐treated patients, insulin was used as monotherapy (28.1%) or in combination with other antihyperglycaemic therapies (71.9%). Mean (standard deviation) total daily insulin doses were 65 ± 49 IU with substantial variability, ranging from less than 10 to 250 or more IU per day; both treatment arms were treated with a similar distribution of insulin doses (Figure S1). Among subsets taking and not taking insulin at baseline, clinical profiles, baseline HbA1c, and concomitant therapies were well balanced between empagliflozin‐ and placebo‐treated patients (Table S1).
3.1. New insulin initiation
In 3633 (52%) participants not on insulin at baseline, empagliflozin reduced the relative need for insulin use versus placebo by 60% (7.1% vs. 16.4%; adjusted HR 0.40 [95% CI 0.32‐0.49]; P < .0001; Figure 1). Reductions in incident insulin use were observed across a range of baseline HbA1c, but were most pronounced among patients with an HbA1c of 8% or higher to less than 9% (adjusted HR 0.28 [95% CI 0.20‐0.39]) compared with an HbA1c of less than 8% (adjusted HR 0.48 [95% CI 0.33‐0.69]), and an HbA1c of 9% or higher (adjusted HR 0.49 [95% CI 0.34‐0.71]); P interaction = .037. Treatment effects were consistent across subgroups defined by type 2 diabetes disease duration: 5 years or less (adjusted HR 0.27; 95% CI 0.17‐0.44), 5‐10 years (adjusted HR 0.40; 95% CI 0.27‐0.60), and longer than 10 years (adjusted HR 0.45; 95% CI 0.34‐0.60); P interaction = .18.
FIGURE 1.
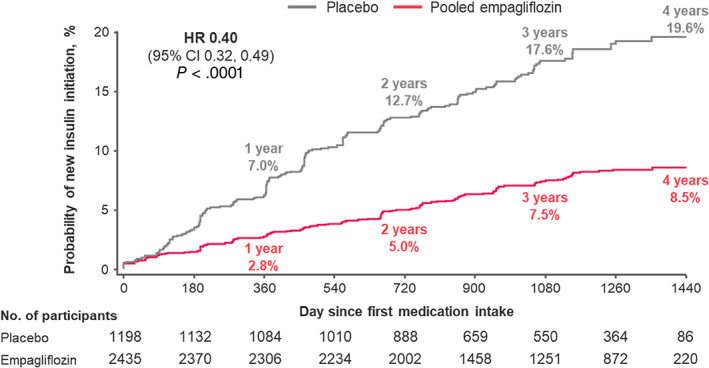
Sustained new insulin initiation among insulin‐naїve participants at baseline (n = 3633). Outcome of sustained insulin initiation was defined to be maintained on ≥2 consecutive visits ≥13 weeks apart. Displayed event rates are based on Kaplan‐Meier estimates. Cox regression model adjusted for baseline HbA1c, time since type 2 diabetes diagnosis, body mass index, estimated glomerular filtration rate, geographic region, and treatment status. CI, confidence interval; HR, hazard ratio
3.2. Substantial insulin dose increase
In 3387 (48%) patients using insulin at baseline, empagliflozin reduced the need for a greater than 20% insulin dose increase by 58% (14.4% vs. 29.3%; adjusted HR 0.42 [95% CI 0.36‐0.49]; P < .0001; Figure 2). Treatment effects on greater than 20% dose increases were consistent across subgroups of baseline HbA1c and type 2 diabetes duration (both P interaction > .05). In sensitivity analysis, consistent benefits with empagliflozin were observed when considering sustained insulin dose increases of more than 10% from baseline (22.8% vs. 41.9%; adjusted HR 0.44 [95% CI: 0.39‐0.50]; P < .0001) or of more than 30% from baseline (9.1% vs. 21.5%; adjusted HR 0.37 [95% CI: 0.31‐0.45]; P < .0001).
FIGURE 2.
