Abstract
Background and purpose
New prophylactics for migraine, targeting calcitonin gene‐related peptide (CGRP), have recently emerged. Real‐world data are important for a comprehensive understanding of treatment response. We assessed the consistency of response to erenumab, a monoclonal CGRP receptor antibody, in a real‐world setting, in order to determine which patients may be considered responders in clinical practice.
Methods
All erenumab‐treated patients (n = 100) completed a time‐locked daily electronic diary, and an automated algorithm was used to monitor treatment response. Monthly migraine days (MMD), non‐migrainous headache days, days of acute medication use (MAMD), well‐being and coping with pain were assessed for a 6‐month period. The primary outcome was reduction in MMD compared to baseline.
Results
The numbers of MMD and MAMD decreased in all months, in both episodic and chronic migraine patients, compared to baseline (p < 0.001), while general well‐being (p < 0.001) and coping with pain (p < 0.001) also improved. Of all patients, 36% had an MMD reduction of ≥50% in ≥3/6 months, and 6% had such a reduction in all 6 months. For a ≥30% MMD reduction, the figures were 60% and 24%, respectively. Almost 90% of patients with an average MMD reduction of ≥30% over the first 3 months had a sustained response in the last 3 months. In addition, 20% of patients without an initial response (average <30%), had a delayed response (average ≥30%) in the last 3 months.
Conclusion
Erenumab was effective in migraine patients who were highly refractory to previous prophylactics. As a practical guideline, we propose that treatment be continued for at least 6 months and that patients with a ≥30% MMD reduction in at least half of the treatment period should be considered to be responders.
Keywords: electronic headache diary, erenumab, migraine, real‐world data, responder
Erenumab shows effectiveness in a highly treatment‐refractory patient population. In addition to reductions in migraine days, severity of migraine headache, accompanying symptoms and acute medication intake, use of erenumab led to an increase in general well‐being and in coping with pain. However, responses to erenumab fluctuated in the course of treatment and were not consistent for each month.

INTRODUCTION
New prophylactic therapeutic options for migraine, targeting calcitonin gene‐related peptide (CGRP), have emerged [1]. Clinical trials with these anti‐CGRP (receptor) antibodies demonstrated that 50% of episodic migraine patients experience a ≥50% reduction in migraine days in response to treatment compared to baseline [2, 3]. The percentage of responders diminishes to 30% when studying patients who previously failed up to four prophylactics [4, 5, 6]. Even though this cut‐off point of a 50% reduction in monthly migraine days (MMD) is presented in trials, in clinical practice it seems less suitable because many patients who consider treatment to be effective will not fulfill this 50% criterion. Another issue is that, in many clinical trials, treatment response is assessed in the last month of treatment or as an average response over several months, but data on consistency of response obtained by looking at every month of the treatment period are rarely provided. Real‐world data, collected independently from the pharmaceutical companies, are important to obtain more comprehensive insight and understanding of response rates and consistency of individual response in order to formulate clinical treatment guidelines. In particular, as it is expected that health insurance reimbursements in most countries will depend on strict criteria, it is of utmost importance to reach consensus on the definition of a “good response” in clinical practice.
In the present study we aimed to assess consistency in response to erenumab in a real‐world setting in order to gain more insight into individual variability of response and to determine which patients may be considered responders.
METHOD
Participants
Migraine patients treated with erenumab at the Leiden Headache Center were included in this study. Because of the restricted availability of erenumab, all patients had ≥8 MMD and had failed on ≥4 migraine prophylactic treatments (with failure defined as ineffective treatment, treatment discontinued because of side effects or treatment contraindicated), including candesartan, beta blockers, valproate and topiramate. Diagnosis, based on International Classification of Headache Disorders (ICHD)‐3 criteria [7], was made by a neurology resident in consultation with a heachache specialist neurologist, or by neurologists themselves. Patients with a second primary headache diagnosis, other than tension‐type headache, were excluded. Patients with a history of cerebrovascular or cardiovascular events were excluded. Comorbidities were recorded for all patients, with a focus on disorders involving pain. This study was approved by the Medical Ethics Committee of the Leiden University Medical Center.
Treatment
In clinical trials, no evidence was provided that erenumab 140 mg led to a response that was clearly distinct from that of a 70 mg dose; therefore, all patients started treatment with erenumab 70 mg (administered subcutaneously once every 4 weeks). Patients administered the initial injection themselves under supervision of a physician or a headache nurse, and subsequent injections were administered at home. After 3 months, patients had a consultation with their treating physician, after which the dose was optionally increased to 140 mg for the subsequent 3 months based on a joint decision between patient and physician. There were no strict criteria for increasing the dose. As there is a strict policy in the Netherlands regarding medication overuse, and polypharmacy is not part of clinical practice [8], none of the patients used additional prophylactic treatment simultaneously with erenumab or had medication overuse before starting treatment. Other preventive medication was tapered off, including a wash‐out period, before starting the baseline diary.
