Abstract
The aim of this article is to discuss how physiology and anatomical background affect the effectiveness of implant‐dependent microinvasive glaucoma surgery (MIGS). First, we provide a micro view of aqueous outflow and tissue behaviour. Second, we review studies exploring the mechanisms of the pressure‐lowering effect of MIGS, as well as tissue behaviour during aqueous flow and tissue motion. We also describe and classify microinvasive surgical procedures and the most important types of implants, as well as their mechanisms of action, implantation techniques and efficacy. Further, we summarize the indications and surgical results presented in recent studies, providing an evidence‐based update on novel and emerging MIGS techniques for the treatment of open‐angle glaucoma. These data can help surgeons to personalize the management of glaucoma and to choose the best MIGS option for individual glaucoma patients.
Keywords: glaucoma, implants, microinvasive surgery, MIGS
Introduction
Trabeculectomy has been the ‘glaucoma surgery of choice’ for years. However, due to the risk of vision‐threatening complications, as well as a decrease in postoperative effectiveness with time, a constant search for safer and more effective surgical techniques has been underway (Soltau et al. 2000; Gedde et al. 2012). For over 10 years, intensive research on minimally invasive methods of glaucoma surgery, termed microinvasive glaucoma surgery (MIGS), has been conducted (SooHoo et al, 2014). Ahmed and Saheb proposed that MIGS treatments should be characterized by the following five properties (Saheb & Ahmed 2012): an ab interno approach through a clear corneal incision that spares the conjunctiva; use of procedures that minimize trauma to the target tissue; an intraocular pressure (IOP)‐lowering efficacy that justifies the approach; a high safety profile that avoids serious complications compared to other glaucoma surgeries; and rapid recovery with minimal impact on the patient's quality of life (Caprioli et al. 2015). Subsequently, in 2014, the American Glaucoma Society and the US Food and Drug Administration (FDA) characterized MIGS as the implantation of a surgical device intended to lower IOP via an outflow mechanism, with either an ab interno or ab externo approach associated with very little or no scleral dissection (Francis et al. 2011).
Microinvasive glaucoma surgery (MIGS) is intended to achieve lower IOP in patients with glaucoma with less surgical time and, ideally, to have a medication‐sparing effect. The surgical techniques involved are based on the physiological aspects of aqueous humour (AH) flow in the eye; furthermore, the choice between various anatomical sites in the eye globe influences the range of the corresponding IOP‐lowering effects. To date, a reduction in IOP is the only proven method to slow the progression of visual field loss (Gedde et al. 2012; Saheb & Ahmed 2012; SooHoo et al, 2014). Because of increasing life expectancy, patients live longer with glaucoma (Brandão & Grieshaber 2013) and are at a risk of glaucoma progression over a longer period. Therefore, it is essential to operate on glaucoma at an early stage and to lower the IOP intensively from the beginning. However, the role of MIGS in the glaucoma treatment algorithm has yet to be fully determined. In this paper, we review the characteristics and clinical outcomes of the most frequently used implants in MIGS. We also discuss their advantages and biases, along with factors that should be considered in future studies.
For this review, we used PubMed to conduct an online search of literature published from 2015 to 2020, using keywords appropriate to this topic. For an overall description of MIGS implants, we included studies with the following keywords: trabecular micro‐bypass stent, iStent Supra® (Glaukos, San Clemente, CA, USA), Schlemm's canal scaffold, Hydrus® (Ivantis, Irvine, CA, USA), suprachoroidal microstent, CyPass® Micro‐Stent (Alcon, Fort Worth, TX, USA), XEN® Gel Stent (Allergan, Irvine, CA, USA), and PRESERFLO® MicroShunt (Santen, Osaka, Japan). To assess clinical outcomes, we included randomized clinical trials (RCTs) comparing MIGS with trabeculectomy or other therapies, observational studies and other methodologies. Research on MIGS as a solo surgery or in conjunction with cataract extraction was also examined.
Physiology of Aqueous Drainage
In order to better explain the mechanism of action of the implants, we present details of the anatomy and physiology of Schlemm's canal (SC) and the mechanisms of AH outflow.
Aqueous humour (AH) is drained from the eye via two physiological pathways (Achache 2001; Morrison & Pollack 2003). The conventional path begins at the level of the irido‐corneal trabecular meshwork (TM) and accounts for approximately 83–96% of drainage (Achache 2001). From the anterior chamber, the AH moves through the TM to SC and the intrascleral connecting channels, which lead to the intrascleral venous plexus, aqueous vessels and venous vessels of the suprascleral space. Aqueous vessels begin as collector channels (CC) in the exterior wall of SC and can be seen on the surface of the eye in the corneal limbus (Morrison & Pollack 2003).
Drainage can also occur by an unconventional suprachoroidal pathway that does not begin in the trabeculum (Morrison & Pollack 2003; Tamm 2009). Small amounts of AH can pass through the cornea and vitreous and thus through the retina and optic disc. However, unconventional drainage mainly takes place through the anterior part of the choroid, also referred to as the suprachoroidal pathway (Pederson et al. 1977; Morrison & Pollack 2003). Drainage via this path takes place through the base of the ciliary muscle (where the AH is produced), which does not have an endothelial barrier to the anterior chamber (Pederson et al. 1977). Use of this drainage pathway decreases with age, from 30% to 35% in young persons (25–30 years old) to 3% in individuals aged over 60 years (Pederson et al. 1977). The reason for this phenomenon is assumed to be the stiffening of tissues, which increases with age (Wang et al. 2017).
Drainage Path Structure and Outflow Resistance
Aqueous humour (AH) flows out of the anterior chamber as a mass stream regulated by a pressure gradient (Pederson et al. 1977) and fills SC. The pressure in SC must be lower than that in the anterior chamber to permit AH flow. This reduction in SC pressure simultaneously requires a one‐way mechanism to prevent backflow of the AH into SC from the episcleral veins. The pressure in these veins is normally lower than that at the entrance of the CCs, which in turn must be lower than that in SC to permit AH flow. The AH flows through the drainage pathways at an average rate of 2.0 µL/min (Johnson & Kamm 1983). In healthy human eyes, outflow facility has a value of 0.40 µL x min/mm Hg at 10 mm Hg (Brubaker 1975), but this value decreases with age (Gaasterland et al. 1978). From a physiological perspective, the trabeculum, particularly the interior wall of SC, and the TM near the CC are the main sources of resistance to AH outflow; the remaining resistance likely comes from the exterior wall and surrounding tissues (Johnson & Kamm 1983; Rosenquist et al. 1989). This area is called the juxtacanalicular space and is assumed to be the primary site of IOP regulation (Goel et al. 2010). Thus, elevated IOP in glaucoma is caused by an increase in outflow resistance in the AH drainage pathway rather than by an increase in AH production (Achache 2001). Moreover, this outflow resistance is not constant—it is a function of IOP and rises as IOP rises (Brubaker 2003).
Schlemm's Canal
Schlemm's Canal (SC) was named in honour of the German anatomist, Friedrich Schlemm, who, in 1830, discovered the canal in the anterior chamber angle through which the AH is taken into the bloodstream (Dvorak‐Theobald 1955; Mansouri & Shaarawy 2015). Schlemm's canal (SC) drains AH from the trabeculum into the suprascleral and conjunctival veins via CCs. It is a circuitous channel 36–40‐mm long and 190–370‐µm wide (Achache 2001). Its interior wall consists of a continuous monolayer of endothelium (Ethier 2002), in which the endocellular route of AH flow is found, represented by giant vacuoles and pores (Achache 2001). Giant vacuoles are potential spaces between the extracellular matrix and the inner wall cells of SC (Ethier 2002). They form dynamically and respond instantaneously to changes in IOP (Epstein & Rohen 1991; Dautriche et al. 2015), and their quantity and size increase as IOP increases. The majority of giant vacuoles are found near CC outlets (Parc et al. 2000), which suggests that a greater pressure gradient is present there due to the increased aqueous flow (Ethier 2002). Pores are structures in the inner wall ranging from 0.6 to 3 µm in size (Ethier 2002; Braakman et al. 2014) and are responsible for approximately 10% of the resistance to AH drainage (Alvarado et al. 2004). They can be found in the walls of giant vacuoles, but may be functionally unrelated to them (Tamm 2009). Pores form the main pathway of aqueous flow through the inner wall of SC.
The interior diameter of SC changes in response to IOP fluctuations (Johnstone & Grant 1973; Johnstone 1979) but is too large to generate significant resistance in the outflow path (Achache 2001). When the IOP rises, the TM expands towards the lumen of the canal, causing it to narrow. At high IOPs, parts of the canal's lumen close, increasing the probability that its walls will collapse and increase resistance in drainage routes (Battista et al. 2008); the canal does not collapse under the influence of physiological increases in IOP in healthy eyes (Ten Hulzen & Johnson 1996). Extensive collapse of the canal only occurs at pressures of 40 mm Hg or higher, with the exception of points where the septa are located (which do not collapse) (Van Buskirk 1982).
The AH flows out of SC through one of the 30 CCs and aqueous veins and then to the system of suprascleral veins (Rosenquist et al, 1989), ophthalmic veins and the general circulation (Morrison & Pollack 2003). Aqueous veins are approximately 1 mm in length and 50 µm in diameter (Rosenquist et al. 1989) (Fig. 1). According to Hagen–Poiseuille's law, the resistance of aqueous veins should be insignificant if they are not collapsed or compressed (Dietlein et al. 2000). Therefore, distal aqueous drainage routes do not appear to play a significant role in generating outflow resistance (Johnson & Kamm 1983).
