Abstract
Introduction
Recombinant coagulation factor VIII (FVIII) products are the standard of care for patients with haemophilia A. The development of modified FVIII products has provided benefit for patients but presented challenges for monitoring FVIII activity.
Aim
This single‐centre study evaluated the Roche FVIII one‐stage clotting assay (OSA) in measuring FVIII activity in plasma samples spiked with seven FVIII products at clinically relevant concentrations.
Methods
FVIII‐deficient plasma samples were spiked with two batches of recombinant FVIII products (octocog alfa, moroctocog alfa, simoctocog alfa, efmoroctocog alfa, damoctocog alfa pegol, rurioctocog alfa pegol, lonoctocog alfa) at 1–120 IU/dL FVIII activity, according to their labelled potency. Measurement was conducted on the cobas t 511/711 analysers using the Roche FVIII OSA and the Technoclone TECHNOCHROM FVIII:C chromogenic substrate assay (CSA).
Results
Using the OSA, FVIII activity was close to labelled potency for most analysed FVIII products including a recombinant FVIII Fc fusion protein. PEGylated FVIII product, damoctocog alfa pegol, was marginally above and single‐chain product, lonoctocog alfa, below the predefined acceptance criteria: for FVIII activity < 25 IU/dL: ± 5 IU/dL; for FVIII activity ≥ 25 IU/dL: ± 20% (relative). The different principles of OSA and CSA led to discrepancies in the estimation of all analysed FVIII products. Additionally, in vitro recovery was increased at lower levels of FVIII activity using the OSA, whereas recovery was more consistent using the CSA.
Conclusion
These data allow the interpretation of FVIII activity results for different FVIII products using the Roche FVIII OSA on the cobas t 511/711 analysers.
Keywords: activated partial thromboplastin time, biological assay, drug monitoring, factor VIII, haemophilia A, recombinant fusion proteins
1. INTRODUCTION
Haemophilia A (HA) is a rare X‐linked bleeding disorder caused by a deficiency of coagulation factor VIII (FVIII). 1 Worldwide, the prevalence at birth of HA is 24.6 cases per 100,000 males. 2 Cloning of the F8 gene that encodes FVIII has improved diagnosis and management of the disease, as well as allowing for therapeutic advances such as the development of recombinant FVIII products and their subsequent modifications. 3 Regular replacement with recombinant FVIII products is the standard of care for patients with HA. 4 The development of extended half‐life FVIII products has provided longer intervals for patients between doses or higher trough levels of FVIII with the same dosing interval. 4
Several FVIII replacement products are available that differ in length and structural modifications of the molecule, for example: full‐length plasma or recombinant products, B‐domain truncated products and the most recent generation of products that includes modifications such as the introduction of a covalent linkage to form a single‐chain product, fusion of the Fc domain of immunoglobulin G1 and nonspecific or site‐specific PEGylation (conjugation of active polyethylene glycol). 5 , 6
The ability to reliably monitor levels of FVIII activity is clinically important during prophylaxis to directly assess peak and trough levels and thus prevent breakthrough bleeding; however, inter‐patient variability exists in the amount of FVIII needed to achieve a desired trough level. 7 Patients’ needs and lifestyles also change over time. Personalised pharmacokinetics using Bayesian analysis with limited sampling can help optimise prophylaxis in patients with HA. 8 In addition, levels of FVIII activity in plasma guide clinical decision‐making in the surgical management of patients with HA. 1 A better understanding of the performance of assays measuring FVIII activity is required; however, the availability of several different, modified FVIII replacement products (especially those with extended half‐life) poses new challenges for laboratory monitoring.
The types of assay for detecting FVIII activity are the one‐stage activated partial thromboplastin time (aPTT) clotting assay (OSA) and the chromogenic substrate assay (CSA), which are based on different assay principles. 9 Discrepancies between OSA and CSA in the measurement of FVIII activity have been described in several studies and determining which assay provides the better indication of ‘true’ FVIII activity is an active area of research. 9 , 10 Discrepancies of up to 50% in FVIII detection between the OSA and CSA have been reported for some FVIII replacement products 11 , 12 ; however, other products can be measured consistently using either assay. 13 Discrepancies can be minimised by using a product‐specific reference standard instead of a plasma standard for calibration. 12 , 14 In addition, intra‐assay differences between OSAs can occur as a result of several variables including the activator type, phospholipid source, FVIII‐deficient plasma and analyser used. 5 , 15
Studies are available from the manufacturers of FVIII replacement products regarding measurement of FVIII activity; however, drawing comparisons between such studies can be problematic due to the inter‐ and intra‐assay discrepancies mentioned above. The evaluation of a single platform to measure samples from patients treated with different FVIII replacement products is representative of a clinical laboratory. The current study aimed to evaluate the performance of the Roche FVIII OSA (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) in measuring plasma samples spiked with a panel of recombinant FVIII replacement products, across clinically relevant ranges of FVIII activity, in a defined set‐up using the cobas t 511/711 analysers.