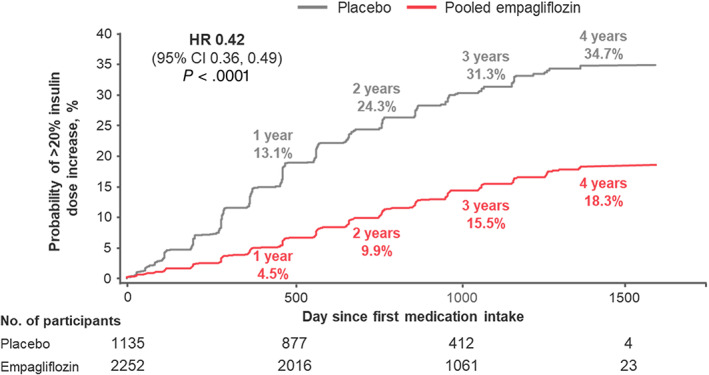
Sustained total daily insulin dose increase by >20% among insulin‐treated participants at baseline (n = 3387). Displayed event rates are based on Kaplan‐Meier estimates. Cox regression model adjusted for baseline HbA1c, time since type 2 diabetes diagnosis, body mass index, estimated glomerular filtration rate, geographic region, and treatment status. CI, confidence interval; HR, hazard ratio
3.3. Insulin discontinuation or substantial dose decrease
In 3387 (48%) patients using insulin at baseline, few patients (three with empagliflozin and none with placebo) discontinued insulin with sustained glycaemic control for the remainder of the study. Empagliflozin increased the proportion of patients achieving sustained greater than 20% insulin dose reductions with sustained glycaemic control compared with placebo (9.2% vs. 4.9%; adjusted HR 1.87 [95% CI: 1.39‐2.51]; P < .0001; Figure 3). Similarly, consistent benefits with empagliflozin were observed when considering sustained insulin dose reductions of more than 10% from baseline (14.0% vs. 7.5%; adjusted HR 1.91 [95% CI: 1.50‐2.43]; P < .0001) or of more than 30% from baseline (5.6% vs. 3.3%; adjusted HR 1.68 [95% CI: 1.17‐2.43]; P = .0055).
FIGURE 3.
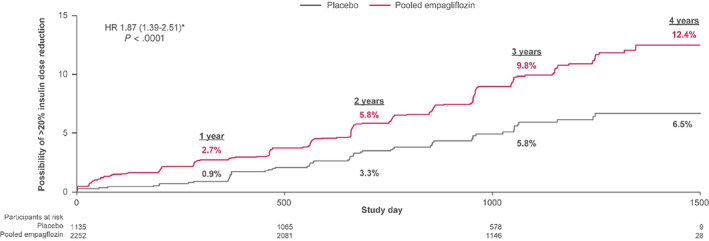
Sustained total daily insulin dose reduction by >20% among insulin‐treated participants at baseline (n = 3387). Dose reductions were considered appropriate if they were accompanied by no subsequent change (defined as <0.2% increase) or a decrease in subsequent HbA1c. Displayed event rates are based on Kaplan‐Meier estimates. *Cox regression model adjusted for baseline HbA1c, time since type 2 diabetes diagnosis, body mass index, estimated glomerular filtration rate, geographic region, and treatment status. CI, confidence interval; HR, hazard ratio
3.4. Projected long‐term benefits of empagliflozin on insulin‐free survival
Among 1198 participants assigned to placebo and not treated with insulin at baseline, 278 (23.2%) either died or were initiated on insulin (sustained over at least two study visits) during follow‐up. Among 2435 participants assigned to empagliflozin not treated with insulin at baseline, 280 (11.5%) experienced this composite endpoint.
