Abstract
Cabozantinib is registered in fixed 60 mg dose. However, 46% to 62% of patients in the registration studies needed a dose reduction due to toxicity. Improved clinical efficacy has been observed in renal cell carcinoma patients (RCC) with a cabozantinib exposure greater than 750 μg/L. In our study we explored the cabozantinib exposure in patients with different tumour types. We included RCC patients from routine care and salivary gland carcinoma (SGC) patients from a phase II study with ≥1 measured C min at steady‐state. The geometric mean (GM) C min at the starting dose, at 40 mg and at best tolerated dose (BTD) were compared between both tumour types. Forty‐seven patients were included. All SGC patients (n = 22) started with 60 mg, while 52% of RCC patients started with 40 mg. GM C min at the start dose was 1456 μg/L (95% CI: 1185‐1789) vs 682 μg/L (95% CI: 572‐812) (P < .001) for SGC and RCC patients, respectively. When dose‐normalised to 40 mg, SGC patients had a significantly higher cabozantinib exposure compared to RCC patients (C min 971 μg/L [95% CI: 790‐1193] vs 669 μg/L [95% CI: 568‐788]) (P = .005). Dose reductions due to toxicity were needed in 91% and 60% of SGC and RCC patients, respectively. Median BTD was between 20 to 30 mg for SGC and 40 mg for RCC patients. GM C min at BTD were comparable between the SGC and the RCC group, 694 μg/L (95% CI: 584‐824) vs 583 μg/L (95% CI: 496‐671) (P = .1). The observed cabozantinib exposure at BTD of approximately 600 μg/L is below the previously proposed target. Surprisingly, a comparable exposure at BTD was reached at different dosages of cabozantinib for SGC patients compared to RCC patients Further research is warranted to identify the optimal exposure and starting dose to balance efficacy and toxicity.
Keywords: cabozantinib, pharmacokinetics, renal cell carcinoma, salivary gland neoplasms, toxicity
Short abstract
What's new?
Cabozantinib, a potent tyrosine kinase inhibitor that targets multiple signaling pathways, is approved for use against advanced renal cell carcinoma (RCC). Variations in cabozantinib clearance, however, warrant further investigation. Here, the authors evaluated cabozantinib exposure in RCC patients and in patients with salivary gland cancer (SGC). SGC patients were found to have significantly higher cabozantinib exposure compared to RCC patients following a 40 mg dose. However, the best‐tolerated cabozantinib exposure was equivalent (~600 μg/L) for both tumor types and was substantially below the previously proposed target. The findings offer insight on exposure, dose, and the balance between efficacy and toxicity for cabozantinib.
Abbreviations
- BTD
best tolerated dose
- CI
confidence interval
- C max
maximum concentration
- C min
trough concentration
- GM
geometric mean
- HCC
hepatocellular carcinoma
- ICI
checkpoint inhibitors
- IMDC
International Metastatic Database Consortium
- IRB
Institutional Review Board
- MET
hepatocyte growth factor receptor
- MTC
medullary thyroid cancer
- OD
once daily
- ORR
overall response rate
- OS
overall survival
- PFS
progression free survival
- PK
pharmacokinetics
- PPI
proton pump inhibitor
- RCC
renal cell carcinoma
- SGC
salivary gland cancer
- TAM
Tyro3, Axl and Mer
- TDM
therapeutic drug monitoring
- TKI
tyrosine kinase inhibitor
- VEGFR
vascular endothelial growth factor receptor
1. INTRODUCTION
Cabozantinib is a potent oral tyrosine kinase inhibitor targeting multiple kinases, including the vascular endothelial growth factor receptor 2 (VEGFR2) and the hepatocyte growth factor receptor (MET). Inhibition of these key signalling pathways affects the tumour vasculature and averts angiogenesis, proliferation and metastasis of cancer cells. 1 A distinctive feature of cabozantinib over other VEGFR inhibitors is that it also targets the Tyro3, Axl and Mer (TAM) family of receptor kinases, which is thought to contribute to an antitumor immunomodulating effect. 2
Cabozantinib capsules (Cometriq) have been approved for treatment of progressive, unresectable locally advanced medullary thyroid cancer (MTC) in a fixed dose of 140 mg once daily (OD). Cabozantinib tablets (Cabometyx) in a fixed dose of 60 mg once daily have been approved for treatment of patients with advanced renal cell carcinoma (RCC) and for patients with hepatocellular carcinoma (HCC) who have previously been treated with sorafenib. 3 Recently, cabozantinib in combination with checkpoint inhibitors (ICI) has shown promising results in RCC patients. 4 , 5 The combination of cabozantinib and nivolumab for patients with metastatic RCC has been approved by the FDA. Furthermore, the combination has been added as a first‐line treatment option for clear cell RCC for all International Metastatic Database Consortium (IMDC) risk groups in the RCC guideline of the European Society for Medical Oncology and in the RCC guideline of the European Association of Urology. 6 , 7 In addition, multiple trials investigating cabozantinib‐ICI combinations for other solid tumours are ongoing (n = 16). Although the tablets and capsules are not deemed bioequivalent based on a small difference in the maximum concentration (C max) reached, both the tablets and capsules showed comparable area under the concentration‐time curve. 8 Moreover, both formulations exhibited dose proportional pharmacokinetics.
