Abstract
Objective
The objective of this study was to assess the effectiveness of a low‐dose intravenous S‐ketamine treatment on refractory pain in patients with Complex Regional Pain Syndrome (CRPS).
Methods
In this retrospective study, patients with CRPS who received intravenous S‐ketamine from March 2010 to April 2019 were included. According to our inpatient protocol, S‐ketamine dose was increased until pain reduction was achieved or side effects were observed. Maximum dose was 14 mg/h and treatment duration was 7 days. Primary outcome parameters were pain scores (Numeric Rating Scale) at baseline (T0), end of infusion (T1), and approximately 4 weeks postinfusion (T2). Patients were categorized as responder/nonresponder at T1 and T2. Patients were considered a responder in case there was pain score reduction of greater than or equal to 2 points or if treatment was reported as successful.
Results
Forty‐eight patients were included. Mean disease duration was 5 years (interquartile range [IQR] = 6 years). Median pain score significantly decreased from 8 (IQR = 2) at T0 to 6 (IQR = 4) at T1 (p < 0.001). At T1, 62% of the patients were responders. At T2, 48% of the patients remained a responder. A significant proportion of the responders at T1 turned into nonresponders at T2 (p = 0.03).
Conclusion
In a group of patients with CRPS with refractory pain, low‐dose intravenous S‐ketamine treatment resulted in effective pain relief during infusion. Although a significant proportion of initial responders became nonresponders at follow‐up, half of the patients were still a responder at ~ 4 weeks postinfusion. Further research is needed to investigate mechanisms responsible for pain relief by S‐ketamine infusions and to ascertain possible predictors of response to the treatment.
Keywords: Complex Regional Pain Syndrome (CRPS), dose, intravenous, pain, S‐ketamine
Key Points.
In a group of patients with CRPS with refractory pain, low‐dose intravenous S‐ketamine treatment resulted in effective pain relief during infusion
Responders started to observe treatment effect within 2 days
The median effective S‐ketamine dose was 6 mg/h
At ~ 4 weeks postinfusion, almost half of the patients were still responders
Further research is needed to understand which pathophysiological mechanisms are targeted by S‐ketamine infusion in order to predict treatment responders
INTRODUCTION
Complex Regional Pain Syndrome (CRPS) type 1 is a syndrome characterized by a continuing (spontaneous and/or evoked) regional pain that is seemingly disproportionate in time or degree to the usual course of pain after trauma or other lesion. 1 The pain is regional, not in a specific nerve territory or dermatome, and usually has a distal predominance. 1 , 2 In addition, CRPS is characterized by a variable progression over time. 2 The estimated incidence of CRPS varies between 5.5 and 26.2 per 100,000 person years 3 , 4 and women are affected at least 3 times more often than men. 4 CRPS is diagnosed using a set of clinical criteria: the International Association for the Study of Pain (IASP) clinical diagnostic criteria. 5 In sum, the diagnosis is based on continuous pain in the affected limb, which is disproportionate to the inciting event and which is accompanied by (a minimum set of) sensory, sudomotor, vasomotor, motor, and trophic abnormalities. 5 , 6
The Dutch CRPS guidelines (last updated in 2014) for the treatment of CRPS define our daily practice. 7 Nociceptive pain in CRPS is treated in a step‐by‐step manner, with weak opioids being the maximum step as strong opioids are not recommended in CRPS. Neuropathic pain is treated with co‐analgesics. These guidelines also recommend treatment with intravenous S‐ketamine for patients with CRPS type I with therapy‐resistant pain. This therapy is therefore regularly prescribed and administered to patients with CRPS with therapy‐resistant pain in our department.