Assuming that empagliflozinʼs effects on insulin therapy during the trial period would persist with long‐term use, the estimated mean ‘insulin‐free survival’ was longer with empagliflozin than placebo at all ages. Lifetime benefits on insulin‐free survival were, understandably, inversely related to baseline age, ranging from 1.4 to 11.3 years (Figure 4). For a 45‐year‐old, estimated insulin‐free survival was 20.1 years with empagliflozin and 10.0 years with placebo (difference: 10.1 years; 95% CI 5.7‐14.5 years; P < .0001). At the age of 60 years, insulin‐free survival was 16.7 versus 10.5 years (difference: 6.2; 95% CI 4.6‐7.8; P < .0001), and at the age of 75 years, it was 9.7 versus 8.1 years (difference: 1.5; 95% CI 0.0‐3.1; P = .056; Figure 5).
FIGURE 4.
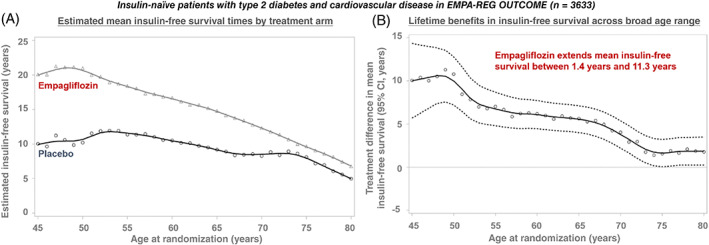
Long‐term benefit of empagliflozin on survival free from insulin initiation in the EMPA‐REG OUTCOME trial. Estimated mean insulin‐free survival times, A, in the empagliflozin and placebo arms are displayed. Treatment differences and 95% CIs are estimated for insulin‐free survival, B, after application of a locally weighted scatterplot‐smoothing procedure to age‐based Kaplan‐Meier estimates
FIGURE 5.
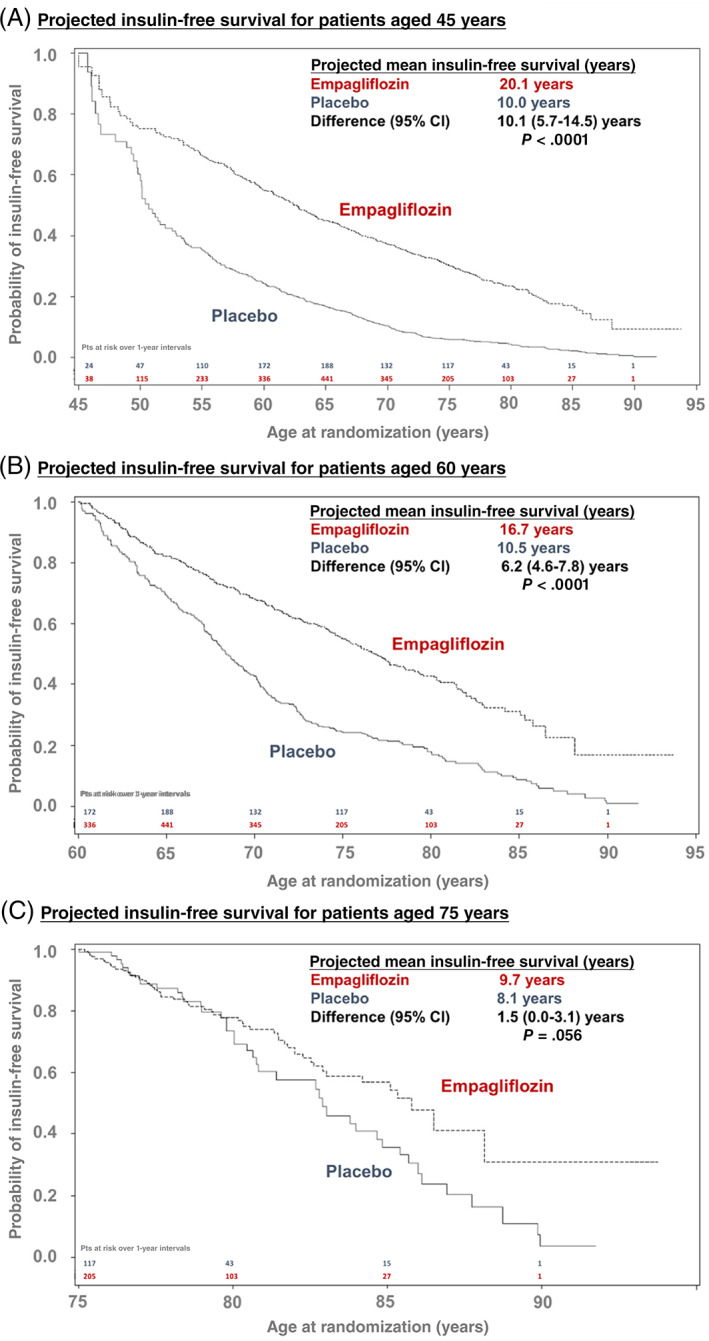
Residual survival free from insulin initiation by age at randomization. Age‐based Kaplan‐Meier curves are displayed for patients aged, A, 45, B, 60, and C, 75 years for insulin‐free survival between the empagliflozin and placebo arms. Area under the survival curve (up to a maximum of 90 years) reflected average time alive and free from insulin initiation. The difference in these survival time estimates is the time that empagliflozin prolongs lifespan without the need for insulin initiation among insulin‐naïve patients
4. DISCUSSION
In the EMPA‐REG OUTCOME trial, empagliflozin markedly delayed time to insulin initiation and reduced the need for large dose increases in patients already using insulin relative to placebo. Among insulin‐treated patients, empagliflozin also facilitated sustained reductions in insulin requirements without subsequent worsening in glycaemic control. These treatment effects on insulin intensification were consistent in sensitivity analyses varying the thresholds to define a substantial dose change, and across subgroups of baseline HbA1c and type 2 diabetes duration. Taken together, in the context of a randomized clinical trial in which the investigators were encouraged to maintain glycaemic equipoise, empagliflozin meaningfully and durably delayed the requirement for insulin or insulin intensification among adults with type 2 diabetes and cardiovascular disease. These adjunctive treatment benefits may help further inform decision calculus in integrating SGLT‐2 inhibitors among eligible patients.
4.1. Glycaemic effects of empagliflozin