2. MATERIALS AND METHODS
2.1. Study design
This single‐centre study was conducted on site at Roche Diagnostics GmbH, Penzberg, Germany, between November 2019 and August 2020. FVIII‐deficient plasma samples were spiked with two batches of recombinant FVIII replacement products according to the labelled activity and FVIII activity was subsequently measured using the OSA and/or CSA. The deviation limits were set by predefined acceptance criteria: for FVIII activity < 25 IU/dL: ± 5 IU/dL; for FVIII activity ≥ 25 IU/dL: ± 20% (relative). Vendor‐purchased patient samples, used as a reference for comparison, were fully compliant with actual ethical approval, based on information provided by the vendor (TRINA BIOREACTIVES AG, Naenikon, Switzerland). All measurements were performed on the cobas t 511/711 analysers, which work in an equivalent manner (Figure S1). 16 , 17
Determination of FVIII activity using a FVIII one‐stage clotting assay (OSA)
FVIII was measured in the OSA using the Factor VIII ready‐for‐use cassette for cobas t analysers (Roche Diagnostics International Ltd); other individual reagents required were sourced from Roche Diagnostics International Ltd and the assay protocol was performed according to the manufacturer's instructions. 18 Sample plasma was diluted and added to human plasma with a FVIII level < 1% of the physiological value, whereas all other coagulation factors were present at levels typically > 50% of the physiological value. Test plasma was incubated at 37°C for 4 min with the aPTT reagent containing phospholipids (purified soy phosphatides) and a contact activator (ellagic acid). The intrinsic coagulation cascade was then initiated by adding CaCl2. The resulting clotting time was interpreted using a calibration curve, obtained with dilutions of a calibrator plasma (Global Cal, Roche Diagnostics International Ltd).
Determination of FVIII activity using a FVIII chromogenic substrate assay (CSA)
For CSA measurements, the TECHNOCHROM FVIII:C assay (Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Austria) was performed on the cobas t 511/711 analysers. The reagents (A, B, substrate‐buffer‐mixture) were prepared as described in the package insert. 19 Samples were diluted 1:40 with NaCl (Roche Diagnostics International Ltd) and reagents A and B were added in equivalent quantities. After incubation at 37°C for 2 min, the substrate‐buffer‐mixture was added in a ratio of 1:5 to the diluted sample. The absorption increase was recorded at 408 nm for 150 s. For the calibration curve, six different dilutions of the Coagulation Reference (Technoclone) were prepared (1:423; 1:130; 1:80; 1:53.3; 1:40; 1:26.6; FVIII activity specified for each batch 60–150 IU/dL) and measured in the same way as a patient sample.
2.2. Samples and sample handling
FVIII replacement products were reconstituted according to the package inserts. FVIII‐deficient plasma samples were spiked with two independent batches of seven different commercially available FVIII replacement products (Table 1) at clinically relevant concentrations (1–120 IU/dL) according to their labelled activity. For all products used in this study, the potency was determined by the manufacturer using a CSA (information derived from the European Medicines Agency [EMA], https://www.ema.europa.eu/en, accessed January 2020). The first batch (Batch #1) of FVIII replacement products were diluted in severe HA plasma (George King Bio‐Medical, Inc., Overland Park, KS, USA) and the second batch (Batch #2) were diluted in FVIII‐immunodepleted plasma (Roche Diagnostics International Ltd). Two different diluents were used due to availability; however, both produce equivalent results with respect to FVIII activity (Figure S2). Batches #1 and #2 were spiked into FVIII‐deficient plasma and measured with the OSA on the cobas t 711 analyser in triplicates. In parallel, Batch #2 samples were measured with the CSA on the cobas t 511 analyser. Regular controls were performed to check for consistent and comparable performance. In addition, a method comparison (OSA cobas t 711 vs. CSA cobas t 511) was performed using native plasma samples (N = 49) (Figure S1). Patient 3.2% citrate plasma samples from individual donors with different FVIII activity were purchased from a commercial vendor (TRINA BIOREACTIVES AG).
TABLE 1.
Characteristics of different FVIII replacement products
| Active substance | Name | Manufacturer | Molecule length | EHL modification | Half‐life (h) a |
|---|---|---|---|---|---|
| Octocog alfa | ADVATE | Shire/Takeda | Full length | – | 12.9 |
| Moroctocog alfa | ReFacto AF | Pfizer | BDT | – | 14.8 |
| Simoctocog alfa | Vihuma | Biotest | BDT | – | 14.7 |
| Efmoroctocog alfa | ELOCTA | Biogen/Sobi | BDT | Fc fusion | 20.9 |
| Damoctocog alfa pegol | Jivi | Bayer | BDT | 60 kDa PEG | 17.4 |
| Rurioctocog alfa pegol | ADYNOVI |
Baxalta/Shire/Takeda |
Full length | 20 kDa PEG | 15.0 |
| Lonoctocog alfa | AFSTYLA | CSL Behring | BDT | Single‐chain b | 14.2 |
BDT, B‐domain truncated; CSA, chromogenic substrate assay; EHL, extended half‐life; Fc, crystallisable fragment; FVIII, factor VIII; h, hours; OSA, one‐stage clotting assay; PEG, polyethylene glycol.