Ketamine, a derivative of phencyclidine (“Angel dust”), was introduced in the 1960s as an anesthetic. 8 Its large margin of safety quickly led to widespread use during the Vietnam war. 8 Ketamine has been coined a “dissociative anesthetic” 8 ; however, its unique methods of action are still incompletely understood. It is thought, that this state of dissociative anesthesia is achieved via a disruption of cortico‐cortical communication. 9
When S‐ketamine is administered in a low dose, it can be used as an analgesic in various acute and chronic pain settings. 10 This analgesic effect of S‐ketamine is achieved through its antagonistic‐actions on the N‐methyl‐D‐aspartate (NMDA) receptor. 11 , 12 , 13 Activation of the NMDA receptor is thought to be a major contributor to the wind‐up phenomenon, which leads to central sensitization. 14 , 15 , 16 , 17 Central sensitization can develop symptoms like spontaneous pain, allodynia, and hyperalgesia to nociceptive stimuli. 15 , 16 , 17 These symptoms can be reduced when the wind‐up phenomenon and central sensitization are blocked by S‐ketamine administration. 14 , 15 , 16 , 17
What is known about the clinical effects of S‐ketamine administration in CRPS is that the analgesic effects have not only been observed during administration, but also seem to last for a period of time after the administration. 18 , 19 A meta‐analysis by Zhao et al. 19 showed that intravenous S‐ketamine therapy can provide clinically effective pain relief in short term for less than 3 months. Zhao and colleagues 19 used a 30% pain relief event rate to evaluate efficacy of S‐ketamine in CRPS and found an effect of 69% immediately after S‐ketamine treatment and an effect of 58% at the 1–3 months of follow‐ups.
In our experience, the effectiveness of S‐ketamine therapy in our patients with CRPS is not as great or as long lasting as described in the meta‐analysis. However, in our infusion protocol, the S‐ketamine dose is relatively lower than the dose used by most studies included in the meta‐analysis by Zhao et al. 19 This could be a reason for the discrepancy between our clinical results and the findings in the meta‐analysis. Therefore, we conducted this retrospective cohort study in which we aimed to assess the effectiveness of our lower dose intravenous S‐ketamine treatment for pain intensity in our patients with CRPS.
METHODS
Ethical approval
Ethical approval for this study was obtained from Medical Ethics Committee of Erasmus University Medical Center Rotterdam (MEC‐2019‐0209).
Patients
We searched the electronic medical record database for patients with CRPS who received a low‐dose intravenous S‐ketamine treatment in the period between March 2010 and April 2019 at the Center for Pain Medicine (CPM) at Erasmus MC University Medical Center. Only patients aged 18 years or older during the time of treatment were included in this study. Of patients who had multiple S‐ketamine treatments during the study period, only data from the first treatment were included in this study. At the CPM, CRPS is diagnosed using the IASP clinical diagnostic criteria for CRPS. 5 Patients with CRPS who were treated with S‐ketamine in an emergency setting, and thus not in an elective setting, were excluded. Furthermore, patients who were admitted to the hospital for combination treatments of S‐ketamine with another drug were also excluded from this study.
Treatment with S‐ketamine
S‐ketamine is administered intravenously in patients with CRPS according to the protocol which was derived from the study by Sigtermans et al. 18 Although a maximum dose of 0.43 mg/kg/h is described in the literature, 18 we maintain a maximum dose of 14 mg/h (0.2 mg/kg/h normalized to a 70 kg patient) on our ward.
A synopsis of our protocol is the total inpatient treatment duration is 7 days. S‐ketamine is started at 3 mg/h. The dose is increased twice a day (usually in the late morning and late afternoon) with steps of 1–2 mg/h. The increase depends on whether the patient experiences pain reduction or side effects. Once the patient notices a reduction in pain, the S‐ketamine dose is not increased further for the duration of admission, we call this the “effective dose.” In case the patient experiences severe side effects, the dose is reduced until the side effects disappear. If the side effects reduce, the S‐ketamine dose is increased again and effect and side effects are closely monitored.
Measurements, data collection, and management
Data for this study were extracted from electronic medical records. Data were obtained at baseline before treatment (T0), at discharge from the hospital (T1), and at the first follow‐up outpatient appointment (T2), which was at a variable time interval, depending on individual appointments of patients in the outpatient clinic. The primary outcome parameters were pain scores (Numeric Rating Scale [NRS]) at T0, T1, and T2.
Patients were categorized as either a responder or nonresponder to S‐ketamine at T1 and T2. Patients were considered a responder if there was a reduction in their pain score of greater than or equal to 2 points from baseline 20 and/or if treatment was reported as successful in the electronic medical record. In case the patients met neither of these 2 conditions, they were categorized as nonresponders.
The following data were also extracted: maximum dose, “effective dose,” side effects, and the day the patient first noticed treatment effect.