Fig. 1.
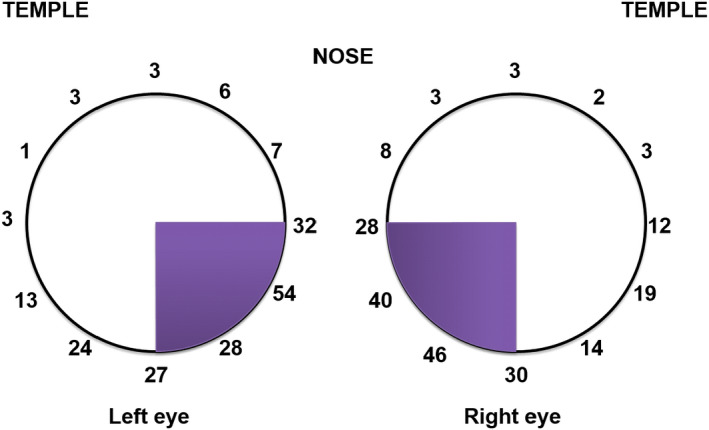
Aqueous vein density.
Scientific Background of the Outflow Mechanism
Intraoperative provocative gonioscopy and channelography were evaluated in a study conducted by Grieshaber et al. (2009); their multinomial regression model results revealed that higher IOP levels are correlated with lower blood reflux rates independently of age. Provocative gonioscopy, during which blood reflux into SC is observed, is the simplest method of assessing the conventional drainage pathway. It also facilitates localization of an uncollapsed collector or aqueous vein (Johnstone et al. 2011), enabling the assessment of the blood reflux pattern. Provocative gonioscopy is performed intraoperatively in an eye with paracentesis‐induced hypotony (Grieshaber et al. 2010). Blood reflux is not provoked by a compression of the episcleral veins by the gonioscopic lens, but rather by the hypotony itself. Using this technique and an assessment of the amount of blood present in the angle, three filling patterns can be determined: no filling, incomplete filling and complete filling (Grieshaber et al. 2010). The technique also provides information related to SC patency and its connection to a patent distal system. A more precise assessment of the distribution of aqueous veins can be obtained through canalography (Video S1) and involves the direct injection of fluorescein into SC using a microcatheter during the canaloplasty procedure (Grieshaber et al. 2009). Episcleral outflow can then be assessed and graded according to the number of vessels that fill with dye in each quadrant. The most frequent point of implantation in SC surgery is the nasal quadrant, mainly because surgical access through a temporal corneal incision is straightforward. The nasal quadrant contains most of the CCs and aqueous veins (Fig. 1; Videos S2 and S3).
Zhou & Smedley (2005) introduced the trabeculum bypass theory, which proposes reducing resistance in this part of the drainage route. They hypothesized the presence of two types of bypasses, permitting either unidirectional or bidirectional flow, incorporated through boundary conditions for solving the equations and deriving the facility of outflow and the reduced IOP. According to their hypothesis, the amount of outflow increases by 13% and 26% in the presence of a unidirectional and bidirectional bypass, respectively. The circumferential flow is significant only in the immediate quadrant of the bypass. The authors also observed increased flow through SC only in the quadrant where the implant was applied. Intraocular pressure (IOP) reduction was dependent on the initial pressure (Zhou & Smedley 2005), and SC and CC dilation significantly lowered the IOP. With trabecular bypass alone, the elevated IOP in primary open‐angle glaucoma (POAG) is expected to drop to the mid‐to‐high teens (15–19 mm Hg). Intraocular pressure (IOP) can be further reduced by another 3–6 mm Hg with moderate SC and CC dilation; however, the circumferential length of the dilated SC affects the efficacy of IOP reduction. In theory, SC dilation using a trabecular bypass is analogous to a partial trabeculotomy in terms of IOP reduction. Within the scope of the concept of segmental flow, trabecular bypass operations may not reduce the IOP as much as do traditional procedures. This is likely because, in patients with POAG, the number of opened CC remains the same when IOP increases (as it does in healthy eyes), causing increased outflow resistance.
Suprachoroidal Space
Prostaglandin analogues (latanoprost, travoprost) can increase suprachoroidal outflow and thus lower the IOP. Certain authors suggest that the suprachoroidal outflow route may undergo greater modification with the use of medications than the conventional route, as described above in the paragraph ‘Physiology of aqueous drainage’ (Toris 2010; Winkler & Fautsch 2014). This is also why attempts are made to use this path in MIGS. Emi et al. (1989) suggested that a negative pressure gradient of 3–4 mm Hg is generated between the suprachoroidal space and the anterior chamber, creating a potential driving force for AH outflow to the suprachoroidal space. The pressure difference between the anterior chamber and the posterior suprachoroidal space increases at higher IOP (Emi 1989).
Cyclodialysis is the separation of the longitudinal muscle of the ciliary body from the scleral spur. Aqueous humour (AH)flows directly through this cleft into the suprachoroidal space and causes hypotony, a phenomenon described for the first time over a century ago (Fuchs 1900). Intentional surgical cyclodialysis has been used for the treatment of glaucoma and was first described by Heine in 1905. Surgical cyclodialysis is achieved by inserting a spatula between the sclera and choroid through a posterior scleral incision into the anterior chamber (Fuchs 1900). Ultrasound biomicroscopy image of cyclodialysis is shown in Fig. 2.
Fig. 2.
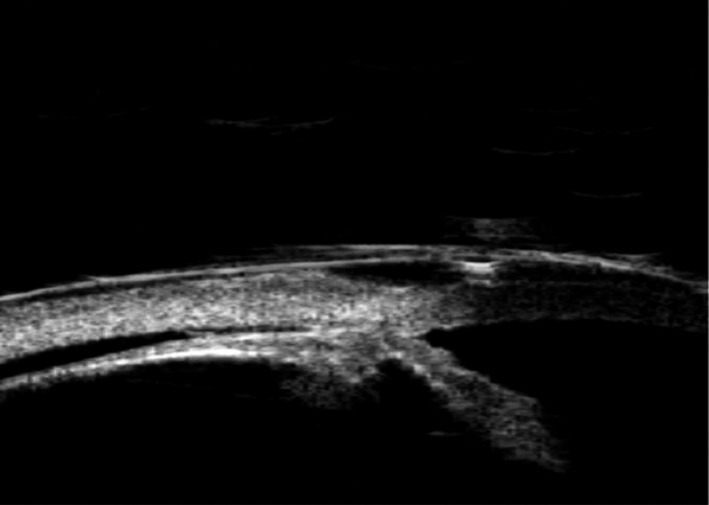
Cyclodialysis is visible in ultrasound biomicroscopy.
However, achieving controlled cyclodialysis for the purpose of therapeutic IOP reduction is difficult and attempts to surgically increase suprachoroidal outflow have had limited success due to various complications (Gentile et al. 1996; Ozdamar et al. 2003; Jordan et al. 2007; Razeghinejad & Spaeth 2011). Suprachoroidal devices may also be considered if trabecular stents fail or if the target pressure cannot be achieved using these stents. Depending on their mechanism of IOP‐lowering action, MIGS implants can be classified as those that improve conventional outflow, those that enhance unconventional outflow, and those that bypass conventional outflow (Saheb & Ahmed 2012; Brandão & Grieshaber 2013; Schmidt et al. 2013). In this review, we focus on six devices in these classifications:
-
SC implants that correct conventional outflow
-
▪
iStent®
-
▪
Hydrus®
-
▪
-
Suprachoroidal space implants that strengthen outflow via the unconventional route
-
▪
CyPass®
-
▪
iStent Supra®
-
▪
-
Implants that bypass physiological aqueous drainage pathways
-
▪
XEN® Gel Stent
-
▪
PRESERFLO® MicroShunt
-
▪
The iStent®
The first‐generation iStent® trabecular micro‐bypass stent (European Union CE certified & FDA approved, 2012) was designed to restore natural physiological outflow by creating a patent bypass to SC through the TM. It targets the increased resistance caused by the juxtacanalicular part of the TM, which is believed to represent the site of greatest resistance to AH outflow in patients with POAG (Johnson 2006). It has an ‘L’‐shaped structure with a snorkel‐shaped inlet on the short side (which sits in the anterior chamber) and an open half‐pipe lumen. At 1.0 mm in length and 0.33 mm in height, a snorkel length of 0.25 mm and a diameter of 120 µm, it is the smallest device approved for use in humans. The convex side of the iStent® sits against the inner wall of SC, with the open half‐pipe against the outer wall. Mathematical models of AH outflow project the size of the lumen to be more than adequate to accommodate the flow induced by the stent (Yuan et al. 2016). Separate orientations of the iStent are available for the right eye (OD) and for the left eye (OS). The iStent® is inserted ab interno through a small temporal clear corneal incision and placed in SC at the lower nasal quadrant (Video S1). Implantation of the stent at this location allows the AH to bypass the obstructed TM and drain directly from the anterior chamber into SC; this also optimizes outflow in the lower nasal quadrant area, which has the highest concentration of CCs (Le & Saheb 2014) (Fig. 1.).