The values were taken from the prescribing information of each product provided on the EMA website (https://www.ema.europa.eu/en, accessed December 2020). The half‐life was calculated based on an adult population (≥ 18 years). Measurements were performed with the CSA except for octocog alfa (OSA).
No consensus whether the degree of half‐life prolongation is sufficient for the EHL classification. 5
For the calculation of the OSA/CSA, Batch #2 OSA and CSA results were used. In addition, untreated patient samples (N = 37) containing native FVIII at different activities (TRINA BIOREACTIVES AG) were used as a reference for comparison with the spiked plasma samples containing recombinant FVIII products.
To assess matrix‐dependent differences in FVIII recovery across a range of concentrations, a calibrator (Global Cal, Roche Diagnostics International Ltd), which was a normal human plasma pool, was diluted in NaCl or severe HA plasma (George King Bio‐Medical) to defined concentrations (1–80 IU/dL). These samples were prepared in duplicate and measured in triplicate using the OSA.
2.3. Comparison of FVIII assay performance with published literature
Publications that reported the performance of different FVIII assays in monitoring different recombinant FVIII products were extracted from the most recent World Federation of Hemophilia (WFH) Guidelines for the Management of Hemophilia. 1 The product simoctocog alfa was not included in the WFH Guidelines; therefore, an independent search of the literature in PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed September 2020) using the term ‘study + simoctocog alfa’ was conducted. Data extraction was carried out by one user. Studies with values of FVIII activity separated by activator type were preferred. Studies analysing post‐infusion patient samples were excluded. If more than one study of the same product met the criteria and provided the same results in terms of over‐ or underestimation, only one study was selected. As there was no full consensus on the absolute or relative difference that is clinically tolerable in FVIII replacement product monitoring within the existing literature, 1 the measured values from each of the studies were recalculated according to the acceptance criteria used in this study. Where the reported values were separated by activator type, the values measured with ellagic acid as the activator were selected. Three different values for low (< 6 IU/dL), medium (20–40 IU/dL) and high (80–100 IU/dL) FVIII activity corresponding to the labelled potency were classified as within (correct), above (overestimated) or under (underestimated) the acceptance criteria used. The results were compared to the measured values of the closest target concentrations.
2.4. Data management and analysis
FVIII activity was read out directly from the analyser and Microsoft Excel was used for data processing. Recovery was calculated as measured FVIII activity divided by the target activity according to manufacturer labelled potency and expressed as percent of target values.
3. RESULTS
3.1. Measurement of FVIII activity using the Roche OSA
Measurement of FVIII activity across the given concentration range (1–120 IU/dL) using the OSA in Batch #1 and Batch #2 is shown in Figures 1A and 1B, respectively. In Batch #1, values for the PEGylated FVIII replacement product damoctocog alfa pegol (Jivi, Bayer) (FVIII activity range, 10–100 IU/dL) were marginally higher than the upper deviation limit. This marginal overestimation was increased in the second batch for values across a FVIII activity range of 10–120 IU/dL. A substantial underestimation was observed for samples spiked with the single‐chain concentrate lonoctocog alfa (AFSTYLA, CSL Behring) above a FVIII activity of 20 IU/dL in both batches. For two of the FVIII replacement products, rurioctocog alfa pegol (ADYNOVI, Baxalta/Shire/Takeda) and simoctocog alfa (Vihuma, Biotest), single concentrations of one batch were marginally above the acceptance limit in Batch #1 and Batch #2, respectively.
FIGURE 1.

Performance of OSA measuring different FVIII replacement products. Two independent batches of seven different FVIII replacement products were diluted in FVIII‐deficient plasma to defined concentrations (1–120 IU/dL) according to their labelled potency and FVIII activity was measured using the OSA. The first batch (Batch #1, A) of FVIII replacement products were diluted in severe HA plasma (George King Bio‐Medical) and the second batch (Batch #2, B) were diluted in FVIII‐immunodepleted plasma (Roche Diagnostics International Ltd). The highest concentration (120 IU/dL) of lonoctocog alfa (AFSTYLA, CSL Behring) was removed from the data set due to a dilution error. The black line represents the ideal agreement and the dotted red lines the lower and upper deviation limits of the predefined acceptance criteria: for FVIII activity < 25 IU/dL: ± 5 IU/dL; for FVIII activity ≥ 25 IU/dL: ± 20% (relative). FVIII, factor VIII; HA, haemophilia A; OSA, one‐stage clotting assay
3.2. Comparison of FVIII activity results using the Roche OSA to similar platforms
Data from previously published studies that measured FVIII activity in plasma spiked with FVIII replacement products using an OSA with ellagic acid as aPTT activator were recalculated according to the acceptance criteria used in this study. Table 2 shows that the results obtained in this study are comparable to those reported in previously published work. 11 , 12 , 13 , 20 , 21
TABLE 2.