Statistical analysis
Descriptive statistics were used to determine the frequency distributions of the variables and to describe measures of central tendency and variability of the continuous parameters dependent on the shape of their distribution. The Shapiro‐Wilk test was used to analyze whether or not continuous parameters were normally distributed. A Related‐Samples Friedman’s Two‐Way analysis of variance by ranks was used for finding a difference in the skewedly distributed pain scores across the 3 moments of measurement. The Wilcoxon signed‐rank was used to assess the difference between these scores before and after treatment and before treatment and at first follow‐up. The McNemar test was performed to compare paired proportions of the non‐responders at T1 and T2. No correction for multiple testing was made. The alpha level for statistical significance was set at the traditional level of 0.05. The study was analyzed using a per‐protocol analysis. Data analysis was performed using IBM SPSS statistics for Windows, version 25.0.
RESULTS
Patients
Data from 60 patients were identified and extracted from electronic medical records. A total of 12 patients were excluded from the study because of 2 reasons. First, 9 patients were admitted to the hospital for a combination treatment of S‐ketamine with another drug. These other drugs were mannitol, tadalafil, or pamidronic acid. Second, 3 patients received S‐ketamine in an emergency setting. Thus, a total of 48 patients were included in this study (Figure 1). Patients’ baseline characteristics are shown in Table 1.
FIGURE 1.
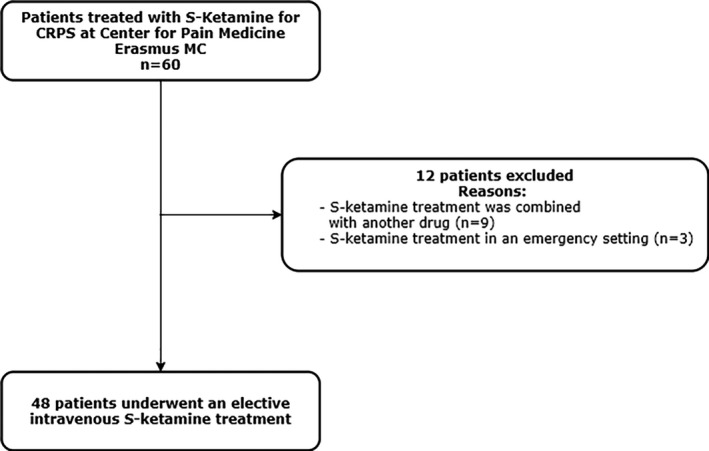
Flowchart of patient selection. MC, Medical Center; CRPS, Complex Regional Pain Syndrome
TABLE 1.
Patients’ baseline characteristics
| n = 48 | |
|---|---|
| Age (years) | 39 (SD ± 14) |
| Gender | |
| Male | 7 (15%) |
| Female | 41 (85%) |
| Duration of diagnosis (years) | |
| Median (IQR) | 5 (6) |
| NRS before treatment | |
| Median (IQR) | 8 (2) |
| Medication at start treatment | |
| Paracetamol | 17 (35%) |
| NSAIDs | 11 (23%) |
| Opioids | 30 (63%) |
| Antidepressants | 12 (25%) |
| Anti‐epileptics | 16 (33%) |
Abbreviations: IQR, interquartile range; NSAIDs, non‐steroidal anti‐inflammatory drugs; NRS, Numeric Rating Scale; SD, standard deviation.
S‐ketamine treatment
Characteristics of the S‐ketamine treatment are depicted in Table 2. The median time interval between discharge (T1) and the first follow‐up appointment (T2) lasted 4 weeks (interquartile range [IQR] = 5).
TABLE 2.
Characteristics of S‐ketamine treatment
| Duration of treatment (days) | 6 (SD ± 2) |
| Maximum dose (mg/h) | 7 (SD ± 3) |
| Side effects | n = 30 (63%) |
| Headache | 6 (20%) |
| Drowsiness | 11 (37%) |
| Dizziness | 14 (47%) |
| Nausea | 8 (27%) |
| Hallucinations | 0 (0%) |
Abbreviations: mg, milligram; h, hour.
Pain scores
At T0, the pain score was available of 42 of the 48 patients and their median NRS before treatment was 8 (IQR = 2). At T1, the pain score of 36 patients was available and their median NRS at discharge was 6 (IQR = 4). At T2, the pain score of 18 patients was available and their median pain score was 7 (IQR = 3).