The pharmacokinetics (PK) of cabozantinib has been characterised in a population PK study which combined exposure data from healthy volunteers and cancer patients with different types of solid malignancies. 9 , 10 Cabozantinib shows large interpatient variability of approximately 50%, which is in line with other tyrosine kinase inhibitors. 11 Interestingly, patients with MTC had a two‐fold higher apparent clearance compared to RCC patients. The underlying cause of this difference remains unclear, but it may suggest a difference in cabozantinib clearance due to factors which are affected differently by different tumour types. With cabozantinib currently being studied for multiple tumour types, this finding could be of importance as for most oral tyrosine kinase inhibitors a correlation between the level of systemic exposure and clinical benefit exists. 12 , 13 , 14 , 15 , 16
For cabozantinib, its exposure response relationship has been investigated in patients with RCC in the phase III METEOR study. 17 , 18 , 19 The 60, 40 and 20 mg dose corresponded to an average cabozantinib steady‐state concentration of 1125, 750 and 375 μg/L, respectively. The exposure reached with the 60 mg dose resulted in improved progression free survival (PFS), reduced tumour growth and increased overall response rate compared to the 40 and 20 mg doses. For a dose of 40 mg instead of 60 mg once daily, the model predicted a modest 1.1‐fold increased risk of disease progression. However, this modest loss in efficacy opposes a 1.4‐fold lower risk of developing adverse events. Nevertheless, the starting dose of 60 mg was selected over 40 mg. 19 In the pivotal trials, adverse events were judged to be manageable with dose interruptions and reductions. The main dose limiting toxicities were fatigue and decreased appetite. 20 However, the proposed cabozantinib target exposure of 1125 μg/L seems poorly tolerated as can be derived from the high percentage of patients necessitating dose reductions in studies (40%‐79%). 20 , 21 , 22 In addition, patients in routine patient care often differ from patients treated within trials in terms of increased age, number of comorbidities, disease severity, pretreatment and lower performance status. 23 , 24 This casts a doubt on the tolerability of the proposed target exposure in these patients. Furthermore, in our clinic the phase II trial of cabozantinib for the treatment of patients with recurrent and/or metastatic salivary gland cancer was terminated prematurely due to severe toxicity, especially severe wound complications within the prior irradiated area of the head and neck. 25 As almost all patients in our study required dose reduction, this may call for a reconsideration of the accurate starting dose of cabozantinib.
In our study, we evaluate cabozantinib exposure in patients with salivary gland cancer and patients with renal cell cancer. Furthermore, potential relationships between cabozantinib exposure, patient characteristics, toxicity and treatment outcomes are explored.
2. METHODS
2.1. Study design and patient population
This observational study was performed using clinical data and cabozantinib trough concentrations (C min) obtained from patients treated with cabozantinib for RCC and SGC between January 2018 and August 2020. Baseline characteristics were retrospectively retrieved from the electronical health records or retrieved from the case report forms at start of cabozantinib treatment. Missing data at baseline were replaced by the closest value with a time interval up to 21 days prior to start of treatment or if not available, were left missing. For cabozantinib treatment, date, dose, time of intake and use of potent cytochrome P‐450 inhibitors or inducers were collected at start and at each measured steady‐state cabozantinib C min level. In addition, date and reason for each dose adjustment were collected. Adverse events necessitating dose reduction or treatment discontinuation were scored as clinically relevant toxicity. Best tolerated dose (BTD) was defined as the latest dose level before treatment discontinuation or at time of data cut off (1 August 2020).
For evaluation of the time on treatment, the time from start until the last day of cabozantinib treatment or data cut off was recorded. Also, the reason for treatment discontinuation was documented. Patient weight at baseline and at the end of cabozantinib treatment was collected.
2.2. Cabozantinib pharmacokinetics
Patients had plasma cabozantinib C min levels measured as part of routine patient care (RCC) or as part of the CABO‐ASAP study (SGC) (NCT03729297). All blood samples were collected in ethylenediamine tetra acetic acid plasma tubes and were subsequently centrifuged on the same day. Samples were stored at 4°C to 8°C until the day of analysis for a maximum of 14 days. A validated high‐performance liquid chromatography coupled with tandem mass spectrometry detection assay was used to determine total cabozantinib concentrations in plasma. 26 Only samples measured at steady‐state were taken into account and patients needed at least one cabozantinib C min level at steady‐state to be included. Steady‐state was defined as cabozantinib treatment at a fixed dose for more than 17 consecutive days based on four times the half‐life of cabozantinib.
No predefined sampling moments were set for measuring cabozantinib plasma concentrations. However, therapeutic drug monitoring is standard of care in our clinic and the first measurement is usually performed approximately 4 weeks after treatment initiation, at the moment of dose reduction and to evaluate the effect of dose adjustment. Patients were instructed to postpone cabozantinib intake till after sample collection on hospital visits. For each sample, the date and time of last intake of cabozantinib and the date and time of the plasma sample collection were recorded. In case the sample was not collected 24 hours after intake, the trough concentration was calculated by the approach described by Wang et al with a minimum of 5 and a maximum of 72 hours between blood collection and last pill intake. 27
Cabozantinib exposures at start, 40 mg and BTD, respectively, were determined using extrapolation of the dose‐normalised average exposure based on all available cabozantinib C min measurements per patient. Cabozantinib shows dose‐proportional exposure over the range of 20 to 140 mg which supports the use of this approach. For each patient, the average dose‐normalised cabozantinib C min exposure per milligram of cabozantinib was calculated as follows: First, each available cabozantinib C min measurement at steady‐state was dose‐normalised by dividing it by the administered dose of cabozantinib used at the moment of measurement (cabozantinib exposure/administered dose of cabozantinib in mg). Subsequently, the average of these dose‐normalised exposures was calculated per patient. This average concentration was thereafter multiplied by the specific dosages of interest for example, starting dose, 40 mg and best tolerated dose. The average cabozantinib exposure over the course of treatment was estimated based on steady‐state exposures. This estimate was calculated by multiplying the number of days at each dose level of cabozantinib and with the corresponding milligrams of cabozantinib. The sum of the amount of cabozantinib was subsequently divided by the duration of treatment in days to yield the average amount of cabozantinib in milligram per day. The average cabozantinib exposure was then calculated by multiplying this average amount of cabozantinib with the dose‐normalised exposure. Correlations between average calculated and measured cabozantinib C min levels at start, 40 mg and BTD were assessed to confirm dose‐proportionality in our cohort.