Comparison of selected studies investigating the effect of FVIII replacement products in OSA with aPTT reagent containing ellagic acid
| Assay performance according to FVIII activity level a | |||||
|---|---|---|---|---|---|
| (target level in IU/dL) | |||||
| Active substance | Activator in aPTT reagent | Low (< 6) | Medium (20–40) | High (80–100) | References |
| Octocog alfa | Ellagic acid | ✓ (5.0) | ✓ (30.0) | ✓ (100.0) | 13 |
| Ellagic acid (current study) | ✓ (5.0) | ✓ (40.0) | ✓ (100.0) | Batch #1 | |
| ✓ (5.0) | ✓ (40.0) | ✓ (100.0) | Batch #2 | ||
| Moroctocog alfa | Ellagic acid (Actin FS) | N.D. | ✓ (20.0) | ↓ (90.0) | 12 |
| Ellagic acid (Actin FSL) | N.D. | ✓ (20.0) | ✓ (90.0) | 12 | |
| Ellagic acid (current study) | ✓ (5.0) | ✓ (20.0) | ✓ (100.0) | Batch #1 | |
| ✓ (5.0) | ✓ (20.0) | ✓ (100.0) | Batch #2 | ||
| Simoctocog alfa | Ellagic acid | ✓ (5.0) | ✓ (30.0) | ✓ (100.0) | 13 |
| Ellagic acid (current study) | ✓ (5.0) | ✓ (40.0) | ✓ (100.0) | Batch #1 | |
| ✓ (5.0) | ↑ (40.0) | ✓ (100.0) | Batch #2 | ||
| Efmoroctocog alfa | Ellagic acid/ Silica/ Kaolin/ Polyphenol c | ✓ (5.4) | ✓ (22.0) | ✓ (87.0) | 27 |
| Ellagic acid (aPTT) | ✓ (5.0) | ✓ (20.0) | ✓ (80.0) | Batch #1 | |
| ✓ (5.0) | ✓ (20.0) | ✓ (80.0) | Batch #2 | ||
| Damoctocog alfa pegol | Ellagic acid (Actin FS) | ✓ (4.3) | ↑ (37.5) | ↑ (86.5) | 20 |
| Ellagic acid (Actin FSL) | ✓ (4.3) | ✓ (37.5) | ✓ (86.5) | 20 | |
| Ellagic acid (current study) | ✓ (5.0) | ↑ (40.0) | ↑ (80.0) | Batch #1 | |
| ✓ (5.0) | ↑ (40.0) | ↑ (80.0) | Batch #2 | ||
| Rurioctocog alfa pegol | Ellagic acid/ Polyphenol b | ✓ (5.0) | ↑ (20.0) | ✓ (80.0) | 21 |
| Ellagic acid (current study) | ✓ (5.0) | ↑ (20.0) | ✓ (80.0) | Batch #1 | |
| ✓ (5.0) | ✓ (20.0) | ✓ (80.0) | Batch #2 | ||
| Lonoctocog alfa | Ellagic acid | ✓ (4.0) | ↓ (30.0) | ↓ (100.0) | 11 |
| Ellagic acid (current study) | ✓ (5.0) | ↓ (40.0) | ↓ (100.0) | Batch #1 | |
| ✓ (5.0) | ↓ (40.0) | ↓ (100.0) | Batch #2 | ||
aPTT, activated partial thromboplastin time; FVIII, factor VIII; OSA, one‐stage clotting assay.
✓: correct; ↑: overestimated; ↓: underestimated; N.D.: no data available. Batch #1 and Batch #2 refer to the different batches of the FVIII products used in this study.
Recalculated based on the following predefined acceptance criteria: for FVIII activity < 25 IU/dL: ± 5 IU/dL; for FVIII activity ≥ 25 IU/dL: ± 20% (relative); target levels based on the labelled potency of samples analysed in the respective study are given in parentheses.
Mean result measured by 10 laboratories using aPTT reagents containing either ellagic acid or polyphenol.
Mean result measured by 30 different laboratories: aPTT activator reagents that were used included ellagic acid (eight laboratories), silica (19 laboratories), kaolin (two laboratories) and polyphenols (one laboratory).