Thus, the median NRS score changed from 8 (IQR = 2) before treatment to 6 (IQR = 4) at discharge and 7 (IQR = 3) at first follow‐up, (p < 0.001); see Figure 2. Pairwise comparison yielded a significant difference between the scores before treatment and both those at discharge (p < 0.001) and those at first follow‐up (p = 0.015).
FIGURE 2.
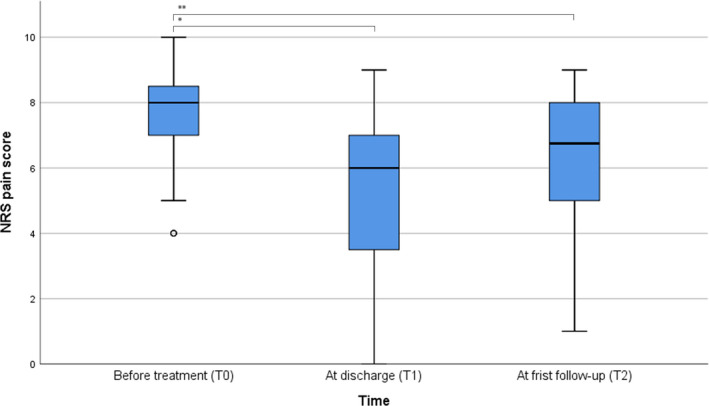
Pain scores of patients with CRPS before start of the S‐ketamine infusion (T0, n = 42), at discharge of the treatment (T1, n = 36), and at first follow‐up visit to the clinic after treatment (T2, n = 18) NRS, Numeric Rating Scale. *p < 0.001, **p = 0.015
Responder versus nonresponder
At T1, data of 47 of the 48 patients were available for classification as responder or nonresponder as of 1 patient, the response to S‐ketamine treatment was unknown. After S‐ketamine infusion at discharge from the hospital (T1), 29 (62%) of 47 patients were classified as responders, and 18 (38%) of 47 patients as nonresponders. At the first follow‐up appointment (T2), data were available for 40 of the 48 patients. At T2, 19 (48%) of these 40 patients were responders, all of them were also responders at T1. At T2, 21 (53%) of the 40 patients were classified as nonresponders. None of the patients who were originally nonresponders to S‐ketamine treatment at T1 became responders at T2. Six of the 40 patients (15%) turned from a responder at T1 into a nonresponder at T2. This implies that a statistically significant proportion of responders at T1 turned into nonresponders at T2 (p = 0.03; see Table 3).
TABLE 3.
(Non) T2 (n [% of total])
| T2 | Total | ||
|---|---|---|---|
| Responder | Nonresponder | ||
| T1 | |||
| Responder | 19 (48) | 6 (15) | 25 (63) |
| Nonresponder | 0 (0) | 15 (38) | 15 (38) |
| Total | 19 (48) | 21 (53) | 40 (100) |
For responders at T1, the median NRS before treatment was 8 (IQR = 1) and at discharge 5 (IQR = 4). Pairwise comparison yielded a significant difference between the scores before treatment and at discharge (p < 0.001). At follow‐up (T2), in 9 of the 19 responders, the NRS was available and for these patients the median NRS score was 6 (IQR = 3). Unfortunately, we encountered too many missing NRS scores at follow‐up to statistically compare these scores with baseline and discharge.
For nonresponders at T1, the median NRS before treatment was 8 (IQR = 2) and at discharge 8 (IQR = 1). Pairwise comparison yielded no significant difference between the scores before treatment and at discharge (p = 0.125).
Responders at T1 started to observe a treatment effect at a median of 2 (IQR = 1) days after starting treatment. The median effective dose of these responders was 6 (IQR = 4) mg/h S‐ketamine.
Regarding demographic parameters, no statistically significant differences were found between responders and nonresponders after S‐ketamine infusion.
DISCUSSION
This retrospective cohort study included patients with CRPS with therapy‐resistant pain who were treated with a low‐dose 7‐day intravenous S‐ketamine infusion. It showed a statistically significant reduction in median pain scores between admission (T0) and discharge (T1) and between admission and follow‐up (T2) at a median of 4 weeks. At T1, 62% of the patients were responders. For a significant proportion of patients, from whom data were available at both T1 and T2, 48% still were a responder at T2.
