Abstract
Background and aims
Opioid agonist treatment is effective but resource intensive to administer safely in custodial settings, leading to significant under‐treatment of opioid dependence in these settings world‐wide. This study assessed the safety of subcutaneous slow‐release depot buprenorphine in custody.
Design
Open‐label, non‐randomized trial.
Setting
Correctional centres in New South Wales, Australia.
Participants
Sixty‐seven men and women, aged ≥ 18 years of various security classifications with a diagnosis of moderate to severe DSM‐5 opioid use disorder currently serving a custodial sentence of ≥ 6 months were recruited between November 2018 and July 2019. Patients not in opioid agonist treatment at recruitment commenced depot buprenorphine; patients already stable on oral methadone treatment were recruited to the comparison arm.
Intervention and comparator
Depot buprenorphine (CAM2038 weekly for 4 weeks then monthly) and daily oral methadone.
Measurements
Safety was assessed by adverse event (AE) monitoring and physical examinations at every visit. Participants were administered a survey assessing self‐reported diversion and substance use at baseline and weeks 4 and 16.
Findings
Retention in depot buprenorphine treatment was 92.3%. Ninety‐four per cent of patients reported at least one adverse event, typically mild and transient. No diversion was identified. The prevalence of self‐reported non‐prescribed opioid use among depot buprenorphine patients decreased significantly between baseline (97%) and week 16 (12%, odds ratio = 0.0035, 95% confidence interval = 0.0007–0.018, P < 0.0001).
Conclusions
This first study of depot buprenorphine in custodial settings showed treatment retention and outcomes comparable to those observed in community settings and for other opioid agonist treatment used in custodial settings, without increased risk of diversion.
Keywords: Buprenorphine, CAM2038, depot, opioid use disorder, prisoners, prisons, safety, slow‐release, subcutaneous
Introduction
People in custody have complex health issues with high levels of comorbidities, including opioid use disorder (OUD) [1]. Injecting drug use and needle and syringe‐sharing during incarceration has been documented globally [2] and are key drivers of HIV, hepatitis C virus (HCV) and other infections in custody [3]; OUD is also associated with elevated risk of mortality following release from custody [4].
There is a substantial body of evidence for the effectiveness of opiate agonist treatment (OAT) with methadone or buprenorphine in custody, including reduced drug use [5], reduced mortality in prison [6] and post‐release [7], increased treatment engagement post‐release [5] and evidence of cost‐effectiveness in custodial settings [8, 9, 10]. Despite these benefits, there remains significant under‐treatment of patients with OUD and suboptimal provision of OAT in custodial settings world‐wide [11, 12].
A key reason for under‐treatment is concern regarding diversion of opioid medications within the prison population [13], requiring more intensive supervision than in community settings. This is a particular concern with sublingual buprenorphine, with diversion the most frequent reason for treatment discontinuation in a randomized controlled trial (RCT) undertaken in prisons in Maryland, USA [14]. Buprenorphine diversion has also been reported in prisons in the United Kingdom [15] and Australia [16].
A potential solution is the introduction of long‐acting injectable depot buprenorphine products administered subcutaneously, weekly or monthly, that have been shown to be safe and efficacious in community settings [17, 18, 19]. Depot medications should ensure limited diversion of opioid medications in custodial settings, and allow greater efficiencies in treatment provision given the need for less frequent dosing [20].
The study reported here is the first, to our knowledge, to examine the safety of a new depot buprenorphine formulation, CAM2038, in custodial settings. The primary objective was to identify any unexpected safety and tolerability considerations of depot buprenorphine in adults in custody. Diversion, substance use and treatment retention were assessed as secondary outcomes.
Methods
Design and setting
This 16‐week non‐randomized open‐label trial assessed a buprenorphine regimen consisting of two depot buprenorphine formulations—CAM2038 q1w (once weekly) and q4w (once monthly) (see details in Supporting information, Appendix S1). The comparator group—patients already stable on oral methadone—reflected ‘business as usual’, with outcomes reported to provide context. Given concerns regarding sublingual buprenorphine diversion and associated violence (i.e. threats, coercion or intimidation to divert doses to others) reported by people with a history of incarceration who were consulted during the planning phase of the study, a gold‐standard RCT design was deemed unethical, as this would have required increased treatment with sublingual buprenorphine to conduct such a trial, therefore exposing more people to diversion‐related harms in this setting. Justice Health and Forensic Mental Health Network (JHFMHN) OAT policy reflected this, which restricted the use of sublingual buprenorphine except where methadone was clinically contraindicated, with limited patient numbers further precluding the feasibility of a randomized design. Between November 2018 and November 2019, patients were recruited and followed‐up in seven correctional centres across six metropolitan and rural areas of NSW, with a mix of adult men and women with a range of security classifications.