Headache diary
We prospectively collected data on headache using a daily electronic diary [9], which included questions about headache presence, headache severity (scores ranging from 1 [mild] to 3 [severe]) and headache characteristics, presence of aura, accompanying symptoms (nausea, vomiting, photophobia and phonophobia), acute pain medication use, a general well‐being question (“How did you feel yesterday?”, scores ranging from 0 to 10) on a daily basis and a coping with pain question (“How well could you cope with the headache yesterday?”, scores ranging from 0–10) in case of a headache. An automated and validated algorithm, based on the ICHD‐3 criteria, or intake of a triptan was used to determine whether headache days fulfilled migraine criteria [7, 9]. Headache days not fulfilling migraine criteria were labelled as non‐migrainous headache days. The diary was time‐locked, meaning patients had 48 h to complete a given diary day. All patients started the headache diary at least 4 weeks before treatment was initiated, which was defined as the baseline period, and continued the diary for at least 6 months after initiating treatment, unless earlier treatment discontinuation occurred. One month was defined as a period of 28 days. Diary compliance had to be ≥80%.
Patient‐reported outcome measures
To incorporate the patients' own perspectives of treatment effect, all patients were asked to fill out the Patient Global Impression of Change (PGIC) questionnaire [10] and the Headache Impact Test (HIT)‐6 [11]. The PGIC is a one‐item questionnaire, in which patients indicate their global perception of change in clinical status since starting treatment on a seven‐point Likert scale, scoring from 1 (no change or worse) to 7 (much better). The HIT‐6 is a six‐item questionnaire that assesses the impact headache has on a patient's daily life (total score range of 36–78), with larger scores reflecting a higher impact. These questionnaires were asked independently, had no effect on the treating physicians decision to increase erenumab dosage, and were not repeated after 6 months.
Adverse events
In addition to the consultations with the treating physicians at the Headache Clinic where adverse effects were reported, patients were interrogated separately by structured interview to investigate adverse events, such as injection site reactions, upper respiratory tract infections and constipation, and had the opportunity to report other possible adverse events.
Statistics
Baseline characteristics, including sex, age, headache diagnosis, failed prophylactics and baseline headache measures were summarized using means, standard deviations, frequencies and proportions. Failure to respond to the prophylactics propranolol and metoprolol was counted as one failure (treatment class: beta blockers). For each patient the number of MMD, monthly (non‐migrainous) headache days (MnmHD) and monthly acute medication use days (MAMD), as well as average monthly general well‐being, and average monthly pain coping were calculated in periods of 1 month (28 days). To investigate the change in severity of migraine, we studied headache severity and accompanying symptoms. We also calculated the number of MMD with nausea, photophobia or phonophobia and the proportion of MMD with severe headache (score 3 on the severity question in the e‐diary). For each of these outcome variables, a linear mixed model was fitted with time (month) as a fixed effect and patient as a random effect, and post hoc pairwise comparisons were conducted with Bonferroni correction for multiple testing. Response per month was determined by calculating the proportion of patients with ≥30%, 50%, 75% and 100% reductions in MMD compared to baseline. Consistency of response was determined by counting the number of months in which a patient experienced at least 30%, 50%, 75% and 100% reductions. These months did not have to be consecutive.
In addition to the response per month, the migraine response was also investigated by calculating the average migraine reduction over several months. For the calculations of sustained and delayed response, we chose a similar approach to that used by Goadsby et al. in the STRIVE study [12]. The average reduction in MMD in months 1 to 3 was compared to the average MMD reduction in months 4 to 6. Sustained responders were defined as patients who achieved an average of ≥50% reduction in MMD during the first 3 months and who continued to have this response during months 4 to 6. Delayed responders had an MMD reduction <50% during the first 3 months of treatment, but achieved an MMD reduction ≥50% during months 4 to 6. We then calculated the number of sustained and delayed responders in regard to a ≥30% reduction in migraine.
To investigate to what extent patients were capable of assessing the treatment effect after 3 months, PGIC score and absolute reduction in HIT‐6 score were correlated using Spearman correlation with migraine reduction in the third month and with the average migraine reduction in the first 3 months.
Adverse events were summarized using descriptive statistics. All calculations and analyses were performed using R version 3.6.2. Two‐sided p values <0.05 were taken to indicate statistical significance (https://www.r‐project.org/).
Missing data
Patients who discontinued erenumab before completion of the 6‐month follow‐up were included in the linear mixed model analyses only for the months in which they used erenumab, and they were added as non‐responders (<30% response) in the subsequent months for the calculation of proportion of responders. Missing diary days were not imputed. For the calculations of sustained and delayed responders, only the patients who completed the 6‐month follow‐up period were included.