The iStent® is manufactured using titanium, a commonly used medical implant material with proven biocompatibility. A heparin coating ensures wetting of the lumen for self‐priming. It is nonferromagnetic and thus safe for magnetic resonance (MR) imaging, although nonclinical testing has demonstrated that iStent® models GTS100R and TS100L are MR‐conditional, that is, safe for use in specified MR environments under specified conditions (https://www.glaukos.com/en-uk/istent-inject-w-procedure/innovative-design/). Specifically, a patient with this device can be safely scanned in an MR system meeting the following conditions: static magnetic field of 3T or less, maximum spatial gradient magnetic field of 4000 gauss/cm (40 T/m), maximum MR system reported and a whole body averaged specific absorption rate of 4 W/kg (first level‐controlled operating mode; http://www.glaukos.com/istent/design). Several stents can be used to achieve a better hypotensive effect (Katz et al. 2015).
The iStent Inject®
The iStent Inject® is a second‐generation trabecular micro‐bypass stent recently introduced to provide additional IOP reduction. Similar to the first‐generation iStent, the iStent Inject® is made of biocompatible, medical‐grade titanium. The system contains an injector preloaded with two heparin‐coated titanium stents, each with a central lumen and four side outlets to permit for multidirectional aqueous outflow. The implants are placed ab interno on two distinct areas of the TM into SC where AH subsequently flows into CCs. The two stents are able to tap into up to 6 clock hours (i.e. half of the total span of the angle) of the CCs. The placement of stents in two separate regions enables access of AH to more CCs, theoretically enabling a more pronounced decline in IOP (Salimi et al. 2019) (Fig. 3). Both generations of the iStent® are contraindicated in eyes with primary‐ or secondary‐angle closure glaucoma, including neovascular glaucoma, as well as in patients with a retrobulbar tumour, thyroid eye disease, Sturge–Weber Syndrome or other type of condition that can cause elevated episcleral venous pressure. Gonioscopy should be performed prior to surgery to exclude peripheral anterior synechiae (PAS), rubeosis and other angle abnormalities or conditions that prohibit adequate angle visualization, which could lead to improper placement of the stent (Videos S2 and S3).
Fig. 3.

iStent® Inject implant.
The iStent Supra®
The iStent Supra® is a third‐generation stent made from polyethersulfone and a coloured titanium sleeve (Fig. 4). It consists of a 4‐mm long tube with an opening at each end and is placed in the suprachoroidal space via ab interno access through a clear corneal incision. It is designed to create a patent lumen from the anterior chamber into the suprachoroidal space and has retention rings to provide stability at the implant location. The iStent Supra® has not yet been commercially released and, thus, limited information is available at present.
Fig. 4.

iStent® Supra implant.
The iStent® first generation is currently the most widely used type of stent. The efficacy and safety of micro‐bypass by itself or in combination with phacoemulsification has been assessed in numerous studies (Fea 2010; Fernández‐Barrientos et al., 2010; Arriola‐Villalobos et al., 2012; Fea et al. 2014). Samuelson et al. (2011) presented the results of a study on iStent® implantation with simultaneous cataract removal surgery (Fig. 5). The study involved a year‐long observation of 240 eyes randomly categorized into two groups: in the first group, patients underwent cataract phacoemulsification surgery with iStent® implantation; only cataract removal was performed in the second group. In the iStent® group, 72% of treatment eyes achieved an unmedicated IOP of ≤21 mm Hg at the 1‐year time point, compared to 50% in the control group. The safety profile was similar in both groups (Samuelson et al. 2011). Fea et al. (2015) conducted a trial of iStent® versus phacoemulsification alone in a randomized setting, with results assessed up to 16 months, finding a statistically significant difference in final IOP between the groups. Ferguson et al. (2016) reported a 21% reduction in postoperative IOP at 24 months in a real‐world study of 350 American eyes that underwent iStent® implantation in combination with cataract surgery, while Gallardo et al. (2016) reported a 31% reduction in IOP after 3 years in a mainly Hispanic population. Craven et al. (2012) reported a statistically significant therapeutic effect in an iStent® group in their 2‐year observational study.
Fig. 5.
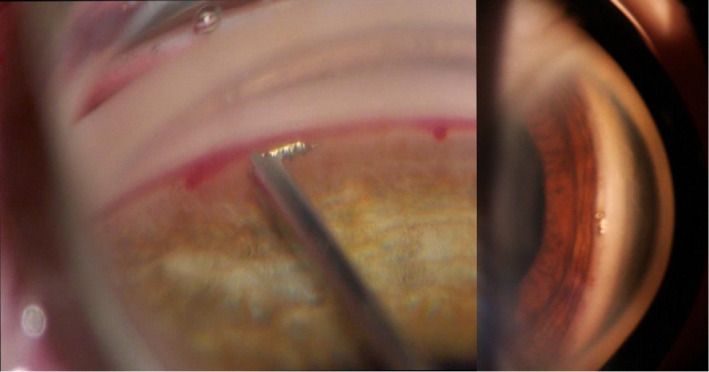
iStent® implant inserted during gonioscopy.
Studies have also confirmed beneficial effects of implanting multiple stents, and that multiple implantation may reduce the number of administered medications (Belovay et al. 2012). In a nonrandomized prospective case series study, Belovay et al. (2012) compared IOP among patients with two and three stents. The authors suggested that the observed dependence of pressure on the number of applied stents indicated that the surgeon can optimize the number of implanted stents according to each patient's target IOP. Additionally, a study involving patients with refractory glaucoma (after prior trabeculectomy) who received either two iStent® trabecular micro‐bypass stents or one iStent Supra® suprachoroidal stent for the treatment of refractory glaucoma, along with postoperative travoprost (Myers et al. 2018). At the 48 months, 97% of the eyes in the first group versus 98% of those in the second group achieved IOP ≤15 and ≤18 mm Hg, respectively, on one medication, indicating that both stents have similar IOP‐lowering effectiveness.
The most frequently described complications after iStent® implantation are hyphema, transitory increase in IOP, corneal oedema, an obstructed stent, implantation‐related difficulties, entrapment of the vitreous, improper stent positioning and the necessity of repeating the procedure (Arriola‐Villalobos et al. 2012; Belovay et al. 2012; Craven et al. 2012; Ferguson et al. 2017; Pillunat et al. 2017; Esfandiari et al. 2019; Le et al. 2019).
The Hydrus® Microstent SC scaffold
The Hydrus® Microstent SC scaffold is a CE‐certified SC scaffold that directly bypasses the TM to drain AH into SC. It is made from nitinol®, a nickel‐titanium alloy, is flexible, biocompatible, and contains three windows over its 8 mm length (Figs 6 and 7) (Mansouri & Shaarawy 2015). The implant is designed to enable increased outflow of AH from the anterior chamber into SC (Grierson et al. 2015) (Video S4). The Hydrus microstent dilates approximately one quadrant of SC (3 clock hours), and the most common site of implantation is the nasal quadrant. The proximal 1 mm inlet section of the microstent remains outside SC in the anterior chamber, ensuring direct inflow of the AH (Gulati et al. 2013). Studies conducted on eyeballs collected from human cadavers have shown significant improvement in outflow after implantation of the Hydrus microstent under different perfusion pressures (Camras et al. 2012). Saheb and Ahmed published data from a 6‐month observation of 28 eyes after phacoemulsification with Hydrus implantation and reported that the average initial IOP dropped from 29 to 15 mm Hg (Saheb & Ahmed 2012). Pfeiffer et al. (2015) showed a statistically significant greater reduction in IOP over a 2‐year period in a Hydrus plus cataract surgery group compared to a surgery alone group. No statistically significant differences in terms of safety were observed. In the COMPARED study, stand‐alone Hydrus implantation in POAG patients resulted in a higher surgical success rate and fewer medications compared with implantation of two iStents®, with the safety profiles being similar for the procedures. In that study, 47% of patients with Hydrus implants and 24% of those with the two iStents were medication‐free at 12 months. The percentage of eyes reaching ≤18 mm Hg without medications was greater in the Hydrus group (30% versus 9%), as was the percentage of eyes reaching a 20% or more reduction in IOP from the washed‐out baseli (40% versus 13%). The mean IOP for eyes without medications was 17.3 ± 3.3 mm Hg in the Hydrus group and 19.2 ± 2.4 mm Hg in the two iStents® group (mean change = −8.2 mm Hg versus −5.1 mm Hg; difference in change, −3.1 mm Hg). Complications in both groups included subconjunctival haematoma, hyphema, PAS and IOP spikes of 10 mm Hg or more (Pfeiffer et al. 2015; Otarola et al. 2020).
Fig. 6.

A Hydrus® implant.
Fig. 7.