Participants
Adults in custody (aged ≥ 18 years) with a full‐time sentence of at least 6 months before earliest release date and a diagnosis of moderate to severe OUD (DSM‐5) [21] were eligible for the study. Patients not in OAT on entry to custody were eligible for the depot buprenorphine arm of the trial (including people who used illicit sublingual buprenorphine–naloxone), while those being treated with oral methadone were eligible for the comparison arm. Currently incarcerated, potentially eligible patients were identified by JHFMHN staff and invited to participate. While this approach relied upon patients reporting a history of OUD and/or illicit sublingual buprenorphine–naloxone to JHFMHN staff, as health‐care provision was distinct from provision of custodial services in NSW, the potential limitation of this approach (e.g. reluctance to disclose substance use) was considered minimal. Exclusion criteria included any known hypersensitivity to methadone or buprenorphine, serious untreated psychiatric comorbidity and exposure to a new investigational drug or device within the last 30 days. Women were screened for pregnancy but were not required to use contraception, given that the risk of pregnancy while in custody is low. Other exclusion criteria included: clinical contraindications to partial/full opioid agonist treatment, current severe medical condition (e.g. hepatic failure or respiratory insufficiency), recent history of suicidal ideation or behaviour, forensic mental health patient not guilty by reason of mental illness, clinically significant laboratory abnormalities, history of Torsades de Pointes, an electrocardiogram demonstrating a clinically significant abnormality (e.g. liver function tests), prescription of strong inhibitors or inducers of CYP 3A4, currently breastfeeding or pregnancy, incarceration during parole and/or enrolment in a drug free or intensive treatment programme (for men).
Study treatment procedures
Depot buprenorphine was initiated according to current Australian guidelines [22]. In brief, after an initial 4‐mg test dose of sublingual buprenorphine–naloxone, participants commenced depot buprenorphine with four once‐weekly injections followed by three once‐monthly injections administered (see details of regimen in Supporting information, Appendix SI).
Outcome measures and assessments
The primary objective was to identify any safety and tolerability considerations of CAM2038 specific to the adult custodial population. Safety was assessed by adverse event (AE) and serious AE (SAE) monitoring, injection‐site examinations and physical examinations. AEs were documented by study medical officers throughout the study until 28 days following the final CAM2038 injection and followed until resolution or trial completion. Treatment emergent adverse events (TEAE) were defined as any event, irrespective of relationship to study drug occurring after the first dose of depot buprenorphine. AEs were coded after database lock by primary system organ class and preferred term, according to the Medical Dictionary for Regulatory Activities, version 18.0. AE study drug relatedness and severity (graded as mild, moderate or severe) was determined by the site delegated clinician (medical officer or nurse practitioner). Injection‐site examinations were performed during each scheduled visit, assessing for injection reactions including: erythema, swelling, pruritus and pain. Participants were also asked about local injection‐site reactions.
Face‐to‐face surveys were administered by trained, external independent researchers at baseline and weeks 4 and 16. At baseline, participants were asked whether they had ever heard about diversion‐associated violence (i.e. threats, coercion or intimidation to divert doses to others) in custodial settings and whether they personally had ever diverted (shared, exchanged or sold methadone or buprenorphine dose) while incarcerated. At weeks 4 and 16, participants were asked whether they had heard about recent diversion‐related violence, including whether they had attempted to remove their depot injection or divert their methadone dose since last interview. The JHFMHN ‘Incident Information Management System’ was reviewed for documented diversion incidents among depot buprenorphine patients during the study period. Non‐medical use of opioids and other substances was measured by self‐report at baseline and weeks 4 and 16 using a subset of the Australian Treatment Outcomes Profile [23].
Statistical methods
All analyses were performed using SAS version 9.4 [24]. Baseline data were presented by treatment group and summarized by means and standard deviations (SDs) where the data were continuous and as frequencies and percentages where the data were categorical.
The non‐randomized design and irreconcilable differences between groups that could not be statistically adjusted for prevented comparisons between treatment groups. Analysis followed the intention‐to‐treat principle. Within each treatment arm, any non‐medical substance use in the last 28 days (yes/no), including non‐prescribed opioids, methamphetamines and any injecting drug use, were treated as binary variables, and modelled using a logistic mixed effects regression model to determine the effects of time (baseline versus 4 weeks; baseline versus week 16). The model included fixed categorical effects for time and a random participant level intercept. Within‐group changes over time were presented as odds ratios (ORs) with 95% confidence intervals (CIs) and P‐values. Within‐group changes from baseline in the frequency of use were assessed using zero‐inflated negative binomial mixed‐effects regression models; conditional count ratios (CCRs) are presented with 95% CIs and P‐values.
A Kaplan–Meier curve presented days in depot buprenorphine treatment from first dose until 28 days post‐final monthly injection or 7 days post‐final weekly injection. Voluntary withdrawals (dropout due to reasons perceived to be related to study drug) were considered failures and involuntary withdrawals (dropout for reasons unrelated to study drug, e.g. administrative transfer to a non‐trial centre or unexpected release) were censored.
Ethical conduct and approval
The trial was approved by JHFMHN and the Aboriginal Health and Medical Research Council (AHMRC) Human Research Ethics Committees (Protocol JH File no. G561/17 and HREC/18/JH/3), as well as the CSNSW Ethics Committee. Participation was voluntary and written and informed consent was obtained from all participants prior to the conduct of any study procedure. Oversight was provided by a data safety monitoring board which met three times throughout the trial to assess progress, safety and efficacy end‐points. Participants were reimbursed for their time in after completing each scheduled researcher‐led questionnaire: $20 Australian dollars (AUD) for the screening and baseline visits, $40 AUD for the 1‐month follow‐up visit and $60 AUD for the week 16 visit, deposited into participants’ bank accounts.
