Abstract
Background and Aims
There are no prospective data on stereotactic body radiation therapy (SBRT) as a bridge to liver transplantation for HCC. This study aimed to evaluate the efficacy and safety of SBRT as bridging therapy, with comparison with transarterial chemoembolization (TACE) and high‐intensity focused ultrasound (HIFU).
Approach and Results
Patients were prospectively enrolled for SBRT under a standardized protocol from July 2015 and compared with a retrospective cohort of patients who underwent TACE or HIFU from 2010. The primary endpoint was tumor control rate at 1 year after bridging therapy. Secondary endpoints included cumulative incidence of dropout, toxicity, and posttransplant survival.
During the study period, 150 patients were evaluated (SBRT, n = 40; TACE, n = 59; HIFU, n = 51). The tumor control rate at 1 year was significantly higher after SBRT compared with TACE and HIFU (92.3%, 43.5%, and 33.3%, respectively; P = 0.02). With competing risk analysis, the cumulative incidence of dropout at 1 and 3 years after listing was lower after SBRT (15.1% and 23.3%) compared with TACE (28.9% and 45.8%; P = 0.034) and HIFU (33.3% and 45.1%; P = 0.032). Time‐to‐progression at 1 and 3 years was also superior after SBRT (10.8%, 18.5% in SBRT, 45%, 54.9% in TACE, and 47.6%, 62.8% in HIFU; P < 0.001). The periprocedural toxicity was similar, without any difference in perioperative complications and patient and recurrence‐free survival rates after transplant. Pathological complete response was more frequent after SBRT compared with TACE and HIFU (48.1% vs. 25% vs. 17.9%, respectively; P = 0.037). In multivariable analysis, tumor size <3 cm, listing alpha‐fetoprotein <200 ng/mL, Child A, and SBRT significantly reduced the risk of dropout.
Conclusions
SBRT was safe, with a significantly higher tumor control rate, reduced the risk of waitlist dropout, and should be used as an alternative to conventional bridging therapies.
Abbreviations
- AFP
alpha‐fetoprotein
- CR
complete response
- DDLT
deceased donor liver transplantation
- ECOG
Eastern Cooperative Oncology Group
- HIFU
high‐intensity focused ultrasound
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- mRECIST
Modified Response Evaluation Criteria in Solid Tumors
- OS
overall survival
- pCR
pathological complete response
- PR
partial response
- RFA
radio frequency ablation
- SBRT
stereotactic body radiation therapy
- SD
stable disease
- TACE
transarterial chemoembolization
- TTP
time‐to‐progression
Liver transplantation (LT) is the best treatment option for selected patients with early HCC.( 1 , 2 ) The implementation of the Model for End‐Stage Liver Disease (MELD) exception points for patients with HCC aimed to alleviate the disparity of access to LT between patients with and without HCC.( 3 ) Given the shortage of donor organs with increasing time on the waitlist, it is recommended that locoregional treatment be applied if the anticipated waiting time is longer than 6 months.( 4 , 5 )
The aims of bridging therapy include the prevention of tumor progression and the reduction in the rate of dropout. Furthermore, the response to bridging therapy might predict posttransplant recurrence.( 6 , 7 ) Transarterial chemoembolization (TACE) is one of the most widely used bridging therapies, and studies on explant pathology have shown tumor necrosis rates of 27%‐57% in patients with HCC within the Milan criteria.( 8 ) However, the use of TACE carries the risk of contrast nephropathy and endothelial injury that could lead to increased risk of hepatic artery complication.( 9 , 10 ) Although other locoregional therapies, such as high‐intensity focused ultrasound (HIFU), increase the eligibility for bridging therapy in candidates with HCC by up to 80%,( 11 ) it has not been shown to reduce dropout rates.( 11 , 12 )
Recently, the use of stereotactic body radiation therapy (SBRT) has been shown to be more effective for local control and prolonged the survival of patients with nonresectable HCC when compared with TACE alone.( 13 ) SBRT employs a few fractions of potent doses of highly conformal radiation therapy with high geometric precision and accuracy in tumor tracking and motion management, thus being able to deliver a high tumoricidal dose to tumors while limiting radiation to surrounding critical normal tissues. Four prospective series have shown a 1‐year local control rate of 87%‐100% in patients with nonresectable HCC.( 14 , 15 , 16 , 17 ) Several retrospective studies on the use of SBRT as a bridge to LT have shown it to be safe, with similar tumor control and dropout rates compared with conventional bridging therapies.( 18 , 19 , 20 ) To the best of our knowledge, there are currently no prospective data on the use of SBRT as bridging therapy for LT candidates. In this study, we investigated the efficacy and safety of SBRT as bridging therapy for candidates with HCC on the LT waitlist.
Patients and Methods
This is a prospective study with patients recruited from Queen Mary Hospital, The University of Hong Kong, from 1 July 2015 to 1 March 2020. The study was approved by the Institutional Review Board of the University of Hong Kong (UW15‐191) and registered at clinicaltrials.gov (NCT03950102). In this prospective study, SBRT was adopted as the primary bridging therapy, and all patients were initially assessed for feasibility of SBRT. Data analysis was carried out in March 2021 to ensure that the time between recruitment to data analysis for all patients was more than 1 year. This study was conducted according to the Declaration of Helsinki and informed consents were obtained from all patients. No donor organs were obtained from executed prisoners or other institutionalized persons.
The outcomes of SBRT was compared with a control group of patients who received TACE or HIFU as bridging therapy, which were the standard bridging therapies at our center before the commencement of this trial.( 11 )
Study Objectives
The primary outcome of this study was the tumor control rate at 1 year. Tumor control was defined as complete response (CR), partial response (PR), or stable disease (SD), whereas objective response was defined as CR or PR according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST).( 21 ) The mRECIST was adopted given that the tumor necrosis achieved by locoregional therapy might not correspond to an immediate reduction in tumor size.( 21 , 22 )
The secondary outcomes included the cumulative incidence of dropout after bridging therapy while accounting for competing risks. Dropout was defined as death before transplant, delisting due to tumor progression, or being too sick for transplant. Other secondary outcomes included the extent of pathological tumor necrosis, in particular, the percentage of pathological CR (pCR) in the liver explant, and the safety of SBRT including treatment toxicities, perioperative complications, and long‐term outcomes after transplant.
The inclusion and exclusion criteria were defined as follows:
Inclusion Criteria
Patients with HCC who were accepted on transplant waitlist;
Child score ≤8;
Adequate hematological function defined as absolute neutrophil count ≥ 1.0 × 109/L, platelet ≥20 × 109/L and hemoglobin ≥8 g/dL;
Eastern Cooperative Oncology Group (ECOG) performance status ≤2.
Exclusion Criteria
Age <18 years old;
Extrahepatic metastasis;
Radiological vascular invasion;
Previous radiotherapy to liver;
Positive pregnancy test;
Unwilling or unable to adhere to study requirements and procedure.