2.3. Statistical analysis
For the primary outcome, geometric mean cabozantinib C min levels at start, at 40 mg and at BTD were calculated for both SGC and RCC patients and the log‐transformed data were compared to an independent samples T‐test. Baseline patient and cabozantinib treatment characteristics were described using descriptive statistics. Associations between previously identified baseline patient characteristics (albumin, gender and body mass index) and cabozantinib exposure were first explored visually followed by linear regression (continuous variables) or with an independent samples T‐test (categorical variables) if deemed of interest. The exposure‐toxicity relationship was explored by comparing log‐transformed cabozantinib exposure at start of treatment and the occurrence of dose reduction (yes/no) with an independent samples T‐test. The relationship between average cabozantinib exposure and relative weight loss was explored visually. The preliminary efficacy was assessed by comparing progression free survival (PFS) and overall survival (OS) for patients with RCC with cabozantinib exposure below and above the median average exposure per day and for patients requiring a dose reduction (yes/no) relative to the starting dose, through Kaplan‐Meier methods using the unstratified log‐rank test.
All statistical analyses were performed using IBM SPSS statistics for Windows version 25.0 (IBM Corp, Armonk, New York). Geometric mean values are reported with 95% confidence intervals (CI). All P values were calculated with two‐sided statistical testing. Outcomes with P values less than .05 were considered to be statistically significant.
3. RESULTS
3.1. Patients characteristics
In total 47 patients were included, 22 patients with SGC and 25 patients with RCC. Baseline characteristics are shown in Table 1. For patients with RCC, most patients received cabozantinib as third line treatment. All patients with RCC received prior anti‐VEGFR therapy and the majority received prior treatment with an immune checkpoint inhibitor. For patients with SGC, the majority underwent surgery and received postoperative radiotherapy. Seven SGC patients (31.8%) received prior systematic therapy in the palliative setting.
TABLE 1.
Baseline characteristics at start of cabozantinib treatment
| RCC patients (n = 25) | SGC patients (n = 22) | |
|---|---|---|
| Age (years) | 67 (37‐81) | 57 (49‐72) |
| Gender | ||
| Male | 21 (84) | 10 (45) |
| KPS | ||
| 90‐100 | 7 (28) | 9 (41) |
| 80‐90 | 9 (36) | 1 (4.5) |
| 70‐80 | 8 (32) | 12 (55) |
| 60‐70 | 1 (4) | 0 |
| Histology | ||
| Clear cell | 20 (80) | NA |
| Papillary | 3 (12) | NA |
| Other RCCs | 2 (8) | NA |
| ACC | NA | 16 (72) |
| SDC | NA | 4 (18) |
| Other SGCs | NA | 2 (9) |
| IMDC risk score | ||
| Intermediate | 13 (52) | NA |
| Poor | 12 (48) | NA |
| Prior treatment | ||
| Surgery | 14 (56) a | 18 (82) |
| Postoperative radiotherapy | NA | 18 (82) |
| Palliative radiotherapy | DNC | 10 (45) |
| Adjuvant systemic therapy | NA | 2 (9) |
| Palliative systemic therapy | 25 (100) | 7 (32) |
| Line of systemic therapy (median, range) | 3 (2‐6) | 2 (1‐5) |
| Prior VEGFR‐I | 25 (100) | 1 (5) |
| Prior ICI | 19 (76) | 0 (0) |
| Weight (kg) | 82 (51‐121) | 76 (54‐111) |
| BMI (kg/m2) | 25.5 (17.8‐36.3) | 25.1 (21.4‐39.9) |
| Haemoglobin (μmol/L) | 7.1 (4.8‐8.7) | 8.2 (6.5‐9.7) |
| Leukocytes (109/L) | 7.9 (4.5‐14.2) | 6.7 (4.1‐11.3) |
| Thrombocytes (109/L) | 337 (210‐984) | 270 (131‐454) |
| Neutrophils (109/L) | 5.9 (3.7‐11.5) | 4.6 (2.3‐8.9) |
| Calcium (mmol/L) | 2.41 (2.24‐2.73) | 2.41 (2.24‐2.65) |
| Serum creatinine (μmol/L) | 121 (52‐228) | 64 (46‐108) |
| ALT (IU/L) | 22 (9‐107) | 23 (12‐76) |
| AST (IU/L) | 24 (18‐49) | 27 (14‐68) |
| Albumin (g/L) | 31.5 (20‐38) | 38.0 (28‐42) |
Note: Data are presented as n (%) for categorical variables and median (range) for continuous variables.
Abbreviations: ACC, adenoid cystic carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DNC, data not collected; ICI, immune checkpoint inhibitor; IMDC, International Metastatic RCC Database Consortium; NA, not applicable; RCC, renal cell carcinoma; SDC, salivary duct carcinoma; SGC, salivary gland cancer; VEGFR‐I, vascular endothelial growth factor inhibitor.
Nephrectomy.