Results
Treatment exposure
Between November 2018 and July 2019, 133 participants were screened for the trial and 129 were enrolled (67 into the depot buprenorphine arm and 62 into the methadone arm). Two patients screened into the depot buprenorphine arm withdrew before receiving a dose of CAM2038 and two patients in the methadone arm did not proceed to enrolment, as they were unable to comply with the requirements of the study (Fig. 1). All 67 patients in the depot buprenorphine arm received at least one 4‐mg test dose of sublingual buprenorphine–naloxone prior to first dose of depot buprenorphine. Of the three patients who required more than one sublingual dose, two received a second dose due to a delay in study drug supply while the third patient received a second dose as a precaution due to nausea and vomiting within 2 hours of first dose. There were no reports of sedation from sublingual BPN doses. During the 16‐week trial period, 259 weekly (16, 24 and 32 mg) and 233 monthly (64, 128 and 160 mg) depot buprenorphine injections were administered. In addition, 23 patients required at least one 8‐mg top‐up or ‘booster’ dose, with seven patients receiving two booster doses and one patient receiving three.
Figure 1.

Enrolment and study flow.
Participants
While similar proportions of depot buprenorphine and methadone patients were male (82 versus 85%, P = 0.602), Australian‐born (94 versus 94%, P = 0.910) and identified as Aboriginal and/or Torres Strait Islander (42 versus 34%, P = 0.354), depot buprenorphine patients were significantly younger [34 (SD = 7.5) versus 38 (SD = 8.9) years, P = 0.004] and were less likely to report not having completed year 10/secondary education (46 versus 68%, P = 0.014) (Table 1). Clinically, depot buprenorphine patients had a significantly lower body mass index (BMI) [29 (SD) = 5.6 versus 31 (SD = 7.7), P = 0.046], and while HCV antibody prevalence was similar between groups, depot buprenorphine patients were much more likely to be HCV RNA‐positive (33 versus 4%, P < 0.0001). Depot buprenorphine patients were significantly less likely to report previous OAT (70 versus 97%, P < 0.001) and had significantly higher visual analogue scale (VAS) opioid craving scores [53 (SD = 32.4) versus 18 (SD = 23), P < 0.001] at baseline.
Table 1.
Demographics and baseline clinical characteristics.
| Characteristic | Depot buprenorphine | Methadone | P‐value |
|---|---|---|---|
| n = 67 (%) | n = 62 (%) | ||
| Male (%) | 55 (82%) | 53 (85%) | 0·602 |
| Age, years, mean (SD) | 34 (7·5) | 38 (8·9) | 0·004 |
| Australian‐born (%) | 63 (94%) | 58 (94%) | 0·910 |
| Aboriginal and/or Torres Strait Islander (%) | 28 (42%) | 21 (34%) | 0·354 |
| Did not complete junior high school, year 10 (%) | 31 (46%) | 42 (68%) | 0·014 |
| BMI, kg/m2, mean (SD) | 29 (5·6) | 31 (7·7) | 0·046 |
| Anti‐HCV+ (%) | 49 (74%) | 52 (84%) | 0·182 |
| Of those anti‐HCV+, HCV RNA detected (%) | 16 (33%) | 2 (4%) | < 0·0001 |
| Anti‐HIV+ (%) | 0 (0%) | 1 (2%) | 0·297 |
| Previous OAT (%) | 70 | 97 | < 0·0001 |
| Mean number previous OAT episodes (SD) | 1·6 (1·9) | 2·4 (1·8) | 0·027 |
| Baseline methadone dose (mg), mean (SD) | NA | 92 (38·6) | – |
| Ever overdosed on opiates (%) | 25 (37%) | 32 (52%) | 0·102 |
| Subjective opioid withdrawal scale (SOWS), mean (SD) | 4 (5·5) | 3 (5·3) | 0·341 |
| Opioid craving (need‐to‐use VAS, mean (SD) | 53 (32·4) | 18 (23·0) | < 0·0001 |
SD = standard deviation; HCV = hepatitis C virus; OAT = opioid agonist therapy; VAS = visual analogue scale; NA = not available.
Safety
All but two depot buprenorphine patients reported at least one TEAE (65/67, 97%), most deemed related to study drug (63/67; 94%, Table 2); the majority were mild (88%) and resolved within one day while the remaining 12% were moderate. The most commonly reported drug‐related TEAEs were injection site pain, constipation, injection‐site swelling, headache, and injection site erythema (Table 3). Fifteen patients (22%) experienced injection site reactions (defined as ≥2 mild injection‐related or ≥1 moderate injection‐related AE); there were no severe injection related AEs. Of the four patients who discontinued treatment due to study drug‐related TEAEs, two ceased due to observed medical conditions (opioid‐related constipation and self‐reported non‐observed drowsiness) and two were due to self‐reported psychiatric and nervous system conditions (in both cases multiple contributing factors were identified including changes to and non‐compliance with psychotrophic medications and ongoing illicit drug use). One patient was also involuntarily withdrawn from treatment due to an SAE (acute HCV) determined to be unrelated to study drug. There were no deaths during the study.