Prioritization of Deceased Donor LT Waitlist and MELD Exception
All patients were evaluated by a multidisciplinary team that consisted of surgeons, clinical oncologists, hepatologists, and radiologists before placement on the deceased donor LT (DDLT) waitlist. Besides eligibility to transplant candidacy, the choice of bridging therapy was also discussed in this meeting. The diagnosis of HCC was made based on typical dynamic imaging according to the American Association for the Study of Liver Disease.( 5 ) The University of California San Francisco (UCSF) criteria were used for DDLT selection.( 2 ) A MELD exception point of 18 was granted to patients with T2 HCC (solitary tumor 2‐5 cm, or 2‐3 tumors and each ≤3 cm) who remained within stage T2 for ≥6 months after diagnosis. An additional 2 MELD scores was granted every 3 months if the tumors remained within T2 stage.( 23 ) The HCC selection criteria and MELD exception allocation system has not changed since 2010 in our center.
Before the commencement of this trial, the standard bridging therapies used were TACE and HIFU. All patients were discussed at the multidisciplinary meeting, and the decision and choice of bridging therapy was made based on patient’s liver function and whether ascites and portal vein thrombosis was present. In this study, patients who underwent SBRT under the prospective study protocol were analyzed in the SBRT group. Patients in the retrospective cohort were analyzed according to the bridging therapy that was decided on in the multidisciplinary meeting at the time of transplant evaluation. All bridging therapies were done after transplant evaluation. The time of enrollment of the study was defined as the time of listing. Radio frequency ablation (RFA) was not used as bridging therapy, as it is primarily used as a curative treatment for patients with HCC not requiring LT.( 24 )
Prospective Cohort—SBRT as Bridging Therapy
Patients were potentially eligible for SBRT if they fulfilled the study inclusion and exclusion criteria. Our center primarily adopted the Radiation Therapy Oncology Group 1112 trial protocol that aimed to deliver to the highest possible tumoricidal radiation dosage to HCC.( 25 , 26 ) The final eligibility for SBRT was confirmed after planning CT, tumor contouring by radiation oncologists, and dosimetrists’ assessment for tumor and organ‐at‐risk (OAR). The procedure and dose constraints of SBRT was presented in the Supporting Information. No patient received palliative RT (i.e., 8 Gy in single fraction).( 27 )
Retrospective Control—TACE
Patients were selected for TACE if they fulfilled the following criteria: absence of main portal vein thrombosis, no significant ascites or recurrent hepatic encephalopathy, Child A, adequate coagulation profile as defined by international normalized ratio <1.5 and platelet ≥80 × 109/L, estimated glomerular filtration rate ≥45 mL/min, and ECOG ≤ 2. Procedure of TACE was standardized as reported.( 13 ) Reassessment imaging was performed after 2 rounds of TACE at 8‐10 weeks interval.
Retrospective Control—HIFU
Patients who had ascites, pleural effusion, borderline liver function, and thrombocytopenia <80 × 109/L would be offered HIFU. The selection criteria for HIFU included absence of recurrent hepatic encephalopathy, bilirubin ≤100 μmol/L, subcutaneous tissue thickness ≤3.5 cm, clearly visualized tumor at screening, and ECOG ≤2. Because fluid medium can facilitate the visualization of tumor and the propagation of ultrasound waves, ascites was not a contraindication for HIFU. For tumor located at the dome of liver, artificial right pleural effusion was usually required. The procedure of HIFU is described in detail in the Supporting Information.
Evaluation of Radiological Disease Control
Contrast imaging was performed every 3 months while on the waiting list for transplant. All radiological reporting was performed according to the mRECIST by at least two radiologists who specialized in HCC and transplant radiology and was reviewed in a biweekly multidisciplinary meeting.( 21 )
Waitlist Dropout and Assessment for Treated HCC
Patients were delisted (i.e., dropout) once HCC stage progressed beyond UCSF criteria. Downstaging policy has not been adopted in our center. In candidates in whom HCC was the sole indication for transplant, absence of active HCC (i.e., CR according to mRECIST and normal alpha‐fetoprotein [AFP] level) consistently for at least 1 year after bridging therapy and actual MELD <15 were considered “treated” and were removed from waitlist. These patients were closely followed up with regular surveillance.
Toxicity and Perioperative Complications After Bridging Therapies
Safety of bridging therapies were measured according to toxicity graded with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. The need and reason for readmission up to 30 days after bridging therapy was recorded. Radiation‐induced liver disease (RILD) was defined as anicteric hepatomegaly and ascites, or elevated liver aminotransferases >5 times the upper limit of normal, or worsening Child score by 2 from baseline that occurred within 3 months after SBRT.( 28 ) Perioperative complications were graded according to the Clavien‐Dindo classification.( 29 ) The extent and severity of peritoneal adhesion as a result of bridging therapies were documented by the operating surgeons.
Explant Histopathology Analysis
All explants were fixed in 10% formalin and processed with hematoxylin and eosin–stained sections. The explant was sliced in 5‐mm contiguous slices to identify small lesions. All liver lesions identified on pretransplant imaging were reviewed by pathologists who specialized in HCC and transplant pathology. Necrosis of tumor was evaluated under microscopy, and pCR was defined as 100% tumor necrosis in the absence of viable tumor cells. For patients who had multiple tumors, pCR was defined as 100% tumor necrosis in all tumor foci found in the explant.
Statistics
Comparison between groups was done using the chi‐squared or Mann‐Whitney U test when appropriate. Dropout was defined as delisting due to any cause except for treated HCC. The cumulative risk of dropout was estimated by competing risk analysis: patients who underwent LT or had treated HCC were considered to have the competing event.( 30 ) Overall survival (OS) was calculated from the time of listing to death from any cause. Time‐to‐progression (TTP) was calculated from the time of listing and from the time of first bridging therapy to radiological progression. Posttransplant OS and recurrence‐free survival rates were assessed from the time of LT to death from any cause and tumor recurrence, respectively. Survival rates were analyzed using the Kaplan‐Meier method and compared using the log‐rank test. The Fine‐Gray competing risk model with subdistribution hazard ratio was used to identify factors that predicted dropout.( 31 ) Patients who had LT or treated HCC were modelled as competing events. Univariable analysis was performed using factors related to patient demographics, tumor stage, liver function, AFP level, and bridging therapy. Significant factors from univariate analysis (P < 0.1) were entered for multivariable analysis. As a result of a longer follow‐up time in the retrospective control, sensitivity analysis was performed to consider the effect of follow‐up time to endpoint events, i.e., progression and survival. Only events that occurred up to the median follow‐up time of the SBRT group were analyzed. Another sensitivity analysis to include only patients who were eligible for all three bridging therapies was performed. Statistical significance was defined as P value <0.05, and all tests were performed two‐tailed. All calculations were done using SPSS version 22 and R 4.0.2.
Sample Size Calculation
Sample size calculation was performed based on the 1‐year tumor control rate after SBRT (87%‐100%) for nonresectable HCC in 4 prospective studies,( 15 , 16 , 17 ) compared with that for TACE and HIFU, which was 41.2%.( 11 ) The overall effective size was 0.46 (87%‐41.2%). In order to detect a 2‐tailed statistical significance of 0.05 and power of 0.8, 40 patients are required. Taking into consideration that approximately 15%‐20% of patients might have screen failure for SBRT, we intended to recruit 47 patients into this study.