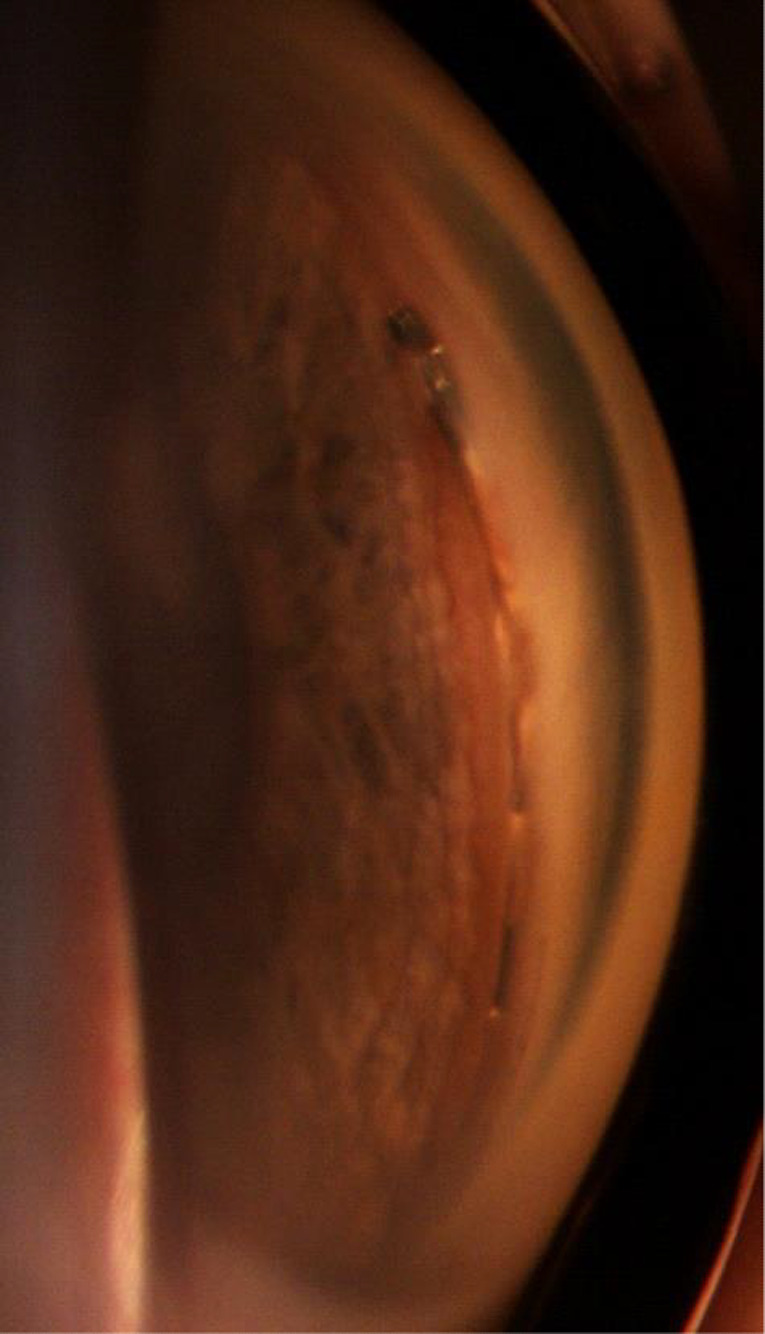
A Hydrus® implant in gonioscopy.
The XEN® Gel Stent
The XEN® Gel Stent (CE certified in 2011): this category of subconjunctival MIGS stents was developed with the aim of improving the predictability and safety profile of bleb‐forming procedures (Do et al. 2020). The stents are FDA approved for use with cataract surgery and stand‐alone procedures (Lewis 2014). They are nonabsorbable implants made from a soft, cross‐linked collagen tube with a length of 6 mm, and interior lumen diameters of 140, 63 and 45 µm in the Xen 140, Xen 63 and Xen 45 versions, respectively. Unlike silicone tube shunts, they do not incite significant inflammation or a foreign body tissue reaction, reducing the risk of fibrous proliferation, progressive inhibition of aqueous flow, and bleb failure (Lewis 2014). Their design is based on the Hagen–Poiseuille equation, with the pressure difference across the tube estimated based on its length and internal lumen diameter to avoid postoperative hypotony (Lewis 2014) (Fig. 8). The lowest AH resistance is offered by XEN® 140. The XEN® 63 lowers the AH resistance up to 2–3 mm Hg and XEN® 45 retains 6–8 mm Hg of resistance for aqueous outflow, warranting the least risk of hypotony (Sheybani et al. 2015; Sheybani et al. 2016). The only model commercially available at the moment is the XEN® 45. However, results from an early study of the XEN® 63 were recently published (Lavin‐Dapena et al. 2020). XEN® stents can be implanted by both ab externo and ab interno approaches. In the ab interno approach, the stent is delivered into the anterior chamber through an inferotemporal clear corneal incision made with a 27‐G sharp bevelled needle tip. The sharp tip is introduced at the TM and advanced through the sclera to exit approximately 2.5–3 mm posterior to the limbus into the subconjunctival space. The internal and external locations are verified, and the anterior chamber is irrigated to ensure flow and bleb formation (Green et al. 2018). The implant connects the anterior chamber to the subconjunctival space transsclerally (Fig. 8). The concept of the transscleral XEN® implant is based on utilizing the outflow route produced by trabeculectomy and bypassing potential points of outflow resistance while conserving the conjunctiva. The bypassing of all potential points of outflow resistance together with the ab interno access eliminate the need to create a scleral flap and reduce the probability of the related complications that can accompany traditional antiglaucoma surgeries (Video S5).
Fig. 8.
A XEN® implant.
Studies evaluating the stent's safety and efficacy in reducing IOP in patients with early‐stage, medium‐stage and advanced‐stage glaucoma are in progress. Initial reports indicate that the XEN® implant enables effective IOP control and a reduction in the number of antiglaucoma medications administered (Lewis 2014), and has a favourable safety profile. Pérez‐Torregrosa et al. (2016) assessed the safety and effectiveness of phacoemulsification combined with XEN® 45 implant surgery in 30 POAG eyes with concomitant cataract over a 12‐month observation period. The surgery was augmented with subconjunctival mitomycin C (MMC). The mean preoperative IOP of 21.2 ± 3.4 mm Hg decreased by 29% at 12 months, and the average number of medications decreased by 95%. Similarly, De Gregorio et al. (2018) implanted XEN® 45 Gel Stents in 41 eyes in combination with phacoemulsification. Complete success, defined as a postoperative IOP ≥6 and ≤17 mm Hg without glaucoma medications, and qualified success, defined as a postoperative IOP ≥6 and ≤17 mm Hg with medication, were achieved in 80% and 98% of the cases, respectively. After 12 months, the mean postoperative IOP was 13.1 ± 2.4 mm Hg (mean IOP reduction of 42%) with a mean of 0.4 ± 0.8 medication classes (p < 0.05 for both IOP and medications).
Several authors have reported similar effectiveness of XEN® implants in lowering IOP (Lewis 2014; Pérez‐Torregrosa et al. 2016; Galal et al. 2017; Smith et al. 2019). Laborda‐Guirao compared the effectiveness of combined XEN® 45 with phacoemulsification surgery with solo procedure in a retrospective study of 80 eyes with 12 months of follow‐up (Laborda‐Guirao et al. 2020). They did not find any significant differences in success rates, number of IOP‐lowering medications or re‐operations between groups. The authors concluded that XEN® implants alone or in conjunction with phacoemulsification are effective in treating patients with advanced POAG. Wagner et al. (2020) retrospectively compared the effectiveness of XEN® to trabeculectomy in 171 eyes with refractory glaucoma. After 12 months of follow‐up, there were no statistically significant differences between the groups in the complete success rates (66% and 59% in the trabeculectomy and XEN® groups, respectively) or in the ratio of needling and complication rates. Results from the longest follow‐up study for XEN® 45 (36 months) were recently published for a cohort of 91 eyes (Gillmann et al. 2020a; Gillmann et al. 2020b). Complete success based on the criterion of IOP ≤15 mm Hg or a 20% reduction from baseline (medication free) was achieved in 29% of eyes at 3 years after surgery. There were no significant differences in efficacy between the XEN® 45 procedure performed solo versus when combined with phacoemulsification. For XEN® 63, the longest follow‐up as of this writing is 5 years, but the study population consisted only of 11 eyes (Lavin‐Dapena et al., 2020). An IOP <18 mm Hg or a reduction of 20% versus baseline was achieved in nine eyes (82%). The main complications of XEN® surgery include choroidal detachment, implant dislocation and extrusion, subconjunctival haemorrhage and encapsulated blebs (Dervenis et al. 2017). The prevention of chronic hypotony has been a hallmark of the device, which utilizes an intrinsic flow‐limiting design based on the tube length and internal lumen diameter. Prospective comparative studies with larger groups of patients and longer follow‐up periods are needed to further assess the value this device (Green et al. 2018; Do et al. 2020).
The PRESERFLO® MicroShunt
The PRESERFLO® MicroShunt, formerly known as the InnFocus MicroShunt, is implanted using the ab externo approach; however, it fulfils the MIGS criteria proposed by the American Glaucoma Society and the US FDA. The surgical technique does not require dissection of a scleral flap (as does trabeculectomy), sclerectomy or iridectomy, or flap suture placement. The bleb is placed more posteriorly and is thicker than in conventional filtering procedures (Beckers & Pinchuk 2019). The recovery time for a trabeculectomy is typically between 4 and 6 weeks, while recovery time from a MicroShunt implantation is generally around 2 weeks; the follow‐up visit is also less intensive. The implant is made of a synthetic polymer of poly(styrene‐block‐isobutylene‐block‐styrene) (SIBS) (Acosta et al. 2006; Pinchuk et al. 2017). In vivo studies have not reported material‐related anterior chamber reactions, bleb encapsulation, neovascularization, or inflammatory or fibrotic responses; the only reported reaction was deposition of type IV collagen near the tube (Pinchuk et al. 2017). The design of the PRESERFLO® MicroShunt device is also based on the Hagen–Poiseuille equation (Arrieta et al. 2011) and is similar to that of the Xen® Gel Stent. The PRESERFLO® shunt tube is 8.5 mm long and 1.1 mm wide, with a 70 µm inner diameter and a 350 µm outer diameter. The tube has two fins that are 4.5 mm from the anterior tip and help secure the device location and prevent anterior migration. The implantation technique also utilizes MMC. An approximately 5‐mm wide fornix‐based conjunctival/Tenon's flap is created, after which a deep sub‐Tenon's pocket (6–8 mm) is formed. After marking the sclera 3 mm from the limbus, a 1‐mm deep scleral pocket is created using a triangular knife. A 25‐gauge needle is inserted into the pocket to exit the anterior chamber through the angle. The device is then advanced through the needle track in the pocket bevel into the anterior chamber, with the proximal end of the shunt extending approximately 2–3 mm into the chamber. Thereafter, the fins are secured within the scleral pocket, and flow is established by gently applying pressure on the eye or by flushing a balanced saline solution through a side‐port (Beckers & Pinchuk 2019). After checking the flow, Tenon's capsule and the conjunctiva are sutured in a watertight fashion (Pillunat et al. 2017).