Table 2.
Summary of treatment emergent adverse events in depot buprenorphine patients.
| Category | n = 67 (%) |
|---|---|
| ≥ 1 TEAE | 65 (97) |
| ≥ 1 drug‐related TEAE | 63 (94) |
| Injection site reaction (≥ 2 mild or ≥ 1 moderate injection‐related AEs) | 15 (22) |
| Non‐injection site AE | 56 (84) |
| ≥ 1 severe AE | 1 (2) |
| Deaths | 0 |
| ≥ 1 SAE | 2 (3) |
| Hospitalization | 2 (3) |
| ≥ 1 drug‐related SAE | 0 |
| Drug‐related AE leading to discontinuation | 4 (6) |
| Overdose | 0 |
TEAE = treatment emergent adverse event; AE = adverse event; SAE = serious adverse event.
Table 3.
Treatment emergent adverse events in > 10% of depot buprenorphine patients.
| Severity | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Injection site pain | 35 (52)* | 35 (52) | 1 (2) | 0 |
| Constipation | 34 (51)* | 26 (39) | 9 (13) | 0 |
| Injection site swelling a | 23 (34) | 22 (33) | 1 (2) | 0 |
| Headache | 19 (28)* | 16 (24) | 4 (6) | 0 |
| Injection site erythema | 15 (22) | 15 (22) | 0 | 0 |
| Nausea | 14 (21) | 13 (19) | 1 (2) | 0 |
| Vomiting | 13 (19) | 10 (15) | 3 (4) | 0 |
| Self‐reported sedation | 10 (15)* | 10 (15) | 1 (2) | 0 |
| Self‐reported urinary hesitancy | 10 (15) | 9 (13) | 1 (2) | 0 |
| Pruritus | 7 (10) | 7 (10) | 0 | 0 |
| Rash | 7 (10) | 6 (9) | 1 (2) | 0 |
Includes injection site mass and induration.
One participant experienced a mild and moderate adverse event (AE).
Two participants in the depot buprenorphine arm (2%) experienced SAEs, neither of which were related to study drug. One incident (impulsive act related to methamphetamine use) involved two severe AEs. The patient was treated in a hospital emergency department for bilateral neck lacerations, mild renal impairment and hypotension and was discharged the same day, and AEs had completely resolved within 72 hours. The other SAE (acute HCV) was moderate, with the acute rise in HCV RNA and hepatic injury deemed to be related to viral hepatitis rather than study drug. The patient reported injecting drug use and sharing injecting equipment 3 days after their first depot buprenorphine injection; they became acutely unwell 5 days after their second dose and was admitted to hospital for 7 days.
As a stable‐in‐treatment population, fewer patients on continuous methadone experienced at least one AE (45 of 62, 73%), with the majority mild (75%) or moderate (23%). Of the two SAEs, one (rhabdomyolysis) was severe with unknown aetiology, where the patient required treatment in hospital and was discharged after 3 days. The other SAE (overdose) was also deemed severe; this patient reported taking 900 mg non‐prescribed quetiapine requiring treatment in ED.
Diversion
At baseline, 78% of the overall sample reported having ever heard of threats, coercion or intimidation related to OAT diversion (84% depot buprenorphine versus 73% methadone arm) and 17% of the overall sample reported diverting methadone or sublingual buprenorphine–naloxone while incarcerated (19% depot buprenorphine versus 15% methadone arm). During follow‐up, small proportions (< 10%) reported having heard about recent intimidation or violence related to OAT diversion at each time‐point. No patient reported having attempted to remove their depot injection (e.g. by aspiration) or divert their methadone doses during the study. Self‐reports were consistent with routinely collected incident reporting system data, in which no documented depot buprenorphine diversion was identified during the trial period.
Substance use
The prevalence of non‐prescribed opioid use in the depot buprenorphine arm significantly decreased between baseline (97%) and week 4 (61%, OR = 0.048, 95% CI = 0.010–0.221, P = 0.0001) and week 16 (12%, OR = 0.0035, 95% CI = 0.0007–0.018, P < 0.0001) (Table 4). The frequency of non‐prescribed opioids also decreased significantly between baseline and week 4 [22.51 (SD = 9.67) to 5.41 (SD = 7.70) days, CCR = 0.35, 95% CI = 0.26–0.48, P < 0.001], but not between weeks 4 and 16 [2.14 (SD = 6.61) days, CCR = 0.67, 95% CI 0.39–1.17, P = 0.1568]. The prevalence of injecting drug use also significantly declined from 81% at baseline to 52% at week 4 (OR = 0.22, 95% CI = 0.09–0.53, P = 0.0008) and to 17% at week 16 (OR = 0.032, 95% CI = 0.012–0.087, P < 0.0001). Again, however, while the frequency of injecting fell significantly between baseline [17.55 (SD = 12.5)] days and week 4 [4.36 (SD = 7.14) days, CCR = 0.35, 95% CI = 0.24–0.50, P < 0.0001], the decline between weeks 4 and 16 [2.51 (SD) = 6.81 days, CCR = 0.61, 95% CI = 0.35–1.05, P = 0.0742] was not statistically significant. No statistically significant change in prevalence (25, 10%, 14%) or frequency (1, 3, 2 days) of methamphetamine use in the past 28 days between baseline and weeks 4 and 16, respectively was observed in the depot buprenorphine arm.