RESULTS
Between January 2019 and March 2020 a total of 100 patients (85 women, 15 men) started erenumab treatment. Five patients discontinued treatment before the 6‐month follow‐up period ended: one patient experienced general malaise, which made her reluctant to continue treatment after 2 months; one patient discontinued after 3 months because of severe daily nausea; and two patients discontinued after 4 months, one because of an allergic skin reaction increasing after every subsequent injection and the other because of severe constipation which required hospital admission. One patient discontinued treatment after five erenumab injections because of lack of efficacy. No patients were excluded because of diary compliance <80%. Of all patients, 56 (56%) increased the erenumab dose to 140 mg after 3 months of treatment. Almost 50% of patients fulfilled the criteria for chronic migraine, the other half experienced episodic migraine, with a high number of MMD but without ≥15 headache days/month in total. The mean number of failed prophylactics was 5. Three patients reported fibromyalgia as a comorbidity, two had rheumatoid arthritis, and seven had a history of arthrosis. Average diary compliance was 97%. Patient baseline characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics (n = 100)
| Women, n (%) | 85 (85) |
| Age, years | 43 ± 12 |
| Migraine without aura, n (%) | 61 (61) |
| Episodic migraine, n (%) | 54 (54) |
| MMD baseline | 14.0 ± 5.9 |
| MnmHD baseline | 2.9 ± 3.9 |
| MAMD baseline | 6.1 ± 3.7 |
| Failed prophylactics | 5.0 ± 1.0 |
A month is defined as 28 days. Baseline = 28 days before starting treatment.
Abbreviations: MAMD, monthly acute medication days; MMD, monthly migraine days; MnmHD, monthly (non‐migrainous) headache days. Values are mean ± SD, unless otherwise indicated.
Headache diary
The mean MMD decreased in all months compared to the baseline period (each month p < 0.001; Table 2). Months 2 to 6 also showed reduced MMD compared to the first month of treatment (month 2: p = 0.05, month 3: p = 0.03, months 4–6: each p < 0.001). The number of MnmHD did not decrease compared to the baseline period. A reduction was found for MAMD for all months compared to the baseline period (each month p < 0.001). The number of migraine days with nausea as an accompanying symptom diminished in every month (each month p < 0.001) compared to baseline, as did the number of migraine days with photophobia (each month p < 0.001) and with phonophobia (p < 0.001). The proportion of migraine days with a severe headache (score 3 on the severity question in the electronic diary) decreased compared to baseline during the course of 6 months (months 2–5: p < 0.001, month 6: p = 0.017). Average monthly general well‐being (reported daily; each month p < 0.001) and average monthly coping with pain (reported when headache was present; each month p < 0.001), improved in all months compared to the baseline month (Table 2). Results for episodic and chronic migraine patients separately are presented in Tables S1 and S2.
TABLE 2.
Monthly treatment response
|
Baseline Mean ± SD |
Month 1 Mean ± SD |
Month 2 Mean ± SD |
Month 3 Mean ± SD |
Month 4 Mean ± SD |
Month 5 Mean ± SD |
Month 6 Mean ± SD |
|
|---|---|---|---|---|---|---|---|
| MMD | 14.0 ± 5.9 | 11.7 ± 7.6** | 10.3 ± 7.5** | 10.2 ± 7.0** | 9.9 ± 7.1** | 9.6 ± 7.5** | 9.2 ± 6.6** |
| MnmHD | 2.9 ± 3.9 | 3.2 ± 3.9 | 3.3 ± 4.3 | 3.2 ± 4.3 | 3.2 ± 4.4 | 3.0 ± 4.0 | 3.0 ± 4.1 |
| MAMD | 6.1 ± 3.7 | 4.6 ± 2.8** | 4.8 ± 3.2** | 4.6 ± 2.8** | 4.5 ± 2.7** | 4.1 ± 2.6** | 4.4 ± 2.6** |
| MMD with nausea | 9.2 ± 6.4 | 7.3 ± 7.1** | 6.5 ± 6.6** | 6.1 ± 6.3** | 5.9 ± 6.5** | 5.8 ± 6.6** | 5.4 ± 5.8** |
| MMD with photophobia | 13.1 ± 6.4 | 10.9 ± 7.9** | 9.6 ± 7.5** | 9.5 ± 7.0** | 9.1 ± 7.2** | 8.8 ± 7.6** | 8.4 ± 6.7** |
| MMD with phonophobia | 13.0 ± 6.5 | 10.6 ± 8.0** | 9.4 ± 7.6** | 9.3 ± 7.2** | 9.0 ± 7.1** | 8.7 ± 7.5** | 8.2 ± 6.7** |
| Proportion of MMD with severe headache, % of MMD | 47.9 ± 28.0 | 35.8 ± 29.0** | 37.5 ± 32.9** | 37.0 ± 32.1** | 36.7 ± 32.8** | 35.6 ± 34.3** | 41.4 ± 36.4* |
| General well‐being | 5.2 ± 1.7 | 5.8 ± 1.8** | 6.0 ± 1.9** | 6.0 ± 1.9** | 6.0 ± 1.9** | 6.1 ± 1.8** | 6.1 ± 1.8** |
| Coping with pain | 4.5 ± 1.5 | 5.0 ± 1.6** | 5.0 ± 1.7** | 4.9 ± 1.7** | 4.9 ± 1.6** | 5.0 ± 1.6** | 4.9 ± 1.6** |
Linear mixed model analysis was based on the data as observed, with no imputation of missing values. p values were calculated for each month compared to baseline. *p < 0.05, ** p < 0.001. A month is defined as 28 days.
MMD with severe headache = migraine days with severe headache as a proportion of all migraine days. General well‐being = monthly average of general well‐being, inquired on daily basis, score range 0–10. Coping with pain = monthly average score for coping with pain, assessed for every migraine and headache day, score range 0–10.