As mentioned in the introduction, the S‐ketamine dose in our infusion protocol is relatively lower than the dose used by most studies included in the meta‐analysis by Zhao et al. 19 This was one of the reasons why our research group believed that the effect of our S‐ketamine infusions was not as large or as long‐lasting as described by Zhao et al. 19 Zhao and colleagues reported an effective treatment by using a 30% pain relief event rate in 69% of the patients immediately after S‐ketamine treatment and in 58% of the patients at the 1–3 month’s follow‐up. 19 Despite our low‐dose regimen, the results of our study still show that about half of our patients benefit from low‐dose S‐ketamine administration for up to 4 weeks on average (T2). This is in line with the 58% of patients with an effective treatment effect at the 1–3 month’s follow‐up described by Zhao et al. 19 However, most studies included in the meta‐analysis do not have a retrospective design and are therefore methodologically not comparable to our study.
For the responders to S‐ketamine treatment, we did a post hoc analysis on pain relief beyond T2. Data on duration of pain reduction were available from 12 of 19 patients who were responders at both T1 and T2. The median duration of their treatment effect was 9 weeks (IQR = 7) and 1 patient even reported a duration effect of 27 weeks. Despite the low number of patients, the treatment effect of 9 weeks is roughly in line with the reported pain relief of 11 weeks by Sigtermans and colleagues. 18 In addition, our results fit in the range of 1–3 months effective pain relief after S‐ketamine infusion reported by the meta‐analysis by Zhao et al. 19
Interestingly, we found that a considerable proportion of responders at T1 turned into nonresponders at T2. For these patients, continued treatment might be necessary and therefore follow‐up therapy with an oral NMDA‐receptor antagonist might be proposed.
Furthermore, it is notable that responders noticed a positive treatment effect within 2 days and that the effective dose—median of 6 mg/h—was relatively low. These findings raise the following questions: (1) whether long hospital admissions are necessary and (2) whether relatively high dosing greater than 14 mg/h is required. First, as an alternative to lengthy inpatient hospital admissions, outpatient day care treatments with multiple clinic visits could be proposed. 21 , 22 , 23 Second, although the current study suggests that an S‐ketamine dose less than 14 mg/h might be sufficient to achieve pain relief, Sigtermans et al. and the consensus guidelines of the United States recommend a higher dose of ~ 22 mg/h for patients with CRPS. 18 , 21 Altogether, there is no sufficient evidence yet to define a “gold standard” protocol.
This study has several limitations. First and foremost, is the retrospective design of this cohort study. We encountered missing data and the outcome assessment was not standardized. Unfortunately, we encountered too much missing data to draw valid conclusions after the 4‐week post‐treatment period. Prospective studies are needed that study the duration of pain relief and other factors, such as return to work, pain medication reduction, and the need of repeating the intravenous S‐ketamine treatments. 21 , 24 Furthermore, our patients were treated in a tertiary referral hospital, were suffering from CRPS for several years, and were therapy‐refractory to several other treatments. Therefore, these results might not be generalizable to patients who are newly diagnosed with CRPS. Another limitation is that we cannot compare our intravenous low‐dose regimen with relatively high‐dose regimens recommended by, for instance, Xu and colleagues. 25 Finally, the focus on pain relief is a common limitation in pain research and deserves more attention. Not only is pain perception a subjective outcome parameter, it is also heavily influenced by multiple interrelated biological, psychological, and social factors. 26 For future research, it would be advisable to obtain objective parameters, such as quantitative sensory testing 27 , 28 and the emerging diagnostic and prognostic biomarkers of small noncoding RNAs (microRNAs) 29 alongside additional subjective parameters, like the quality of life and the measurement of the global perceived effect of the treatment. 30 , 31
In the search for optimal pain management, Woolf et al. 32 advocated as early as 1999 for a shift in pain medicine from a symptom‐based treatment toward a mechanism‐based treatment. For CRPS treatment, it is currently advised to target the underlying pathophysiological mechanisms deemed most prominent during history taking and physical examination. 33 , 34 However, in clinical practice, S‐ketamine is still prescribed for therapy‐resistant pain without thorough consideration of the pathophysiological mechanism causing the pain. In addition, awareness of the mechanisms underlying pain reduction by S‐ketamine need to be explored because they can be used to predict response on the treatment. If more insights on these mechanisms were to be revealed, S‐ketamine might be prescribed in a more mechanism‐based and individually tailored manner.