Results
During study period, 47 patients were evaluated for SBRT. Seven (14.9%) patients were unsuitable for SBRT: 3 were due to mean liver dose exceeding the dose acceptance criteria, 2 had tumors with close proximity to duodenum and stomach, and 2 had discrepancy of tumor position on planning and treatment CT because of irregular breathing pattern and ascites. Among these 7 patients, 5 had TACE and 2 had HIFU as bridging therapy.
From January 2010, 192 patients with HCC were accepted on the waitlist and 150 (78.1%) underwent bridging therapies (Fig. 1). Of these, 40 patients underwent SBRT during the study period, and 51 and 59 patients had HIFU and TACE, respectively. The median time of bridging therapy was done at 39 days after listing in the SBRT group, 32 days in the HFU, and 30.5 days in the TACE group (P = 0.809). At the time of analysis, all patients had a recruitment to follow‐up time for at least 1 year. Forty‐two (21.9%) patients had poor liver function and were deemed unsuitable for any treatment.
FIG. 1.
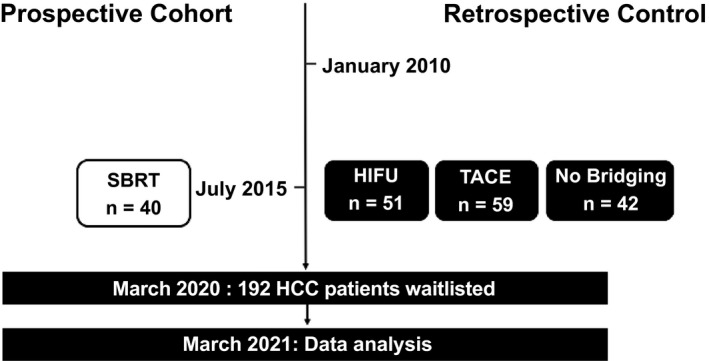
Patient recruitment and distribution.
Patient demographics were presented in Table 1. Patients with TACE had significantly better liver function compared with SBRT and HIFU as reflected by the MELD score (10 vs. 13 vs. 12, respectively; P < 0.001) and proportion of Child A stage (61% vs. 52.5% vs. 33.3%, respectively; P = 0.001). There was no difference in tumor number and AFP level at listing. There was a trend for larger tumor size in the SBRT group compared with the TACE group (2.8 cm vs. 2.2 cm, respectively; P = 0.055) without any difference between SBRT and HIFU groups (2.8 cm vs. 2.6 cm, respectively; P = 0.911).
TABLE 1.
Clinical Characteristics of All Patients
| At Listing | SBRT (n = 40) | TACE (n = 59) | HIFU (n = 51) | P Value |
|---|---|---|---|---|
| Age (years) | 59.6 (36‐69) | 58.1 (42‐69) | 59.5 (38‐68) | 0.617 |
| Sex, male, (n, %) | 26 (65.0) | 50 (84.7) | 41 (80.4) | 0.025 |
| BMI (kg/m2) | 26.1 (19.7‐41.9) | 25.0 (18.1‐44.3) | 24.0 (15.6‐34.1) | 0.144 |
| HBV (n, %) | 34 (85.0) | 45 (76.3) | 41 (80.4) | 0.287 |
| HCV (n, %) | 3 (7.5) | 8 (13.6) | 9 (17.6) | 0.462 |
| MELD at listing | 13 (11‐15) | 10 (9‐12) | 12 (10‐16) | <0.001 |
| Child‐Turcotte‐Pugh (n, %) | 0.001 | |||
| Child A | 21 (52.5) | 36 (61.0) | 17 (33.3) | |
| Child B | 19 (47.5) | 21 (35.6) | 22 (43.1) | |
| Radiological tumor number (n, %) | 0.136 | |||
| 1 | 28 (70.0) | 33 (55.9) | 40 (78.4) | |
| 2 | 8 (20.0) | 19 (32.2) | 9 (17.6) | |
| 3 | 4 (10.0) | 7 (11.9) | 2 (3.9) | |
| Radiological tumor size (cm) | 2.8 (1.1‐5.7) | 2.2 (0.8‐6.3) | 2.6 (0.7‐5.1) | 0.072 |
| Radiological within Milan (n, %) | 33 (82.5) | 52 (88.1) | 48 (94.1) | 0.491 |
| AFP at listing (ng/mL) | 9.5 (2‐11,772) | 19.0 (2‐7,320) | 15.0 (1‐159,370) | 0.405 |
| AFP at listing (n, %) | 0.141 | |||
| <200 ng/mL | 34 (85.0) | 43 (72.9) | 41 (80.4) | |
| ≥200 ng/mL | 6 (15.0) | 16 (27.1) | 10 (19.6) |
Variables were presented as median (range) or number (percentage).
Abbreviation: BMI, body mass index.
Bridging Therapy With SBRT/TACE/HIFU
The dosimetry details of SBRT are summarized in Table 2. There were 56 SBRT treated HCC in 40 patients. The median dose delivered was 50 Gys in 5 fractions. All patients completed SBRT without premature termination. The median number of TACE performed per patient was 3 (range, 1‐9). In patients with HIFU, 5 required artificial ascites/pleural effusion, and none had ≥grade 3 perioperative complication.
TABLE 2.
SBRT Dose Summary
| Median Dose (Gy) | 50 (35‐50) |
| Median number of fractions | 5 |
| Technique (n, %) | |
| Gating | 23 (57.5) |
| 4D free breathing | 8 (20) |
| Free breathing | 2 (5) |
| ABC (BH) | 7 (17.5) |
| Number of lesion treated | |
| 1 | 28 (70) |
| 2 | 8( 20) |
| 3 | 4 (10) |
| Tumor size (cm) | 2.80 (1‐5.7) |
| Liver volume (mL) | 969.03 (625.45‐1,734.81) |
| Liver volume minus GTV (mL) | 955.06 (622.62‐1,646.25) |
| GTV volume (mL) | 11.11 (0.53‐136.8) |
| Total PTV volume (mL) | 61.58 (9.96‐199.04) |
| Dose to 95% of the planning target volume (Gy) | 50.06 (36.20‐103.41) |
| Liver minus GTV mean dose (Gy) | 14.94 (6.92‐61.25) |
| D800cc liver minus GTV median (Gy) | 1.50 (0.16‐16.94) |
| Liver minus GTV (Veff) | 814.66 (118.20‐1,646.25) |
Variables were presented as median (range) or number (percentage).
Abbreviations: ABC, active breathing control; BH, breath hold; GTV, gross tumor volume; PTV, planning target volume.