Abbreviations: MAMD, acute medication days; MMD, monthly migraine days; MnmHD, monthly (non‐migrainous) headache days.
Consistency of response
We used the total study population (n = 100) to assess consistency of response. The number of patients with ≥50% reduction in MMD compared to the baseline month fluctuated in the 6‐month follow‐up period between 22 (22%) and 43 (43%). For episodic migraine patients this number fluctuated between 16 (29%) and 33 (61%), and for chronic migraine patients between 5 (11%) and 13 (28%). The number of patients with ≥30% reduction fluctuated between 47 (47%) and 59 (59%; Figure 1). For episodic migraine patients this number fluctuated between 34 (63%) and 40 (74%), and for chronic migraine patients between 13 (28%) and 20 (43%).
FIGURE 1.
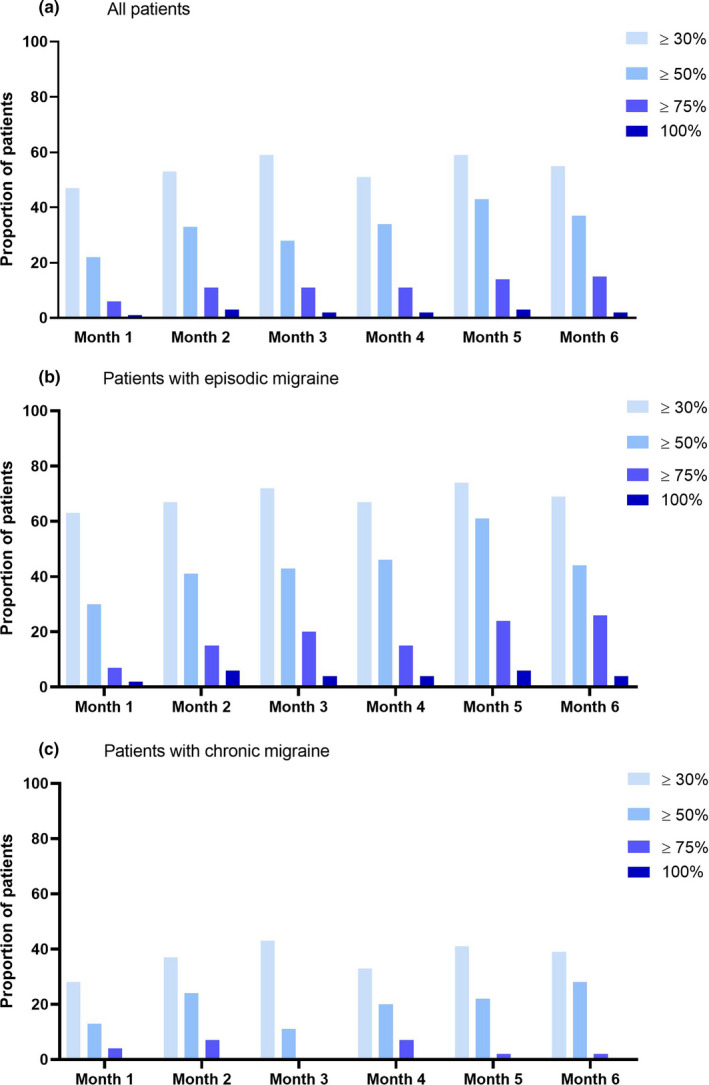
Response rate by month for (a) all patients (n = 100), (b) episodic migraine patients (n = 54) and (c) chronic migraine patients (n = 46). Proportions of patients with, respectively, ≥30%, ≥50%, ≥75% and 100% reductions in migraine days compared to the baseline month, are shown, presented by month. A month is defined as 28 days. Total number of patients is n = 100 (all patients), and 56 (56%) increased erenumab dosage after 3 months from 70 mg to 140 mg. For patients who discontinued erenumab before 6 months, the months without medication were counted as <30% reduction
Of all patients, 36% experienced ≥50% MMD reduction in at least 3 out of 6 months, while 6% of patients had ≥50% MMD reduction in all 6 months (Figure 2). For ≥30% MMD reduction this was 60% and 24%, respectively. Of the patients with episodic migraine 26/54 (48%) had ≥50% MMD reduction and 42/54 (78%) had ≥30% MMD reduction in at least 3 out of 6 months; for chronic migraine the equivalent figures were 10/46 (22%) and 18/46 patients (39%), respectively.
FIGURE 2.