3.3. Measurement of FVIII activity using the Roche OSA and TECHNOCHROM CSA
In order to investigate whether the differences in FVIII activity of the FVIII replacement products were specific to the OSA, FVIII activity across the concentration range (1–120 IU/dL) was measured in samples from Batch #2 using both the OSA and CSA. Most of the CSA results were above the upper acceptance limit (for FVIII activity < 25 IU/dL: ± 5 IU/dL; for FVIII activity ≥ 25 IU/dL: ± 20% [relative]) (Figure S3). The mean ratio for each FVIII replacement product was calculated with the OSA and CSA results of samples adjusted across a range of FVIII activities (5–100 IU/dL), according to their labelled potency. The OSA/CSA ratio of each product was dependent on the FVIII level. At 100 IU/dL FVIII activity, the observed range of discrepancy between the OSA and CSA results was approximately 20–50% (Figure 2). The OSA/CSA ratio from 37 native patient samples across a range of 4.24–102.00 IU/dL FVIII activity (measured using the OSA) showed lower deviation (OSA/CSA ratio, 0.84–1.23), relative to the spiked plasma samples (OSA/CSA ratio, 0.52–1.98) (Figure 2).
FIGURE 2.

OSA/CSA ratio for different FVIII replacement products. Freshly prepared FVIII‐immunodepleted plasma samples (Roche Diagnostics International Ltd) spiked with FVIII recombinant products from Batch #2 were measured using both the OSA and CSA. The ratio for each FVIII replacement product was calculated with OSA and CSA results of samples adjusted to 5 IU/dL, 20 IU/dL, 60 IU/dL and 100 IU/dL FVIII activity, according to their labelled potency. The grey box indicates the minimum and maximum OSA/CSA ratios of native patient samples (N = 37) across a range of 4.24–102.00 IU/dL FVIII activity (measured using the OSA). CSA, chromogenic substrate assay; FVIII, factor VIII; HA, haemophilia A; OSA, one‐stage clotting assay
3.4. Percentage recovery of FVIII replacement products over FVIII concentration range
The percentage recovery of the different FVIII replacement products was calculated as measured FVIII activity divided by the target activity according to manufacturer labelled potency and expressed as percent of target values (Figure 3). In all analysed FVIII products, the percentage recovery was increased at lower levels of FVIII activity using the OSA. In contrast, the recovery of CSA results was nearly constant across the analysed range of FVIII activity.
FIGURE 3.
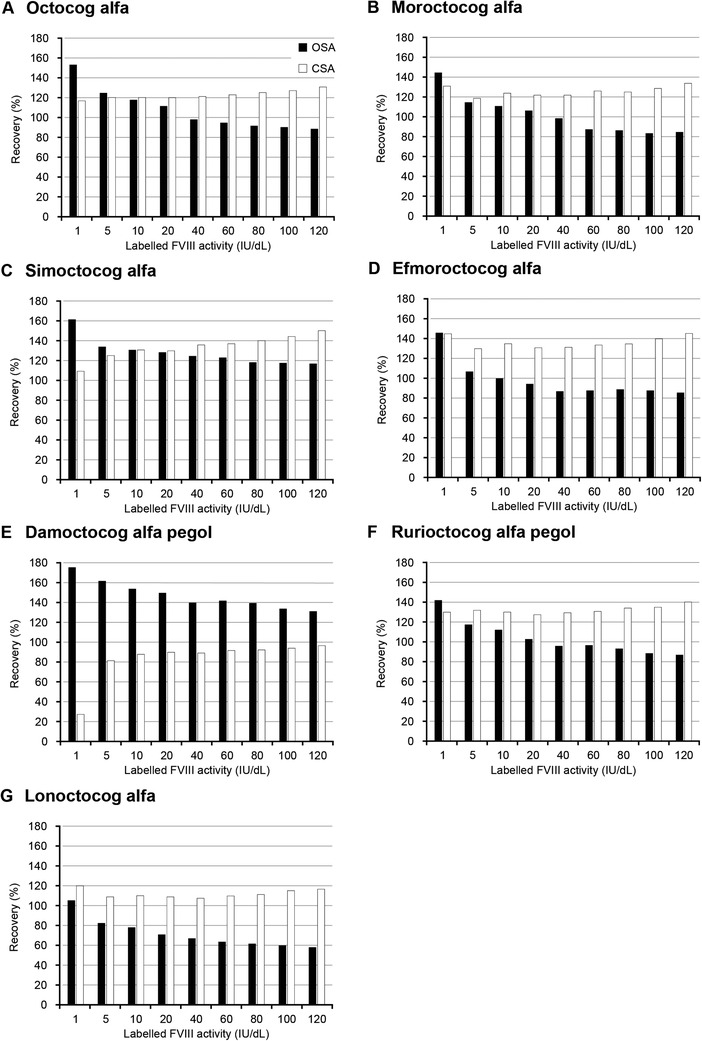
Percentage recovery of different FVIII replacement products using the OSA and CSA. The FVIII replacement products from Batch #2 were diluted in FVIII‐immunodepleted plasma (Roche Diagnostics International Ltd) according to their labelled potency (1–120 IU/dL). The samples were measured with the OSA and CSA and the recovery to target activity according to labelled potency was calculated. Recovery was calculated as measured FVIII activity divided by the target activity according to manufacturer labelled potency and expressed as percent of target values. White bars represent CSA and black bars are OSA. CSA, chromogenic substrate assay; FVIII, factor VIII; OSA, one‐stage clotting assay
To determine whether this assay‐dependent effect was attributable to the dilution matrix, a calibrator plasma was diluted in either NaCl or severe HA plasma (Figure S4). The dilutions in NaCl showed a constant recovery of 100 ± 4%. However, for dilutions in severe HA plasma, the same pattern (increasing recovery with lower FVIII activity) was observed as seen for the OSA values of the plasma spiked with replacement FVIII products.