Evaluation of Treatment Response After Bridging Therapy
The mRECIST among the SBRT, TACE, and HIFU groups at 3, 6, 9, and 12 months after bridging therapy are shown in Fig. 2A. The primary endpoint of tumor control rate at 1 year was the highest after SBRT compared with TACE and HIFU (92.3% vs. 43.5% vs. 33.3%, respectively; P = 0.02). In fact, tumor control rate was consistently better after SBRT when compared with TACE and HIFU at every time point within the first year after bridging therapy: 3 months (91.7% vs. 69.6% vs. 72.3%, respectively; P = 0.025), 6 months (96% vs. 67.3% vs. 59.5%, respectively; P = 0.029), and 9 months (90% vs. 50% vs. 51.9%, respectively; P = 0.009).
FIG. 2.
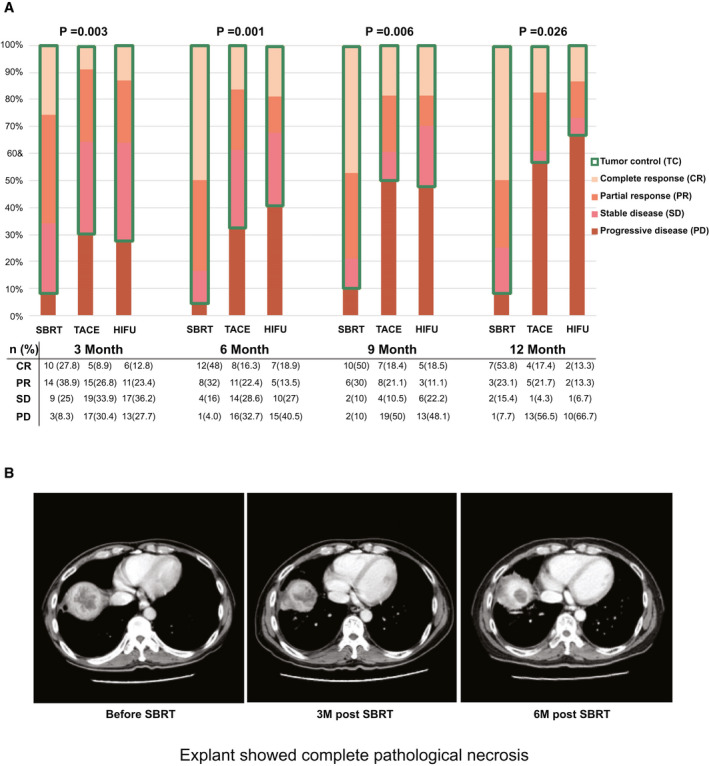
(A) The mRECIST among the SBRT, TACE, and HIFU groups. (B) CT images before, 3 months after, and 6 months after SBRT.
The rate of radiological CR was the highest after SBRT at 3, 6, 9, and 12 months when compared with TACE and HIFU. Except at 3 months, radiological CR was observed in approximately half of the patients with SBRT (48%‐53.8%), and it was significantly better when compared with TACE or HIFU (Fig. 2A). The objective response was significantly higher in the SBRT group at 3 to 12 months after bridging therapy (Supporting Table S1). An example of radiological response after SBRT is illustrated in Fig. 2B.
Need for Additional Bridging Therapy
Two patients in the SBRT group required additional bridging therapy. The first patient had a new HCC focus that was found 3 months after the first SBRT. Because of the short time interval from the first SBRT, he had one cycle of TACE and received SBRT again at 7 months after the first SBRT. The second patient also had PD due to development of a new HCC focus and had the second SBRT 9 months afterward. Fifteen patients with HIFU received TACE (3 due to PD, 5 due to PR, and 7 due to SD) at a median of 4 months (range, 3‐22). Eleven patients with TACE received HIFU (5 due to PD, 1 due to PR, and 6 due to SD) at a median of 5 months (range, 2‐17.5).
Dropout Risk
The median follow‐up time for the whole cohort, SBRT group, TACE group, and HIFU group from the time of listing was 42 months (range, 0.5‐137.7), 39.3 months (range, 2.8‐100.4), 47.8 months (range, 1.5‐133.7), and 48.7 months (range, 0.5‐129.1), respectively (P = 0.073 for comparison between the 3 groups). The cumulative incidence of dropout with competing risk analysis for the SBRT group was 15.1%, 20.5%, and 23.3% at 1, 2, and 3 years, respectively, after listing, and the incidence was significantly lower than for those in the TACE group (28.9%, 35.6%, and 45.8%, respectively; P = 0.034) and the HIFU group (33.3%, 41.2%, and 45.1%, respectively; P = 0.032) (Fig. 3A).
FIG. 3.
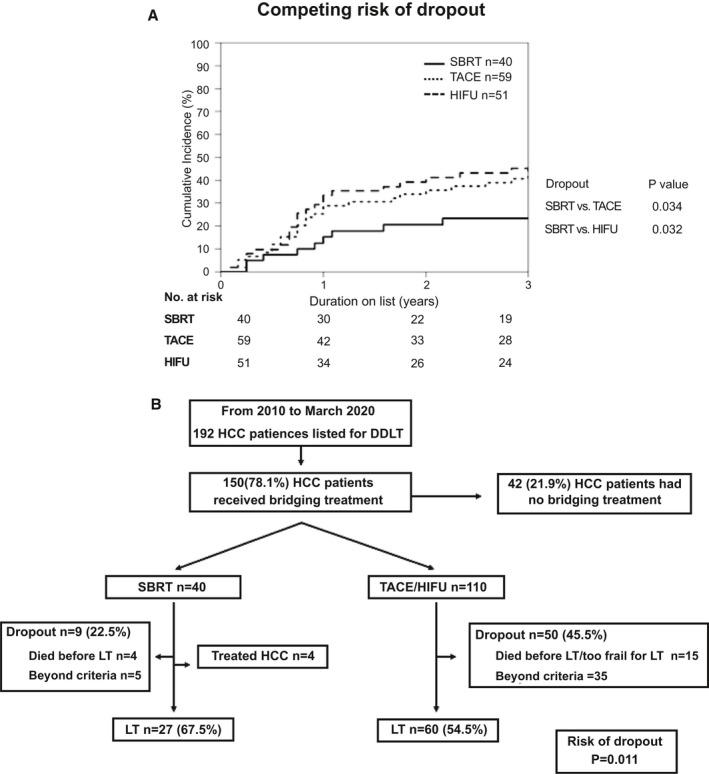
(A) Cumulative incidence of waitlist dropout among the SBRT, TACE, and HIFU groups. The cumulative incidence of dropout was estimated while accounting for competing risk. (B) Waitlist outcomes of all patients.
At time of study analysis, the overall dropout risk was lower in the SBRT group compared with patients with TACE and HIFU (9/40 [22.5%] vs. 50/110 [45.5%]; P = 0.011) (Fig. 3B). Four patients in the SBRT group fulfilled the criteria for treated HCC and were delisted. At the time of last follow‐up, none had HCC recurrence after a median follow‐up period of 32 months (range, 26‐44). On the other hand, no patients in the TACE or HIFU groups fulfilled the criteria for treated HCC. Among the 9 patients delisted from the SBRT group, 5 were due to tumor progression and 4 died on waitlist because of ruptured cerebral aneurysm, spontaneous bacterial peritonitis, necrotizing fasciitis of lower limb, and hepatic encephalopathy at 45, 335, 357, and 609 days after SBRT, respectively. Tumor progression (70%) was the predominate reason accounted for delisting in the TACE/HIFU group. Fifteen patients died on waitlist or were too frail for LT. Of these, 9 had complications of liver cirrhosis, 2 had cerebrovascular accident, 2 were deemed too frail for LT, 1 had perforated peptic ulcer, and 1 died of unknown cause.