3.2. Cabozantinib treatment
Patients with SGC all received the 60 mg starting dose as was mandated by the study protocol. For patients with RCC, the selection of the starting dose was at the physicians' discretion. The majority of patients with RCC (48%) started with 40 mg. Cabozantinib treatment characteristics are depicted in Table 2. Median duration of treatment was 22 (4‐151) weeks for RCC and 29.5 (3‐55) weeks for SGC. The SGC phase II study was terminated prematurely due to the occurrence of severe wound complications in previously irradiated areas. Dose reductions relative to the starting dose were needed in 20 (91%) patients with SGC and 15 (60%) patients with RCC. All dose reductions were related to toxicity. Median best tolerated dose, defined as the latest dose level before treatment discontinuation, was between 20 and 30 mg in patients with SGC and 40 mg in patients with RCC. Median average cabozantinib dose intensity over the duration of treatment was approximately 40 mg in both tumour types. In the RCC groups, 16 patients (64%) discontinued treatment due to progressive disease and 5 (20%) due to toxicity. One patient had liver test elevations, one patient had a nonhealing wound on the lower extremity, one patient had a gastrointestinal mucosal lesion and two patients had a gastrointestinal perforation.
TABLE 2.
Cabozantinib treatment details
| RCC patients (n = 25) | SGC patients (n = 22) | |
|---|---|---|
| Dose at start of treatment | ||
| 60 | 8 (32) | 22 (100) |
| 40 | 13 (52) | 0 |
| 20 | 4 (16) | 0 |
| Median treatment duration (weeks) | 22 (4‐151) | 29.5 (3‐55) |
| Dose reduction during treatment | 15 (60) | 20 (91) |
| Reason of treatment discontinuation | 21 (84) a | 22 (100) |
| Toxicity | 5 (20) | 3 (14) |
| Progressive disease | 16 (64) | 6 (27) |
| Death | 0 | 0 |
| Other b | 0 | 13 (59) |
| Best tolerated cabozantinib dose (mg) | ||
| 20 | 5 (20) | 11 (50) |
| 27 c | 1 (4) | 0 |
| 30 c | 3 (12) | 2 (9) |
| 40 | 13 (52) | 7 (32) |
| 50 c | 1 (4) | 0 |
| 60 | 2 (8) | 2 (9) |
| Median average dose intensity (mg) | 39 (20‐60) | 38 (24‐60) |
| Total number of samples at steady state | 42 | 37 |
| Median number of samples per patient | 1 (1‐5) | 1 (1‐3) |
Four patients still on treatment at data cut‐off.
Stop of study due to safety concerns.
Cabozantinib dose reached via an alternative dosing schedule (eg, 20 and 40 mg used alternately).
3.3. Cabozantinib exposure at start, 40 mg and at BTD
In total 79 cabozantinib samples at steady‐state were available, 37 of patients with SGC and 42 of patients with mRCC. The number of samples per patient ranged from 1 to 5. Cabozantinib C min levels were measured for 13 (59%) patients with SGC at the start dose level of 60 mg, 9 (41%) at 40 mg and 16 (73%) at best tolerated dose. For patients with RCC, these percentages were 68%, 76% and 80% at start, 40 mg and at BTD, respectively. The average dose‐normalised cabozantinib C min based on all available measurement was used to calculate the cabozantinib C min level at a specific dose level for each individual patient. There was a good correlation between measured and calculated cabozantinib C min levels (R 2 > 0.89, Figure S1A‐C).
At the start dose, patients with SGC had approximately two‐fold higher GM cabozantinib exposure compared to patient with RCC (1456 μg/L [95% CI 1185‐1789] vs 682 μg/L [95% CI 572‐812], P < .001, for patients with SGC and RCC, respectively) (Figure 1). After dose normalisation to 40 mg, patients with SGC still had a significantly higher cabozantinib exposure compared to patients with RCC (GM C min 971 μg/L [95% CI 790‐1193] vs 669 μg/L [95% CI 568‐788] (P = .005). Cabozantinib GM C min levels at BTD were comparable between the SGC group and the RCC group, 694 μg/L (95% CI 584‐824) versus 583 μg/L (95% CI 496‐671) (P = .1) as depicted in Figure 1. At BTD, 64% of SGC patients and 80% of RCC patients had an exposure below the proposed target of >750 μg/L.
FIGURE 1.
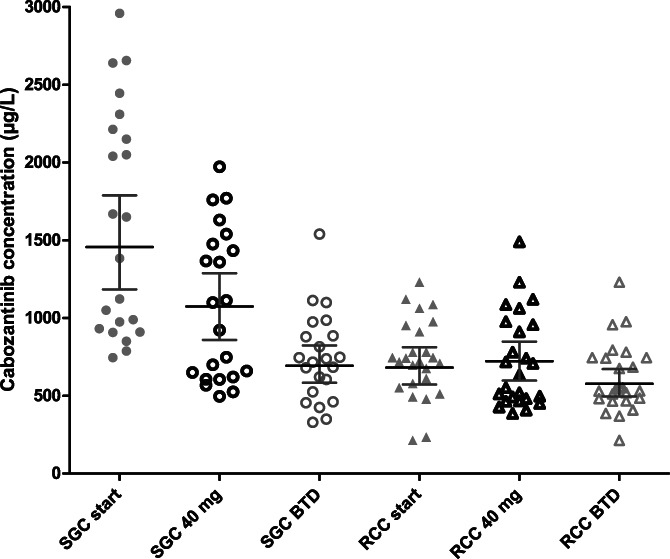
Scatter plots of the individual cabozantinib C min levels at start, 40 mg and at BTD for patients with SGC and RCC. Data are presented with geometric mean (95% CI). BTD, best tolerated dose; CI, confidence interval; C min, trough concentration, RCC, metastatic renal cell carcinoma; SGC, salivary gland cancer
3.4. Cabozantinib exposure and patient characteristics
To explore the potential relationship between patient baseline characteristics and cabozantinib exposure, previously identified demographic variables that differed in our cohorts at baseline (albumin and gender) were visually assessed via scatter plots (Figure S2). No apparent correlation between cabozantinib exposure and albumin levels was observed. A higher cabozantinib exposure was observed for female patients compared to their male counterparts. In female patients with SGC GM cabozantinib C min at 40 mg was 1254 μg/L (95% CI 996‐1577) compared to 714 μg/L (95% CI 540‐945) in male patients (P = .002). For patients with RCC, no difference could be observed.