4. DISCUSSION
This study evaluated the performance of the Roche FVIII OSA in measuring plasma samples spiked with a panel of seven recombinant FVIII replacement products at clinically relevant concentrations. FVIII activity measured with the OSA was close to the labelled potency for most of the analysed FVIII replacement products, apart from a marginal overestimation for damoctocog alfa pegol (Jivi, Bayer) and underestimation for lonoctocog alfa (AFSTYLA, CSL Behring). A review of published literature showed that the measurement of FVIII activity of different FVIII products using the OSA was comparable to other commercially available platforms. There were notable discrepancies in the measurement of FVIII activity of the replacement products using the OSA versus the CSA. In the OSA, the percentage recovery to the labelled potency increased with decreasing FVIII activity of the recombinant FVIII replacement product in a matrix‐dependent effect, while in the CSA the recovery was nearly constant over the entire concentration range. This study adds to the current body of knowledge by providing data allowing the interpretation of FVIII assay results using the Roche FVIII OSA on the cobas t 511/711 analysers for seven different FVIII replacement products at clinically relevant concentrations.
For damoctocog alfa pegol (Jivi, Bayer), a slight overestimation of FVIII activity was observed using the OSA. This was also seen in another study using an ellagic acid containing aPTT reagent (Actin FS). 20 The performance of damoctocog alfa pegol (Jivi, Bayer) in the OSA seemed to be reagent‐specific, which could be explained by an interaction of the 60 kDa PEG residue with components of the aPTT reagent. 20 , 22 FVIII activity of the single‐chain FVIII product lonoctocog alfa (AFSTYLA, CSL Behring) was underestimated using the OSA. It is known that the covalent linkage between the heavy and light chains results in an increased affinity to bind von Willebrand factor (vWF). 23 A single‐chain FVIII with an Fc fusion showed a prolonged clotting time compared with a two‐chain FVIII with an Fc fusion in the presence of vWF, but an equivalent clotting time in the absence of vWF. 24 Therefore, the prolonged clotting time of lonoctocog alfa (AFSTYLA, CSL Behring) may be due to a delayed release of recombinant FVIII from vWF during activation. 24 A slower activation rate would have no influence on the CSA result because measurement only starts after the activation step. Due to this underestimation, the manufacturer of lonoctocog alfa (AFSTYLA, CSL Behring) recommends a correction factor of 2 be applied to all FVIII results measured with the OSA. Since not all concentrations show the same percentage deviation, a uniform multiplication by a single factor does not result in a full correction over the entire range, as shown in this study; therefore, this correction factor should be used with caution. 25 Neither the B‐domain truncation nor the Fc fusion appeared to have any influence on the performance of the Roche FVIII OSA, thus the OSA tested appears to be reliable in detecting this range of FVIII replacement products.
In this study, the different assay principles of the OSA and CSA led to notable differences in the estimation of all FVIII products analysed. In contrast, native plasma samples showed lower deviation. The difference in the OSA/CSA ratio between plasma samples spiked with FVIII replacement products and native samples could be explained by the calibration curve being performed with normal plasma containing native FVIII. Therefore, product‐specific calibration standards would reduce the difference between OSA and CSA results for the FVIII replacement products, as shown in a previously published study using moroctocog alfa (ReFacto AF, Pfizer). 26 However, the implementation of product‐specific standards in clinical laboratory practice is complicated by the variety of available FVIII replacement products.
The percentage recovery increased at lower concentrations of FVIII activity in the OSA; this underlies the observation that the OSA/CSA ratio was dependent upon the level of FVIII activity. As this pattern was also seen with octocog alfa (ADVATE, Shire/Takeda), the only unmodified product in the panel tested, product modifications can be excluded as a variable. This observation was made in several previous studies and declared as relative overestimation at low levels. 21 , 27 , 28 When measuring normal plasma diluted in severe HA plasma, the same pattern is seen. In contrast, dilutions in NaCl showed a constant recovery over the analysed range. Therefore, this effect may be caused by the different matrices used for calibration and dilution of FVIII and not by the FVIII replacement products themselves.