Safety and Adverse Effects After Bridging Therapy
There was no 30‐day mortality after bridging therapy in all 3 groups, and no patient required urgent transplant for acute decompensation. Fever, fatigue, and creatinine were similar after SBRT, TACE, and HIFU. Grade 2 toxicity in bilirubin was more commonly observed in the SBRT and HIFU group, and hematological derangement including thrombocytopenia and leucopenia was more commonly seen after SBRT, but none was associated with clinically adverse events. The need for readmission within 30 days after bridging therapy was also similar between the 3 groups (Supporting Tables S2 and S3).
Outcomes After Transplant and Explant Analysis
The median follow‐up time for the whole cohort, SBRT group, TACE group, and HIFU group from the time of transplant was 60.6 months (range, 0.6‐124.5), 40.6 months (range, 0.9‐60.3), 71.6 months (range, 8.3‐124.5), and 80.5 months (range, 0.6‐118), respectively; P < 0.001. Twenty‐seven (67.5%), 32 (54.2%), and 28 (54.9%) patients in the SBRT, TACE, and HIFU groups underwent DDLT, respectively. At the time of transplant, there was no difference in tumor stage, size, number, and AFP level, although patients with TACE had lower median MELD score (10 vs. 12 in SBRT and HIFU, P = 0.001). There was no difference in hospital mortality, severe perioperative complication rate, vascular and biliary complication, and hospital and intensive care unit stay among the 3 groups (Table 3). For patients who received SBRT, there was no perihilar or peritumoral adhesion reported from the operating surgeons.
TABLE 3.
Characteristics of All Patients Who Have Undergone Transplantation
| At Transplant | SBRT (n = 27) | TACE (n = 32) | HIFU (n = 28) | P Value |
|---|---|---|---|---|
| MELD at transplant | 12 (8‐33) | 10 (8‐12) | 12 (11‐15) | 0.001 |
| AFP at transplant (ng/mL) | 6 (3‐339) | 7.5 (1‐1,286) | 12 (2‐21,984) | 0.177 |
| Radiological tumor number | 1.0 (0‐3) | 1.0 (0‐3) | 1.0 (0‐3) | 0.167 |
| Radiological tumor size (cm) | 2.0 (0‐5.7) | 1.6 (0‐5.8) | 2.1 (0‐4.3) | 0.214 |
| Radiological within Milan (n, %) | 25 (92.6) | 29 (90.6) | 26 (92.9) | 0.941 |
| Time on waiting list (days) | 421 (89‐944) | 255.5 (21‐1,280) | 241 (1‐1,909) | 0.21 |
| Perioperative outcomes | ||||
| Hospital mortality (n, %) | 1 (3.8) | 0 (0) | 1 (3.6) | 0.527 |
| Postoperative complication ≥Clavien Grade 3a* (n, %) | 7 (26.9) | 4 (12.5) | 8 (28.6) | 0.253 |
| Overall complication rate (n, %) | 18 (69.2) | 16 (50.0) | 19 (67.9) | 0.232 |
| Vascular thrombosis (n, %) | 1 (3.7) | 0 (0) | 0 (0) | 0.106 |
| Early biliary leak/ stricture (n, %) | 0 (0) | 0 (0) | 0 (0) | — |
| Blood loss (L) | 3.0 (0.75‐15.0) | 3.0 (0.5‐11.7) | 3.9 (0.7‐20.0) | 0.806 |
| Intraoperative packed cell transfusion (units) | 6.0 (0‐21) | 5.0 (0‐29) | 4.5 (0‐31) | 0.91 |
| Intraoperative FFP transfusion (units) | 5.0 (0‐21) | 4.0 (0‐17) | 6.0 (0‐17) | 0.554 |
| Intraoperative platelet transfusion (units) | 6.0 (0‐28) | 4.0 (0‐16) | 8.0 (0‐24) | 0.313 |
| ICU stay (days) | 3.0 (2‐52) | 3.0 (2‐7) | 3.0 (2‐32) | 0.611 |
| Hospital stay (days) | 16.0 (10‐174) | 15.0 (10‐69) | 15.0 (8‐132) | 0.79 |
| Explant pathological analysis | ||||
| Pathological tumor number | 1.0 (0‐multiple) | 2.0 (0‐multiple) | 1.0 (0‐multiple) | 0.666 |
| Pathological tumor size (cm) | 2.0 (0.8‐4.5) | 2.5 (0.3‐6.3) | 2.5 (0.3‐6.3) | 0.045 |
| Pathological complete necrosis (n, %) | 13 (48.1) | 8 (25.0) | 5 (17.9) | 0.037 |
| Tumor differentiation (n, %) † | 0.361 | |||
| Well differentiated | 2 (7.7) | 2 (6.3) | 3 (10.7) | |
| Moderately differentiated | 12 (46.2) | 21 (65.6) | 19 (67.9) | |
| Poorly differentiated | 3 (11.5) | 1 (3.1) | 1 (3.6) | |
| Vascular invasion (n, %) † | ||||
| Microvascular | 6 (22.2) | 8 (25.0) | 7 (25.0) | 0.079 |
| Macrovascular | 1 (3.7) | 0 (0) | 1 (3.6) | 0.529 |
| Pathological within Milan (n, %) | 22 (81.5) | 24 (75.0) | 21 (75.0) | 0.442 |
Variables were presented as median (range) or number (percentage).
Clavien‐Dindo classification.
Pathological details were not available in patients who had complete necrosis.
Abbreviations: FFP, fresh frozen plasma; ICU, intensive care unit.
In explant pathological analysis, there was no difference in tumor number, differentiation, and vascular invasion among the 3 groups. Although patients with SBRT had the largest radiological tumor size at the time of listing, they had smaller tumors (2 cm vs. 2.5 cm in the TACE and HIFU groups; P = 0.045) and a higher rate of pCR (48.1% in the SBRT group vs. 25% in the TACE group vs. 17.9% in the HIFU group; P = 0.037) (Table 3).
Survival and Recurrence
Patients who underwent SBRT as bridging therapy had a better TTP on the waitlist. The TTP at 1, 2, and 3 years from the time of listing was significantly lower in the SBRT group (10.8%, 18.5%, and 18.5% in the SBRT group, 45%, 50.6%, and 54.9% in the TACE group, and 47.6%, 62.8%, and 62.8% in the HIFU group; P < 0.001), as shown in Fig. 4A. Because some patients had bridging therapy before listing, the TTP from the time of first bridging therapy was also performed, the benefits of SBRT in reducing the TTP remained unchanged (Supporting Fig. S1). The 1, 2, and 3‐year OS rates from the time of listing were 84.9%, 76.4%, and 73%, respectively, in the SBRT group, 88.1%, 72.7%, and 65.6%, respectively, in the TACE group, and 80.4%, 60.8%, and 54.9%, respectively, in the HIFU group (P = 0.295) (Fig. 4A).