Table 4.
Within‐group changes in prevalence and frequency of past 28‐day drug use from baseline (odds ratio or conditional count ratio, 95% confidence intervals).
| Prevalence and frequency of use | Depot buprenorphine | Methadone | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depot buprenorphine (n = 67) | Methadone (n = 62) | 4 weeks | 16 weeks | 4 weeks | 16 weeks | |||||||||
| Baseline | 4 weeks | 16 weeks | Baseline | 4 weeks | 16 weeks | Estimate | P‐value | Estimate | P‐value | Estimate | P‐value | Estimate | P‐value | |
| Non‐prescribed opioids, n (%) | 65 (97.01%) | 41 (61.19%) | 8 (12.31%) | 1 (1.61%) | 1 (1.61%) | 0 (0%) | 0.048 (0.010, 0.221) | 0.0001 | 0.0035 (0.0007, 0.018) | < 0.0001 | ||||
| Any injecting drug use, n (%) | 54 (80.60%) | 35 (52.24%) | 11 (16.92%) | 4 (6.45%) | 4 (6.45%) | 3 (4.84%) | 0.22 (0.09, 0.53) | 0.0008 | 0.032 (0.012, 0.087) | < 0.0001 | 1.00 (0.22, 4.52) | 1.0000 | 0.74 (0.15, 3.70) | 0.7081 |
| Methamphetamine, n (%) | 17 (25.37%) | 7 (10.45%) | 9 (13.85%) | 5 (8.06%) | 4 (6.45%) | 3 (4.84%) | 0.33 (0.12, 0.91) | 0.0321 | 0.50 (0.19, 1.32) | 0.1633 | 0.77 (0.19, 3.19) | 0.7210 | 0.57 (0.12, 2.66) | 0.4760 |
| Mean days used non‐prescribed opioids (SD) | 22.51 (9.67) | 5.41 (7.70) | 2.14 (6.61) | 0.02 (0.13) | 0.02 (0.13) | 0.00 (0.00) | 0.35 (0.26, 0.48) | < 0.0001 | 0.67 (0.39, 1.17) | 0.1568 | ||||
| Mean days any injecting drug use (SD) | 17.55 (12.50) | 4.36 (7.14) | 2.51 (6.81) | 0.24 (1.07) | 0.11 (0.48) | 0.25 (1.36) | 0.35 (0.24, 0.50) | < 0.0001 | 0.61 (0.35, 1.05) | 0.0742 | 0.29 (0.05, 1.60) | 0.1530 | 1.43 (0.33, 6.15) | 0.6298 |
| Mean days used methamphetamine (SD) | 0.54 (1.13) | 0.48 (2.78) | 0.56 (1.67) | 0.29 (1.08) | 0.11 (0.48) | 0.23 (1.35) | 3.03 (0.78, 11.77) | 0.1077 | 3.46 (0.88, 13.52) | 0.0744 | 0.52 (0.07, 3.95) | 0.5225 | 2.74 (0.35, 21.66) | 0.3353 |
SD = standard deviation.
Among methadone patients, the prevalence of non‐prescribed opioids (0.3, 0.3, 0.0%), injecting drug use (1, 1, 1%) and methamphetamine use (1, 1, 1%) did not statistically change between baseline and weeks 4 and 16, respectively. Similarly, we did not find a statistically significant change in frequency of non‐prescribed opioid use or injecting drug use of methamphetamine use over time, both of which were used less than once in the past 28 days by methadone patients (Table 4).
Retention
In survival analysis, five participants were considered failures in depot buprenorphine treatment; four for treatment‐related reasons as described above, with the fifth withdrawing from treatment for transfer to a non‐trial site located closer to family. Seven participants were censored before 113 days, including six patients who were involuntarily withdrawn from treatment for reasons unrelated to the study drug [including for custodial services administrative transfer to non‐trial sites (n = 3) and unexpected release from custody (n = 2)], with the seventh involuntarily withdrawn due to the unrelated SAE. Treatment retention probability was 92.3% (Fig. 2).
Figure 2.
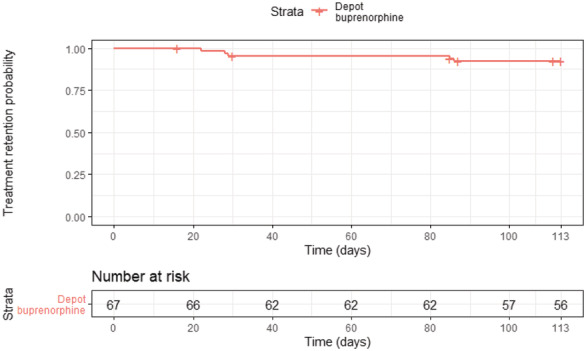
Kaplan–Meier estimates of injectable depot buprenorphine treatment retention.