The reductions in insulin requirements over time observed with empagliflozin may partially reflect its glucose‐lowering potential. In EMPA‐REG OUTCOME, empagliflozin lowered mean HbA1c by −0.57% (95% CI: −0.60% to −0.53%) compared with placebo by 12 weeks, during a period when antihyperglycaemic therapies were to be unaltered. 15 These glycaemic effects were similarly observed among patients who were and were not on insulin at baseline (Figure S2). 17 Glycaemic effects were safely achieved without an increment in the risk of hypoglycaemic events or diabetic ketoacidosis, even among high‐risk insulin‐treated patients, 17 which may partially reflect changes in background antihyperglycaemic therapies or dosing. After 12 weeks, for the remainder of the trial, investigators followed local guidelines to maintain glycaemic control; insulin dose reduction after the addition of empagliflozin is thus conceivable to maintain glycaemic equipoise. Other oral antihyperglycaemic therapies lower insulin requirements among insulin‐treated patients with type 2 diabetes. 20 Indeed, several early‐phase randomized clinical trials of SGLT‐2 inhibitors among insulin‐treated patients have corroborated reductions in daily insulin requirements. 21 Beyond additional glycaemic control, insulin requirement reductions may also reflect an underlying benefit of SGLT‐2 inhibition on either insulin secretion or insulin action, or both. 12 , 13
4.2. Lower rates of insulin initiation
We further show lower rates of new insulin initiation with an SGLT‐2 inhibitor among previously insulin‐naïve patients with type 2 diabetes. Glucagon‐like peptide‐1 receptor agonists have greater glycaemic effects and more directly stimulate endogenous pancreatic insulin release, and have been shown to lower future insulin use and intensification. 22 , 23 In trials designed to maintain glycaemic equipoise, some degree of unbalanced drop‐in of additional glucose‐lowering therapies are to be expected in the placebo group. 22 , 24 However, direct glycaemic effects of empagliflozin may not entirely explain the substantial between‐arm differential use of these additional therapies (including insulin) observed in EMPA‐REG OUTCOME. Reductions in insulin requirements were observed early and maintained over time between empagliflozin and placebo arms. SGLT‐2 inhibitors may thus have other effects in contributing to reductions and postponement in insulin requirements beyond simple glucose‐lowering alone. Insulin levels and secretion decline in response to lower plasma glucose concentrations among SGLT‐2 inhibitor‐treated patients. However, in experimental models 25 , 26 , 27 , 28 and early human studies, 12 , 13 SGLT‐2 inhibitors may improve β‐cell function and peripheral insulin sensitivity; these metabolic changes are apparent even after single doses of SGLT‐2 inhibitors. 12 Posited mechanisms underlying these effects include weight loss, reductions in liver fat, lowering tissue inflammation, modulation of adipocyte‐derived hormonal activity, and increased β‐cell proliferation. 29 , 30 , 31 Alternative study designs (outside of a trial targeting glycaemic equipoise) would be needed to determine the clinical contributions of these other mechanisms underlying lower insulin requirements with SGLT‐2 inhibition.