3.5. Cabozantinib exposure and toxicity
An explorative analysis was performed between the occurrences of clinically relevant toxicity, defined as an adverse event necessitating dose reduction or treatment discontinuation, and cabozantinib exposure in patients which RCC. Since nearly all patients with SGC (91%) required dose reductions, no comparison could be made between patients with and without dose reduction for this group. In patients with RCC, a trend was observed for a higher cabozantinib C min level for patients who needed a dose reduction relative to the start dose, that is, 769 μg/L (95% CI 663‐893) versus 568 μg/L (95% CI 384‐842), respectively (P = .079) (Figure S3).
Patient weight was collected at start of cabozantinib treatment and at discontinuation or last moment of follow‐up. Median percentage weight loss from baseline was 6.2% (range: −2.6 to 34) and 7.2% (range: −0.5 to 18.7) in patients with SGC and RCC, respectively. No correlation was observed between cabozantinib exposure and weight loss (Figure S4).
3.6. Cabozantinib exposure and response
Median estimated average cabozantinib exposure was 573 μg/L (range: 225‐1088) in patients with RCC. Median PFS was 34.0 weeks (95% CI 24.2‐43.8). In the preliminary efficacy analysis, no difference in PFS was observed between patients with an average cabozantinib exposure above and below the median average cabozantinib exposure (19.0 weeks, 95% CI 0‐45.7 vs 34 weeks, 95% CI 32.6‐35.5, respectively). Interestingly, a longer PFS was observed for patients with a dose reduction relative to the start dose compared to patients without a dose reduction (41.0 weeks, 95% CI 13.5‐68.5 vs 20.0 weeks, 95% CI 9.6‐30.4, P = .023, respectively). In the exploratory OS analysis, no difference could be observed for patients with an average cabozantinib exposure above and below the median and start dose of 60 mg or below 60 mg (Figures S5 to S8).
4. DISCUSSION
In this observational study, we observed a significantly higher cabozantinib exposure in patients with SGC compared to those with RCC, also after correcting for differences in the dose administered. Dose reductions due to toxicity were needed in a large percentage of patients. The cabozantinib exposure reached at the best tolerated dose level was comparable between both groups of patients. However, this exposure lies well below the previously proposed target of >750 μg/L for which improved treatment outcomes have been observed.
Cabozantinib pharmacokinetics have previously been characterised in patients with various solid tumours. 9 , 17 Female gender has been associated with a 21% decrease in cabozantinib clearance compared to male gender. 17 This difference was however not deemed clinically significant given the high interpatient variability of cabozantinib. In our study, the female gender was more evenly distributed in patients with SGC compared to patients with RCC (55% vs, 16%, respectively) and this may partly explain the higher geometric mean cabozantinib C min levels observed in the SGC group. This potential gender effect warrants further research in larger cohorts as indications for cabozantinib are extending and females are often underrepresented in clinical trials. 28 Another possible explanation for the observed difference in cabozantinib concentrations in SGC and RCC patients may be the difference in serum albumin concentrations. Previously, lower albumin concentrations have been associated with lower maximal cabozantinib concentrations. 29 Therefore, the higher albumin concentrations in patients with SGC may be an explanation for the higher cabozantinib concentrations observed in these patients. Interestingly, a two‐fold lower cabozantinib exposure was previously reported in patients with MTC, which possibly indicates that specific shared characteristics within a tumour type may affect cabozantinib exposure. However, for other registered tyrosine kinase inhibitors targeting VEGFR, no apparent differences have been reported in pharmacokinetics between groups of patients with different tumour types, excluding cancers affecting organs known to be involved in drug metabolism, such as hepatocellular carcinoma and thyroid cancer. 30 , 31 , 32 Cabozantinib exposure is not affected by renal impairment, so the difference in serum creatinine between both groups is unlikely to be of relevance. 29 Prior treatment with VEGFR inhibitors in RCC patients could potentially have contributed to a lower absorption from the gastrointestinal tract. Theoretically, reduced salivary function and changes in salivary composition after irradiation may lead to changes in the digestive process and hence increased drug absorption and/or reduced metabolism in SGC patients. 33 , 34 However, prior studies of TKIs in SGC have not yet reported pharmacokinetic data to support this hypothesis. 35 , 36
Similar to the registration trials, the majority of patients required dose reduction due to toxicity. Median best tolerated dose was between 20 and 30 mg for SGC patients and 40 mg for RCC patients. However, at best tolerated dose, cabozantinib exposure was comparable between patients with SGC and RCC (694 vs 583 μg/L, respectively). The slightly higher exposure in SGC patients is most likely the result of the fixed starting dose of 60 mg once daily. In contrast to real world patients, use of a lower starting dose was not allowed. In addition, as the study was terminated early, not all patients may have received dose reduction and therefore the best tolerated dose and corresponding exposure could have been overestimated. Nevertheless, our finding in RCC patients is line with a previous study of Cerbone et al, who observed a significantly higher median cabozantinib C min in routinely treated RCC patients with Grade 3 to 4 toxicity versus those without toxicity (623 vs 452 μg/L, respectively). 37 Furthermore, they reported a median C min of 405 and 521 μg/L in patients with and without progressive disease, respectively. This indicates a narrow range in which efficacy and tolerability need to be balanced.