Discussion
To the best of our knowledge, this is the first study internationally to demonstrate that depot buprenorphine preparations (CAM2038 administrated weekly and monthly) are safe and well tolerated for patients with moderate to severe OUD in custodial settings. Treatment retention was high, there were no treatment discontinuations related to injection site AEs and no reports of medication diversion. Consistent with previous community‐based studies of depot buprenorphine, most AEs were mild and resolved within 1 day, while observed SAEs, severe AEs, hospitalizations and discontinuations were uncommon and comparable to published studies of depot buprenorphine occurring in community settings [17, 18, 19].
At baseline, a majority (78%) of participants were familiar with intimidation or violence related to OAT diversion and just fewer than one‐fifth (17%) reported having ever diverted their OAT in custody. This finding is consistent with previous reports conducted in NSW, including a small (n = 60) community‐based survey of people with a history of incarceration which found that 25% reported diverting their OAT dose [16], and the most recent Network Patient Health Survey [25] which found that illicit methadone or buprenorphine were the most commonly reported injected drugs in prison. Diversion was also directly observed in an RCT of OAT in the United States [14]. With no reports of tampering or diversion during the current study, results suggest that depot buprenorphine may support the widespread delivery of OAT while protecting those most vulnerable from diversion [13].
A significant decline in self‐reported non‐prescribed opioid use and injecting drug use in the depot buprenorphine group was observed. Over time, the rate that depot buprenorphine patients reported substance use began to resemble that of the stable in‐treatment oral methadone patients. This finding is consistent with general population data indicating that many patients take several weeks or even months to stabilize in treatment and highlights the importance of providing access to treatment for those with OUD not already on treatment on entry to custody.
Reporting of mild AEs was common, with the majority of depot buprenorphine patients reporting at least one treatment‐related AE, considerably higher than community‐based studies which have routinely reported 10–20% of participants experiencing TEAEs [17, 18, 19]. This may have been driven by many factors, including the complexity of the patient population with high levels of comorbidity, as well as the regular monitoring required for the trial. Participants on depot buprenorphine were reviewed frequently post‐injection to assess safety and whether there were attempts of diversion. This may have increased opportunities for patient AE reporting, due to the often‐daily patient/clinician contact provided during study to participants.
Strengths of the current study were that it was conducted in multiple sites across a range of security settings, and successfully oversampled female as well as Aboriginal patients. Women in custody have particularly complex and often unmet health needs [26] and Aboriginal people are over‐represented in Australian opioid treatment [27] and custodial populations [26].
Limitations of the study included no matched control group, with patients who were stable on methadone used as a comparison group instead. Direct transfer from methadone to depot buprenorphine has not been formally reported and would have complicated enrolment procedures. We chose self‐report rather than urine toxicology to monitor drug use to address patient confidentiality concerns in custodial settings, noting that self‐reported drug use is generally valid, particularly when independent of treatment process or potential adverse consequences [28]. Custodial settings often employ urine drug screening to monitor drug use in prisons with sanctions (e.g. loss of visits or other punishments) when positive drug tests are returned, making it unlikely that a participant who is likely to yield a positive result would voluntarily submit to this testing.
Conclusion and implications
These results suggest that the benefits of use of injectable depot buprenorphine in custody are comparable to those observed in community settings [17, 18, 19], as well as other forms of OAT in this setting [5]. Injectable depot buprenorphine treatment is an efficient treatment to administer compared to oral/sublingual OAT, and therefore should be considered for use in order to safely expand access to treatment in custodial settings, especially in those not already stable on methadone treatment or who would otherwise not receive treatment.
Overall, the findings support implementation of depot buprenorphine in custody, which will have widespread implications given the lack of access to OAT in these settings world‐wide. In the United States, Macmadua and colleagues estimated 1840 lives would have been saved nationally per annum in 2016 if all clinically indicated patients had received treatment for opioid dependence while in custody [29]. Future research is required to assess the supports required for people released from custody, including treatment transfer to community providers to ensure continuity of care. The benefits of commencing depot buprenorphine treatment in custody for the post‐release period may identify further advantages of this formulation for this high‐risk cohort and high‐risk period. Depot treatment may also offer other benefits in custodial settings where frequent lockdowns occur, which may limit access to daily treatment options, such as during the current pandemic [30].
Trial registration
https://www.anzctr.org.au ACTRN12618000942257.
Declaration of interests
A.J.D. reports grants from Braeburn/Camurus AB, to conduct clinical studies with buprenorphine products and travel support to Hunter New England Local Health District, which employs A.J.D. He is an honorary investigator on an Indivior‐funded study of buprenorphine products and has served on an advisory board for Mundipharma. P.S.H. reports grants from Braeburn/Camurus AB, to conduct clinical studies with buprenorphine products and has served on advisory boards for Lundbeck, Indivior, AbbVie and Gilead. N.L. reports grants from Braeburn and Camurus AB to conduct sponsored and investigator‐led clinical studies with CAM2038. N.L. served on Advisory Boards for Mundipharma, Indivior and Chiesi Pharmaceuticals. M.C. was employed by the NSW Ministry of Health, sponsors of the study. All other authors declare no competing interests.