FIG. 4.
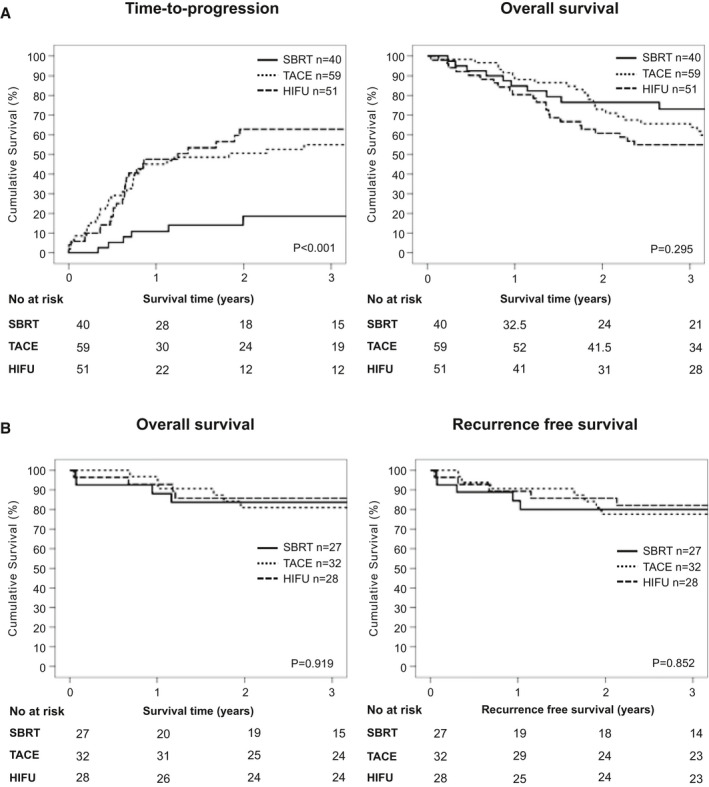
(A) TTP and OS from the time of listing among the SBRT, TACE, and HIFU groups. (B) Overall and recurrence‐free survival after transplant among the SBRT, TACE, and HIFU groups.
The survival and recurrence rates after transplant were similar between the 3 groups. The OS at 1, 2, and 3 years was 88.2%, 83.8% and 83.8%, respectively, in the SBRT group, 96.9%, 80.9%, and 80.9%, respectively, in the TACE group, and 92.9%, 85.7%, and 85.7%, respectively, in the HIFU group (P = 0.919). The recurrence‐free survival rates at 1, 2, and 3 years were 84.4%, 80%, and 80%, respectively, in the SBRT group, 90.6%, 77.7%, and 77.7%, respectively, in the TACE group, and 89.3%, 85.7%, and 82.1%, respectively, in the HIFU group (P = 0.852; Fig. 4B).
Only 18/150 (12%) patients had AFP >1,000 at time of listing. Based on the data from published series, AFP ≥200 ng/mL was used in multivariable analyses.( 32 , 33 , 34 ) In multivariable analyses, tumor size >3 cm, AFP ≥200 ng/mL at listing, and Child B or C predicted a higher risk of dropout, whereas SBRT reduced the risk of dropout (Table 4).
TABLE 4.
Univariable and Multivariable Analyses of Prognostic Factors Affecting Dropout
| Variables | Dropout | |||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (n = 150) | 1.003 (0.963‐1.046) | 0.87 | ||
| Sex (n = 150) | ||||
| Female (n = 33) | Ref | |||
| Male (n = 117) | 1.774 (0.870‐3.618) | 0.115 | ||
| BMI (kg/m2) (n = 150) | 1.010 (0.954‐1.068) | 0.74 | ||
| Disease etiology (n = 150) | ||||
| Non‐HBV (n = 30) | Ref | |||
| HBV (n = 120) | 1.430 (0.735‐2.783) | 0.292 | ||
| Tumor number at listing | ||||
| 1 (n = 101) | Ref | |||
| ≥2 (n = 49) | 1.644 (0.966‐2.799) | 0.067 | ||
| Size of the largest tumor at listing (cm) (n = 150) | ||||
| <3 cm (n = 95) | ||||
| ≥3 cm (n = 55) | Ref | Ref | ||
| 1.978 (1.174‐3.333) | 0.01 | 2.031 (1.183‐3.486) | 0.01 | |
| AFP at listing (n = 150) | ||||
| <200 ng/mL (n = 118) | Ref | Ref | ||
| ≥200 ng/mL (n = 32) | 1.862 (1.042‐3.327) | 0.036 | 2.489 (1.293‐4.790) | 0.006 |
| Child grade at listing (n = 150) | ||||
| Grade A (n = 74) | Ref | Ref | ||
| Grade B/C (n = 76) | 1.939 (1.135‐3.314) | 0.015 | 2.561 (1.405‐4.668) | 0.002 |
| Bridging treatment (n = 150) | ||||
| SBRT (n = 40) | Ref | Ref | ||
| HIFU (n = 51) | 2.485 (1.144‐5.398) | 0.022 | 3.026 (1.369‐6.686) | 0.006 |
| TACE (n = 59) | 2.006 (0.932‐4.319) | 0.075 | 2.324 (1.067‐5.061) | 0.034 |
Abbreviation: BMI, body mass index.
Sensitivity Analysis
Sensitivity analysis was performed by considering events up to the median follow‐up time of the SBRT group. TTP and OS from the time of listing was significantly better in the SBRT group and was consistent with the primary analysis (Supporting Fig. S2). Patients who were eligible for all three bridging therapies were also analyzed in a separate sensitivity analysis. The clinical characteristics are listed in Supporting Table S4. The results were similar to the primary analysis. The mRECIST and tumor control from 3 to 12 months after bridging therapies are shown in Supporting Fig. S3. Patients who received SBRT had better TTP when compared with HIFU and TACE, whereas the OS was comparable (Supporting Fig. S4). With competing risk analysis, the SBRT group had a lower incidence of dropout, as shown in Supporting Fig. S5.
Discussion
This study investigates the use of SBRT as bridging therapy for candidates with HCC on the LT waitlist. In the present study, SBRT showed a better radiological tumor control when compared with other bridging therapies at every time point after treatment. The use of SBRT was associated with a lower risk of dropout and with a higher rate of pCR in explant histopathology when compared with TACE and HIFU. The findings add to the growing evidence that SBRT is safe and effective and can be used as an alternative bridging therapy for candidates with HCC.