The observed percentage of dose reductions of 60% in our study is in line with the 57% reported in a cohort of 410 RCC patients treated with cabozantinib in an early access programme in France (CABOREAL). 38 In CABOREAL study, a significantly longer OS was observed for patients who initiated cabozantinib treatment with 60 mg. The CABOREAL study did not report PFS. However, in our preliminary analysis on efficacy, we did not observe an effect of the 60 mg starting dose or a higher average cabozantinib exposure on PFS or OS, but it is important to note that our analysis a based on a limited number of heterogenous patients in terms of pretreatment and tumour histology. The median age in our cohort was 67 years. The observed median PFS of approximately 7.8 months in our study is in line with the reported median of 8.1 months for patients with clear cell RCC aged 65 to 74 in the METEOR phase 3 trial and just above the observed median of 7.0 months in a cohort of nonclear cell RCC patients. 39 , 40 Interestingly, we observed a longer PFS for patients with a dose reduction relative to the start dose, which may suggest a tipping point between optimal exposure versus long‐term tolerable exposure. However, this finding needs to be evaluated in a larger group of patients.
The phase II study of cabozantinib in SGC patients was terminated prematurely due to severe wound complications in six (24%) patients, mainly in areas which received prior radiotherapy. 25 In our RCC cohort, five patients discontinued cabozantinib treatment due to toxicity. Four out of these five patients developed moderate to severe wound (healing) complications likely related to cabozantinib treatment. These percentages exceed the incidence of 1% to 3% reported in the registration studies. No clear associations could be made between cabozantinib exposure and the occurrence of these complications. Therefore, these complications may be a result of individual sensitivity and the presence of risk factors associated with wound complications or inclusion of patients that were not eligible for the registration studies. Cabozantinib is a strong inhibitor of VEGFR‐2, but the additional inhibition of C‐MET and TAM kinases may be an explanation for the high percentage of wound complications reported, as these kinases also play an important role in tissue repair and maintaining vascular integrity. 41 , 42 Clarification of these risk factors in future studies is of major importance to be able to identify patients at risk and ideally prevent these patients from severe toxicity.
Our study has some potential limitations. First, the CABO‐ASAP study was a phase II study whereas the RCC patients were collected from routine patient care. In general, trials participants are fitter and medication adherence is higher compared to patients in routine care and this may have contributed to a higher observed cabozantinib exposure in SGC patients. 43 , 44 However, this is likely of limited influence as the exposure at best tolerated dose was comparable between both groups. Furthermore, the exposure in RCC patients at 40 mg was just slightly lower than the reported average cabozantinib exposure in the phase III trial. 17 Another limitation is the small number of cabozantinib blood samples per patient in our analysis. The pharmacokinetic analysis in the phase II CABO‐ASAP was not an objective of the study. Consequently, we did not have a C min level at each dose level of every patient. Cabozantinib samples of RCC patients were collected from routine care and although therapeutic drug monitoring is standard of care in our clinic, C min levels were not available for all received dose levels, as patients sometimes required a rapid dose adjustment before a visit for PK evaluation could be scheduled. To be able to compare cabozantinib exposure between dose levels, we performed extrapolation based on the dose‐normalised average exposure of all available C min levels. This was allowed as cabozantinib has been reported to exhibit dose proportional pharmacokinetics in the range of 20 to 140 mg. In addition, we showed an adequate correlation between the measured and calculated average cabozantinib C min levels which supports the legitimacy of our approach. However, for some TKIs a decrease in exposure over time has been reported, possibly due to a decrease in absorption. 45 , 46 Cabozantinib samples taken at a later time during treatment may therefore distort this dose proportionality. Based on our data we could not detect a decrease in cabozantinib exposure over time and therefore believe that this does not affect our conclusions. Finally, even though the observations are based on limited number of patients, they show large similarity with the reported percentage of dose reductions and cabozantinib exposure in the registration studies. 18 , 22 Therefore, the pharmacology of cabozantinib in this relatively small group of patient appears to be representative for clinical practice.
5. CONCLUSION
In our study, we observed an unexpected higher cabozantinib exposure in patients with SGC compared to those with RCC even after correcting for dose differences between both groups. However, the cabozantinib exposure at BTD were comparable between both groups. At BTD the majority of patients did not reach the proposed target exposure of >750 μg/L. For most patients with RCC, the cabozantinib level at BTD corresponded to a dose of 40 mg. Therefore, a 40 mg start dose followed by dose‐adjustment based on exposure and tolerability may be preferred over the 60 mg dose to avoid unnecessary toxicity yet the effect of this approach on efficacy requires more research. Future studies should focus on identifying an optimal and tolerable exposure‐response target value for cabozantinib and elucidate factors that contribute to the differences in exposure, in order to individualise and improve treatment outcomes.
CONFLICT OF INTEREST
All mentioned relationships are outside the submitted work. FGAJ has been on an advisory board for Amgen and Genzyme. IMED received a research grant from Novartis. NPvE has received research grants from Novartis, Astellas, Janssen‐Cilag, Pfizer, Ipsen, has been on an advisory board for Pfizer, and received honoraria from Bayer and Sanofi. CMLvH has received research grants form AstraZeneca, Bristol Meyers Squibb, Merck Sharp and Dohme, Merck, Ipsen, Sanofi, and Novartis, has been on an advisory board for Bayer, Bristol‐Meyers Squibb, Ipsen, Merck Sharp and Dohme and Regeneron. SFM received honoraria from Pfizer, Roche and Merck Sharp and Dohme and Bristol Meyers Squibb. The other authors declare no conflicts of interest. (SDK, MU, WvB).
ETHICS STATEMENT
The current study has been performed in accordance with the Declaration of Helsinki (October 2013). The CABO‐ASAP (NCT03729297) phase II study has been approved by the medical ethical comity and the Institutional Review Board of the Radboudumc (IRC) and all patients gave written informed consent. The analysis in patients with RCC has been approved by the IRB of the Radboudumc (December 2018; 2018‐4617) and with their consent no informed consent was required, as we used data from routine care.