The PRESERFLO® MicroShunt is registered in Europe and was released in 2019 for the surgical treatment of patients with early‐to‐advanced POAG, but it has not yet been approved by the US FDA. Published evidence is limited; however, study results show that a mean IOP reduction of 30–55% from baseline can be achieved, with a substantial reduction in glaucoma medications (Canadian Agency for Drugs & Technologies in Health 2019). The longest retrospective observational study to date reports 3‐year outcomes in 23 mixed‐race patients with POAG in the Dominican Republic who received a PRESERFLO® MicroShunt (14 eyes with the shunt alone, nine eyes with concomitant cataract surgery). The authors revealed that the qualified success rate (IOP ≤14 mm Hg and IOP reduction ≥20%) was 100% in the shunt alone procedure and 95% for the combined surgeries; the mean medicated IOP was reduced from 23.8 ± 5.3 to 10.7 ± 2.8 mm Hg in the group without phacoemulsification and to 10.7 ± 3.5 mm Hg in the combined surgery group. Further, the mean number of glaucoma medications was reduced from 2.4 ± 0.9 to 0.3 ± 0.7 in the former group and to 0.7 ± 1.1 in the latter group (Batlle et al. 2016).
Adverse events occur in 10–25% of cases and include hyphema (<10%), hypotony (10–16%), a shallow anterior chamber (4–13%), choroidal detachment or effusion (<9%), the device touching the iris (13%) and exposure of the Tenon's capsule (9%) (Sadruddin et al. 2019). As it is a bleb‐dependent procedure, bleb needling may be required in 2–10% of cases, usually within 9 months of follow‐up (Beckers & Pinchuk 2019). However, the device may substantially reduce IOP in contrast to most MIGS procedures, which are associated with only modest reductions in IOP; therefore, it can target patients with moderate‐to‐severe and refractory glaucoma. Only a few clinical trials with the PRESERFLO® MicroShunt are currently underway (NCT01881425, NCT00772330, NCT01563237, NCT02177123; http://www.clinicaltrials.gov/).
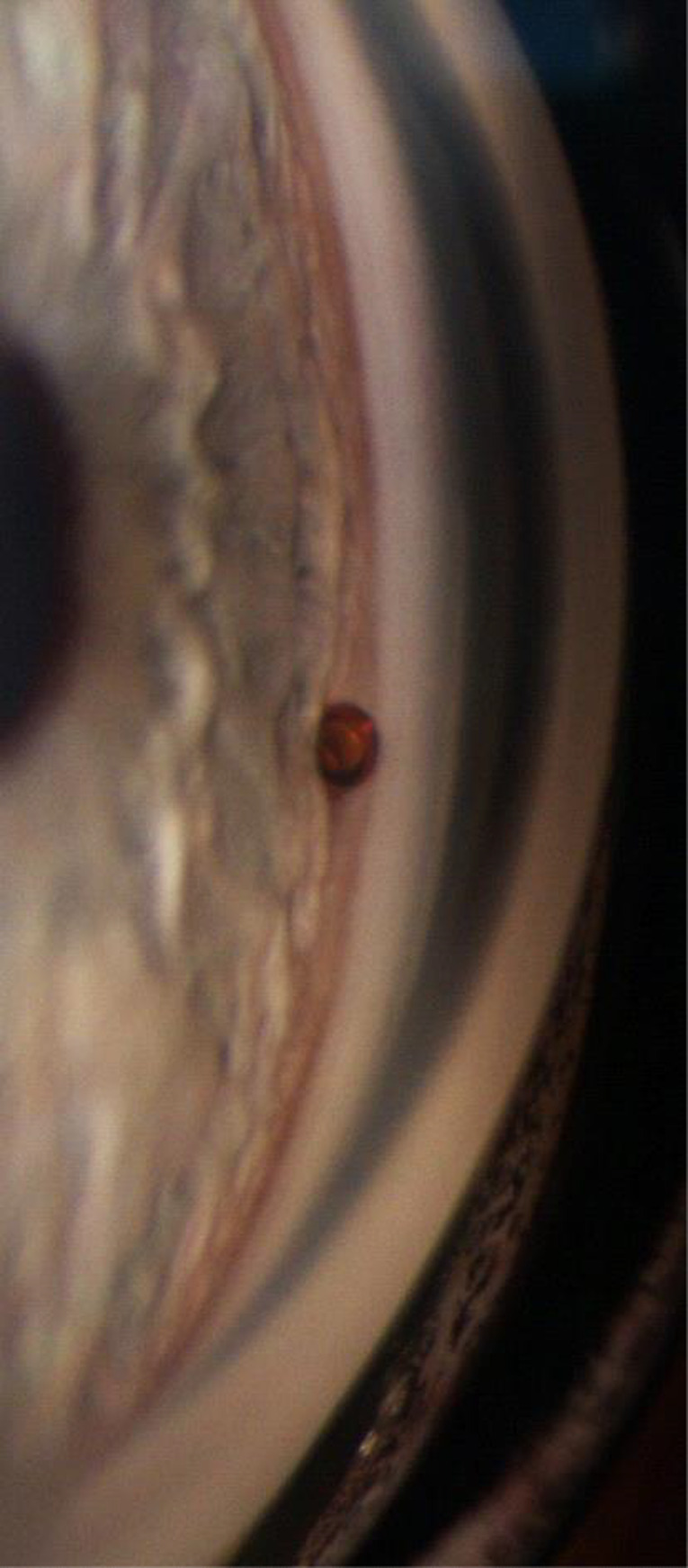
The CyPass Micro‐Stent
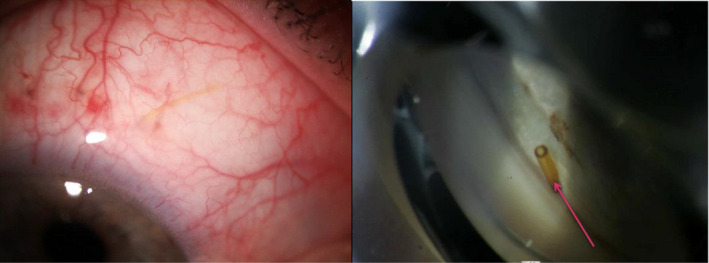
The CyPass Micro‐Stent is implanted ab interno into the suprachoroidal space and was designed to achieve controlled AH outflow from the anterior chamber into the suprachoroidal space (Figs 9, 10, 11). The FDA withdrew the device from the market in 2018 (https://www.fda.gov/medical‐devices/safety‐communications/update‐potential‐eye‐damage‐alcon‐cypass‐micro‐stent‐used‐treat‐open‐angle‐glaucoma‐fda‐safety) after the detection of a dramatic rise in endothelial cell loss (ECL) among patients who received the microstent during cataract surgery, compared with patients who underwent cataract surgery alone (Compass‐XT clinical trial, NCT02700984, https://clinicaltrials.gov/). The damage likely originated from the device's positioning within the anterior chamber's angle. The ECL correlated with the number of retention rings noted on clinical examination by gonioscopy, particularly when two or more retention rings were visible.
Fig. 9.
A CyPass® implant in a gonioscopy
Fig. 10.
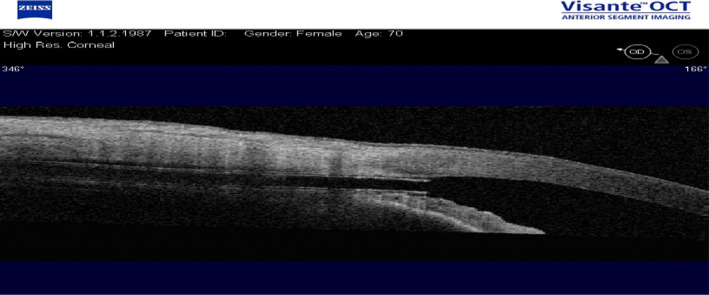
A CyPass® implant observed using an OCT Visante.
Fig. 11.

A CyPass® implant.
Discussion
The most likely point for the highest AH resistance outflow is the internal wall of SC, namely the TM. Accordingly, stent implantation through MIGS should be an effective method of treating POAG, as its IOP‐lowering effect is based on bypassing this crucial site of AH outflow resistance. This is supported by the above mentioned studies: IOP is reduced on average by 10–26% by the iStent® (Spiegel et al. 2009; Seiboldet al. 2016), up to 40% by the Hydrus® (Otarola et al. 2020), and 29–42% with the Xen® (De Gregorio et al. 2018); the PRESERFLO® MicroShunt is even better, averaging 30–55%. These studies differ widely in reported results and evidence. Notably, most involved combined procedures, rather than stand‐alone implantations.