Various mechanisms of action have been proposed for the therapeutic effects of ketamine. The most prominent rationale is the antagonism of the NMDA receptor causing a weakening of central neuroplastic changes like the wind‐up phenomenon and central sensitization. 14 , 15 , 35 However, effects of ketamine on CRPS might not be restricted to only its antagonistic actions on the NMDA receptor. For instance, Sorel et al. 36 showed that the analgesic effect of ketamine in CRPS is related to changes in GABA/glutamate balance in the cerebral cortex. Furthermore, ketamine also has immunomodulatory and anti‐inflammatory properties. 37 , 38 Therefore, ketamine might modulate inflammation and interact with the closely linked immune and nervous system in the pathophysiology of CRPS. Finally, ketamine has been prescribed for its antidepressant properties. 39 , 40
Further research is needed in order to determine the underlying mechanism(s) for which it is beneficial to give S‐ketamine to patients with CRPS and to predict responsiveness to S‐ketamine treatment. Given the exploratory nature of this retrospective study, we did not consider it achievable to look for predictors of response to S‐ketamine. In search of predictors, Birklein et al. and our research group previously mentioned the potential use of systemic microRNAs as a biomarker in the diagnosis and management of CRPS. 29 , 41 These small noncoding RNA molecules can negatively regulate gene expression and their dysregulation is revealed in several diseases. 42 , 43 For patients with CRPS, it seems that microRNA profiling could be used as a prognostic biomarker to identify responders to treatment with ketamine. 44 Besides microRNAs, there are other potential biomarkers, however, results are preliminary and more research is needed for their validation. 41
Besides intravenous administration of S‐ketamine, other routes of administration have to be studied for future recommendations. With regard to iontophoresis, Vranken et al. 45 administered ketamine via iontophoresis to patients with intractable central pain, but this did not result in pain reduction after 1 week. For treatment of chronic neuropathic pain states with topical ketamine, studies show conflicting results. 46 In a small double‐blind placebo‐controlled trial, Finch et al. 47 reported that topical ketamine does not result in pain relief in patients with CRPS, but does cause a reduction in allodynia. Furthermore, promising results are available for sublingual ketamine in depression and should be studied further for CRPS. 48 , 49 , 50
In addition, we would also like to highlight the responsibility to reduce health care costs and acknowledge that a 7‐day inpatient S‐ketamine infusion has considerable economic implications. Ideally, intravenous S‐ketamine therapy should result in long‐term pain relief and, for instance, decrease opioid consumption, improve functionality, and help patients return to work. Unfortunately, no economic studies have been conducted to determine the cost‐effectiveness for treating CRPS with S‐ketamine and these studies are warranted in the future.
CONCLUSION
This study showed that our low‐dose intravenous S‐ketamine treatment resulted in effective pain relief for a subgroup of patients with CRPS with refractory pain. At ~ 4 weeks after infusion, almost half of the patients were still responders. It is notable that responders to low‐dose intravenous S‐ketamine treatment started to observe treatment effect within 2 days and that the median effective S‐ketamine dose was 6 mg/h. More research is needed to understand which pathophysiological mechanisms are targeted by S‐ketamine infusion in order to predict treatment responders.
CONFLICT OF INTEREST
None of the authors have any conflict of interest.