Supporting information
Figure S1 Correlation between measured and calculated average cabozantinib C min levels for (A) the 40 mg dose level, (B) the dose level at start and (C) BTD level
Figure S2 Scatter plot of gender (A) and baseline albumin levels (B) vs calculated cabozantinib C min levels at 40 mg
Figure S3 Scatter plot of the calculated individual cabozantinib C min levels at the start dose for RCC patients who did and did not receive a dose reduction relative to the starting dose
Figure S4 Scatter plot of percentage weight loss from baseline vs average cabozantinib C min exposure
Figure S5 Kaplan‐Meier curve of progression free survival for patients with advanced RCC with an exposure above and below the median average exposure of 573 μg/L
Figure S6 Kaplan‐Meier curve of progression free survival for patients with advanced RCC with an without dose reductions relative to the starting dose level
Figure S7 Kaplan‐Meier curve of overall survival for patients with advanced RCC with an exposure above and below the median average exposure of 573 μg/L
Figure S8 Kaplan‐Meier curve of overall survival for patients with advanced RCC with a 60 mg starting dose and those with a lower than 60 mg stating dose
Krens SD, van Boxtel W, Uijen MJM, et al. Exposure‐toxicity relationship of cabozantinib in patients with renal cell cancer and salivary gland cancer. Int. J. Cancer. 2022;150(2):308-316. doi: 10.1002/ijc.33797
DATA AVAILABILITY STATEMENT
The datasets generated and analysed for the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298‐2308. [DOI] [PubMed] [Google Scholar]
- 2. Bergerot P, Lamb P, Wang E, Pal SK. Cabozantinib in combination with immunotherapy for advanced renal cell carcinoma and urothelial carcinoma: rationale and clinical evidence. Mol Cancer Ther. 2019;18(12):2185‐2193. [DOI] [PubMed] [Google Scholar]
- 3. FDA . Cabometyx prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208692s008lbl.pdf. Accessed December 1, 2020.
- 4. Choueiri TK, Powles T, Burotto M, et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first‐line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2020;31:S1159. [Google Scholar]
- 5. Agarwal N, Vaishampayan U, Green M, et al. 872P: phase Ib study (COSMIC‐021) of cabozantinib in combination with atezolizumab: results of the dose escalation stage in patients (pts) with treatment‐naïve advanced renal cell carcinoma (RCC). Ann Oncol. 2018;29:viii308. [Google Scholar]
- 6. Powles T. Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first‐line clear cell renal cancer. Ann Oncol. 2020. 10.1016/j.annonc.2020.11.016 [DOI] [PubMed] [Google Scholar]
- 7. Bedke J, Albiges L, Capitanio U, et al. Updated European Association of Urology guidelines on renal cell carcinoma: nivolumab plus cabozantinib joins immune checkpoint inhibition combination therapies for treatment‐naïve metastatic clear‐cell renal cell carcinoma. Eur Urol. 2021;79(3):339‐342. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen L, Benrimoh N, Xie Y, Offman E, Lacy S. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anticancer Drugs. 2016;27(7):669‐678. [DOI] [PubMed] [Google Scholar]
- 9. Lacy S, Yang B, Nielsen J, Miles D, Nguyen L, Hutmacher M. A population pharmacokinetic model of cabozantinib in healthy volunteers and patients with various cancer types. Cancer Chemother Pharmacol. 2018;81(6):1071‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen L, Chapel S, Tran BD, Lacy S. Updated population pharmacokinetic model of cabozantinib integrating various cancer types including hepatocellular carcinoma. J Clin Pharmacol. 2019;59(11):1551‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Wit D, Guchelaar HJ, den Hartigh J, Gelderblom H, van Erp NP. Individualized dosing of tyrosine kinase inhibitors: are we there yet? Drug Discov Today. 2015;20(1):18‐36. [DOI] [PubMed] [Google Scholar]
- 12. Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta‐analysis. Cancer Chemother Pharmacol. 2010;66(2):357‐371. [DOI] [PubMed] [Google Scholar]
- 13. Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first‐line metastatic renal‐cell carcinoma: a randomised double‐blind phase 2 trial. Lancet Oncol. 2013;14(12):1233‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suttle AB, Ball HA, Molimard M, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111(10):1909‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27(19):3141‐3147. [DOI] [PubMed] [Google Scholar]
- 16. FDA . Cometriq prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203756s009lbl.pdf. Accessed December 1, 2020.