An ideal bridging therapy should be effective, noninvasive, and safe for candidates with HCC. The data from the present study showed that SBRT is close to, if not an ideal bridging therapy. SBRT is completely noninvasive without an increased risk of periprocedural toxicities, RILD, and perioperative complications. Most importantly, SBRT was shown to be very effective. The high dose radiation given in divided doses achieved a “radio‐ablative” effect. Objective response was achieved in 63.9%‐80% patients with SBRT comparing with 26.7%‐39.5% in patients who had other conventional bridging therapies. Within the first year of SBRT, tumor control was maintained at 90%. This was in great contrast to patients with TACE or HIFU in whom the effect of tumor control reduced with time: 50%‐51.9% at 9 months to 33.3%‐43.5% at 12 months. SBRT was performed as an outpatient procedure, and most patients did not need additional bridging therapy. This was in contrast to TACE and HIFU, wherein both required hospitalization with patients often require additional bridging therapies. Early economic analysis suggested that SBRT might cost less than TACE as bridging therapy.( 35 ) Importantly, 4 patients were removed from the waitlist because of treated HCC with no recurrence to date. Such phenomenon was not observed in patients in the TACE or HIFU groups. For those who received DDLT, the pCR rate was 48.1%, and pathological tumor size was significantly smaller in the SBRT group despite a larger tumor size at listing. These findings shed insights on the effectiveness of SBRT as “radio‐ablative,” which may be curative for selected patients with HCC. Although RFA is used as bridging therapy in many centers, this modality is reserved as curative treatment for patients with HCC not requiring LT.( 24 ) Data from a randomized controlled trial at our center showed that for HCC within the Milan criteria, RFA offered similar long‐term outcomes to liver resection. If the HCC was treated by RFA, then the patient would not be assessed for LT in the first instance. In the present study, poor liver function, large tumor size, and TACE or HIFU as bridging therapy were predictors for dropout. Patients who had ascites received HIFU as bridging therapy, and the underlying more advanced liver disease might contribute to a higher dropout rate in the HIFU group.
The cumulative risk of dropout was significantly lower in the SBRT group, and in sensitivity analysis, the beneficial effect of SBRT to TTP remained unchanged. Despite the use of retrospective control, patients with HCC included in the study were homogenous because the selection criteria, MELD exception point system, and availability of deceased organs in our center remained unchanged since 2010. The posttransplant overall and recurrence‐free survival rates were similar after SBRT compared with other bridging therapies. However, given the higher rate of tumor control on waitlist and a higher pCR in explant, SBRT might lead to better posttransplant survival in the long term.( 6 , 36 , 37 ) The sample size and follow‐up time of our study were not powered to test this hypothesis, but it will be of interest to follow up the long‐term survival and recurrence in these patients. Furthermore, tumor control was seen in 90% of patients with SBRT within the first year, and such an effect was sustained, suggesting that the use of SBRT as downstage therapy should be recommended, given tumor progression accounts for the majority of dropout in downstage protocols.( 38 ) At the time when this study protocol was drafted, only a few small case series on SBRT as a bridge to LT were available, and data on dropout rate were lacking.( 18 , 39 , 40 ) Therefore, tumor control rate at 1 year was taken as the primary endpoint based on the prospective studies on SBRT for nonresectable HCC. The mRECIST after bridging therapies were reported every 3 months in the first year after listing; therefore, most patients would have reached study endpoint, i.e., transplant, delist, or death. The findings in the present study serve as a call for a multicenter randomized controlled trial because a standardized algorithm to guide the choice of bridging therapy for candidates with HCC is needed. Also, the best criteria to assess treatment response after bridging treatments remain controversial. The mRECIST was used in this study, but a recent paper suggested that the Response Evaluation Criteria in Solid Tumors (RECIST) might be more closely correlated with pathological necrosis.( 41 ) The prediction of pathological necrosis by the RECIST and mRECIST is beyond the scope of the present study, but it is certainly an important question that warrants a detailed evaluation. Anonymization of radiologists was not feasible because of the use of lipiodol in TACE, and patients who had gross ascites were offered HIFU. Nonetheless, crucial clinical decisions including MELD exception point and delisting were made based on radiology reports, and all reports were reviewed by at least two radiologists; therefore, the chance of bias was minimal.
In our study, 7 patients (14.9%) had screening failure for SBRT because of the mean liver dose exceeding the dose acceptance criteria, position discrepancy, and radiation dose constraints to OAR. Because SBRT involves high dose radiation, precision is crucial, and the radiation directed to target HCC must be within a 2 mm discrepancy between planning and treatment CT. Therefore, in our experience, patients with significant ascites or pleural effusion are unsuitable for SBRT. As with all forms of radiation, marrow suppression is a potential side effect. Pancytopenia is extremely common in LT candidates. In the present study, a lower platelet cutoff of 20 × 109/L was adopted, which was much lower than the conventional SBRT protocol of 50‐70 × 109/L.( 25 ) This may explain the higher grade 3/4 toxicity in thrombocytopenia and leucopenia among the patients with SBRT, but all patients recovered spontaneously without any clinically significant event.
Our study has several limitations. Firstly, this was a nonrandomized trial in a single center. The nonrandomized nature and the use of retrospective control might lead to bias. Nevertheless, all patients with SBRT were enrolled under a standardized protocol, and the techniques for TACE and HIFU were also standardized. In addition, there was no change in HCC selection, MELD exception system, tumor response evaluation, and organ availability within the study period, and therefore the chance of bias would be minimal. Secondly, the effect of SBRT was compared with both TACE and HIFU, but HIFU is not widely available worldwide.( 11 ) Nonetheless, the mRECIST at 3, 6, 9, and 12 months were similar, and the TTP curves overlapped in patients with TACE and HIFU, suggesting a similar tumor control ability. Lastly, the dropout rate in our study was high, as it was conducted in a region with a low organ donation rate. Our recent paper showed that the dropout rate was as high as 43.4%, even for candidates with high MELD and hepatorenal syndrome.( 42 ) The limited availability of deceased organs stresses the importance of an effective bridging therapy.
In conclusion, SBRT was safe and effective as bridging therapy in waitlisted patients with HCC. Compared with other bridging therapies, SBRT reduced the risk of dropout, offered a better tumor control rate at 1 year, and achieved a higher rate of pCR in explant histopathology. Therefore, SBRT should be incorporated in the current bridging therapy algorithm, in addition to TACE and HIFU, as a safe and effective alternative for candidates with HCC.
Author Contributions
T.C.L.W. was responsible for concept and design of study, data collection, drafting of manuscript, statistical analysis, and final review of the paper. V.H.F.L., A.L.Y.L., H.H.P., V.L., K.O.L., T.Y.C., A.S.Y.F., S.W.M.L., E.C.Y.W., J.W.C.D., A.C.Y.C., T.T.C., J.Y.Y.F., M.Y.L., R.M.W.Y., T.W.L., and C.M.L. were responsible for study design, data collection, statistical analysis, and critical review of manuscript.
Supporting information
Supplementary Material
Supported by General Research Fund (GRF 17149816) and Theme‐based Research Scheme (T12‐703‐19R) of the Research Grant Council, Hong Kong.
Submission declaration: This study was presented in Rising Star Symposium at the ILTS 25th Annual International Congress 2019 and as an oral abstract at the ILTS 27th Annual International Congress in 2021.
Potential conflict of interest: Dr. Pang is employed by Genentech and owns stock in Roche.
Clinical trial number: NCT03950102.