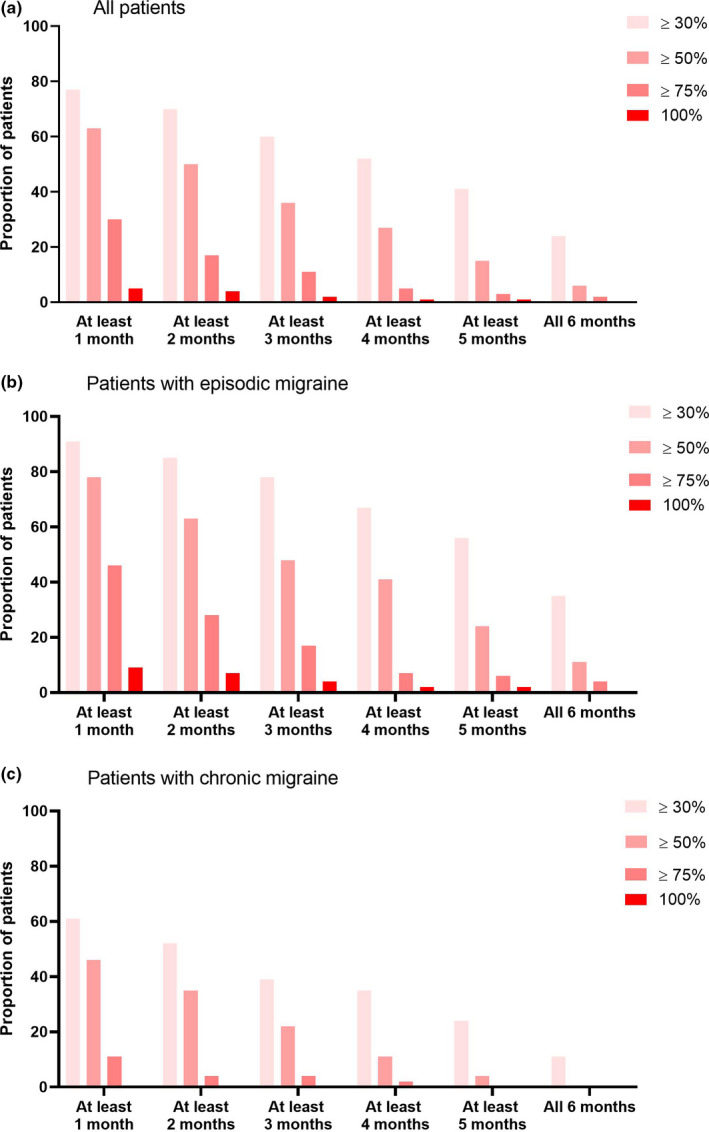
Consistency of response by number of months for (a) all patients (n = 100). (b) episodic migraine patients (n = 54) and (c) chronic migraine patients (n = 46). Proportions of patients with, respectively, ≥30%, ≥50%, ≥75% and 100% reduction in migraine days compared to the baseline month, are shown, presented by number of months (cumulative) with this response. A month is defined as 28 days. For patients who discontinued erenumab before 6 months, the months without medication were counted as <30% reduction.
Sustained and delayed response
An average MMD reduction of ≥50% in the first 3 treatment months was seen in 25 patients. Of these 25 patients, 18 (72%) maintained this reduction in months 4 to 6 (sustained responders; Table 3). Of the 70 patients who had an average MMD reduction in the first 3 months of <50%, 16 (23%) did have an average of ≥50% MMD reduction in months 4 to 6 (delayed responders). There were 54 patients with an average MMD reduction in the first 3 months of ≥30%. Of these 54 patients, 48 (89%) maintained this response during months 4 to 6 (sustained responders). Of the 41 patients with an average MMD reduction in the first 3 months of <30%, eight (20%) had an average MMD reduction in months 4 to 6 of ≥30% (delayed responders). The majority of patients (11/16) who were defined as delayed 50% responders, had an increase in erenumab dosage from 70 mg to 140 mg after 3 months of treatment.
TABLE 3.
Sustained and delayed response to erenumab in patients who continued with erenumab 70 mg (n = 42) and patients who escalated the dosage to 140 mg (n = 53)
| Patients who continued with 70 mg dose | Patients escalating dose to 140 mg | Total | |
|---|---|---|---|
| Average reduction MMD in months 1–3 ≥50% | |||
| N | 21 | 4 | 25 |
| Sustained responders, n (%) | 15 (71) | 3 (75) | 18 (72) |
| Average reduction MMD in months 1–3 <50% | |||
| N | 21 | 49 | 70 |
| Delayed responders, n (%) | 5 (24) | 11 (22) | 16 (23) |
| Average reduction MMD in months 1–3 ≥30% | |||
| N | 35 | 19 | 54 |
| Sustained responders, n (%) | 31 (89) | 17 (89) | 48 (89) |
| Average reduction MMD in months 1–3 <30% | |||
| N | 7 | 34 | 41 |
| Delayed responders, n (%) | 1 (14) | 7 (21) | 8 (20) |
N total = 95 (all patients who completed the 6‐month follow‐up period). Sustained responders = patients who achieved an average of ≥50% or ≥30% reduction in MMD during the first 3 months and during the last 3 months of treatment. Delayed responders = patients who did not achieve an average of ≥50% or ≥30% reduction in MMD during the first 3 months of treatment, but did during the last 3 months of treatment.
Abbreviation: MMD, monthly migraine days.
Patient‐reported outcome
After 3 months of treatment, a total of 90 patients completed the PGIC and the HIT‐6. The median (interquartile range) PGIC score was 5 (4–6), which corresponds to a moderate improvement. A moderate correlation was found between PGIC score and reduction in MMD in the third month after starting treatment (r = 0.59, p < 0.001; Figure 3) as well as between the PGIC score and the average MMD reduction over the first 3 months of treatment (r = 0.64, p < 0.001, not shown). The mean HIT‐6 score diminished from 67 ± 3.4 at baseline to 62 ± 6.1 after 3 months (p < 0.001). The change in HIT‐6 was moderately correlated with the MMD reduction in the third month after treatment initiation (r = 0.43, p < 0.001; Figure 3) as well as with the average MMD reduction over the first 3 months of treatment (r = 0.46, p < 0.001, not shown).