- 17. Lacy S, Nielsen J, Yang B, Miles D, Nguyen L, Hutmacher M. Population exposure‐response analysis of cabozantinib efficacy and safety endpoints in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2018;81(6):1061‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open‐label, phase 3 trial. Lancet Oncol. 2016;17(7):917‐927. [DOI] [PubMed] [Google Scholar]
- 19. Castellano D, Pablo Maroto J, Benzaghou F, et al. Exposure‐response modeling of cabozantinib in patients with renal cell carcinoma: implications for patient care. Cancer Treat Rev. 2020;89:102062. [DOI] [PubMed] [Google Scholar]
- 20. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med. 2015;373(19):1814‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639‐3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitchell AP, Harrison MR, Walker MS, George DJ, Abernethy AP, Hirsch BR. Clinical trial participants with metastatic renal cell carcinoma differ from patients treated in real‐world practice. J Oncol Pract. 2015;11(6):491‐497. [DOI] [PubMed] [Google Scholar]
- 24. Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383‐1389. [DOI] [PubMed] [Google Scholar]
- 25. Boxtel WV, Uijen M, Driessen C, et al. A phase II study on the efficacy and toxicity of cabozantinib in recurrent/metastatic salivary gland cancer patients. J Clin Oncol. 2020;38:6529‐6529. [Google Scholar]
- 26. Krens SD, van der Meulen E, Jansman FGA, Burger DM, van Erp NP. Quantification of cobimetinib, cabozantinib, dabrafenib, niraparib, olaparib, vemurafenib, regorafenib and its metabolite regorafenib M2 in human plasma by UPLC‐MS/MS. Biomed Chromatogr. 2020;34(3):e4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Chia YL, Nedelman J, Schran H, Mahon FX, Molimard M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31(5):579‐584. [DOI] [PubMed] [Google Scholar]
- 28. Mendis S, Anand S, Karasinska JM, et al. Sex representation in clinical trials associated with FDA cancer drug approvals differs between solid and hematologic malignancies. Oncologist. 2021;26(2):107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen L, Holland J, Ramies D, et al. Effect of renal and hepatic impairment on the pharmacokinetics of cabozantinib. J Clin Pharmacol. 2016;56(9):1130‐1140. [DOI] [PubMed] [Google Scholar]
- 30. Ikeda M, Okusaka T, Mitsunaga S, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016;22(6):1385‐1394. [DOI] [PubMed] [Google Scholar]
- 31. Dadu R, Waguespack SG, Sherman SI, et al. Efficacy and tolerability of different starting doses of sorafenib in patients with differentiated thyroid cancer. Oncologist. 2014;19(5):477‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukudo M, Ito T, Mizuno T, et al. Exposure‐toxicity relationship of sorafenib in Japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clin Pharmacokinet. 2014;53(2):185‐196. [DOI] [PubMed] [Google Scholar]
- 33. de Barros PC, Polizello AC, Spadaro AC. Clinical and biochemical evaluation of the saliva of patients with xerostomia induced by radiotherapy. Braz Oral Res. 2004;18(1):69‐74. [DOI] [PubMed] [Google Scholar]
- 34. Valdez IH, Fox PC. Interactions of the salivary and gastrointestinal systems. I. The role of saliva in digestion. Dig Dis. 1991;9(3):125‐132. [DOI] [PubMed] [Google Scholar]
- 35. Zhu G, Zhang L, Li R, Dou S, Yang W, Zhang C. Phase II trial of apatinib in patients with recurrent and/or metastatic adenoid cystic carcinoma of the head and neck: updated analysis. J Clin Oncol. 2018;36:6026‐6026. [Google Scholar]
- 36. Tchekmedyian V, Sherman EJ, Dunn L, et al. Phase II study of lenvatinib in patients with progressive, recurrent or metastatic adenoid cystic carcinoma. J Clin Oncol. 2019;37(18):1529‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerbone L, Di Nunno V, Carril L, et al. 743P activity of systemic therapies after cabozantinib (CABO) in patients (pts) with metastatic renal cell carcinoma (mRCC). Ann Oncol. 2020;31:S577. [Google Scholar]
- 38. Albiges L, Fléchon A, Chevreau C, et al. Real‐world evidence of cabozantinib in patients with metastatic renal cell carcinoma: results from the CABOREAL early access program. Eur J Cancer. 2020;142:102‐111. [DOI] [PubMed] [Google Scholar]
- 39. Donskov F, Motzer RJ, Voog E, et al. Outcomes based on age in the phase III METEOR trial of cabozantinib versus everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2020;126:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martínez Chanzá N, Xie W, Asim Bilen M, et al. Cabozantinib in advanced non‐clear‐cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. 2019;20(4):581‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rothlin CV, Carrera‐Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xian CJ, Couper R, Howarth GS, Read LC, Kallincos NC. Increased expression of HGF and c‐met in rat small intestine during recovery from methotrexate‐induced mucositis. Br J Cancer. 2000;82(4):945‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Margolis J, Princic N, Doan J, Lenhart G, Motzer RJ. Analysis of real‐world treatment adherence in a cohort of 2,395 patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2016;34:517‐517.26729440 [Google Scholar]
- 44. Byfield SA, McPheeters JT, Burton TM, Nagar SP, Hackshaw MD. Persistence and compliance among U.S. patients receiving pazopanib or sunitinib as first‐line therapy for advanced renal cell carcinoma: a retrospective claims analysis. J Manag Care Spec Pharm. 2015;21(6):515‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eechoute K, Fransson MN, Reyners AK, et al. A long‐term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res. 2012;18:5780‐5787. [DOI] [PubMed] [Google Scholar]
- 46. Arrondeau J, Mir O, Boudou‐Rouquette P, et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30(5):2046‐2049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Correlation between measured and calculated average cabozantinib C min levels for (A) the 40 mg dose level, (B) the dose level at start and (C) BTD level
Figure S2 Scatter plot of gender (A) and baseline albumin levels (B) vs calculated cabozantinib C min levels at 40 mg
Figure S3 Scatter plot of the calculated individual cabozantinib C min levels at the start dose for RCC patients who did and did not receive a dose reduction relative to the starting dose
Figure S4 Scatter plot of percentage weight loss from baseline vs average cabozantinib C min exposure
Figure S5 Kaplan‐Meier curve of progression free survival for patients with advanced RCC with an exposure above and below the median average exposure of 573 μg/L
Figure S6 Kaplan‐Meier curve of progression free survival for patients with advanced RCC with an without dose reductions relative to the starting dose level
Figure S7 Kaplan‐Meier curve of overall survival for patients with advanced RCC with an exposure above and below the median average exposure of 573 μg/L
Figure S8 Kaplan‐Meier curve of overall survival for patients with advanced RCC with a 60 mg starting dose and those with a lower than 60 mg stating dose
Data Availability Statement
The datasets generated and analysed for the current study are available from the corresponding author on reasonable request.