A key strength of this study is the comparison of a panel of FVIII replacement products at clinically relevant concentrations using a single system, modelling the situation of a clinical laboratory. In this study, samples were prepared ex vivo; thus, while the concentration and panel of products was chosen with the aim of modelling post‐infusion patient samples as closely as possible, it cannot be excluded that patient samples may be assessed differently. The FVIII replacement product panel in this study was not exhaustive and particular note should be given to the bispecific antibody emicizumab (Hemlibra, Chugai Pharmaceutical Co., Ltd, Tokyo, Japan) that does not require activation by thrombin and can, therefore, shorten aPTT resulting in overestimated FVIII activity using a standard OSA. 29 Regarding CSA for measurement of emicizumab, assays using human FIXa and FX could be used to quantify emicizumab; however, CSA using the bovine equivalent were insensitive to emicizumab and could not be used for quantification of FVIII activity. 29 Based upon the findings of this study, future work should focus on validation in post‐infusion patient samples treated with different FVIII replacement products.
These data support the use of the Roche FVIII OSA on cobas t 511/711 analysers to accurately measure the activity of a panel of FVIII replacement products in plasma samples. In this study, FVIII activity was close to the labelled potency for most analysed FVIII products using the OSA; therefore, a product‐specific reference standard is not required but could be used to reduce the discrepancies between the OSA and CSA.
CONFLICT OF INTERESTS
CK, IH and NR are employees of Roche Diagnostics GmbH. SD is an employee of Roche Diagnostics International Ltd. AT received research grants or honoraria for lectures or consultancy from Bayer, Biotest, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, SOBI and Takeda.
AUTHOR CONTRIBUTIONS
CK, IH, AT and NR contributed to the study conception/design. CK and IH were involved in data acquisition. All authors participated in data analysis and/or interpretation, writing and/or critically reviewing the manuscript and approving the final version for submission.
Supporting information
SUPPLEMENTARY INFORMATION
ACKNOWLEDGEMENTS
This study was supported by Roche Diagnostics GmbH. Third‐party medical writing support, under the direction of the authors, was provided by Heather Small, PhD of Ashfield MedComms (Macclesfield, UK), an Ashfield Health company, and was funded by Roche Diagnostics International Ltd (Rotkreuz, Switzerland). COBAS and COBAS T are trademarks of Roche. All other product names and trademarks are the property of their respective owners.
Ketteler C, Hoffmann I, Davidson S, Tiede A, Richter N Monitoring of different factor VIII replacement products using a factor VIII one‐stage clotting assay on cobas t 511/711 analysers. Haemophilia. 2021;27:e704–e712. 10.1111/hae.14416
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the main figures or in the supplementary material of this article.
REFERENCES
- 1. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. WFH Guidelines for the Management of Hemophilia. 2020;26(S6):1‐158. [DOI] [PubMed] [Google Scholar]
- 2. Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta‐analytic approach using national registries. Ann Intern Med. 2019;171(8):540‐546. [DOI] [PubMed] [Google Scholar]
- 3. Konkle BA, Johnsen JM, Wheeler M, et al. Genotypes, phenotypes and whole genome sequence: approaches from the My Life Our Future haemophilia project. Haemophilia. 2018;24(6):87‐94. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aledort L, Mannucci PM, Schramm W, Tarantino M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(6):479‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half‐life FVIII and factor IX replacement therapies. Semin Thromb Hemost. 2017;43(3):331‐337. [DOI] [PubMed] [Google Scholar]
- 6. Schiavoni M, Napolitano M, Giuffrida G, et al. Status of recombinant factor VIII concentrate treatment for hemophilia A in Italy: characteristics and clinical benefits. Front Med (Lausanne). 2019;6(261). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coppola A, Franchini M. Target of prophylaxis in severe haemophilia: more than factor levels. Blood Transfus. 2013;11(3):327‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Megías‐Vericat JE, Bonanad S, Haya S, et al. Bayesian pharmacokinetic‐guided prophylaxis with recombinant factor VIII in severe or moderate haemophilia A. Thromb Res. 2019;174:151‐162. [DOI] [PubMed] [Google Scholar]
- 9. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one‐stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 2016;14(2):248‐261. [DOI] [PubMed] [Google Scholar]
- 10. Muller J, Goldmann G, Marquardt N, Potzsch B, Oldenburg J. Extended half‐life factor VIII/Factor IX products: assay discrepancies and implications for hemophilia management. Hamostaseologie. 2020;40:S15‐S20. S 01. [DOI] [PubMed] [Google Scholar]