An advantage of the mentioned techniques is ab interno access—access from the anterior chamber of the eye. It allows preservation of the conjunctiva and eliminates scar formation; furthermore, it enables future additional conjunctival surgery. The clear corneal incision used in cataract surgery makes it possible to expand the scope of the procedure to include MIGS without the need for additional incisions in the limbus. This has a small but significant influence on the patient's postoperative quality of life, and therefore, MIGS is often used in combination with phacoemulsification and intraocular lens implantation (Craven et al. 2012; Pillunat et al. 2017).
One limitation of all these procedures is that the postoperative IOP cannot fall below the episcleral venous pressure (EVP). The exact value of the EVP is difficult to evaluate, generally ranging from 7.6 mm Hg to 9.1 mm Hg (Zeimer et al. 1983; Sultan & Blondeau 2003) although it can be even higher in some glaucoma patients (Pillunat et al. 2017).
An open issue is the long‐term, IOP‐lowering effect of MIGS. Recent studies on human TM samples obtained intraoperatively from patients with previous implantation of a trabecular micro‐bypass stent (iStent®) suggest that inflammatory and fibrotic changes occur in the areas surrounding the device. These changes suggest a possible aetiology for device failure over time (Capitena Young et al., 2018). In the study with the longest follow‐up period (53.7 ± 9.3 months), the mean IOP was reduced from 19.4 ± 1.9 mm Hg preoperatively to 16.3 ± 4.2 mm Hg at the end of the observation period, indicating a 16% decrease (Arriola‐Villalobos et al. 2012). Conversely, after a 4‐year follow‐up, Fea et al. (2015) reported that the difference between the initial and final mean IOPs was not statistically significant. No SIBS obstruction have been reported for in vivo studies; however, accumulation of type IV collagen near the tubes has been observed (Sadruddin et al. 2019). At present, long‐term data regarding PRESERFLO® MicroShunt efficacy and the other aforementioned implants are not available.
Another concern involves the effect of implants on the endothelium. The effects of CyPass and ExPress shunts and Ahmed valves on the endothelium have been studied in clinical trials (NCT02700984, https://clinicaltrials.gov/; Saheb et al. 2014; Konopińska et al. 2015). Their influence on the cornea depends on various factors, including the distance from the rear surface of the cornea, the implant material, perioperative trauma and the patient's condition before surgery (Hayashi et al. 1996). Correct positioning of the implant during surgery might reduce the associated risk. In studies of animal models, it was noted that the material of the drainage device affects the degree of cell loss (Lim 2003). The exact mechanism by which implants may damage the endothelium is not fully understood. Various theories have associated damage with an increased fluid flow around the tip of the tube, inflammatory reactions in the anterior chamber, transitory contact between the tube and the cornea or between the tube and the uvea, or immune responses evoked by the presence of a foreign body in the eye (McDermott et al. 1993). Others suggest that persistently elevated IOP directly or indirectly induces hypoxia, thereby damaging the endothelium (Ollivier et al. 2003). Fiore et al. (1989) suggested that the mechanism of endothelial damage may be associated with toxic effects of medications and preservatives contained in ophthalmic drops and with the duration of treatment; these may make the anterior chamber shallower during and after the operation or change the composition of fluids related directly to the sub‐Tenon's space. Some researchers believe that patients taking three or four concurrent antiglaucoma medications have a lower endothelium cell count compared to those taking only one or two (Lass et al. 1998). In a recent prospective study of the iStent Inject®, the ECL had decreased by 15% at 12 months; however, this study did not include a control group (Gillmann et al. 2020a; Gillmann et al. 2020b). Further research on ECL after MIGS implants and with long follow‐up efficacy is clearly needed.
In most cases of MIGS, implants are positioned in the nasal quadrant as, statistically, it is the site of most CCs (Grieshaber et al. 2009). Huang et al. raised the issue of the uneven distribution of CCs around SC. In their study, canalography showed a segmental distribution of CCs, which also varied among patients. This result suggests that the hypotensive success of the iStent® may depend on the distribution of CCs (Huang et al. 2017; Huang et al. 2018). Individualizing and optimizing the site of micro‐bypass stent implantation by choosing the area with the largest number of CCs may become common practice.
Implant‐dependant MIGS has evolved rapidly over the past decade and is demonstrating effectiveness in reducing IOP and improving the management of glaucoma patients. They offer the desired reduction in medication usage in glaucoma patients, since not only importance is to reduce the IOP level, but as important is reducing the medication burden for patients. Further rigorous and standardized studies are needed for clinicians to better predict which patients will benefit most from each type of microdevice. Further research is also needed to determine the best approach for the appropriate primary mode of action (e.g. decrease AH production, increase trabecular or uveoscleral outflow) in cases where adjunctive medication is required.
Supporting information
Video S1. iStent® implantation.
Video S2. iStent inject® implantation.
Video S3. iStent inject® implantation 2.
Video S4. Hydrus® implantation.
Video S5. Xen® implantation.
We would like to thank Editage for English language editing.
References
- Achache F (2001): Anatomical features of outflow pathway. In Mermoud, A & Shaarawy, T (eds.). Non‐penetrating glaucoma surgery. London: M. Dunitz, pp. 21–32. [Google Scholar]
- Acosta AC, Espana EM, Yamamoto H et al. (2006): A newly designed glaucoma drainage implant made of poly(styrene‐b‐isobutylene‐b‐styrene): biocompatibility and function in normal rabbit eyes. Arch Ophthalmol 124: 1742–1749. [DOI] [PubMed] [Google Scholar]
- Alvarado JA, Betanzos A, Franse‐Carman L, Chen J & González‐Mariscal L (2004): Endothelia of Schlemm's canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am J Physiol Cell Physiol 286: C621–C634. [DOI] [PubMed] [Google Scholar]
- Arrieta EA, Aly M, Parrish R, Dubovy S, Pinchuk L, Kato Y, Fantes F & Parel J‐M (2011): Clinicopathologic correlations of poly‐(styrene‐b‐isobutylene‐b‐styrene) glaucoma drainage devices of different internal diameters in rabbits. Ophthalmic Surg Lasers Imaging 42: 338–345. [DOI] [PubMed] [Google Scholar]
- Arriola‐Villalobos P, Martínez‐de‐la‐Casa JM, Díaz‐Valle D, Fernández‐Pérez C, García‐Sánchez J & García‐Feijoó J (2012): Combined iStent trabecular micro‐bypass stent implantation and phacoemulsification for coexistent open‐angle glaucoma and cataract: a long‐term study. Br J Ophthalmol 96: 645–649. [DOI] [PubMed] [Google Scholar]
- Batlle JF, Fantes F, Riss I et al. (2016): Three‐year follow‐up of a novel aqueous humor microshunt. J Glaucoma 25: e58–e65. [DOI] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR & Gong H (2008): Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci 49: 5346–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers JM & Pinchuk L (2019): Minimally invasive glaucoma surgery with a new ab‐externo subconjunctival bypass – current status and review of literature. Eur Ophthalmic Rev 13: 27–30. [Google Scholar]
- Belovay GW, Naqi A, Chan BJ, Rateb M & Ahmed IIK (2012): Using multiple trabecular micro‐bypass stents in cataract patients to treat open‐angle glaucoma. J Cataract Refract Surg 38: 1911–1917. [DOI] [PubMed] [Google Scholar]
- Braakman ST, Pedrigi RM, Read AT, Smith JAE, Stamer WD, Ethier CR & Overby DR (2014): Biomechanical strain as a trigger for pore formation in Schlemm's canal endothelial cells. Exp Eye Res 127: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão LM & Grieshaber MC (2013): Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol 2013: 705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker RF (1975): The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol 14: 286–292. [PubMed] [Google Scholar]
- Brubaker RF (2003): Targeting outflow facility in glaucoma management. Surv Ophthalmol 48: S17–S20. [DOI] [PubMed] [Google Scholar]
- Camras LJ, Yuan F, Fan S, Samuelson TW, Ahmed IK, Schieber AT & Toris CB (2012): A novel Schlemm's canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci 53: 6115–6121. [DOI] [PubMed] [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (2019): Optimal use of minimally invasive glaucoma surgery: recommendations. 8 1c. Ottawa: Canadian Agency for Drugs and Technologies in Health. [PubMed] [Google Scholar]
- Capitena Young CE, Ammar DA, Seibold LK, Pantcheva MB, SooHoo JR & Kahook MY (2018): Histopathologic examination of trabecular meshwork changes after trabecular bypass stent implantation. J Glaucoma 27: 606–609. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Kim JH, Friedman DS et al. (2015): Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology 122: 1795–1801. [DOI] [PubMed] [Google Scholar]