Author contributions
Adrian J. Dunlop: Conceptualization (lead); funding acquisition (lead); methodology; supervision(lead); visualization; draft preparation (equal); review & editing (lead).
Bethany White: Data curation; investigation; project administration; visualization; original draft reparation (lead); review and editing.
Jillian Roberts: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision. Michelle Cretikos: Conceptualization; funding acquisition; investigation; methodology; resources. Dena Attalla: formal analysis; investigation; project administration. Rod Ling: Data curation; formal analysis; methodology; project administration. Andrew Searles: Data curation; formal analysis; investigation; supervision. Judith Mackson: Conceptualization; formal analysis; funding acquisition; investigation. Michael F. Doyle: Conceptualization; formal analysis; methodology. Elizabeth McEntyre: Conceptualization; formal analysis; methodology. John Attia: Conceptualization; formal analysis; methodology. Christopher Oldmeadow: Formal analysis; investigation; methodology; validation. Mark V. Howard: Conceptualization; investigation; methodology. Terry Murrell: Conceptualization; investigation; methodology; project administration; resources. Paul Steven Haber: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; supervision. Nicholas Lintzeris: Conceptualization; formal analysis; funding acquisition; investigation; methodology.
Supporting information
Data S1. Appendix S1—study treatment procedures.
Acknowledgements
We wish to thank all the JHFMHN Drug and Alcohol management, clinical, pharmacy and frontline staff, as well as CSNSW custodial staff and management, who supported the trial. We are grateful to members of our Aboriginal Advisory Group and Data Safety Monitoring Board for their time, expertise and contributions. We give a special thanks and acknowledge Monique Hourn for trial monitoring, and researcher officers Sophia Little, Kerryn Butler, Jeff Stormer and Fatema Sake who conducted all research interviews. We would also acknowledge Wedyan Meshreky's contribution to study development and Erin Nolan for data analysis. Finally, we gratefully acknowledge study participants, without whom this work would not have been possible.
Dunlop A. J., White B., Roberts J., Cretikos M., Attalla D., Ling R., Searles A., Mackson J., Doyle M. F., McEntyre E., Attia J., Oldmeadow C., Howard M. V., Murrell T., Haber P. S., and Lintzeris N. (2022) Treatment of opioid dependence with depot buprenorphine (CAM2038) in custodial settings, Addiction 117: 382–391. doi: 10.1111/add.15627.
Please see complete list of authors and affiliations at the end of the paper.
Contributor Information
Adrian J. Dunlop, Email: adrian.dunlop@health.nsw.gov.au.
Nicholas Lintzeris, Email: nicholas.lintzeris@health.nsw.gov.au.
References
- 1. Fazel S., Yoon I. A., Hayes A. J. Substance use disorders in prisoners: an updated systematic review and meta‐regression analysis in recently incarcerated men and women. Addiction 2017; 112: 1725–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moazen B., Saeedi Moghaddam S., Silbernagl M. A., Lotfizadeh M., Bosworth R. J., Alammehrjerdi Z., et al. Prevalence of drug injection, sexual activity, tattooing, and piercing among prison inmates. Epidemiol Rev 2018; 40: 58–69. [DOI] [PubMed] [Google Scholar]
- 3. Dolan K., Wirtz A. L., Moazen B., Ndeffo‐Mbah M., Galvani A., Kinner S. A., et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet 2016; 388: 1089–1102. [DOI] [PubMed] [Google Scholar]
- 4. Merrall E. L. C., Kariminia A., Binswanger I. A., Hobbs M. S., Farrell M., Marsden J., et al. Meta‐analysis of drug‐related deaths soon after release from prison. Addiction 2010; 105: 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore K. E., Roberts W., Reid H. H., Smith K. M., Oberleitner L. M., Mckee S. A. Effectiveness of medication assisted treatment for opioid use in prison and jail settings: a meta‐analysis and systematic review. J Subst Abuse Treat 2019; 99: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larney S., Gisev N., Farrell M., Dobbins T., Burns L., Gibson A., et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open 2014; 4: e004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marsden J., Stillwell G., Jones H., Cooper A., Eastwood B., Farrell M., et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction 2017; 112(8): 1408–1418. 10.1111/add.13779 [DOI] [PubMed] [Google Scholar]
- 8. Gisev N., Shanahan M., Weatherburn D. J., Mattick R. P., Larney S., Burns L., et al. A cost‐effectiveness analysis of opioid substitution therapy upon prison release in reducing mortality among people with a history of opioid dependence. Addiction 2015; 110: 1975–1984. [DOI] [PubMed] [Google Scholar]
- 9. Zaric G. S., Brennan A. W., Varenbut M., Daiter J. M. The cost of providing methadone maintenance treatment in Ontario, Canada. Am J Drug Alcohol Abuse 2012; 38: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warren E., Viney R., Shearer J., Shanahan M., Wodak A., Dolan K. Value for money in drug treatment: economic evaluation of prison methadone. Drug Alcohol Depend 2006; 84: 160–166. [DOI] [PubMed] [Google Scholar]
- 11. European Monitoring Centre for Drugs and Drug Addiction European Drug Report 2019: Trends and Developments. Luxembourg: Publications Office of the European Union; 2019. https://www.emcdda.europa.eu/system/files/publications/11364/20191724_TDAT19001ENN_PDF.pdf (accessed 4 March 2020). [Google Scholar]
- 12. Harm Reduction International . Global State of Harm Reduction: 2019 updates: 2020. Available at: https://www.hri.global/global‐state‐of‐harm‐reduction‐2019 (accessed 21 April 2020).