- 11. St Ledger K, Feussner A, Kalina U, et al. International comparative field study evaluating the assay performance of AFSTYLA in plasma samples at clinical hemostasis laboratories. J Thromb Haemost. 2018;16(3):555‐564. [DOI] [PubMed] [Google Scholar]
- 12. Ingerslev J, Jankowski MA, Weston SB, Charles LA, The Refacto Field Study Participant . Collaborative field study on the utility of a BDD factor VIII concentrate standard in the estimation of BDDr Factor VIII:c activity in hemophilic plasma using one‐stage clotting assays. J Thromb Haemost. 2004;2(4):623‐628. [DOI] [PubMed] [Google Scholar]
- 13. Tiefenbacher S, Albisetti M, Baker P, et al. Estimation of Nuwiq(®) (simoctocog alfa) activity using one‐stage and chromogenic assays‐results from an international comparative field study. Haemophilia. 2019;25(4):708‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morfini M, Cinotti S, Bellatreccia A, et al. A multicenter pharmacokinetic study of the B‐domain deleted recombinant factor VIII concentrate using different assays and standards. J Thromb Haemost. 2003;1(11):2283‐2289. [DOI] [PubMed] [Google Scholar]
- 15. Peyvandi F, Kenet G, Pekrul I, et al. Laboratory testing in hemophilia: impact of factor and non‐factor replacement therapy on coagulation assays. J Thromb Haemost. 2020;18(6):1242‐1255. [DOI] [PubMed] [Google Scholar]
- 16. Kitchen S, Geisen U, Kappelmayer J, et al. Evaluating the analytical performance of five new coagulation assays for the measurement of prothrombin time and activated thromboplastin time. Int J Lab Hematol. 2018;40(6):645‐654. [DOI] [PubMed] [Google Scholar]
- 17. Kitchen S, De Maat M, Nagler M, et al. System performance evaluation of the cobas t 711 and cobas t 511 coagulation analyzers in routine laboratory settings. Blood Coagul Fibrinolysis. 2020;31(7):459‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roche Diagnostics GH. FVIII [Method Sheet] V1.0. 2020. https://pim‐eservices.roche.com/eLD/api/downloads/baf79854‐0313‐ea11‐fa90‐005056a772fd?countryIsoCode=gb. Mannheim, Germany. 2019. [Google Scholar]
- 19. Technoclone. TECHNOCHROM FVIII:C [Method Sheet] V1.0. In. Vienna, Austria. 2016. [Google Scholar]
- 20. Church N, Leong L, Katterle Y, et al. Factor VIII activity of BAY 94‐9027 is accurately measured with most commonly used assays: results from an international laboratory study. Haemophilia. 2018;24(5):823‐832. [DOI] [PubMed] [Google Scholar]
- 21. Turecek PL, Romeder‐Finger S, Apostol C, et al. A world‐wide survey and field study in clinical haemostasis laboratories to evaluate FVIII: c activity assay variability of ADYNOVATE and OBIZUR in comparison with ADVATE. Haemophilia. 2016;22(6):957‐965. [DOI] [PubMed] [Google Scholar]
- 22. Gu J‐M, Ramsey P, Evans V, et al. Evaluation of the activated partial thromboplastin time assay for clinical monitoring of PEG ylated recombinant factor VIII (BAY 94‐9027) for haemophilia A. Haemophilia. 2014;20(4):593‐600. [DOI] [PubMed] [Google Scholar]
- 23. Zollner S, Raquet E, Claar P, et al. Non‐clinical pharmacokinetics and pharmacodynamics of rVIII‐SingleChain, a novel recombinant single‐chain factor VIII. Thromb Res. 2014;134(1):125‐131. [DOI] [PubMed] [Google Scholar]
- 24. Buyue Y, Liu T, Kulman JD, et al. A single chain variant of factor VIII Fc fusion protein retains normal in vivo efficacy but exhibits altered in vitro activity. PLoS One. 2014;9(11):e113600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowyer A, Key N, Dalton D, Kitchen S, Makris M. The coagulation laboratory monitoring of Afstyla single‐chain FVIII concentrate. Haemophilia. 2017;23(5):e469‐e470. [DOI] [PubMed] [Google Scholar]
- 26. Pouplard C, Ternisien C, Desconclois C, et al. Discrepancies between one stage assay and chromogenic substrate assay in patients treated with recombinant or plasma‐derived FVIII and usefulness of a specific standard in ReFacto AF®‐treated patients. Haemophilia. 2016;22(2):e101‐e103. [DOI] [PubMed] [Google Scholar]
- 27. Sommer JM, Moore N, Mcguffie‐Valentine B, et al. Comparative field study evaluating the activity of recombinant factor VIII Fc fusion protein in plasma samples at clinical haemostasis laboratories. Haemophilia. 2014;20(2):294‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viuff D, Barrowcliffe Tw, Saugstrup T, Ezban M, Lillicrap D. International comparative field study of N8 evaluating factor VIII assay performance. Haemophilia. 2011;17(4):695‐702. [DOI] [PubMed] [Google Scholar]
- 29. Bowyer A, Kitchen S, Maclean R. Effects of emicizumab on APTT, one‐stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia. 2020;26(3):536‐542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFORMATION
Data Availability Statement
The data that support the findings of this study are available in the main figures or in the supplementary material of this article.