- Craven RE, Katz JL, Wells JM & Giamporcaro JE; iStent Study Group (2012): Cataract surgery with trabecular micro‐bypass stent implantation in patients with mild‐to‐moderate open‐angle glaucoma and cataract: two‐year follow‐up. J Cataract Refract Surg 38: 1339–1345. [DOI] [PubMed] [Google Scholar]
- Dautriche CN, Tian Y, Xie Y & Sharfstein ST (2015): A closer look at Schlemm's canal cell physiology: implications for biomimetics. J Funct Biomater 6: 963–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio A, Pedrotti E, Russo L & Morselli S (2018): Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol 38: 1129–1134. [DOI] [PubMed] [Google Scholar]
- Dervenis N, Mikropoulou AM, Dervenis P & Lewis A (2017): Dislocation of a previously successful XEN glaucoma implant into the anterior chamber: a case report. BMC Ophthalmol 17: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietlein TS, Jacobi PC, Lüke C & Krieglstein GK (2000): Morphological variability of the trabecular meshwork in glaucoma patients: implications for non‐perforating glaucoma surgery. Br J Ophthalmol 84: 1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AT, Parikh H & Panarelli JF (2020): Subconjunctival microinvasive glaucoma surgeries: an update on the Xen gel stent and the PreserFlo MicroShunt. Curr Opin Ophthalmol 31: 132–138. [DOI] [PubMed] [Google Scholar]
- Dvorak‐Theobald G (1955): Further studies on the canal of Schlemm; its anastomoses and anatomic relations. Am J Ophthalmol 39: 65–89. [DOI] [PubMed] [Google Scholar]
- Emi K, Pederson JE & Toris CB (1989): Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci 30: 233–238. [PubMed] [Google Scholar]
- Epstein DL & Rohen JW (1991): Morphology of the trabecular meshwork and inner‐wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci 32: 160–171. [PubMed] [Google Scholar]
- Esfandiari H, Taubenslag K, Shah P et al. (2019): Two‐year data comparison of ab interno trabeculectomy and trabecular bypass stenting using exact matching. J Cataract Refract Surg 45: 608–614. [DOI] [PubMed] [Google Scholar]
- Ethier CR (2002): The inner wall of Schlemm's canal. Exp Eye Res 74: 161–172. [DOI] [PubMed] [Google Scholar]
- Fea AM (2010): Phacoemulsification versus phacoemulsification with micro‐bypass stent implantation in primary open‐angle glaucoma: randomized double‐masked clinical trial. J Cataract Refract Surg 36: 407–412. [DOI] [PubMed] [Google Scholar]
- Fea AM, Belda JI, Rękas M et al. (2014): Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open‐angle glaucoma. Clin Ophthalmol 8: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fea AM, Consolandi G, Zola M, Pignata G, Cannizzo P, Lavia C, Rolle T & Grignolo FM (2015): Micro‐bypass implantation for primary open‐angle glaucoma combined with phacoemulsification: 4‐year follow‐up. J Ophthalmol 2015 : 795357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson TJ, Berdahl JP, Schweitzer JA & Sudhagoni RG (2016): Clinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open‐angle glaucoma and cataract. Clin Ophthalmol 10: 1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson TJ, Swan R, Ibach M, Schweitzer J, Sudhagoni R & Berdahl JP (2017): Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg 43: 622–626. [DOI] [PubMed] [Google Scholar]
- Fernández‐Barrientos Y, García‐Feijoó J, Martínez‐de‐la‐Casa JM, Pablo LE, Fernández‐Pérez C & García Sánchez J (2010): Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci 51: 3327–3332. [DOI] [PubMed] [Google Scholar]
- Fiore PM, Richter CU, Arzeno G, Arrigg CA, Shingleton BJ, Bellows AR & Hutchinson BT (1989): The effect of anterior chamber depth on endothelial cell count after filtration surgery. Arch Ophthalmol 107: 1609–1611. [DOI] [PubMed] [Google Scholar]
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR & Smith SD (2011): Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 118: 1466–1480. [DOI] [PubMed] [Google Scholar]
- Fuchs E (1900): Detachment of the choroid after cataract operation. Arch f Ophth 1: 199. [Google Scholar]
- Gaasterland D, Kupfer C, Milton R, Ross K, McCain L & MacLellan H (1978): Studies of aqueous humour dynamics in man VI. Effect of age upon parameters of intraocular pressure in normal human eyes. Exp Eye Res 26: 651–656. [DOI] [PubMed] [Google Scholar]
- Galal A, Bilgic A, Eltanamly R & Osman A (2017): XEN glaucoma implant with mitomycin C 1‐year follow‐up: result and complications. J Ophthalmol 2017: 5457246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo M, Supnet RA, Giamporcaro JE & Hornbreak DM (2016): Outcomes of combined trabecular micro‐bypass and phacoemulsification in a predominantly Hispanic patient population. Clin Ophthalmol 10: 1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ & Schiffman JC (2012): Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow‐up. Am J Ophthalmol 153: 804.e1–814.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile RC, Pavlin CJ, Liebmann JM, Easterbrook M, Tello C, Foster FS & Ritch R (1996): Diagnosis of traumatic cyclodialysis by ultrasound biomicroscopy. Ophthalmic Surg Lasers 27: 97–105. [PubMed] [Google Scholar]
- Gillmann K, Bravetti GE, Rao HL, Mermoud A & Mansouri K (2020): Combined and stand‐alone XEN 45 gel stent implantation: 3‐year outcomes and success predictors. Acta Ophthalmol. 10.1111/aos.14605 [DOI] [PubMed] [Google Scholar]
- Gillmann K, Mansouri K, Ambresin A, Bravetti GE & Mermoud A (2020): A prospective analysis of iStent Inject microstent implantation: surgical outcomes, endothelial cell density, and device position at 12 Months. J Glaucoma 29: 639–647. [DOI] [PubMed] [Google Scholar]
- Goel M, Picciani RG, Lee RK & Bhattacharya SK (2010): Aqueous humor dynamics: a review. Open Ophthalmol J 4: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W, Lind JT & Sheybani A (2018): Review of the Xen Gel Stent and InnFocus MicroShunt. Curr Opin Ophthalmol 29: 162–170. [DOI] [PubMed] [Google Scholar]
- Grierson I, Saheb H, Kahook MY, Johnstone MA, Ahmed IIK, Schieber AT & Toris CB (2015): A novel Schlemm's canal scaffold: histologic observations. J Glaucoma 24: 460–468. [DOI] [PubMed] [Google Scholar]
- Grieshaber MC, Pienaar A, Olivier J & Stegmann R (2009): Channelography: imaging of the aqueous outflow pathway with flexible microcatheter and fluorescein in canaloplasty. Klin Monbl Augenheilkd 226: 245–248. [DOI] [PubMed] [Google Scholar]
- Grieshaber MC, Pienaar A, Olivier J & Stegmann R (2010): Clinical evaluation of the aqueous outflow system in primary open‐angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci 51: 1498–1504. [DOI] [PubMed] [Google Scholar]
- Gulati V, Fan S, Hays CL, Samuelson TW, Ahmed IIK & Toris CB (2013): A novel 8‐mm Schlemm's canal scaffold reduces outflow resistance in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci 54: 1698–1704. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Hayashi H, Nakao F & Hayashi F (1996): Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg 22: 1079–1084. [DOI] [PubMed] [Google Scholar]
- Heine L (1905): Die cyklodialyse, eine neue glaukomoperation. Dtsch med Wschr 31: 824. [Google Scholar]
- Huang AS, Camp A, Xu BY, Penteado RC & Weinreb RN (2017): Aqueous angiography: aqueous humor outflow imaging in live human subjects. Ophthalmology 124: 1249–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AS, Penteado RC, Saha SK, Do JL, Ngai P, Hu Z & Weinreb RN (2018): Fluorescein aqueous angiography in live normal human eyes. J Glaucoma 27: 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M (2006): What controls aqueous humour outflow resistance? Exp Eye Res 82: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MC & Kamm RD (1983): The role of Schlemm's canal in aqueous outflow from the human eye. Invest Ophthalmol Vis Sci 24: 320–325. [PubMed] [Google Scholar]
- Johnstone MA (1979): Pressure‐dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm's canal. Invest Ophthalmol Vis Sci 18: 44–51. [PubMed] [Google Scholar]
- Johnstone MA & Grant WG (1973): Pressure‐dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol 75: 365–383. [DOI] [PubMed] [Google Scholar]
- Johnstone M, Martin E & Jamil A (2011): Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp Eye Res 92: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan JF, Dietlein TS, Dinslage S, Lüke C, Konen W & Krieglstein GK (2007): Cyclodialysis ab interno as a surgical approach to intractable glaucoma. Graefes Arch Clin Exp Ophthalmol 245: 1071–1076. [DOI] [PubMed] [Google Scholar]
- Katz LJ, Erb C, Carceller GA, Fea AM, Voskanyan L, Wells JM & Giamporcaro JE (2015): Prospective, randomized study of one, two, or three trabecular bypass stents in open‐angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol 9: 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopińska J, Deniziak M, Saeed E, Bartczak A, Zalewska R, Mariak Z & Rekas M (2015): Prospective randomized study comparing combined Phaco‐ExPress and phacotrabeculectomy in open angle glaucoma treatment: 12‐month follow‐up. J Ophthalmol 2015: 720109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda‐Guirao T, Cubero‐Parra JM & Hidalgo‐Torres A (2020): Efficacy and safety of XEN 45 gel stent alone or in combination with phacoemulsification in advanced open angle glaucoma patients: 1‐year retrospective study. Int J Ophthalmol 13: 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass JH, Khosrof SA, Laurence JK, Horwitz B, Ghosh K & Adamsons I (1998): A double‐masked, randomized, 1‐year study comparing the corneal effects of dorzolamide, timolol, and betaxolol. Dorzolamide Corneal Effects Study Group. Arch Ophthalmol 116: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Lavin‐Dapena C, Cordero‐Ros R, D'Anna O & Mogollón I (2020): XEN 63 gel stent device in glaucoma surgery: a 5‐years follow‐up prospective study. Eur J Ophthalmol 18: 1120672120952033. [DOI] [PubMed] [Google Scholar]