FIGURE 3.
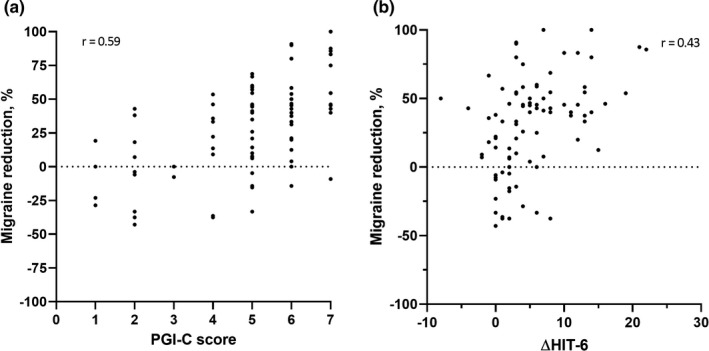
Scatterplot correlation migraine reduction and (a) Patient Global Impression of Change (PGIC) score and (b) change in Headache Impact Test (HIT)‐6 score. PGIC, scoring from 1 (no change or worse) to 7 (much better), was assessed after 3 months of treatment. HIT scores (total range 36–78) are shown as absolute reductions from baseline to 3‐month follow‐up. Migraine reduction is shown in the third month after starting treatment compared to baseline. A month is defined as 28 days. N = 90 (all patients who completed the PGIC and the HIT‐6 at both baseline and 3‐month follow‐up)
Adverse events
Adverse events were reported by 93 patients (93%). Most commonly reported were abdominal complaints (n = 72 [72%]), including constipation (n = 65 [65%]), followed by fatigue (n = 43 [43%]) and injection site reactions (n = 27 [27%]; Table 4).
TABLE 4.
Adverse events
| Adverse events | Frequency, n (%) | Adverse events | Frequency, n (%) |
|---|---|---|---|
| None | 7 (7) | Upper respiratory tract infection | 26 (26) |
| Abdominal discomfort | 72 (72) | Common cold | 22 (22) |
| Constipation | 65 (65) | Coughing | 4 (4) |
| Nausea | 9 (9) | Pharyngeal pain | 5 (5) |
| Diarrhea | 8 (8) | Dizziness | 23 (23) |
| Change in appetite | 2 (2) | Light‐headedness | 20 (20) |
| Stomachache | 2 (2) | Vertigo | 6 (6) |
| Feeling bloated | 2 (2) | Urinary tract infection | 8 (8) |
| Fatigue | 43 (43) | Palpitations/tachycardia | 7 (7) |
| Injection site reaction | 27 (27) | Pruritus | 7 (7) |
| Pain | 15 (15) | Mood disturbance | 7 (7) |
| Swelling | 10 (10) | Anxiety | 5 (5) |
| Redness | 7 (7) | Trouble sleeping | 5 (5) |
| Musculoskeletal system | 27 (27) | Dysregulated menstrual cycle | 3 (3) |
| Myalgia | 12 (12) | Hair loss | 3 (3) |
| Muscle cramps | 5 (5) | Nightmares | 2 (2) |
| Neck pain | 5 (5) | Lower respiratory tract infection | 1 (1) |
| Back pain | 7 (7) | Pneumonia | 1 (1) |
Adverse events are presented as number and percentage of all patients (n = 100) reporting adverse events and divided in categories and subcategories. As patients could report more than one adverse event per category, numbers of subcategories could exceed the total number of adverse events per category.
DISCUSSION
The present study shows that erenumab can be effective in highly treatment‐refractory migraine patients, with a small additional effect of 140 mg compared to 70 mg. A reduction in migraine days, but not in non‐migrainous headache days, was observed, along with a reduction in intake of acute medication, headache severity and accompanying symptoms. As none of the patients had acute medication overuse or additional prophylactics, we observed the pure response to erenumab. We showed that there was a wide range in migraine reduction, and on an individual level that response was not consistent for each month. This makes the definition of a responder in clinical practice not as clear‐cut as is suggested in clinical trials. Moreover, in clinical practice the patient's perception of treatment effectiveness is a factor most neurologists take into account in the decision to continue or discontinue treatment. In the present study, the decision was shared based on patients' and physicians' impression of effectiveness. Previously published expert opinion‐based clinical guidelines suggest treatment with monoclonal antibodies be stopped after 6 to 12 months of treatment [13], but no clear indication is provided with regard to what treatment response is clinically meaningful. We would like to propose that (European) clinical guidelines define a responder in clinical practice by considering the cut‐off value for response, the timing of this response and the duration of treatment.