4.3. Estimating lifetime benefits of empagliflozin
While traditional regression methods offer insights regarding treatment effects during the duration of trial follow‐up, preventative therapies such as SGLT‐2 inhibitors are anticipated to be implemented over lifetime use and the need for insulin may accrue over time, especially among younger adults. Given the implications for costs of care and therapeutic complexity, patients and clinicians may be interested in quantifying the long‐term delay in initiation of insulin with SGLT‐2 inhibitors. We adapted actuarial methods to forecast treatment benefits if empagliflozin was used long‐term and if the effects on insulin initiation would be sustained. These age‐based methods and reports of alternative results have been employed to therapeutic effects of non‐pharmacologic 32 and pharmacologic 19 , 33 , 34 , 35 therapies. The estimated survival projections have been previously validated in a trial of heart failure with known long‐term follow‐up. 33 Among placebo‐treated participants, our estimates of time to insulin initiation appear reasonably well aligned with population‐based studies with long‐term follow‐up. A recent large US electronic health records study used restricted mean survival times to estimate time to insulin initiation among more than 1 million US patients with type 2 diabetes across a broad age range. Estimated time to insulin initiation varied by pathway and sequence of other antihyperglycaemic therapies, but was on average 6.5 years. 36 Other studies similarly estimated median times to insulin intensification of ~6‐8 years among patients with diabetes on multiple oral antihyperglycaemic therapies, 37 , 38 , 39 which are comparable with our estimates among placebo‐treated older adults in EMPA‐REG OUTCOME.
Young patients with type 2 diabetes face substantial longitudinal risks of disease progression and shortened life expectancy, 40 , 41 an especially concerning observation in light of recent trends in type 2 diabetes diagnoses occurring at younger ages. 42 Given greater anticipated duration of empagliflozin use and longer expected survival, we estimate substantial extension in years alive and without need for insulin among younger adults with type 2 diabetes with lifetime use of empagliflozin.
We recognize that many with type 2 diabetes will require insulin for glycaemic control, and that a marked, inappropriate reduction in insulin could unmask deficiency of endogenous insulin, which could in turn potentiate diabetic ketoacidosis. These data are not intended to inform clinical decisions around anticipatory upfront insulin dosing changes upon SGLT‐2 inhibitor initiation.