Mangnus TJP, Dirckx M, Bharwani KD, de Vos CC, Frankema SPG, Stronks DL, et al. Effect of intravenous low‐dose S‐ketamine on pain in patients with Complex Regional Pain Syndrome: A retrospective cohort study. Pain Pract. 2021;21:890–897. 10.1111/papr.13056
REFERENCES
- 1. Harden RN, Bruehl S, Stanton‐Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–31. [DOI] [PubMed] [Google Scholar]
- 2. Veldman PHJM, Reynen HM, Arntz IE, Goris RJA. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–6. [DOI] [PubMed] [Google Scholar]
- 3. Sandroni P, Benrud‐Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population‐based study. Pain. 2003;103:199–207. [DOI] [PubMed] [Google Scholar]
- 4. de Mos M, De Bruijn AGJ, Huygen F, Dieleman JP, Stricker BHC, Sturkenboom M. The incidence of complex regional pain syndrome: a population‐based study. Pain. 2007;129:12–20. [DOI] [PubMed] [Google Scholar]
- 5. Harden RN, Bruehl S, Perez RSGM, Birklein F, Marinus J, Maihofner C, et al. Development of a severity score for CRPS. Pain. 2010;151:870–6. [DOI] [PubMed] [Google Scholar]
- 6. de Boer RDH, Marinus J, van Hilten JJ, Huygen FJ, van Eijs F, van Kleef M, et al. Distribution of signs and symptoms of complex regional pain syndrome type I in patients meeting the diagnostic criteria of the International Association for the Study of Pain. Eur J Pain. 2011;15(830):830.e831–838. [DOI] [PubMed] [Google Scholar]
- 7. Perez RS, Zollinger PE, Dijkstra PU, Thomassen‐Hilgersom IL, Zuurmond WW, Rosenbrand KCJ, et al. Evidence based guidelines for complex regional pain syndrome type 1. BMC Neurol. 2010;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Domino EF, Warner DS. Taming the ketamine tiger. Anesthesiology. 2010;113:678–84. [DOI] [PubMed] [Google Scholar]
- 9. Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60:341–8. [DOI] [PubMed] [Google Scholar]
- 11. Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N‐methyl‐aspartate. Br J Pharmacol. 1983;79:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomson AM, West DC, Lodge D. An N‐methylaspartate receptor‐mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–81. [DOI] [PubMed] [Google Scholar]
- 13. Fisher K, Coderre TJ, Hagen NA. Targeting the N‐methyl‐D‐aspartate receptor for chronic pain management: preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–73. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Maher DP, Cohen SP. Emerging concepts on the use of ketamine for chronic pain. Expert Rev Clin Pharmacol. 2020;13:135–46. [DOI] [PubMed] [Google Scholar]
- 15. Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N‐methyl‐D‐aspartic acid receptor activation; implications for the treatment of post‐injury pain hypersensitivity states. Pain. 1991;44:293–9. [DOI] [PubMed] [Google Scholar]
- 16. Davies SN, Lodge D. Evidence for involvement of N‐methylaspartate receptors in ‘wind‐up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–6. [DOI] [PubMed] [Google Scholar]
- 17. Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–8. [DOI] [PubMed] [Google Scholar]
- 18. Sigtermans MJ, Van Hilten JJ, Bauer MCR, Arbous MS, Marinus J, Sarton EY, et al. Ketamine produces effective and long‐term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–11. [DOI] [PubMed] [Google Scholar]
- 19. Zhao J, Wang Y, Wang D. The effect of ketamine infusion in the treatment of complex regional pain syndrome: a systemic review and meta‐analysis. Curr Pain Headache Rep. 2018;22:12. [DOI] [PubMed] [Google Scholar]
- 20. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain. 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 21. Cohen SP, Bhatia A, Buvanendran A, Schwenk ES, Wasan AD, Hurley RW, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional anesthesia and pain medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43:521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double‐blind placebo controlled study. Pain. 2009;147:107–15. [DOI] [PubMed] [Google Scholar]
- 23. Maher DP, Chen L, Mao J. Intravenous ketamine infusions for neuropathic pain management: a promising therapy in need of optimization. Anest Analg. 2017;124:661–74. [DOI] [PubMed] [Google Scholar]