Generally a 50% reduction in migraine days is regarded as a relevant response to preventive treatment in episodic migraine [14]. For chronic migraine, a reduction of 30% is considered to be of clinical relevance [15]. Episodic migraine patients who failed up to four migraine prophylactics showed a lower response rate [6, 16]. Taking into account that the patients who will probably be eligible for monoclonal CGRP treatment are difficult‐to‐treat patients, with no other therapeutic options and highly frequent attacks, a 30% reduction could be considered relevant. This is supported by the fact that, in our patient population, 42% of patients chose to continue the 70‐mg dose, and not to increase this to 140 mg, even if they did not reach the 50% reduction. In addition, our data evidently demonstrate that, on an individual level, 54% of patients already had a ≥30% and 25% a ≥50% response to the lower dose.
In clinical trials of erenumab, the third month after starting treatment is often used to determine the response to treatment [2, 6], and sometimes the average of 3 months is used [16]. The International Headache Society guidelines for controlled trials for preventive treatment of episodic migraine present a definition for persistence of response which includes patients who experience a ≥50% reduction in the first 4 weeks after initiating treatment and continue to have this response in the following months [17]. However, as is shown in the present study and in earlier trials of erenumab [16], MMD may still decrease after initial failure of response. Additionally, a longitudinal epidemiological study in migraine patients demonstrated a high level of variability in MHD [18]. Importantly, the present study demonstrates that patients do not consistently reach a ≥30% reduction for each month. Even though 80% of patients reached a ≥30% reduction in at least 1 month, only 24% of patients reached this response for all 6 months. So, in daily clinical practice, factors such as the natural fluctuation of migraine frequency and late onset of response need to be considered.
A recent publication on efficacy of erenumab presented the sustained and delayed response to erenumab after 1 year of treatment [12]. Although the duration of the study was different from that of the present study, the results seem comparable. Approximately 85% of patients with an initial response experienced a sustained response after 1 year. Furthermore, 30–40% of patients without an initial response had a delayed response, which is somewhat higher than observed in our present study. This could be useful information that may help physicians manage long‐term expectations in clinical practice. However, decisions on (dis)continuation of treatment need to be taken after a much shorter treatment period.
We would like to make some recommendations for clinical guidelines. Firstly, starting treatment with erenumab on a dosage of 70 mg may be a suitable approach, as a large proportion of patients respond well to this dose and choose to continue with it. Evaluation of response should take place after 3 months, and increasing dosage to 140 mg should be considered even if patients experience little to no response to 70 mg. Secondly, if there is no medical necessity to discontinue treatment, we recommend continuing erenumab for at least 6 months. We recommend that a patient be considered a 'responder' if he/she has a ≥30% reduction in at least half of the treatment period. As retrospective self‐reported MMD are subject to recall bias [9], we strongly advise an electronic headache diary. Discrepancy between the objective diary and subjective self‐reported response was highlighted in the present study by the wide range in migraine reduction and associated PGIC and HIT‐6 scores after 3 months of treatment. Nevertheless, it seems important to incorporate patients' perception of treatment effectiveness, using a validated instrument or by simply asking for patients' perception of treatment effect.
A striking percentage of patients in the present study reported constipation as an adverse event, ranging from very mild and resolved with some dietary adjustments, to severe (hospitalization needed in one patient). This percentage was higher than reported in clinical trials [2, 6] and in previously reported real‐world data [19, 20, 21]. This might be because adverse events were specifically inquired about, with a risk of nocebo effect [22]. Even though we observed a higher rate of adverse events, the low number of patients who discontinued treatment because of side effects still represents a high tolerability. CGRP (mainly ßCGRP) affects the contractile activity of intestinal circular muscle [23]. A study on expression of the CALCRL gene, encoding one of the components of the CGRP receptor, indicated that the enteric neurons have a five times higher expression of CALCRL than vascular cells or sensory neurons [24], which supports the rationale for constipation as a side effect for erenumab.
A strength of the present study is the electronic headache diary, with use of an automated algorithm based on ICHD‐3 criteria. The time‐lock makes the data less susceptible to reporting bias. As monoclonal CGRP treatment is costly, it may well be that healthcare insurance companies will require detailed information on effectiveness in order to reimburse treatment.
The fact that none of the patients had medication overuse headache or used additional prophylactic treatments could be seen as a limitation of this study. Not every region has the same policy regarding detoxification and combining migraine prophylactics, and thus our data may not reflect the general clinical practice for every headache center in the world. However, as our aim was to make a recommendation for the clinical guidelines, observing the purest response to erenumab is important. Moreover, the National Health Care Institute in the Netherlands will not approve monoclonal antibodies for patients with medication overuse. Furthermore, only erenumab was studied, making the results not directly applicable to the other monoclonal antibody treatments. Similar studies regarding other monoclonal antibodies could contribute to recommendations for the clinical guidelines.
In conclusion, erenumab showed effectiveness in a highly treatment‐refractory patient population. In addition to reductions in migraine days, severity of migraine headache, accompanying symptoms and acute medication intake, use of erenumab led to an increase in general well‐being and coping with pain. However, responses to erenumab fluctuated in the course of treatment. As a recommendation for clinical guidelines, we advise that treatment is continued for at least 6 months and a patient is considered to be a responder if they have a ≥30% reduction in migraine days in at least half of the treatment period. A detailed electronic headache diary appears to be essential to reliably assess treatment response.