4.4. Study limitations
This post hoc analysis is subject to certain limitations. While analyses of primary and secondary clinical outcomes by baseline insulin use were prespecified, assessment of subsequent insulin intensification as studied here was exploratory. Insulin use and dosing was assessed during scheduled or unscheduled visits, and detailed information regarding treatment patterns was not available between visits. Given the strict inclusion and exclusion criteria of the trial, together with study procedures with frequent study visits, these data may not be generalizable to all populations, especially patients without ASCVD and those with lesser health access. No data were available regarding insulin type, use pattern, or complexity of regimens.
In a clinical trial, with regular participant visits, and a focus on glycaemia, it is probable that insulin initiation was more frequent than would be expected in real‐world clinical practice. Scheduled ambulatory visits would not however be anticipated to be unbalanced between treatment arms in a blinded trial. However, there may have been an imbalance in unscheduled visitations that may have provided additional opportunities for treatment intensification. SGLT‐2 inhibitors are known to reduce all‐cause hospitalizations, 43 and in particular hospitalizations for heart failure, in at‐risk patients with type 2 diabetes. Hospitalizations occurred more frequently in placebo‐treated participants in EMPA‐REG OUTCOME and may represent important sites of care in which insulin is initiated or dosing is intensified. 44
The actuarial methods assume consistent treatment adherence and magnitude of treatment effects as observed during the trial over lifetime use. Because β‐cell dysfunction continues after the diagnosis of diabetes, it is possible that any insulin‐sparing effects of empagliflozin would be lost over time. Moreover, interval non‐adherence might also be anticipated to attenuate projected gains in insulin‐free survival. Finally, the long‐term actuarial analysis required accounting of competing risks of mortality and thus consideration of a composite of time to insulin initiation or death, despite the two components occurring with different frequency and of unequal patient importance.
Despite these limitations, these data from a large cardiovascular outcomes trial represent the most comprehensive and detailed evaluation of the effects of SGLT‐2 inhibition on insulin initiation or intensification, extending prior concordant observations with another member of this therapeutic class. 24
In conclusion, in EMPA‐REG OUTCOME, empagliflozin substantially reduces rates of insulin initiation or intensification among patients with type 2 diabetes and cardiovascular disease. Reductions in insulin needs and dosing may attenuate treatment attendant adverse effects, including weight gain and hypoglycaemia, which may be especially problematic for patients with co‐morbid cardiovascular disease. Further data from studies with alternative designs that do not actively encourage glycaemic equipoise are needed to corroborate the treatment effects of SGLT‐2 inhibitors on longitudinal insulin requirements. If replicated, the long‐term implications of these effects on improvement in patient satisfaction, quality of life, and costs of care require further study.
CONFLICT OF INTEREST
MV has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa; speaker engagements with Novartis and Roche Diagnostics; and participates on clinical endpoint committees for studies sponsored by Galmed and Novartis. SEI has served as a consultant, speaker, or as a member of clinical trial steering committees for BI, AZ, NN, Sanofi/Lexicon Pharmaceuticals, Merck, vTv Therapeutics, and Abbott/Alere. NS has consulted for Amgen, BI, Eli Lilly (EL), Napp, Novo Nordisk (NN), Pfizer, and Sanofi; and has received grant support from BI. DHF has received financial support from Amgen, AZ, BI, EL, Merck & Co., and Sanofi. SV is President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not‐for‐profit physician organization; holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and has also received grants and personal fees for speaker honoraria and advisory board participation from AZ, Amgen, Bayer, BI, EL, Janssen, and Merck. He has received grants and personal fees for advisory board participation from Amgen, grants from Bristol‐Myers Squibb, personal fees for speaker honoraria and advisory board participation from EL, NN, and Sanofi; and personal fees for speaker honoraria from EOCI Pharmacomm Ltd, Novartis, Sun Pharmaceuticals, and Toronto Knowledge Translation Working Group. CW has received financial support from AZ, Bayer, BI, EL, MSD, and Mundipharma. BZ has received financial support from AZ, BI, EL, Janssen, Merck, NN, and Sanofi. JB has received research support from the National Institutes of Health, Patient Centered Outcomes Research, and the European Union; and has served as a consultant to Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AZ, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sanofi, Sequana Medical, V‐Wave Limited, and Vifor. APO, MB, JTG, and MM are employees of BI.