- 13. Bi‐Mohammed Z., Wright N. M., Hearty P., King N., Gavin H. Prescription opioid abuse in prison settings: a systematic review of prevalence, practice and treatment responses. Drug Alcohol Depend 2017; 171: 122–131. [DOI] [PubMed] [Google Scholar]
- 14. Gordon M. S., Kinlock T. W., Schwartz R. P., Fitzgerald T. T., O'Grady K. E., Vocci F. J. A randomized controlled trial of prison‐initiated buprenorphine: prison outcomes and community treatment entry. Drug Alcohol Depend 2014; 142: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wright N. M., Mohammed Z., Hughes G. J. Comparative prices of diverted buprenorphine/naloxone and buprenorphine in a UK prison setting: a cross‐sectional survey of drug using prisoners. Drug Alcohol Depend 2014; 144: 254–258. [DOI] [PubMed] [Google Scholar]
- 16. White N., Ali R., Larance B., Zador D., Mattick R. P., Degenhardt L. The extramedical use and diversion of opioid substitution medications and other medications in prison settings in Australia following the introduction of buprenorphine–naloxone film. Drug Alcohol Rev 2016; 35: 76–82. [DOI] [PubMed] [Google Scholar]
- 17. Frost M., Bailey G. L., Lintzeris N., Strang J., Dunlop A., Nunes E. V., et al. Long‐term safety of a weekly and monthly subcutaneous buprenorphine depot (CAM2038) in the treatment of adult out‐patients with opioid use disorder. Addiction 2019; 114: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lofwall M. R., Walsh S. L., Nunes E. V., Bailey G. L., Sigmon S. C., Kampman K. M., et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med 2018; 178: 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haight B. R., Learned S. M., Laffont C. M., Fudala P. J., Zhao Y., Garofalo A. S., et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2019; 393: 778–790. [DOI] [PubMed] [Google Scholar]
- 20. Vorspan F., Hjelmström P., Simon N., Benyamina A., Dervaux A., Brousse G., et al. What place for prolonged‐release buprenorphine depot‐formulation Buvidal® in the treatment arsenal of opioid dependence? Insights from the French experience on buprenorphine. Expert Opin Drug Deliv 2019; 16: 907–914. [DOI] [PubMed] [Google Scholar]
- 21. American Psychiatric Association (APA) Manual of Mental Disorders, 5th edn: DSM‐5. Philadelphia, PA: APA; 2013. [Google Scholar]
- 22. Lintzeris N., Dunlop A., Masters D. Clinical Guidelines for Use of Depot Buprenorphine (Buvidal® and Sublocade®) in the Treatment of Opioid Dependence. Sydney, Australia: NSW Ministry of Health; 2019. https://www.health.nsw.gov.au/aod/Publications/full‐depot‐bupe‐interim‐gl.pdf (accessed 5 January 2020). [Google Scholar]
- 23. Ryan A., Holmes J., Hunt V., Dunlop A., Mammen K., Holland R., et al. Validation and implementation of the Australian treatment outcomes profile in specialist drug and alcohol settings. Drug Alcohol Rev 2014; 33: 33–42. [DOI] [PubMed] [Google Scholar]
- 24. SAS Institute Inc SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute, Inc; 2013. [Google Scholar]
- 25. Justice Health and Forensic Mental Health Network Network Patient Health Survey. Malabar, Australia: Justice Health and Forensic Mental Health Network; 2017. [Google Scholar]
- 26. Australian Institute of Health and Welfare (AIHW) The Health of Australian Prisoners. Canberra, Australia: AIHW; 2018. https://www.aihw.gov.au/reports/prisoners/health‐australia‐prisoners‐2018/summary (accessed 20 March 2020). [Google Scholar]
- 27. Australian Institute of Health and Welfare (AIHW) National Opioid Pharmacotherapy Statistics Annual Data Collection (NOPSAD). Canberra, Australia: AIHW; 2019. https://www.aihw.gov.au/reports/australias‐health/alcohol‐and‐other‐drug‐treatment‐services (accessed 4 March 2020). [Google Scholar]
- 28. Darke S. Self‐report among injecting drug users: a review. Drug Alcohol Depend 1998; 51: 253–263. [DOI] [PubMed] [Google Scholar]
- 29. Macmadu A., Goedel W. C., Adams J. W., Brinkley‐Rubinstein L., Green T. C., Clarke J. G., et al. Estimating the impact of wide scale uptake of screening and medications for opioid use disorder in US prisons and jails. Drug Alcohol Depend 2020; 208: 107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saloner B., Parish K., Ward J. A., Dilaura G., Dolovich S. COVID‐19 cases and deaths in federal and state prisons. JAMA 2020; 324: 602–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Appendix S1—study treatment procedures.