- Le JT, Bicket AK, Wang L & Li T (2019): Ab interno trabecular bypass surgery with iStent for open‐angle glaucoma. Cochrane Database Syst Rev 3: CD012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K & Saheb H (2014): iStent trabecular micro‐bypass stent for open‐angle glaucoma. Clin Ophthalmol 8: 1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RA (2014): Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg 40: 1301–1306. [DOI] [PubMed] [Google Scholar]
- Lim KS (2003): Corneal endothelial cell damage from glaucoma drainage device materials. Cornea 22: 352–354. [DOI] [PubMed] [Google Scholar]
- Mansouri K & Shaarawy T (2015): Update on Schlemm's canal based procedures. Middle East Afr J Ophthalmol 22: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott ML, Swendris RP, Shin DH, Juzych MS & Cowden JW (1993): Corneal endothelial cell counts after Molteno implantation. Am J Ophthalmol 115: 93–96. [DOI] [PubMed] [Google Scholar]
- Morrison JC & Pollack IP (eds.) (2003): Glaucoma: science and practice. New York, NY: Thieme. Available at: http://4eyes.gr/images/4eyes/pdf/glaucoma/Glaucoma__Science_and_Practice.pdf. (Accessed on 17 Dec 2020). [Google Scholar]
- Myers JS, Masood I, Hornbeak DM et al. (2018): Prospective evaluation of two iStent® trabecular stents, one iStent Supra® suprachoroidal stent, and postoperative prostaglandin in refractory glaucoma: 4‐year outcomes. Adv Ther 35: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier FJ, Brooks DE, Komaromy AM, Kallberg ME, Andrew SE, Sapp Hl, Sherwood Mb & Dawson WW (2003): Corneal thickness and endothelial cell density measured by non‐contact specular microscopy and pachymetry in Rhesus macaques (Macaca mulatta) with laser‐induced ocular hypertension. Exp Eye Res 76: 671–677. [DOI] [PubMed] [Google Scholar]
- Otarola F, Virgili G, Shah A, Hu K, Bunce C & Gazzard G (2020): Ab interno trabecular bypass surgery with Schlemm´s canal microstent (Hydrus) for open angle glaucoma. Cochrane Database Syst Rev 3: CD012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar A, Aras C & Karacorlu M (2003): Suprachoroidal seton implantation in refractory glaucoma: a novel surgical technique. J Glaucoma 12: 354–359. [DOI] [PubMed] [Google Scholar]
- Parc CE, Johnson DH & Brilakis HS (2000): Giant vacuoles are found preferentially near collector channels. Invest Ophthalmol Vis Sci 41: 2984–2990. [PubMed] [Google Scholar]
- Pederson JE, Gaasterland DE & MacLellan HM (1977): Uveoscleral aqueous outflow in the rhesus monkey: importance of uveal reabsorption. Invest Ophthalmol Vis Sci 16: 1008–1017. [PubMed] [Google Scholar]
- Pérez‐Torregrosa VT, Olate‐Pérez Á, Cerdà‐Ibáñez M, Gargallo‐Benedicto A, Osorio‐Alayo V, Barreiro‐Rego A & Duch‐Samper A (2016): Cirugía combinada mediante facoemulsificación e implante XEN45 con acceso temporal y 2 únicas incisiones. Arch Soc Esp Oftalmol 91: 415–421. [DOI] [PubMed] [Google Scholar]
- Pfeiffer N, Garcia‐Feijoo J, Martinez‐de‐la‐Casa JM et al. (2015): A randomized trial of a Schlemm's canal microstent with phacoemulsification for reducing intraocular pressure in open‐angle glaucoma. Ophthalmology 122: 1283–1293. [DOI] [PubMed] [Google Scholar]
- Pillunat LE, Erb C, Jünemann AG & Kimmich F (2017): Micro‐invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol 11: 1583–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk L, Riss I, Batlle JF et al. (2017): The development of a micro‐shunt made from poly(styrene‐block‐isobutylene‐block‐styrene) to treat glaucoma. J Biomed Mater Res B Appl Biomater 105: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razeghinejad MR & Spaeth GL (2011): A history of the surgical management of glaucoma. Optom Vis Sci 88: E39–E47. [DOI] [PubMed] [Google Scholar]
- Rosenquist R, Epstein D, Melamed S, Johnson M & Grant WM (1989): Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res 8: 1233–1240. [DOI] [PubMed] [Google Scholar]
- Sadruddin O, Pinchuk L, Angeles R & Palmberg P (2019): Ab externo implantation of the MicroShunt, a poly (styrene‐block‐isobutylene‐ block‐styrene) surgical device for the treatment of primary open‐angle glaucoma: a review. Eye Vis (Lond) 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheb H & Ahmed IIK (2012): Micro‐invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 23: 96–104. [DOI] [PubMed] [Google Scholar]
- Saheb H, Gedde SJ, Schiffman JC, Feuer WJ; Tube Versus Trabeculectomy Study Group (2014): Outcomes of glaucoma reoperations in the Tube Versus Trabeculectomy (TVT) Study. Am J Ophthalmol 157: 1179.e2–1189.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi A, Lapointe J & Harasymowycz P (2019): One‐year outcomes of second‐generation trabecular microbypass stents (iStent Inject) implantation with cataract surgery in different glaucoma subtypes and severities. Ophthalmol Ther 8: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson TW, Katz LJ, Wells JM, Duh Y‐J, Giamporcaro JE; US iStent Study Group (2011): Randomized evaluation of the trabecular micro‐bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 118: 459–467. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Kastner C, Sternberg K, Allemann R, Löbler M, Guthoff R & Schmitz KP (2013): New concepts for glaucoma implants–controlled aqueous humor drainage, encapsulation prevention and local drug delivery. Curr Pharm Biotechnol 14: 98–111. [PubMed] [Google Scholar]
- Seibold LK, Gamett KM, Kennedy JB, Mulvahill MJ, Kroehl ME, SooHoo JR, Pantcheva MB & Kahook MY (2016): Outcomes after combined phacoemulsification and trabecular microbypass stent implantation in controlled open‐angle glaucoma. J Cataract Refract Surg 42: 1332–1338. [DOI] [PubMed] [Google Scholar]
- Sheybani A, Dick HB & Ahmed IIK (2016): Early clinical results of a novel ab interno gel stent for the surgical treatment of open‐angle glaucoma. J Glaucoma 25: e691–e696. [DOI] [PubMed] [Google Scholar]
- Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H & Ahmed IIK (2015): Phacoemulsification combined with a new ab interno gel stent to treat open‐angle glaucoma: pilot study. J Cataract Refract Surg 41: 1905–1909. [DOI] [PubMed] [Google Scholar]
- Smith M, Charles R, Abdel‐Hay A et al. (2019): 1‐year outcomes of the Xen45 glaucoma implant. Eye (Lond) 33: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltau JB, Rothman RF, Budenz DL, Greenfield DS, Feuer W, Liebmann JM & Ritch R (2000): Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol 118: 338–342. [DOI] [PubMed] [Google Scholar]
- SooHoo JR, Seibold LK, Radcliffe NM & Kahook MY (2014): Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol 49: 528–533. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Wetzel W, Neuhann T, Stürmer J, Höh H, García‐Feijoo J, Martínez‐De‐La‐Casa JM & García‐Sánchez J (2009): Coexistent primary open‐angle glaucoma and cataract: interim analysis of a trabecular micro‐bypass stent and concurrent cataract surgery. Eur J Ophthalmol 19: 393–399. [DOI] [PubMed] [Google Scholar]
- Sultan M & Blondeau P (2003): Episcleral venous pressure in younger and older subjects in the sitting and supine positions. J Glaucoma 12: 370–373. [DOI] [PubMed] [Google Scholar]
- Tamm ER (2009): The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res 88: 648–655. [DOI] [PubMed] [Google Scholar]
- Ten Hulzen RD & Johnson DH (1996): Effect of fixation pressure on juxtacanalicular tissue and Schlemm's canal. Invest Ophthalmol Vis Sci 37: 114–124. [PubMed] [Google Scholar]
- Toris CB (2010): Pharmacotherapies for glaucoma. Curr Mol Med 10: 824–840. [DOI] [PubMed] [Google Scholar]
- Van Buskirk EM (1982): Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest Ophthalmol Vis Sci 22: 625–632. [PubMed] [Google Scholar]
- Wagner FM, Schuster AK, Emmerich J, Chronopoulos P & Hoffmann EM (2020): Efficacy and safety of XEN®‐Implantation vs. trabeculectomy: data of a "real‐world" setting. PLoS One 15: e0231614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Read AT, Sulchek T & Ethier CR (2017): Trabecular meshwork stiffness in glaucoma. Exp Eye Res 158: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler NS & Fautsch MP (2014): Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther 30: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Schieber AT, Camras LJ, Harasymowycz PJ, Herndon LW & Allingham RR (2016): Mathematical modeling of outflow facility increase with trabecular meshwork bypass and Schlemm canal dilation. J Glaucoma 25: 355–364. [DOI] [PubMed] [Google Scholar]
- Zeimer RC, Gieser DK, Wilensky JT, Noth JM, Mori MM & Odunukwe EE (1983): A practical venomanometer. Measurement of episcleral venous pressure and assessment of the normal range. Arch Ophthalmol 101: 1447–1449. [DOI] [PubMed] [Google Scholar]
- Zhou J & Smedley GT (2005): A trabecular bypass flow hypothesis. J Glaucoma 14: 74–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. iStent® implantation.
Video S2. iStent inject® implantation.
Video S3. iStent inject® implantation 2.
Video S4. Hydrus® implantation.
Video S5. Xen® implantation.