- 24. Grieve S, Perez RSGM, Birklein F, Brunner F, Bruehl S, Harden N, et al. Recommendations for a first Core Outcome Measurement set for complex regional PAin syndrome Clinical sTudies (COMPACT). Pain. 2017;158:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu J, Herndon C, Anderson S, Getson P, Foorsov V, Harbut RE, et al. Intravenous ketamine infusion for complex regional pain syndrome: survey, consensus, and a reference protocol. Pain Med. 2019;20:323–34. [DOI] [PubMed] [Google Scholar]
- 26. Miller ET, Abu‐Alhaija DM. Cultural influences on pain perception and management. Pain Manag Nursing. 2019;20:183–4. [DOI] [PubMed] [Google Scholar]
- 27. Kirkpatrick AF, Saghafi A, Yang K, Qiu P, Alexander J, Bavry E, et al. Optimizing the treatment of CRPS with ketamine. Clin J Pain. 2020;36:516–23. [DOI] [PubMed] [Google Scholar]
- 28. Bosma RL, Cheng JC, Rogachov A, Kim JA, Hemington KS, Osborne NR, et al. Brain dynamics and temporal summation of pain predicts neuropathic pain relief from ketamine infusion. Anesthesiology. 2018;129:1015–24. [DOI] [PubMed] [Google Scholar]
- 29. Birklein F, Ajit SK, Goebel A, Perez RSGM, Sommer C. Complex regional pain syndrome—phenotypic characteristics and potential biomarkers. Nat Rev Neurol. 2018;14:272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hudak PL, Wright JG. The characteristics of patient satisfaction measures. Spine. 2000;25:3167–77. [DOI] [PubMed] [Google Scholar]
- 31. Group TE . EuroQol‐a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 32. Woolf CJ, Decosterd I. Implications of recent advances in the understanding of pain pathophysiology for the assessment of pain in patients. Pain. 1999;82:S141–7. [DOI] [PubMed] [Google Scholar]
- 33. Gierthmühlen J, Binder A, Baron R. Mechanism‐based treatment in complex regional pain syndromes. Nat Rev Neurol. 2014;10:518–28. [DOI] [PubMed] [Google Scholar]
- 34. Bharwani KD, Dirckx M, Huygen FJPM. Complex regional pain syndrome: diagnosis and treatment. BJA Educ. 2017;17(8):262–68. [Google Scholar]
- 35. Schwartzman RJ, Alexander GM, Grothusen JR. The use of ketamine in complex regional pain syndrome: possible mechanisms. Expert Rev Neurother. 2011;11:719–34. [DOI] [PubMed] [Google Scholar]
- 36. Sorel M, Zrek N, Locko B, Armessen C, Ayache SS, Lefaucheur J‐P. A reappraisal of the mechanisms of action of ketamine to treat complex regional pain syndrome in the light of cortical excitability changes. Clin Neurophysiol. 2018;129:990–1000. [DOI] [PubMed] [Google Scholar]
- 37. De Kock M, Loix S, Lavand'homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loix S, De Kock M, Henin P. The anti‐inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62:47–58. [PubMed] [Google Scholar]
- 39. Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, et al. What is the mechanism of Ketamine's rapid‐onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther. 2017;42:147–54. [DOI] [PubMed] [Google Scholar]
- 40. Pochwat B, Nowak G, Szewczyk B. An update on NMDA antagonists in depression. Expert Rev Neurother. 2019;19:1055–67. [DOI] [PubMed] [Google Scholar]
- 41. Bharwani KD, Dik WA, Dirckx M, Huygen FJPM. Highlighting the role of biomarkers of inflammation in the diagnosis and management of complex regional pain syndrome. Mol Diagn Ther. 2019;23:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Douglas SR, Shenoda BB, Qureshi RA, Sacan A, Alexander GM, Perreault M, et al. Analgesic response to intravenous ketamine is linked to a circulating microRNA signature in female patients with complex regional pain syndrome. J Pain. 2015;16:814–24. [DOI] [PubMed] [Google Scholar]
- 45. Vranken JH, Dijkgraaf MGW, Kruis MR, Van Dasselaar NT, Van der Vegt MH. Iontophoretic administration of S (+)‐ketamine in patients with intractable central pain: a placebo‐controlled trial. Pain. 2005;118:224–31. [DOI] [PubMed] [Google Scholar]
- 46. Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: a double‐blind placebo‐controlled trial of topical ketamine. Pain. 2009;146:18–25. [DOI] [PubMed] [Google Scholar]
- 48. Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. 2013;16:2111–7. [DOI] [PubMed] [Google Scholar]
- 49. Nguyen L, Marshalek PJ, Weaver CB, Cramer KJ, Pollard SE, Matsumoto RR. Off‐label use of transmucosal ketamine as a rapid‐acting antidepressant: a retrospective chart review. Neuropsychiatr Dis Treat. 2015;11:2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swainson J, Khullar A. Sublingual ketamine: an option for increasing accessibility of ketamine treatments for depression? J Clin Psychiatry. 2020;81(1):19. [DOI] [PubMed] [Google Scholar]