References
- 1. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 2. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394‐1403. [DOI] [PubMed] [Google Scholar]
- 3. Organ Procurement and Transplantation Network . Aligning OPTN policies with the 2013 PHS guideline for reducing transmission of HIV, HBV, and HCV through solid organ transplantation. https://optn.transplant.hrsa.gov/media/1140/policy_notice_12‐2014.pdf. Published March 2014. Accessed May 31, 2020.
- 4. Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11‐e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agopian VG, Harlander‐Locke MP, Ruiz RM, Klintmalm GB, Senguttuvan S, Florman SS, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US Multicenter HCC Transplant Consortium. Ann Surg 2017;266:525‐535. [DOI] [PubMed] [Google Scholar]
- 7. Kardashian A, Florman SS, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, et al. Liver transplantation outcomes in a U.S. multicenter cohort of 789 patients with hepatocellular carcinoma presenting beyond Milan criteria. Hepatology 2020;72:2014‐2028. [DOI] [PubMed] [Google Scholar]
- 8. Pompili M, Francica G, Ponziani F, Iezzi R, Avolio A. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol 2013;19:7515‐7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panaro F, Ramos J, Gallix B, Mercier G, Herrero A, Niampa H, et al. Hepatic artery complications following liver transplantation. Does preoperative chemoembolization impact the postoperative course? Clin Transplant 2014;28:598‐605. [DOI] [PubMed] [Google Scholar]
- 10. Goel A, Mehta N, Guy J, Fidelman N, Yao F, Roberts J, et al. Hepatic artery and biliary complications in liver transplant recipients undergoing pretransplant transarterial chemoembolization. Liver Transpl 2014;20:1221‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chok KSH, Cheung TT, Lo RCL, Chu FSK, Tsang SHY, Chan ACY, et al. Pilot study of high‐intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients wait‐listed for liver transplantation. Liver Transpl 2014;20:912‐921. [DOI] [PubMed] [Google Scholar]
- 12. Cheung TT, Fan ST, Chan SC, Chok KS, Chu FS, Jenkins CR, et al. High‐intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol 2013;19:3083‐3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong TCL, Chiang CL, Lee AS, Lee VHF, Yeung CSY, Ho CHM, et al. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: a propensity score matched analysis. Surg Oncol 2019;28:228‐235. [DOI] [PubMed] [Google Scholar]
- 14. Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218‐225. [DOI] [PubMed] [Google Scholar]
- 15. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447‐e453. [DOI] [PubMed] [Google Scholar]
- 16. Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012;118:5424‐5431. [DOI] [PubMed] [Google Scholar]
- 17. Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RKS, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631‐1639. [DOI] [PubMed] [Google Scholar]
- 18. Katz A, Chawla S, Qu Z, Kashyap R, Milano M, Hezel A. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys 2012;83:895‐900. [DOI] [PubMed] [Google Scholar]
- 19. Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention‐to‐treat analysis. J Hepatol 2017;67:92‐99. [DOI] [PubMed] [Google Scholar]
- 20. Gresswell S, Tobillo R, Hasan S, Uemura T, Machado L, Thai N, et al. Stereotactic body radiotherapy used as a bridge to liver transplant in patients with hepatocellular carcinoma and Child‐Pugh score >/=8 cirrhosis. J Radiosurg SBRT 2018;5:261‐267. [PMC free article] [PubMed] [Google Scholar]
- 21. Lencioni R, Llovet J. Modified RECIST(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona‐2000 EASL Conference. J Hepatol 2001;35:421‐430. [DOI] [PubMed] [Google Scholar]
- 23. Chan SC, Sharr WW, Chok KSH, Chan ACY, Lo CM. Wait and transplant for stage 2 hepatocellular carcinoma with deceased‐donor liver grafts. Transplantation 2013;96:995‐999. [DOI] [PubMed] [Google Scholar]
- 24. Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early‐stage hepatocellular carcinoma. Br J Surg 2017;104:1775‐1784. [DOI] [PubMed] [Google Scholar]
- 25. Radiation Therapy Oncology Group RTOG 1112 protocol . https://www.nrgoncology.org/Clinical‐Trials/Protocol/rtog‐1112?filter=rtog‐1112. Published November 01, 2013. Accessed June 04, 2020.
- 26. Dawson LA, Eccles C, Craig T. Individualized image guided iso‐NTCP based liver cancer SBRT. Acta Oncol 2006;45:856‐864. [DOI] [PubMed] [Google Scholar]
- 27. Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell R, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980‐3986. [DOI] [PubMed] [Google Scholar]
- 28. Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation‐associated liver injury. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S94‐S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg 2009;250:187‐196. [DOI] [PubMed] [Google Scholar]
- 30. Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J 2004;4:103‐112. [Google Scholar]
- 31. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496‐509. [Google Scholar]
- 32. Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg 2017;265:557‐564. [DOI] [PubMed] [Google Scholar]
- 33. Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128‐139. [DOI] [PubMed] [Google Scholar]
- 34. An J, Na SK, Shim JH, Park YS, Jun MJ, Lee JH, et al. Histological expression of methionine adenosyl transferase (MAT) 2A as a post‐surgical prognostic surrogate in patients with hepatocellular carcinoma. J Surg Oncol 2018;117:892‐901. [DOI] [PubMed] [Google Scholar]
- 35. Nugent FW, Packard A, Hunter K, Qamar A, Stuart KE, Gunturu K, et al. Economic analysis of transarterial chemoembolization (TACE) versus stereotactic body radiotherapy (SBRT) for the treatment of hepatocellular carcinoma (HCC). J Clin Oncol 2018;36(4_suppl):508. [Google Scholar]
- 36. Lee DD, Samoylova M, Mehta N, Musto KR, Roberts JP, Yao FY, et al. The mRECIST classification provides insight into tumor biology for patients with hepatocellular carcinoma awaiting liver transplantation. Liver Transpl 2019;25:228‐241. [DOI] [PubMed] [Google Scholar]
- 37. Lai Q, Vitale A, Iesari S, Finkenstedt A, Mennini G, Onali S, et al. The intention‐to‐treat effect of bridging treatments in the setting of Milan criteria‐in patients waiting for liver transplantation. Liver Transpl 2019;25:1023‐1033. [DOI] [PubMed] [Google Scholar]
- 38. Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long‐term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int 2010;23:299‐306. [DOI] [PubMed] [Google Scholar]
- 40. O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long‐term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl 2012;18:949‐954. [DOI] [PubMed] [Google Scholar]
- 41. Mastrocostas K, Fischer S, Munoz‐Schuffenegger P, Jang HJ, Dawson LA, Liu ZA, et al. Radiological tumor response and histopathological correlation of hepatocellular carcinoma treated with stereotactic body radiation therapy as a bridge to liver transplantation. Abdom Radiol (NY) 2021;46:1572‐1585. [DOI] [PubMed] [Google Scholar]
- 42. Wong TC, Fung JY, Pang HH, Leung CK, Li HF, Sin SL, et al. Analysis of survival benefits of living versus deceased donor liver transplant in high model for end‐stage liver disease and hepatorenal syndrome. Hepatology 2021;74:2441–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