AUTHOR CONTRIBUTIONS
Study conception and design: MV, SEI, APO, MB, BZ, and JB. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: MV. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: MM. Study supervision: JB. MV and JB had full access to the study data and take responsibility for the integrity and accuracy of its analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14535.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
The EMPA‐REG OUTCOME trial was sponsored by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Elevate Scientific Solutions, Horsham, UK, in finalizing the figures for publication.
Vaduganathan M, Inzucchi SE, Sattar N, et al. Effects of empagliflozin on insulin initiation or intensification in patients with type 2 diabetes and cardiovascular disease: Findings from the EMPA‐REG OUTCOME trial. Diabetes Obes Metab. 2021;23(12):2775‐2784. doi: 10.1111/dom.14535
Contributor Information
Muthiah Vaduganathan, Email: mvaduganathan@bwh.harvard.edu.
Javed Butler, Email: jbutler4@umc.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018‐30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 2. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 3. Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 4. Origin Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319‐328. [DOI] [PubMed] [Google Scholar]
- 5. Braffett BH, Dagogo‐Jack S, Bebu I, et al. Association of insulin dose, cardiometabolic risk factors, and cardiovascular disease in type 1 diabetes during 30 years of follow‐up in the DCCT/EDIC study. Diabetes Care. 2019;42(4):657‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosmi F, Shen L, Magnoli M, et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. 2018;20(5):888‐895. [DOI] [PubMed] [Google Scholar]
- 7. Rossello X, Ferreira JP, McMurray JJ, et al. Editorʼs choice‐ impact of insulin‐treated diabetes on cardiovascular outcomes following high‐risk myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2019;8(3):231‐241. [DOI] [PubMed] [Google Scholar]
- 8. Shen L, Rorth R, Cosmi D, et al. Insulin treatment and clinical outcomes in patients with diabetes and heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21(8):974‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sumarsono A, Everett BM, McGuire DK, et al. Trends in aggregate use and associated expenditures of antihyperglycemic therapies among US Medicare beneficiaries between 2012 and 2017. JAMA Intern Med. 2020;180(1):141‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fitchett D, Inzucchi SE, Wanner C, et al. Relationship between hypoglycaemia, cardiovascular outcomes, and empagliflozin treatment in the EMPA‐REG OUTCOME(R) trial. Eur Heart J. 2020;41(2):209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138(17):1904‐1907. [DOI] [PubMed] [Google Scholar]
- 12. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merovci A, Abdul‐Ghani M, Mari A, et al. Effect of dapagliflozin with and without acipimox on insulin sensitivity and insulin secretion in T2DM males. J Clin Endocrinol Metab. 2016;101(3):1249‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 16. Cooper ME, Inzucchi SE, Zinman B, et al. Glucose control and the effect of empagliflozin on kidney outcomes in type 2 diabetes: an analysis from the EMPA‐REG OUTCOME trial. Am J Kidney Dis. 2019;74(5):713‐715. [DOI] [PubMed] [Google Scholar]
- 17. Inzucchi SE, Fitchett D, Jurisic‐Erzen D, et al. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose‐lowering therapy? Diabetes Obes Metab. 2020;22(4):631‐639. [DOI] [PubMed] [Google Scholar]
- 18. Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo‐controlled cardiovascular outcome trial of empagliflozin (EMPA‐REG OUTCOME). Cardiovasc Diabetol. 2014;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Claggett B, Lachin JM, Hantel S, et al. Long‐term benefit of empagliflozin on life expectancy in patients with type 2 diabetes mellitus and established cardiovascular disease. Circulation. 2018;138(15):1599‐1601. [DOI] [PubMed] [Google Scholar]
- 20. Charbonnel B, DeFronzo R, Davidson J, et al. Pioglitazone use in combination with insulin in the prospective pioglitazone clinical trial in macrovascular events study (PROactive19). J Clin Endocrinol Metab. 2010;95(5):2163‐2171. [DOI] [PubMed] [Google Scholar]