CONFLICT OF INTEREST
S.d.V.L. declares no competing interests relevant to the manuscript. G.M.T. reports consultancy support from Novartis, Allergan, Lilly, and Teva, and independent support from Dutch Organization for Scientific Research, the Dutch Heart and Brain Foundations, IRRF and Dioraphte. A.M.v.d.B. reports consultancy or industry support from Novartis, Lilly and Teva, and independent support from the Dutch Research Council (NWO) and the Dutch Heart and Brain Foundations. I.E.V. reports independent support from the Dutch Brain Council and the Dutch Brain Foundation. T.v.d.H. declares no competing interests relevant to the manuscript.
AUTHOR CONTRIBUTIONS
SdVL and GT contributed to the study design. SdVL was responsible for running the study. SdVL carried out the statistical analyses. All authors contributed to the interpretation of the results. SdVL made the figures and wrote the initial draft of the manuscript. IV, TvdH, AMvdB and GT critically revised the article, and all authors approved the final version for submission.
Supporting information
de Vries Lentsch S, Verhagen IE, van den Hoek TC, MaassenVanDenBrink A, Terwindt GM. Treatment with the monoclonal calcitonin gene‐related peptide receptor antibody erenumab: A real‐life study. Eur J Neurol. 2021;28:4194–4203. 10.1111/ene.15075
Antoinette MaassenVanDenBrink and Gisela M. Terwindt shared last authors.
Contributor Information
Simone de Vries Lentsch, Email: s.de_vries_lentsch@lumc.nl.
Gisela M. Terwindt, Email: g.m.terwindt@lumc.nl.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia. 2019;39(3):445‐458. [DOI] [PubMed] [Google Scholar]
- 2. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026‐1037. [DOI] [PubMed] [Google Scholar]
- 3. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE‐2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442‐1454. [DOI] [PubMed] [Google Scholar]
- 4. Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet. 2019;394(10203):1030‐1040. [DOI] [PubMed] [Google Scholar]
- 5. Mulleners WM, Kim B‐K, Láinez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814‐825. [DOI] [PubMed] [Google Scholar]
- 6. Reuter U, Goadsby PJ, Lanteri‐Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two‐to‐four previous preventive treatments were unsuccessful: a randomised, double‐blind, placebo‐controlled, phase 3b study. Lancet. 2018;392(10161):2280‐2287. [DOI] [PubMed] [Google Scholar]
- 7. Headache Classification Committee of the International Headache Society . The international classification of headache disorders. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 8. Pijpers JA, Louter MA, de Bruin ME, et al. Detoxification in medication‐overuse headache, a retrospective controlled follow‐up study: does care by a headache nurse lead to cure? Cephalalgia. 2016;36(2):122‐130. [DOI] [PubMed] [Google Scholar]
- 9. van Casteren D, Verhagen I, de Boer I, et al. E‐diary use in clinical headache practice: a prospective observational study. Cephalalgia. 2021. 10.1177/03331024211010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipton RB, Cohen JM, Gandhi SK, Yang R, Yeung PP, Buse DC. Effect of fremanezumab on quality of life and productivity in patients with chronic migraine. Neurology. 2020;95(7):e878‐e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rendas‐Baum R, Yang M, Varon SF, Bloudek LM, DeGryse RE, Kosinski M. Validation of the Headache Impact Test (HIT‐6) in patients with chronic migraine. Health Qual Life Outcomes. 2014;12. 10.1186/s12955-014-0117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goadsby PJ, Reuter U, Hallstrom Y, et al. One‐year sustained efficacy of erenumab in episodic migraine: results of the STRIVE study. Neurology. 2020;95(5):e469‐e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silberstein SD. Preventive migraine treatment. Continuum (Minneapolis, Minn). 2015;21(4):973‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tassorelli C, Diener HC, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38(5):815‐832. [DOI] [PubMed] [Google Scholar]
- 16. Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123‐2132. [DOI] [PubMed] [Google Scholar]
- 17. Diener HC, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020;40:1026–1044. [DOI] [PubMed] [Google Scholar]
- 18. Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raffaelli B, Kalantzis R, Mecklenburg J, et al. Erenumab in chronic migraine patients who previously failed five first‐line oral prophylactics and onabotulinumtoxinA: a dual‐center retrospective observational study. Front Neurol. 2020;11:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheffler A, Messel O, Wurthmann S, et al. Erenumab in highly therapy‐refractory migraine patients: first German real‐world evidence. J Headache Pain. 2020;21(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ornello R, Casalena A, Frattale I, et al. Real‐life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitsikostas DD. Nocebo in headache. Curr Opin Neurol. 2016;29(3):331‐336. [DOI] [PubMed] [Google Scholar]
- 23. Holzer P, Bartho L, Matusak O, Bauer V. Calcitonin gene‐related peptide action on intestinal circular muscle. Am J Physiol. 1989;256:546‐552. [DOI] [PubMed] [Google Scholar]
- 24. Vgontzas A, Renthal W. Predicting erenumab adverse events with single‐cell genomics. Lancet. 2020;396(10244):95‐96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