- 21. Yang Y, Chen S, Pan H, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: systematic review and meta‐analysis of randomized controlled trials. Medicine. 2017;96(21):e6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bethel MA, Stevens SR, Buse JB, et al. Exploring the possible impact of unbalanced open‐label drop‐in of glucose‐lowering medications on EXSCEL outcomes. Circulation. 2020;141(17):1360‐1370. [DOI] [PubMed] [Google Scholar]
- 23. Zinman B, Nauck MA, Bosch‐Traberg H, et al. Liraglutide and glycaemic outcomes in the LEADER trial. Diabetes Ther. 2018;9(6):2383‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthews DR, Wysham C, Davies M, et al. Effects of canagliflozin on initiation of insulin and other antihyperglycaemic agents in the CANVAS Program. Diabetes Obes Metab. 2020;22(11):2199‐2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta‐cell function. Diabetes. 2011;60(3):890‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Obata A, Kubota N, Kubota T, et al. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. 2016;157(3):1029‐1042. [DOI] [PubMed] [Google Scholar]
- 27. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79(5):1510‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu L, Nagata N, Nagashimada M, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet‐induced obese mice. EBioMedicine. 2017;20:137‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrannini E. Sodium‐glucose co‐transporters and their inhibition: clinical physiology. Cell Metab. 2017;26(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 30. Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA‐REG OUTCOME(R) trial. Diabetologia. 2018;61(10):2155‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor R, Al‐Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7(9):726‐736. [DOI] [PubMed] [Google Scholar]
- 32. Vaduganathan M, Claggett BL, Juraschek SP, Solomon SD. Assessment of long‐term benefit of intensive blood pressure control on residual life span: secondary analysis of the systolic blood pressure intervention trial (SPRINT). JAMA Cardiol. 2020;5(5):576‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claggett B, Packer M, McMurray JJ, et al. Estimating the long‐term treatment benefits of sacubitril‐valsartan. N Engl J Med. 2015;373(23):2289‐2290. [DOI] [PubMed] [Google Scholar]
- 34. Stienen S, Ferreira JP, Vincent J, et al. Estimated long‐term survival with eplerenone. J Am Coll Cardiol. 2019;73(18):2357‐2359. [DOI] [PubMed] [Google Scholar]
- 35. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121‐128. [DOI] [PubMed] [Google Scholar]
- 36. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long‐term trends in antidiabetes drug usage in the U.S.: real‐world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 37. Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57(539):455‐460. [PMC free article] [PubMed] [Google Scholar]
- 38. Khunti K, Millar‐Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 39. Khunti S, Davies MJ, Khunti K. Clinical inertia in the management of type 2 diabetes mellitus: a focused literature review. Br J Diabetes. 2015;15:65‐69. [Google Scholar]
- 40. Koye DN, Ling J, Dibato J, Khunti K, Montvida O, Paul SK. Temporal trend in young‐onset type 2 diabetes‐macrovascular and mortality risk: study of U.K. primary care electronic medical records. Diabetes Care. 2020;43(9):2208‐2216. [DOI] [PubMed] [Google Scholar]
- 41. Sattar N, Rawshani A, Franzen S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139(19):2228‐2237. [DOI] [PubMed] [Google Scholar]
- 42. Koopman RJ, Mainous AG 3rd, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med. 2005;3(1):60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inzucchi SE, Zinman B, Wanner C, et al. 131‐LB: empagliflozin reduces the total burden of all‐cause hospitalizations (ACH) and mortality in EMPA‐REG outcome. Diabetes. 2020;69(suppl 1):131‐LB.31740442 [Google Scholar]
- 44. Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non‐critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16‐38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


